Retbindin: A riboflavin Binding Protein, Is Critical for Photoreceptor Homeostasis and Survival in Models of Retinal Degeneration
Abstract
1. Introduction
2. Results
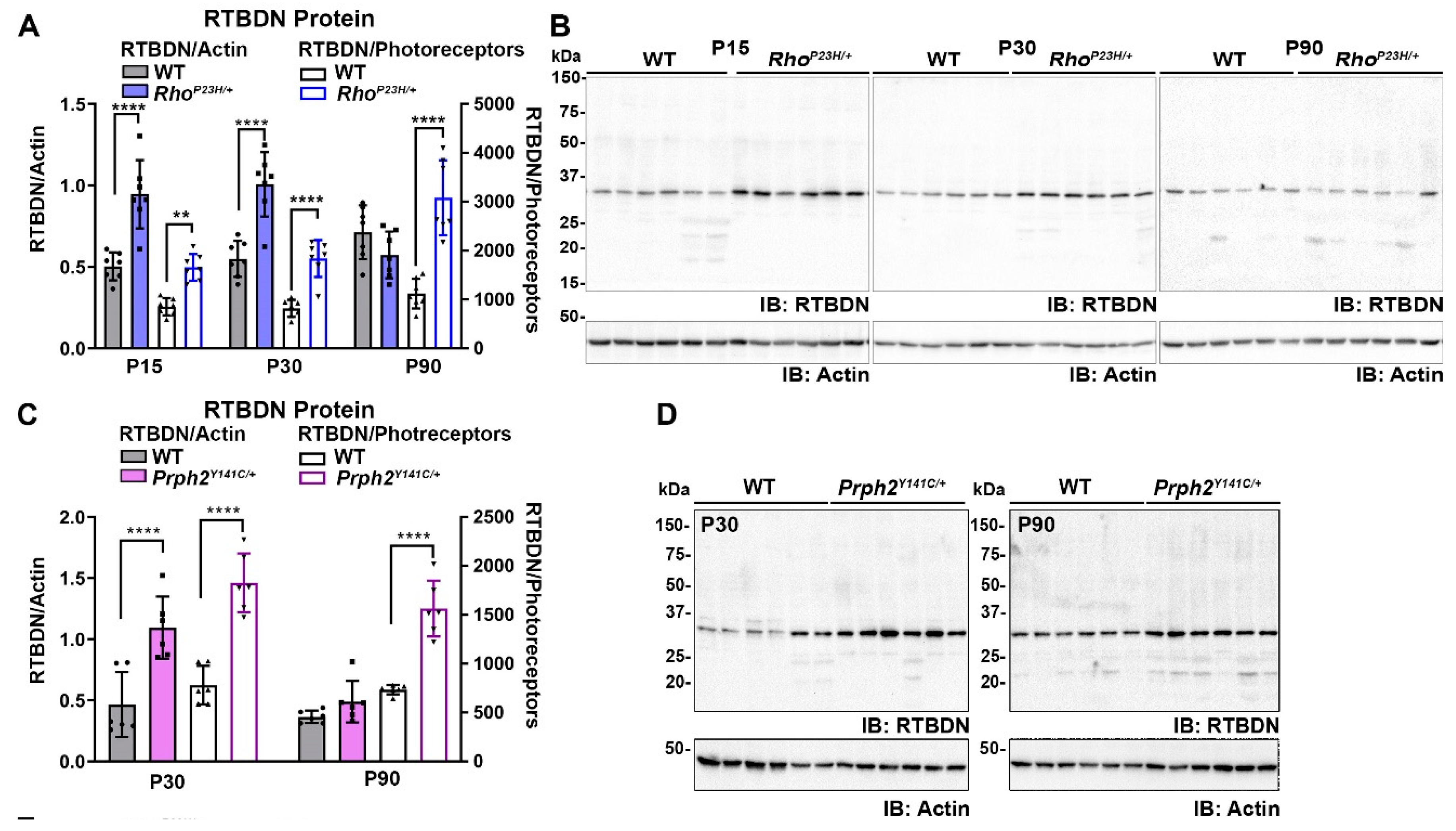
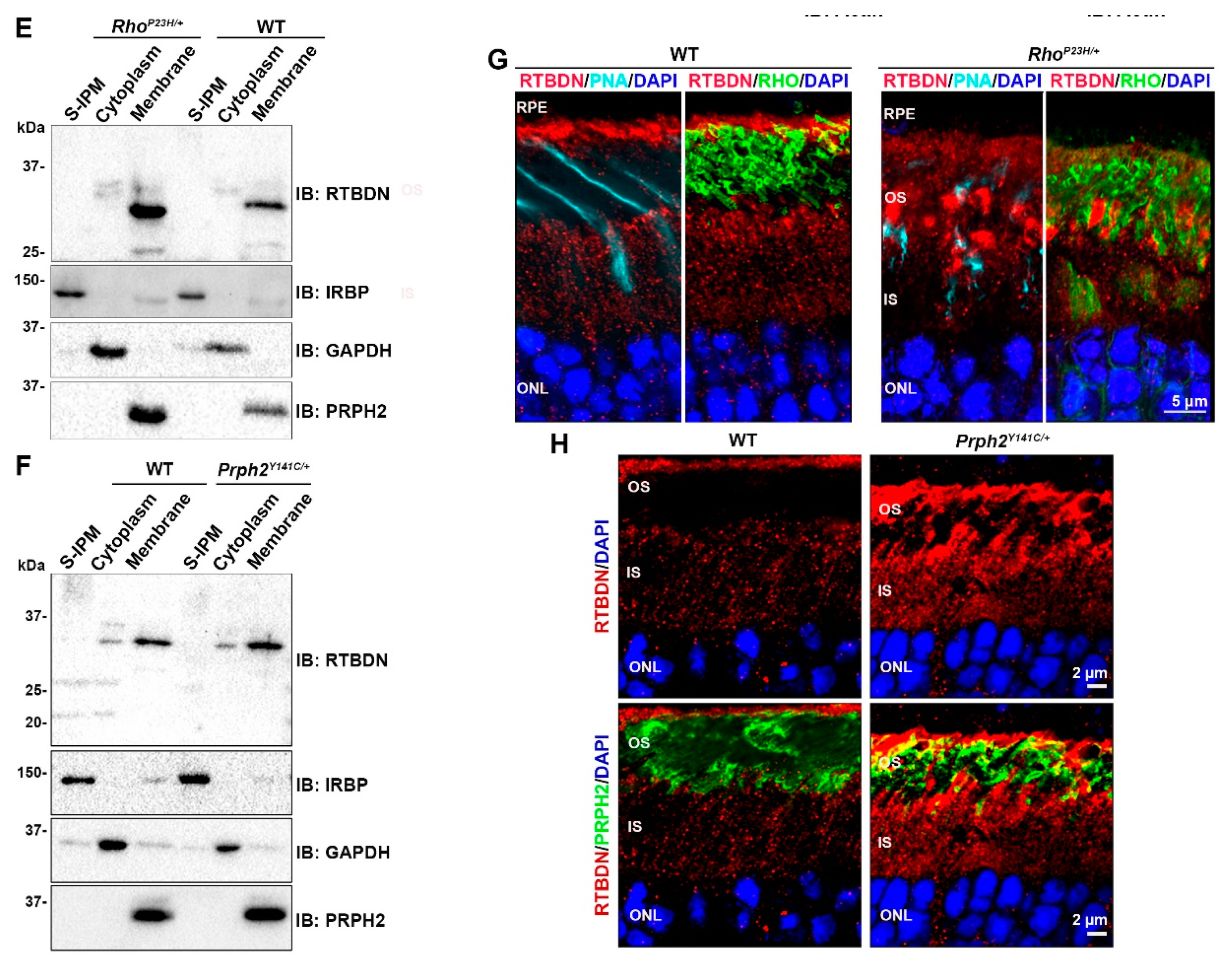
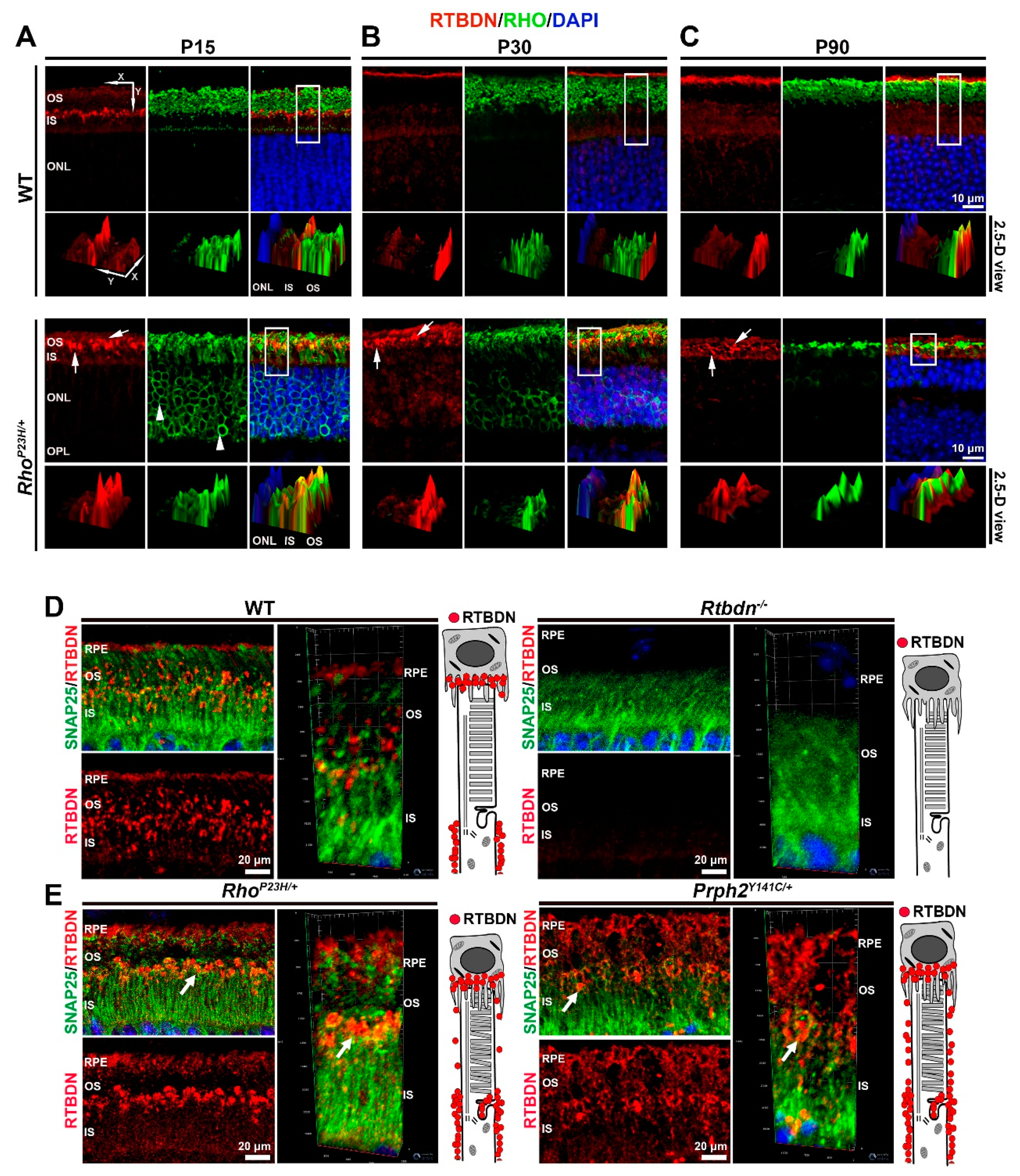
2.1. RTBDN Is Upregulated in the RhoP23H/+ and Prph2Y141C/+ Retina
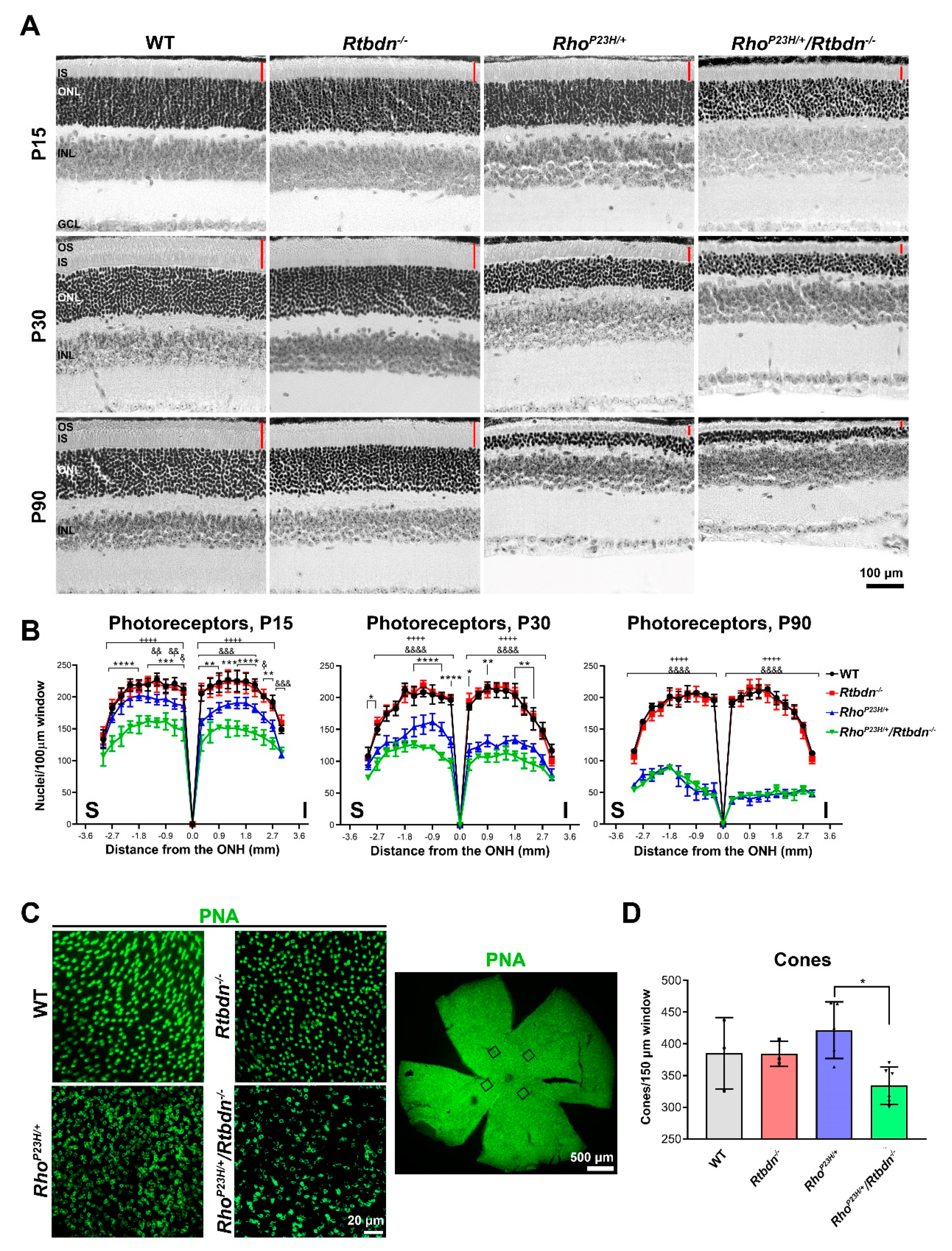
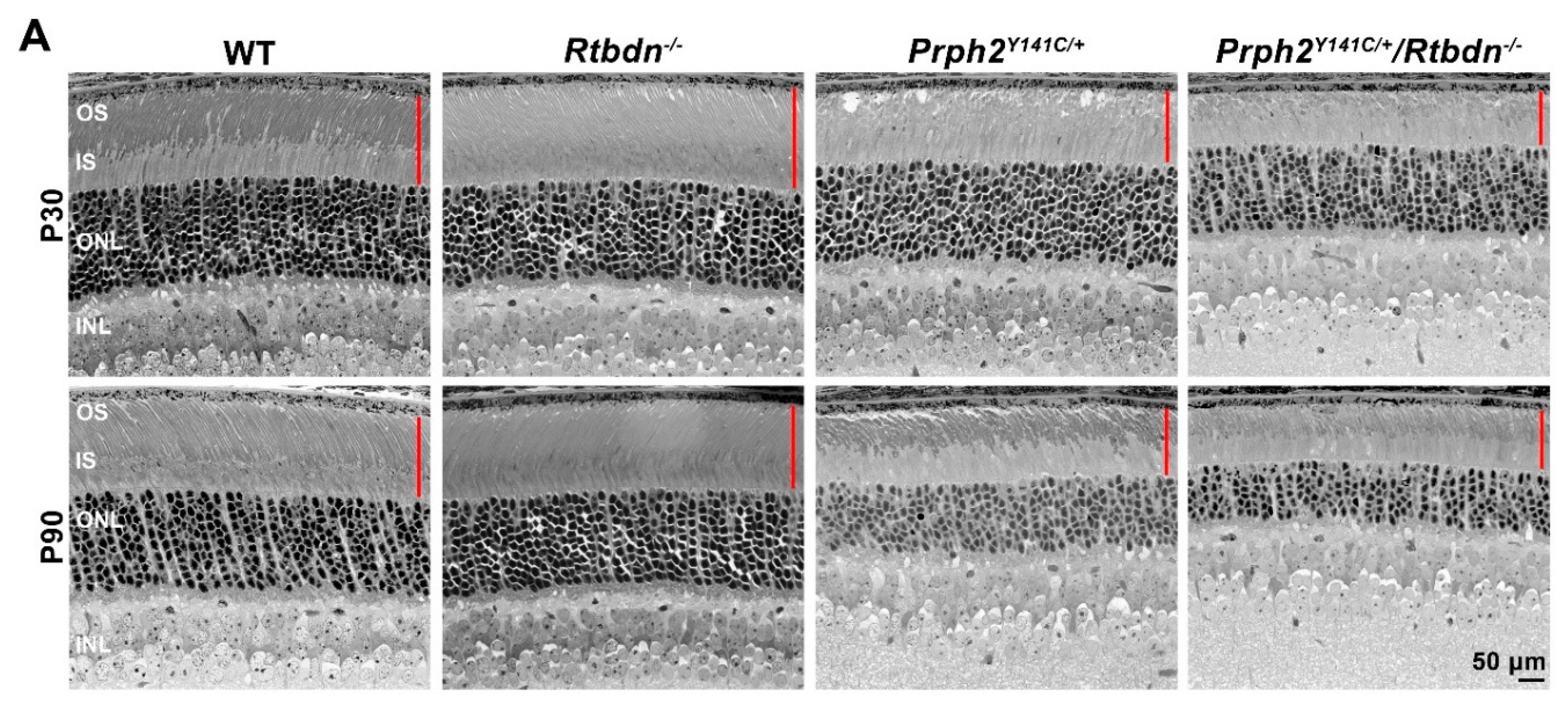
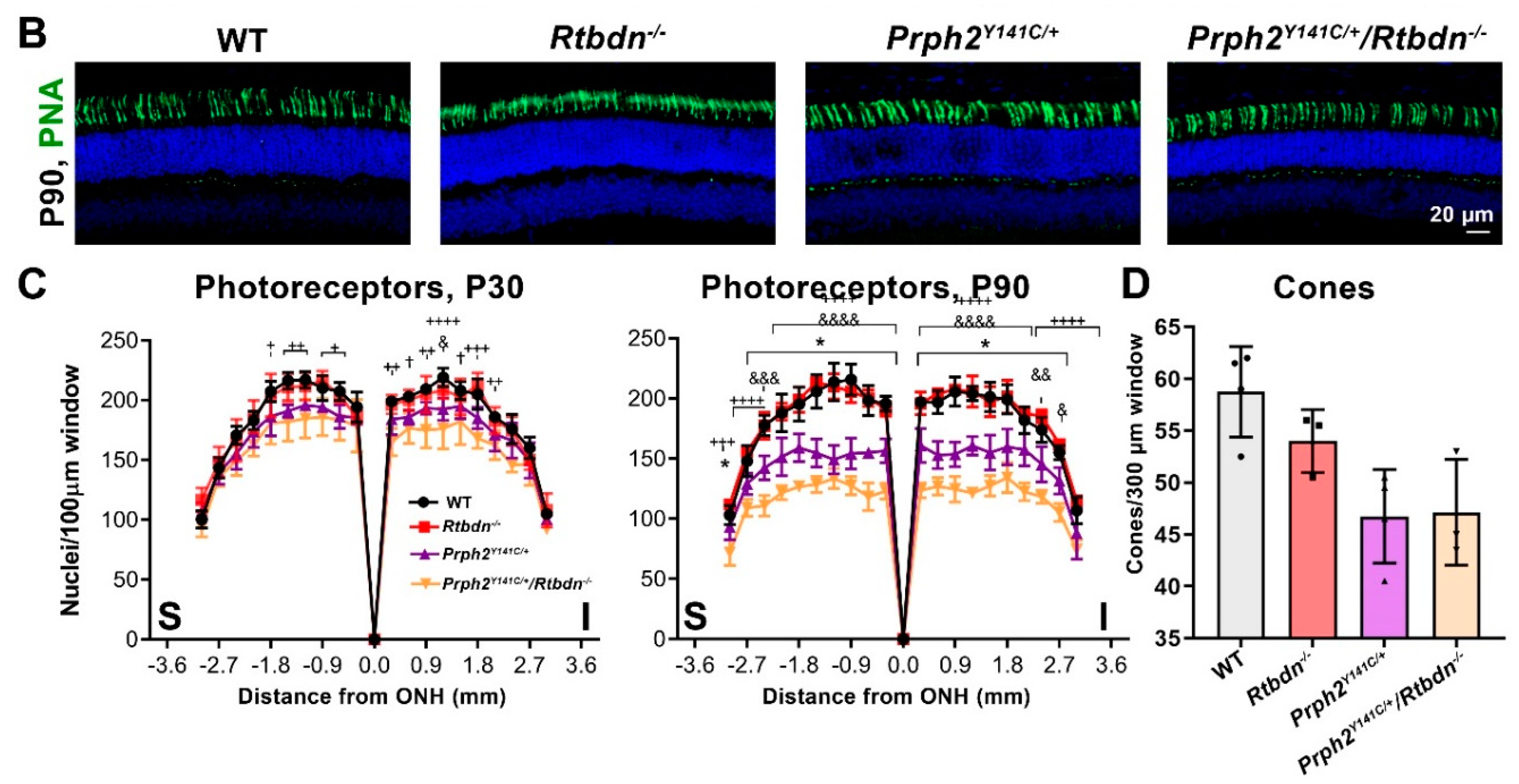
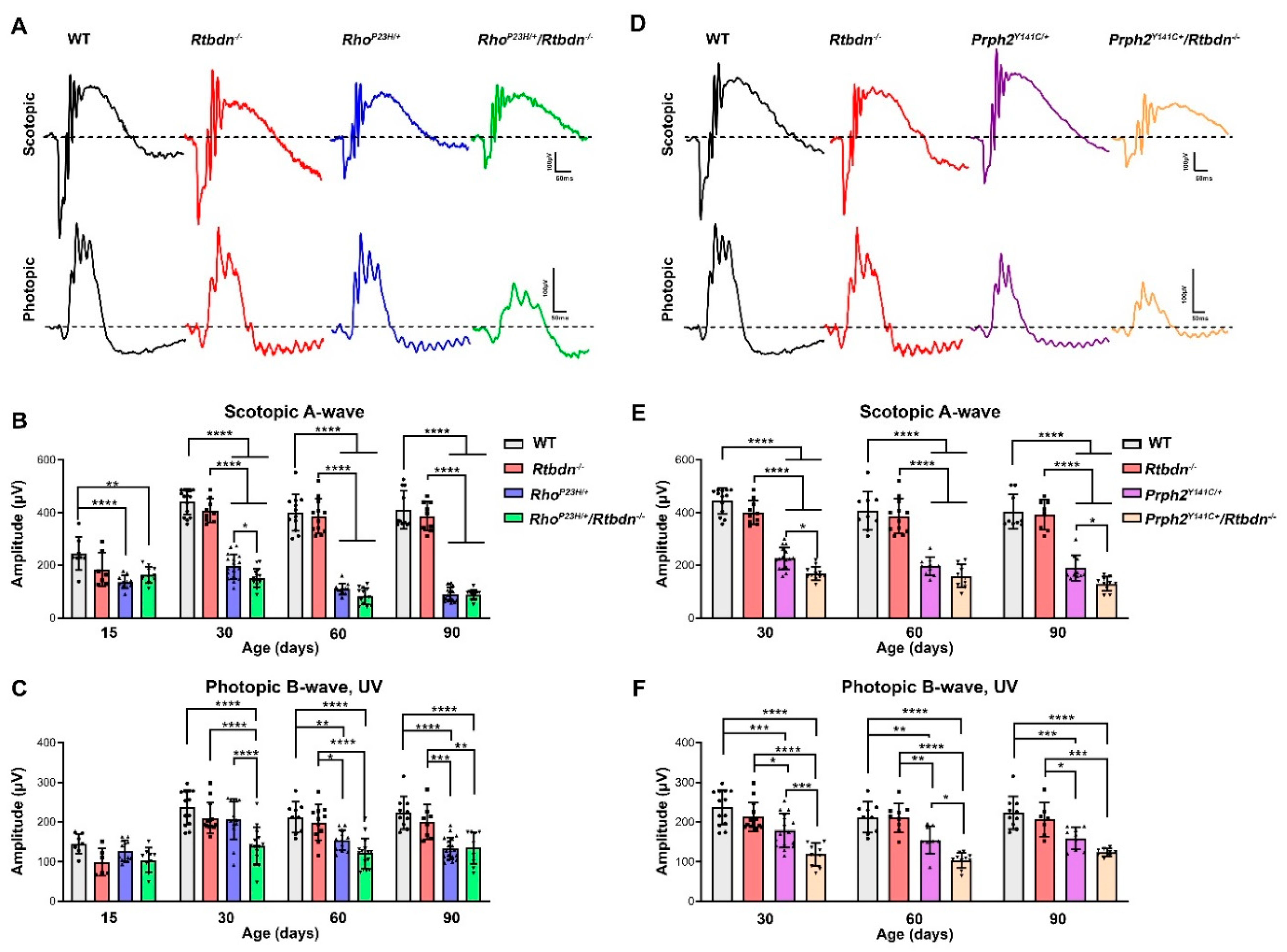
2.2. Elimination of Rtbdn Exacerbates Structural and Functional Degeneration in the RhoP23H/+ and Prph2Y141C/+ Retinas
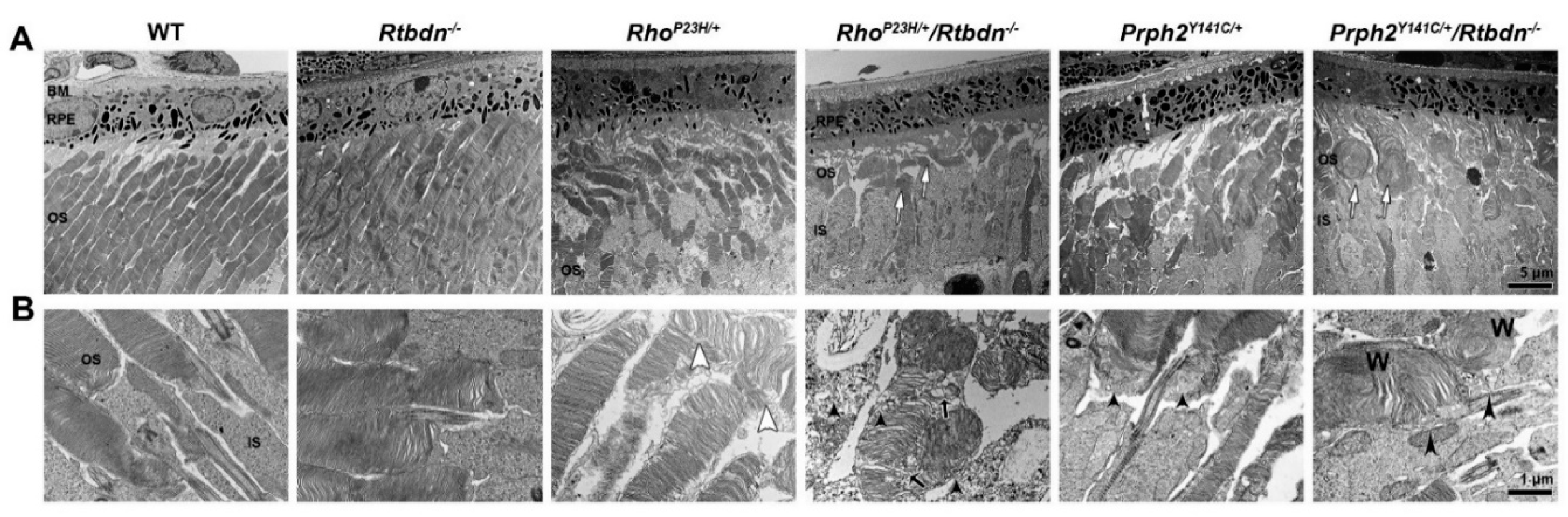
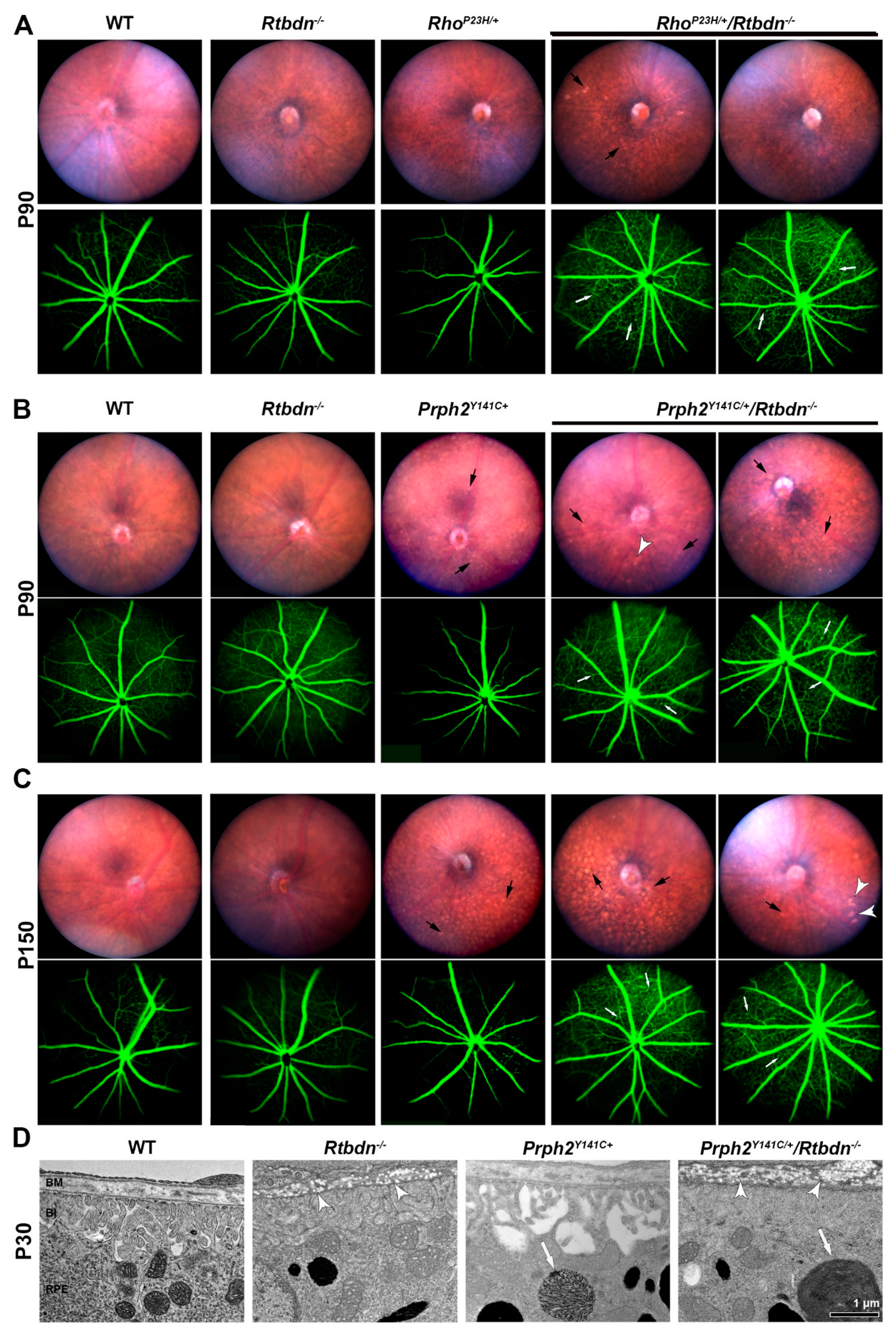
2.3. Ablation of Rtbdn in the RhoP23H/+ and Prph2Y141C/+ Leads to Non-Photoreceptor Phenotypes in the Retina
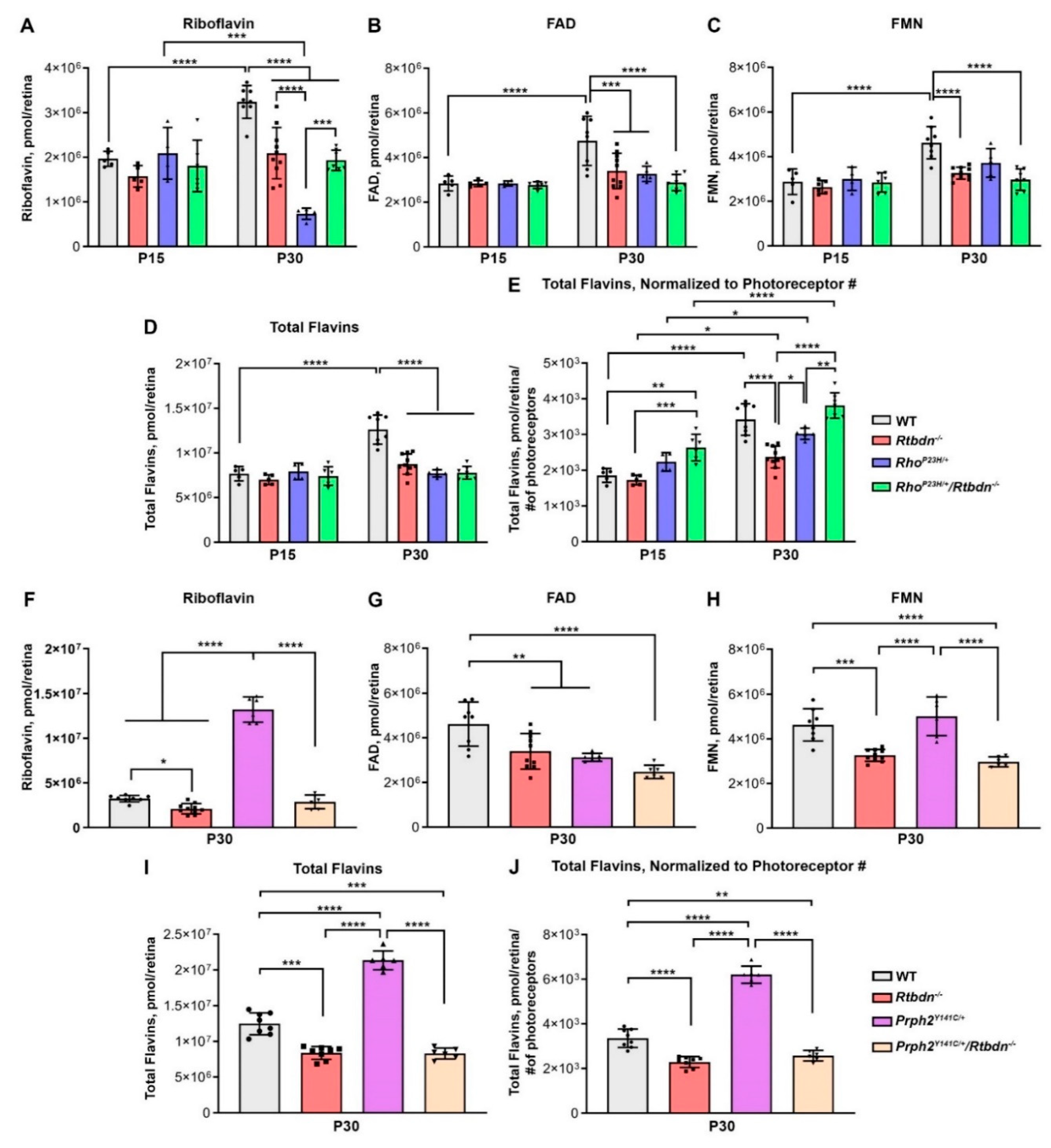
2.4. Lack of RTBDN in IRD Models Alters Flavin Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Immunoblotting
4.3. Immunofluorescence Analysis
4.4. Electroretinography
4.5. Light Histology and Morphometry
4.6. Cone Counts
4.7. Transmission Electron Microscopy
4.8. Fundus and Fluorescein Angiogram Imaging
4.9. Flavin Quantification by HPLC
4.10. Quantification of ATP Levels in Retina
4.11. Statistical Analysis
4.12. Reproducibility
4.13. Data Availability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kelley, R.A.; Al-Ubaidi, M.R.; Naash, M.I. Retbindin is an extracellular riboflavin-binding protein found at the photoreceptor/retinal pigment epithelium interface. J. Biol. Chem. 2015, 290, 5041–5052. [Google Scholar] [CrossRef]
- Wistow, G.; Bernstein, S.L.; Wyatt, M.K.; Ray, S.; Behal, A.; Touchman, J.W.; Bouffard, G.; Smith, D.; Peterson, K. Expressed sequence tag analysis of human retina for the NEIBank Project: Retbindin, an abundant, novel retinal cDNA and alternative splicing of other retina-preferred gene transcripts. Mol. Vis. 2002, 8, 196–204. [Google Scholar]
- Barile, M.; Giancaspero, T.A.; Brizio, C.; Panebianco, C.; Indiveri, C.; Galluccio, M.; Vergani, L.; Eberini, I.; Gianazza, E. Biosynthesis of flavin cofactors in man: Implications in health and disease. Curr. Pharm. Des. 2013, 19, 2649–2675. [Google Scholar] [CrossRef]
- Powers, H.J.; Corfe, B.M.; Nakano, E. Riboflavin in development and cell fate. Subcell. Biochem. 2012, 56, 229–245. [Google Scholar]
- Futterman, S.; Kinoshita, J.H. Metabolism of the retina. I. Respiration of cattle retina. J. Biol. Chem. 1959, 234, 723–726. [Google Scholar]
- Batey, D.W.; Eckhert, C.D. Analysis of flavins in ocular tissues of the rabbit. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1981–1985. [Google Scholar]
- Batey, D.W.; Eckhert, C.D. Identification of FAD, FMN, and riboflavin in the retina by microextraction and high-performance liquid chromatography. Anal. Biochem. 1990, 188, 164–167. [Google Scholar] [CrossRef]
- Kelley, R.A.; Al-Ubaidi, M.R.; Sinha, T.; Genc, A.M.; Makia, M.S.; Ikelle, L.; Naash, M.I. Ablation of the riboflavin-binding protein retbindin reduces flavin levels and leads to progressive and dose-dependent degeneration of rods and cones. J. Biol. Chem. 2017, 292, 21023–21034. [Google Scholar] [CrossRef]
- Wang, L.; Cano, M.; Datta, S.; Wei, H.; Ebrahimi, K.B.; Gorashi, Y.; Garlanda, C.; Handa, J.T. Pentraxin 3 recruits complement factor H to protect against oxidative stress-induced complement and inflammasome overactivation. J. Pathol. 2016, 240, 495–506. [Google Scholar] [CrossRef]
- Ding, X.Q.; Nour, M.; Ritter, L.M.; Goldberg, A.F.; Fliesler, S.J.; Naash, M.I. The R172W mutation in peripherin/rds causes a cone-rod dystrophy in transgenic mice. Hum. Mol. Genet. 2004, 13, 2075–2087. [Google Scholar] [CrossRef]
- Genc, A.M.; Makia, M.S.; Sinha, T.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Elimination of a Retinal Riboflavin Binding Protein Exacerbates Degeneration in a Model of Cone-Rod Dystrophy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef] [PubMed]
- Sakami, S.; Maeda, T.; Bereta, G.; Okano, K.; Golczak, M.; Sumaroka, A.; Roman, A.J.; Cideciyan, A.V.; Jacobson, S.G.; Palczewski, K. Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J. Biol. Chem. 2011, 286, 10551–10567. [Google Scholar] [CrossRef] [PubMed]
- Stuck, M.W.; Conley, S.M.; Naash, M.I. The Y141C knockin mutation in RDS leads to complex phenotypes in the mouse. Hum. Mol. Genet. 2014, 23, 6260–6274. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Dryja, T.P.; McGee, T.L.; Hahn, L.B.; Cowley, G.S.; Olsson, J.E.; Reichel, E.; Sandberg, M.A.; Berson, E.L. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N. Engl. J. Med. 1990, 323, 1302–1307. [Google Scholar] [CrossRef]
- Sohocki, M.M.; Daiger, S.P.; Bowne, S.J.; Rodriquez, J.A.; Northrup, H.; Heckenlively, J.R.; Birch, D.G.; Mintz-Hittner, H.; Ruiz, R.S.; Lewis, R.A.; et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum. Mutat. 2001, 17, 42–51. [Google Scholar] [CrossRef]
- Sung, C.H.; Davenport, C.M.; Hennessey, J.C.; Maumenee, I.H.; Jacobson, S.G.; Heckenlively, J.R.; Nowakowski, R.; Fishman, G.; Gouras, P.; Nathans, J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 1991, 88, 6481–6485. [Google Scholar] [CrossRef]
- Goto, Y.; Peachey, N.S.; Ripps, H.; Naash, M.I. Functional abnormalities in transgenic mice expressing a mutant rhodopsin gene. Investig. Ophthalmol. Vis. Sci. 1995, 36, 62–71. [Google Scholar]
- Price, B.A.; Sandoval, I.M.; Chan, F.; Simons, D.L.; Wu, S.M.; Wensel, T.G.; Wilson, J.H. Mislocalization and degradation of human P23H-rhodopsin-GFP in a knockin mouse model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9728–9736. [Google Scholar] [CrossRef]
- Naash, M.I.; Hollyfield, J.G.; Al-Ubaidi, M.R.; Baehr, W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc. Natl. Acad. Sci. USA 1993, 90, 5499–5503. [Google Scholar] [CrossRef]
- Francis, P.J.; Schultz, D.W.; Gregory, A.M.; Schain, M.B.; Barra, R.; Majewski, J.; Ott, J.; Acott, T.; Weleber, R.G.; Klein, M.L. Genetic and phenotypic heterogeneity in pattern dystrophy. Br. J. Ophthalmol. 2005, 89, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, V.; Tran, H.V.; Gaillard, M.C.; Schorderet, D.F.; Munier, F.L. Pattern dystrophy with high intrafamilial variability associated with Y141C mutation in the peripherin/RDS gene and successful treatment of subfoveal CNV related to multifocal pattern type with anti-VEGF (ranibizumab) intravitreal injections. Retina 2012, 32, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.J.; den Hollander, A.I.; Hoyng, C.B.; Cremers, F.P.; Klevering, B.J.; Keunen, J.E. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog. Retin. Eye Res. 2008, 27, 213–235. [Google Scholar] [CrossRef]
- Khani, S.C.; Karoukis, A.J.; Young, J.E.; Ambasudhan, R.; Burch, T.; Stockton, R.; Lewis, R.A.; Sullivan, L.S.; Daiger, S.P.; Reichel, E.; et al. Late-onset autosomal dominant macular dystrophy with choroidal neovascularization and nonexudative maculopathy associated with mutation in the RDS gene. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3570–3577. [Google Scholar] [CrossRef]
- Sakami, S.; Kolesnikov, A.V.; Kefalov, V.J.; Palczewski, K. P23H opsin knock-in mice reveal a novel step in retinal rod disc morphogenesis. Hum. Mol. Genet. 2014, 23, 1723–1741. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Pahlberg, J.; Boyd, K.K.; Kerov, V.; Kolandaivelu, S.; Ramamurthy, V.; Sampath, A.P.; Artemyev, N.O. Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12468–12473. [Google Scholar] [CrossRef]
- Chen, J.; Simon, M.I.; Matthes, M.T.; Yasumura, D.; LaVail, M.M. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness). Investig. Ophthalmol. Vis. Sci. 1999, 40, 2978–2982. [Google Scholar]
- Song, X.; Vishnivetskiy, S.A.; Seo, J.; Chen, J.; Gurevich, E.V.; Gurevich, V.V. Arrestin-1 expression level in rods: Balancing functional performance and photoreceptor health. Neuroscience 2011, 174, 37–49. [Google Scholar] [CrossRef]
- Liu, H.; Tang, J.; Du, Y.; Saadane, A.; Tonade, D.; Samuels, I.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells Influence Retinal Vascular Degeneration in Mouse Models of Retinal Degeneration and Diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4272–4281. [Google Scholar] [CrossRef]
- Tode, J.; Richert, E.; Koinzer, S.; Klettner, A.; von der Burchard, C.; Brinkmann, R.; Lucius, R.; Roider, J. Thermal Stimulation of the Retina Reduces Bruch’s Membrane Thickness in Age Related Macular Degeneration Mouse Models. Transl. Vis. Sci. Technol. 2018, 7, 2. [Google Scholar] [CrossRef]
- Rudolf, M.; Winkler, B.; Aherrahou, Z.; Doehring, L.C.; Kaczmarek, P.; Schmidt-Erfurth, U. Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch’s membrane of LDL receptor knockout mice. Br. J. Ophthalmol. 2005, 89, 1627–1630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curcio, C.A.; Millican, C.L.; Bailey, T.; Kruth, H.S. Accumulation of cholesterol with age in human Bruch’s membrane. Investig. Ophthalmol. Vis. Sci. 2001, 42, 265–274. [Google Scholar]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of daylight vision in retinal degeneration: Are oxidative stress and metabolic dysregulation to blame? J. Biol. Chem. 2012, 287, 1642–1648. [Google Scholar] [CrossRef]
- Griciuc, A.; Roux, M.J.; Merl, J.; Giangrande, A.; Hauck, S.M.; Aron, L.; Ueffing, M. Proteomic survey reveals altered energetic patterns and metabolic failure prior to retinal degeneration. J. Neurosci. 2014, 34, 2797–2812. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J. The interplay between metabolic homeostasis and neurodegeneration: Insights into the neurometabolic nature of amyotrophic lateral sclerosis. Cell. Regen. 2015, 4, 5. [Google Scholar] [CrossRef]
- Sinha, T.; Makia, M.; Du, J.; Naash, M.I.; Al-Ubaidi, M.R. Flavin homeostasis in the mouse retina during aging and degeneration. J. Nutr. Biochem. 2018, 62, 123–133. [Google Scholar] [CrossRef]
- Khaydukov, E.V.; Mironova, K.E.; Semchishen, V.A.; Generalova, A.N.; Nechaev, A.V.; Khochenkov, D.A.; Stepanova, E.V.; Lebedev, O.I.; Zvyagin, A.V.; Deyev, S.M.; et al. Riboflavin photoactivation by upconversion nanoparticles for cancer treatment. Sci. Rep. 2016, 6, 35103. [Google Scholar] [CrossRef]
- Huvaere, K.; Cardoso, D.R.; Homem-de-Mello, P.; Westermann, S.; Skibsted, L.H. Light-induced oxidation of unsaturated lipids as sensitized by flavins. J. Phys. Chem. B 2010, 114, 5583–5593. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Batabyal, S.; Polley, N.; Pal, S.K. Vitamin B2 in nanoscopic environments under visible light: Photosensitized antioxidant or phototoxic drug? J. Phys. Chem. A 2014, 118, 3934–3943. [Google Scholar] [CrossRef]
- Kelley, R.A.; Al-Ubaidi, M.R.; Naash, M.I. Retbindin Is Capable of Protecting Photoreceptors from Flavin-Sensitized Light-Mediated Cell Death In Vitro. Adv. Exp. Med. Biol. 2018, 1074, 485–490. [Google Scholar]
- Conley, S.M.; Stuck, M.W.; Watson, J.N.; Naash, M.I. Rom1 converts Y141C-Prph2-associated pattern dystrophy to retinitis pigmentosa. Hum. Mol. Genet. 2017, 26, 509–518. [Google Scholar] [CrossRef]
- Winkler, B.S. The electroretinogram of the isolated rat retina. Vision Res. 1972, 12, 1183–1198. [Google Scholar] [CrossRef]
- Kelley, R.A.; Al-Ubaidi, M.R.; Naash, M.I. The Potential Role of Flavins and Retbindin in Retinal Function and Homeostasis. Adv. Exp. Med. Biol. 2016, 854, 643–648. [Google Scholar]
- Conley, S.M.; Stuck, M.W.; Burnett, J.L.; Chakraborty, D.; Azadi, S.; Fliesler, S.J.; Naash, M.I. Insights into the mechanisms of macular degeneration associated with the R172W mutation in RDS. Hum. Mol. Genet. 2014, 23, 3102–3114. [Google Scholar] [CrossRef]
- Redmond, T.M.; Wiggert, B.; Robey, F.A.; Nguyen, N.Y.; Lewis, M.S.; Lee, L.; Chader, G.J. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry 1985, 24, 787–793. [Google Scholar] [CrossRef]
- Cheng, T.; Peachey, N.S.; Li, S.; Goto, Y.; Cao, Y.; Naash, M.I. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J. Neurosci. 1997, 17, 8118–8128. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Ding, X.Q.; Conley, S.M.; Fliesler, S.J.; Naash, M.I. Differential requirements for retinal degeneration slow intermolecular disulfide-linked oligomerization in rods versus cones. Hum. Mol. Genet. 2009, 18, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Koirala, A.; Conley, S.M.; Naash, M.I. A review of therapeutic prospects of non-viral gene therapy in the retinal pigment epithelium. Biomaterials 2013, 34, 7158–7167. [Google Scholar] [CrossRef]
| Antigen | Species | Clone | Application/Concentration | Source |
|---|---|---|---|---|
| Retbindin | Rabbit | 1:500 (WB,IF) | In-House [1,44] | |
| Prph2 | Mouse | 2B7 | 1:1000 (WB, IF) | In house, [45] Available from Millipore Cat# MABN2395 |
| IRBP | Rabbit | 1:1000 (WB) | Gift from Dr. Gregory Liou [46] | |
| GAPDH | Mouse | 1:1000 (WB) | Abcam Cat# Ab8245, RRID:AB_2107448 | |
| Rhodopsin | Mouse | 1D4 | 1:1000 (IF) | Gift from R. Molday Can be purchased: Santa Cruz Biotechnology Cat# sc-57432, RRID:AB_785511 |
| SNAP25 | Mouse | SMI-81 | 1:1000 (WB, IF) | Covance Cat# SMI-81R-100, RRID:AB_510034 |
| Actin-HRP | Mouse | AC-15 | 1:50,000 (WB) | Sigma-Aldrich Cat# A3854, RRID:AB_262011 |
| Rabbit IgG HRP | Goat | 1:25,000 (WB) | Milipore Cat# AP187P, RRID:AB_92625 | |
| Mouse IgG HRP | Goat | 1:25,000 (WB) | Millipore Cat# AP181P, RRID:AB_11214094 | |
| Rabbit IgG Alexa Flour 647 | Goat | 1:1000 (IF) | Thermo Fisher Scientific Cat# A21245, RRID: AB_2535813 | |
| Mouse IgG Alexa Flour 555 | Donkey | 1:1000 (IF) | Thermo Fisher Scientific Cat# A-31570, RRID:AB_2536180 | |
| PNA Alexa Fluor 488 | 1:500 (IF) | Thermo Fisher Scientific Cat# L21409, RRID:AB_2315178 | ||
| DAPI (stain) | 1:1000 (IF) | Thermo Fisher Scientific Cat# 62248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genc, A.M.; Makia, M.S.; Sinha, T.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Retbindin: A riboflavin Binding Protein, Is Critical for Photoreceptor Homeostasis and Survival in Models of Retinal Degeneration. Int. J. Mol. Sci. 2020, 21, 8083. https://doi.org/10.3390/ijms21218083
Genc AM, Makia MS, Sinha T, Conley SM, Al-Ubaidi MR, Naash MI. Retbindin: A riboflavin Binding Protein, Is Critical for Photoreceptor Homeostasis and Survival in Models of Retinal Degeneration. International Journal of Molecular Sciences. 2020; 21(21):8083. https://doi.org/10.3390/ijms21218083
Chicago/Turabian StyleGenc, Ayse M., Mustafa S. Makia, Tirthankar Sinha, Shannon M. Conley, Muayyad R. Al-Ubaidi, and Muna I. Naash. 2020. "Retbindin: A riboflavin Binding Protein, Is Critical for Photoreceptor Homeostasis and Survival in Models of Retinal Degeneration" International Journal of Molecular Sciences 21, no. 21: 8083. https://doi.org/10.3390/ijms21218083
APA StyleGenc, A. M., Makia, M. S., Sinha, T., Conley, S. M., Al-Ubaidi, M. R., & Naash, M. I. (2020). Retbindin: A riboflavin Binding Protein, Is Critical for Photoreceptor Homeostasis and Survival in Models of Retinal Degeneration. International Journal of Molecular Sciences, 21(21), 8083. https://doi.org/10.3390/ijms21218083





