Vasectomy and Photoperiodic Regimen Modify the Protein Profile, Hormonal Content and Antioxidant Enzymes Activity of Ram Seminal Plasma
Abstract
:1. Introduction
2. Results
2.1. Vasectomy Modifies the Protein Profile of Ram Seminal Plasma
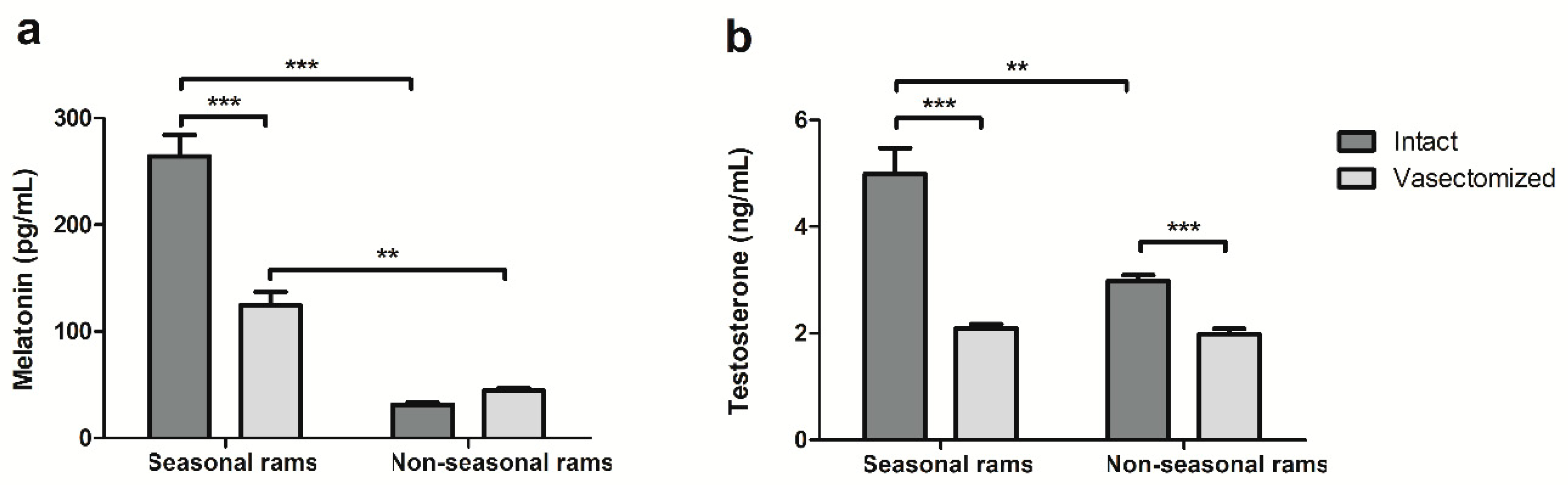
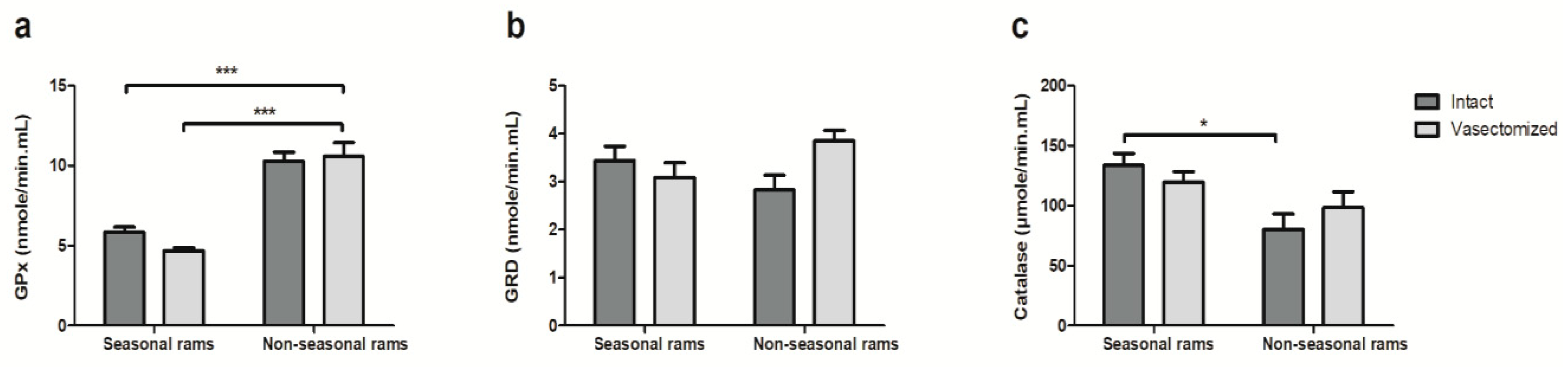
2.2. Vasectomy Decreases Melatonin and Testosterone Concentrations, but Not Antioxidant Enzyme Activity in Ram Seminal Plasma
3. Discussion
4. Materials and Methods
4.1. Animals and Seminal Plasma Extraction
4.1.1. Rams in the Mediterranean Climate
4.1.2. Rams under Equatorial Photoperiod
4.2. Seminal Plasma Protein Analyses
4.3. Seminal Plasma Hormonal Analyses
4.3.1. Melatonin Evaluation
4.3.2. Testosterone Evaluation
4.4. Seminal Plasma Antioxidant Enzymes Activity
4.4.1. Glutathione Peroxidase (GPx)
4.4.2. Glutathione Reductase (GRD)
4.4.3. Catalase
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SP | Seminal plasma |
| GPx | Glutathione peroxidase |
| GRD | Glutathione reductase |
| SOD | Superoxide dismutase |
| DIGE | Difference gel electrophoresis |
Appendix A
| Intact | Vasectomized | |||
|---|---|---|---|---|
| Creole | Romney Marsh | Creole | Romney Marsh | |
| Total protein (mg/mL) | 36.51 ± 2.30 | 42.69 ± 2.18 | 52.05 ± 2.98 | 55.01 ± 10.12 |
| Melatonin (pg/mL) | 32.78 ± 3.38 | 30.15 ± 1.50 | 46.63 ± 3.05 | 42.51 ± 4.52 |
| Testosterone (ng/mL) | 3.06 ± 0.14 | 2.89 ± 0.18 | 1.92 ± 0.13 | 2.05 ± 0.14 |
| Glutathione peroxidase (nmole/min mL) | 10.44 ± 0.82 | 10.15 ± 0.79 | 12.68 ± 1.43 | 9.58 ± 0.71 |
| Glutathione reductase (nmole/min mL) | 2.41 ± 0.46 | 2.73 ± 0.42 | 4.50 ± 0.43 | 3.60 ± 0.29 |
| Catalase (µmole/min mL) | 68.84 ± 16.00 | 91.21 ± 19.50 | 104.04 ± 20.06 | 93.75 ± 17.71 |
References
- Gatti, J.-L.; Druart, X.; Guérin, Y.; Dacheux, F.; Dacheux, J.-L. A 105- to 94-Kilodalton Protein in the Epididymal Fluids of Domestic Mammals Is Angiotensin I-Converting Enzyme (ACE); Evidence That Sperm Are the Source of This ACE1. Biol. Reprod. 1999, 60, 937–945. [Google Scholar] [CrossRef] [Green Version]
- McGraw, L.A.; Suarez, S.S.; Wolfner, M.F. On a matter of seminal importance. Bioessays 2015, 37, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Juyena, N.S.; Stelletta, C. Seminal Plasma: An Essential Attribute to Spermatozoa. J. Androl. 2012, 33, 536–551. [Google Scholar] [CrossRef] [Green Version]
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal plasma proteins as markers of sperm fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef]
- Velho, A.L.C.; Menezes, E.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Metabolomic markers of fertility in bull seminal plasma. PLoS ONE 2018, 13, e0195279. [Google Scholar] [CrossRef]
- Robertson, S.A.; Sharkey, D.J. Seminal fluid and fertility in women. Fertil. Steril. 2016, 106, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Archana, K.; Sridharn, T.B.; Kamini, A.R. Role of Seminal Plasma Proteins in Effective Zygote Formation- A Success Road to Pregnancy. Protein Pept. Lett. 2019, 26, 238–250. [Google Scholar] [CrossRef]
- Novak, S.; Ruiz-Sánchez, A.; Dixon, W.T.; Foxcroft, G.R.; Dyck, M.K. Seminal Plasma Proteins as Potential Markers of Relative Fertility in Boars. J. Androl. 2010, 31, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Arangasamy, A.; Kastelic, J.P. Sperm and seminal plasma proteomics of high- versus low-fertility Holstein bulls. Theriogenology 2019, 126, 41–48. [Google Scholar] [CrossRef]
- Viana, A.G.A.; Martins, A.M.A.; Pontes, A.H.; Fontes, W.; Castro, M.S.; Ricart, C.A.O.; Sousa, M.V.; Kaya, A.; Topper, E.; Memili, E.; et al. Proteomic landscape of seminal plasma associated with dairy bull fertility. Sci. Rep. 2018, 8, 16323. [Google Scholar] [CrossRef]
- Waheed, M.M.; Ghoneim, I.M.; Alhaider, A.K. Seminal plasma and serum fertility biomarkers in dromedary camels (Camelus dromedarius). Theriogenology 2015, 83, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Batruch, I.; Lecker, I.; Kagedan, D.; Smith, C.R.; Mullen, B.J.; Grober, E.; Lo, K.C.; Diamandis, E.P.; Jarvi, K.A. Proteomic Analysis of Seminal Plasma from Normal Volunteers and Post-Vasectomy Patients Identifies over 2000 Proteins and Candidate Biomarkers of the Urogenital System. J. Proteome Res. 2011, 10, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Campbell, A.; Sandoval, S.; Tibary, A. Effects of Vasectomy on Seminal Plasma Alkaline Phosphatase in Male Alpacas (Vicugña pacos). Reprod. Domest. Anim. 2013, 48, 995–1000. [Google Scholar] [CrossRef]
- De Souza, F.F.; Martins, M.I.M.; Lopes, M.D. Vasectomy effect on canine seminal plasma biochemical components and their correlation with seminal parameters. Theriogenology 2006, 66, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj Ghaoui, R.; Thomson, P.; Evans, G.; Maxwell, W. The Origin of Membrane Vesicles in Ram Seminal Plasma. Reprod. Domest. Anim. 2006, 41, 98–105. [Google Scholar] [CrossRef]
- Ghaoui, R.E.-H.; Gillan, L.; Thomson, P.C.; Evans, G.; Maxwell, W.M.C. Effect of Seminal Plasma Fractions From Entire and Vasectomized Rams on the Motility Characteristics, Membrane Status, and In Vitro Fertility of Ram Spermatozoa. J. Androl. 2007, 28, 109–122. [Google Scholar] [CrossRef]
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Macanovic, B.; Vucetic, M.; Jankovic, A.; Stancic, A.; Buzadzic, B.; Garalejic, E.; Korac, A.; Korac, B.; Otasevic, V. Correlation between Sperm Parameters and Protein Expression of Antioxidative Defense Enzymes in Seminal Plasma: A Pilot Study. Dis. Markers 2015, 2015, 436236. [Google Scholar] [CrossRef]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Vicente-Carrillo, A.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Glutathione Peroxidase 5 Is Expressed by the Entire Pig Male Genital Tract and Once in the Seminal Plasma Contributes to Sperm Survival and In Vivo Fertility. PLoS ONE 2016, 11, e0162958. [Google Scholar] [CrossRef]
- Chen, H.; Chow, P.H.; Cheng, S.K.; Cheung, A.L.M.; Cheng, L.Y.L.; O, W.-S. Male Genital Tract Antioxidant Enzymes: Their Source, Function in the Female, and Ability to Preserve Sperm DNA Integrity in the Golden Hamster. J. Androl. 2003, 24, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Cooper, T.G.; De Geyter, M.; De Geyter, C.; Rolf, C.; Kamischke, A.; Nieschlag, E. Studies on the origin of redox enzymes in seminal plasma and their relationship with results of in-vitro fertilization. Mol. Hum. Reprod. 1998, 4, 835–839. [Google Scholar] [CrossRef]
- Casao, A.; Cebrian, I.; Asumpcao, M.; Perez-Pe, R.; Abecia, J.; Forcada, F.; Cebrian-Perez, J.; Muino-Blanco, T. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod. Biol. Endocrinol. 2010, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Senger, P.L. Pathways to Pregnancy and Parturition, 2nd ed.; Current Conceptions, Inc.: Redmond, OR, USA, 2003. [Google Scholar]
- Arendt, J. Melatonin and the Mammalian Pineal Gland; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Gonzalez-Arto, M.; Hamilton, T.R.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Perez-Pe, R.; Muino-Blanco, T.; Cebrian-Perez, J.A.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Luboshitzky, R.; Kaplan-Zverling, M.; Shen-Orr, Z.; Nave, R.; Herer, P. Seminal plasma androgen/oestrogen balance in infertile men. Int. J. Androl. 2002, 25, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kratz, E.M.; Piwowar, A.; Zeman, M.; Stebelová, K.; Thalhammer, T. Decreased melatonin levels and increased levels of advanced oxidation protein products in the seminal plasma are related to male infertility. Reprod. Fertil. Dev. 2016, 28, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Manchester, L.C.; Tan, D.X. Peripheral reproductive organ health and melatonin: Ready for prime time. Int. J. Mol. Sci. 2013, 14, 7231–7272. [Google Scholar] [CrossRef] [Green Version]
- Loren, P.; Sánchez, R.; Arias, M.-E.; Felmer, R.; Risopatrón, J.; Cheuquemán, C. Melatonin Scavenger Properties against Oxidative and Nitrosative Stress: Impact on Gamete Handling and In Vitro Embryo Production in Humans and Other Mammals. Int. J. Mol. Sci. 2017, 18, 1119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Bai, Q.; Yuan, Y.; Liu, P.; Qiao, J. Assessment of Seminal Estradiol and Testosterone Levels as Predictors of Human Spermatogenesis. J. Androl. 2010, 31, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Almeida, O.F.; Lincoln, G.A. Photoperiodic regulation of reproductive activity in the ram: Evidence for the involvement of circadian rhythms in melatonin and prolactin secretion. Biol. Reprod. 1982, 27, 1062–1075. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, G.A.; Almeida, O.F.; Arendt, J. Role of melatonin and circadian rhythms in seasonal reproduction in rams. J. Reprod. Fertil. Suppl. 1981, 30, 23–31. [Google Scholar] [CrossRef]
- Marti, E.; Mara, L.; Marti, J.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Seasonal variations in antioxidant enzyme activity in ram seminal plasma. Theriogenology 2007, 67, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Serna, M.; Torres-Ruda, F.; Cardozo, J.A.; Grajales-Lombana, H.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T.; Pérez-Pé, R.; Casao, A. Changes in melatonin concentrations in seminal plasma are not correlated with testosterone or antioxidant enzyme activity when rams are located in areas with an equatorial photoperiod. Anim. Reprod. Sci. 2019, 200, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Serna, M.; Cardozo, J.A.; Grajales-Lombana, H.; Cebrian-Perez, J.A.; Muino-Blanco, T. Sperm quality and seminal plasma proteins in three sheep breeds under high altitude and tropical conditions. Span. J. Agric. Res. 2018, 16, e0403. [Google Scholar] [CrossRef] [Green Version]
- Pearl, C.A.; Roser, J.F. Lactoferrin expression and secretion in the stallion epididymis. Reprod. Biol. 2014, 14, 148–154. [Google Scholar] [CrossRef]
- Moura, A.A.; Souza, C.E.; Stanley, B.A.; Chapman, D.A.; Killian, G.J. Proteomics of cauda epididymal fluid from mature Holstein bulls. J. Proteom. 2010, 73, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.A.; Scopes, R.K. Phosphoglycerate Kinase B from Ram Testis. Eur. J. Biochem. 1978, 85, 89–95. [Google Scholar] [CrossRef]
- Soleilhavoup, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Kohnke, P.L.; Dacheux, J.L.; Guérin, Y.; Gatti, J.L.; de Graaf, S.P.; Druart, X. Ram seminal plasma proteome and its impact on liquid preservation of spermatozoa. J. Proteom. 2014, 109, 245–260. [Google Scholar] [CrossRef]
- Krassnigg, F.; Niederhauser, H.; Fink, E.; Frick, J.; Schill, W.-B. Angiotensin converting enzyme in human seminal plasma is synthesized by the testis, epididymis and prostate. Int. J. Androl. 1989, 12, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, A.; Hozbor, F.; Sanchez, E.; Fornés, M.W.; Alberio, R.H.; Cesari, A. Conserved ram seminal plasma proteins bind to the sperm membrane and repair cryopreservation damage. Theriogenology 2011, 76, 436–447. [Google Scholar] [CrossRef]
- Danshina, P.V.; Geyer, C.B.; Dai, Q.; Goulding, E.H.; Willis, W.D.; Kitto, G.B.; McCarrey, J.R.; Eddy, E.M.; O’Brien, D.A. Phosphoglycerate Kinase 2 (PGK2) Is Essential for Sperm Function and Male Fertility in Mice1. Biol. Reprod. 2010, 82, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, Q.; Wang, W.; Liu, F. Aberrant expression of sperm-specific glycolytic enzymes are associated with poor sperm quality. Mol. Med. Rep. 2019, 19, 2471–2478. [Google Scholar] [CrossRef] [Green Version]
- Rickard, J.P.; Leahy, T.; Soleilhavoup, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Lynch, G.W.; Druart, X.; de Graaf, S.P. The identification of proteomic markers of sperm freezing resilience in ram seminal plasma. J. Proteom. 2015, 126, 303–311. [Google Scholar] [CrossRef]
- Frenette, G.; Thabet, M.; Sullivan, R. Polyol Pathway in Human Epididymis and Semen. J. Androl. 2006, 27, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondoh, G.; Tojo, H.; Nakatani, Y.; Komazawa, N.; Murata, C.; Yamagata, K.; Maeda, Y.; Kinoshita, T.; Okabe, M.; Taguchi, R.; et al. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 2005, 11, 160–166. [Google Scholar] [CrossRef] [PubMed]
- González-Cadavid, V.; Martins, J.A.M.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.L.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Moura, A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckett, W.M.; Luckas, M.J.M.; Gazvani, M.R.; Aird, I.A.; Lewis-Jones, D.I. Seminal Plasma Lactoferrin Concentrations in Normal and Abnormal Semen Samples. J. Androl. 1997, 18, 302–304. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.R.; Shi, H.J.; Ma, D.; Zhao, H.X.; Lin, B.; Li, R.S. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J. Androl. 2009, 11, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.-Z.; Li, J.-Y.; Li, F.; Wang, H.-Y.; Shi, G.-X. Human ribonuclease 9, a member of ribonuclease A superfamily, specifically expressed in epididymis, is a novel sperm-binding protein. Asian J. Androl. 2009, 11, 240–251. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, H.; Zhang, C.; Li, N.; Lin, Y.; Wang, W. Cloning, expression and location of RNase9 in human epididymis. BMC Res. Notes 2008, 1, 111. [Google Scholar] [CrossRef] [Green Version]
- Penttinen, J.; Pujianto, D.A.; Sipilä, P.; Huhtaniemi, I.; Poutanen, M. Discovery in Silico and Characterization in Vitro of Novel Genes Exclusively Expressed in the Mouse Epididymis. Mol. Endocrinol. 2003, 17, 2138–2151. [Google Scholar] [CrossRef]
- Zhu, C.-F.; Liu, Q.; Zhang, L.; Yuan, H.-X.; Zhen, W.; Zhang, J.-S.; Chen, Z.-J.; Hall, S.H.; French, F.S.; Zhang, Y.-L. RNase9, an Androgen-Dependent Member of the RNase A Family, Is Specifically Expressed in the Rat Epididymis1. Biol. Reprod. 2007, 76, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennella, M.R.F.; Jones, R. The Activity of Some Nucleolytic Enzymes in Semen and in the Secretions of the Male Reproductive Tract. Andrologia 1977, 9, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arto, M.; Vicente-Carrillo, A.; Martinez-Pastor, F.; Fernandez-Alegre, E.; Roca, J.; Miro, J.; Rigau, T.; Rodriguez-Gil, J.E.; Perez-Pe, R.; Muino-Blanco, T.; et al. Melatonin receptors MT1 and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.; Lima-Cabello, E.; López, L.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Barrell, G.K.; Thrun, L.A.; Brown, M.E.; Viguié, C.; Karsch, F.J. Importance of Photoperiodic Signal Quality to Entrainment of the Circannual Reproductive Rhythm of the Ewe1. Biol. Reprod. 2000, 63, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Hedman, M.; de la Torre, B.; Jensen, F.; Pedersen, P.H.; Diczfalusy, E. Effect of vasectomy on the steroid profile of human seminal plasma. Int. J. Androl. 1983, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Navrátil, S.; Forejtek, P. Effect of bilateral vasectomy on testosterone levels in the seminal and blood plasmas of boars. Vet. Med. 1979, 24, 239–243. [Google Scholar]
- Cooper, T.G.; Waites, G.M.H. Testosterone in rete testis fluid and blood of rams and rats. J. Endocrinol. 1974, 62, 619–629. [Google Scholar] [CrossRef]
- Georgiadis, E.I.; Matzoros, C.; Aliferis, C.; Batrinos, M. Are Adrenal and Testicular Androgen Levels Correlated? Horm. Metab. Res. 1992, 24, 488–491. [Google Scholar] [CrossRef]
- D’Occhio, M.J.; Schanbacher, B.D.; Kinder, J.E. Profiles of luteinizing hormone, follicle-stimulating hormone, testosterone and prolactin in rams of diverse breeds: Effects of contrasting short (8L:16D) and long (16L:8D) photoperiods. Biol. Reprod. 1984, 30, 1039–1054. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, G.A.; Almeida, O.F.X.; Klandorf, H.; Cunningham, R.A. Hourly fluctuations in the blood levels of melatonin, prolactine, luteinizing hormone, follicle-stimulatin hormone, testosterone, tri-iodothyronine, thyroxine and cortisol in rams under artificial photoperiods, and the effects of cranial sympathectomy. J. Endocrinol. 1982, 92, 237–250. [Google Scholar] [CrossRef]
- Zini, A.; Fischer, M.; Mak, V.; Phang, D.; Jarvi, K. Catalase-like and superoxide dismutase-like activities in human seminal plasma. Urol. Res. 2002, 30, 321–323. [Google Scholar] [CrossRef]
- Ollero, M.; Muino-Blanco, T.; Lopez-Perez, M.J.; Cebrian-Perez, J.A. Viability of ram spermatozoa in relation to the abstinence period and successive ejaculations. Int. J. Androl. 1996, 19, 287–292. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
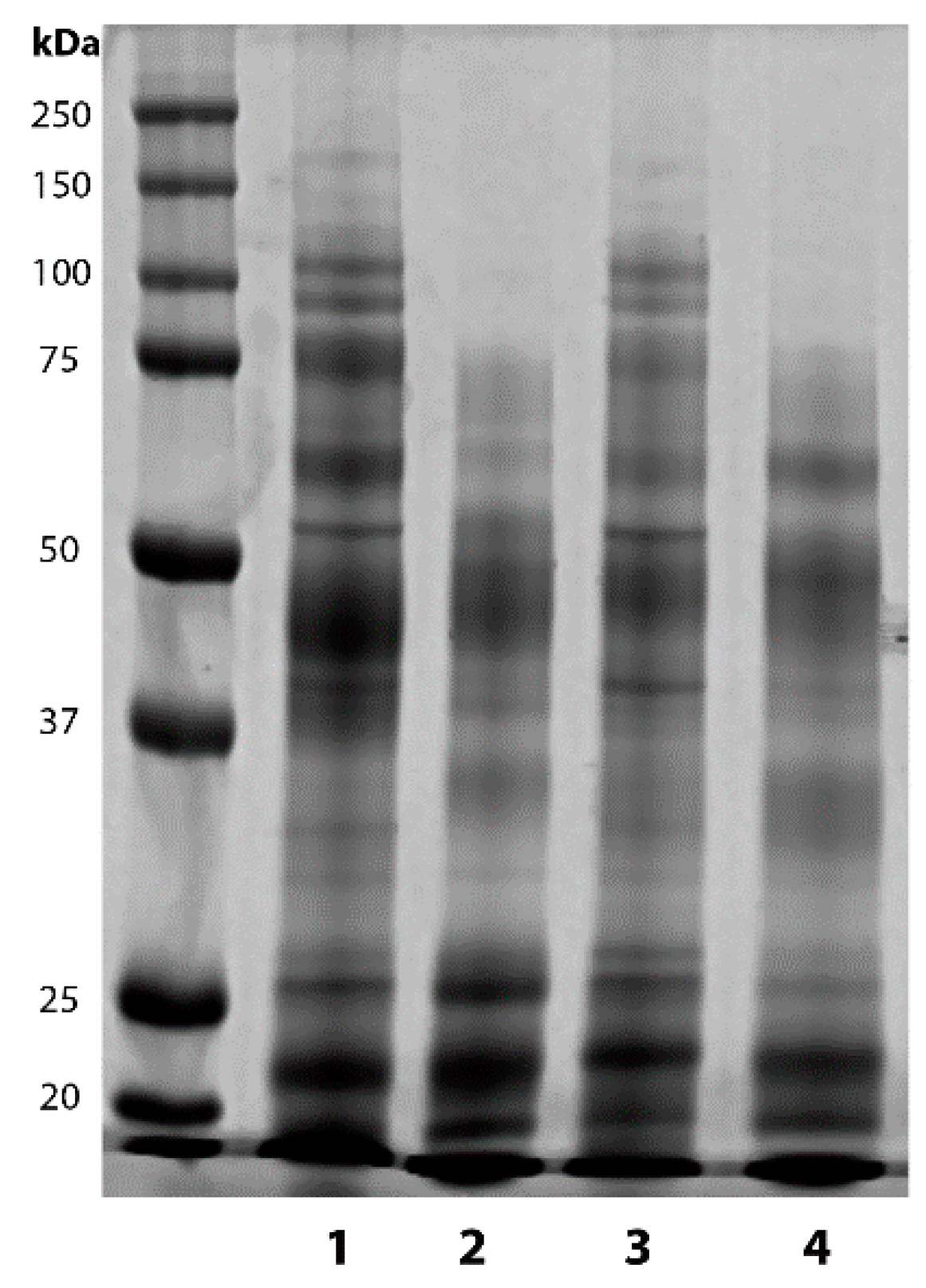
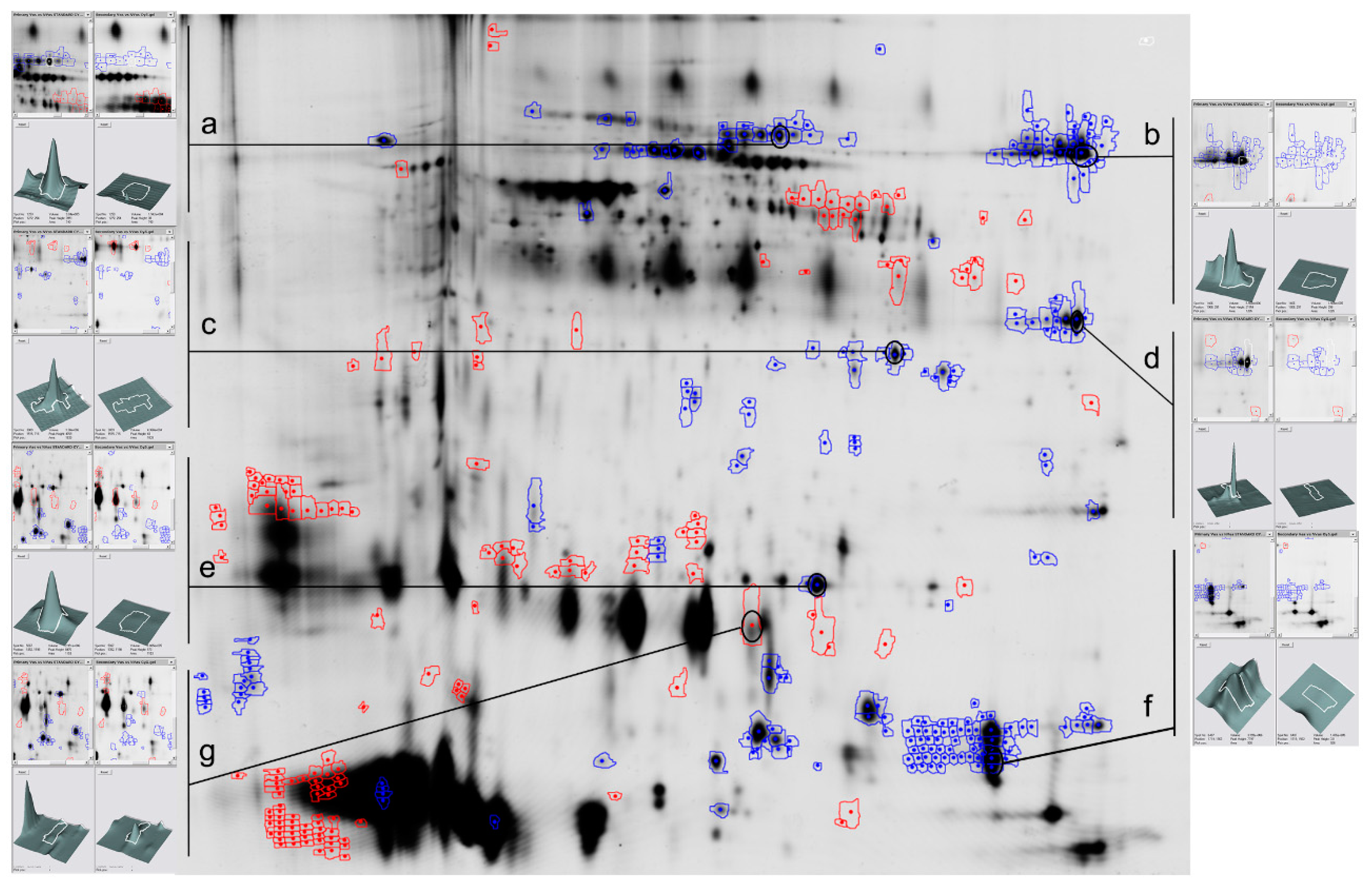
| Band Intensity × 103 (Arbitrary Units) | ||||
|---|---|---|---|---|
| Total Protein (mg/mL) | High MW Bands (250–75 kDa) | Medium MW Bands (75–37 kDa) | Low MW Bands (37–10 kDa) | |
| Intact seasonal rams | 34.20 ± 5.42 a | 3.90 ± 1.43 a | 11.25 ± 3.54 | 9.95 ± 2.80 |
| Vasectomized seasonal rams | 64.63 ± 6.36 b | 0.44 ± 0.08 b | 7.57 ± 1.0 | 11.80 ± 1.46 |
| Intact non-seasonal rams | 39.60 ± 1.66 a | 3.54 ± 0.53 a | 9.45 ± 1.21 | 12.20 ± 1.47 |
| Vasectomized non-seasonal rams | 53.40 ± 2.10 b | 0.60 ± 0.13 b | 10.56 ± 5.29 | 10.85 ± 3.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvajal-Serna, M.; Fatnassi, M.; Torres-Ruda, F.; Cardozo, J.A.; Grajales-Lombana, H.; Hammadi, M.; Abecia, J.A.; Muiño-Blanco, T.; Pérez-Pe, R.; Cebrián-Pérez, J.Á.; et al. Vasectomy and Photoperiodic Regimen Modify the Protein Profile, Hormonal Content and Antioxidant Enzymes Activity of Ram Seminal Plasma. Int. J. Mol. Sci. 2020, 21, 8063. https://doi.org/10.3390/ijms21218063
Carvajal-Serna M, Fatnassi M, Torres-Ruda F, Cardozo JA, Grajales-Lombana H, Hammadi M, Abecia JA, Muiño-Blanco T, Pérez-Pe R, Cebrián-Pérez JÁ, et al. Vasectomy and Photoperiodic Regimen Modify the Protein Profile, Hormonal Content and Antioxidant Enzymes Activity of Ram Seminal Plasma. International Journal of Molecular Sciences. 2020; 21(21):8063. https://doi.org/10.3390/ijms21218063
Chicago/Turabian StyleCarvajal-Serna, Melissa, Meriem Fatnassi, Felipe Torres-Ruda, Jaime Antonio Cardozo, Henry Grajales-Lombana, Mohamed Hammadi, Jose Alfonso Abecia, Teresa Muiño-Blanco, Rosaura Pérez-Pe, Jose Álvaro Cebrián-Pérez, and et al. 2020. "Vasectomy and Photoperiodic Regimen Modify the Protein Profile, Hormonal Content and Antioxidant Enzymes Activity of Ram Seminal Plasma" International Journal of Molecular Sciences 21, no. 21: 8063. https://doi.org/10.3390/ijms21218063
APA StyleCarvajal-Serna, M., Fatnassi, M., Torres-Ruda, F., Cardozo, J. A., Grajales-Lombana, H., Hammadi, M., Abecia, J. A., Muiño-Blanco, T., Pérez-Pe, R., Cebrián-Pérez, J. Á., & Casao, A. (2020). Vasectomy and Photoperiodic Regimen Modify the Protein Profile, Hormonal Content and Antioxidant Enzymes Activity of Ram Seminal Plasma. International Journal of Molecular Sciences, 21(21), 8063. https://doi.org/10.3390/ijms21218063







