Dendritic Cells: Critical Regulators of Allergic Asthma
Abstract
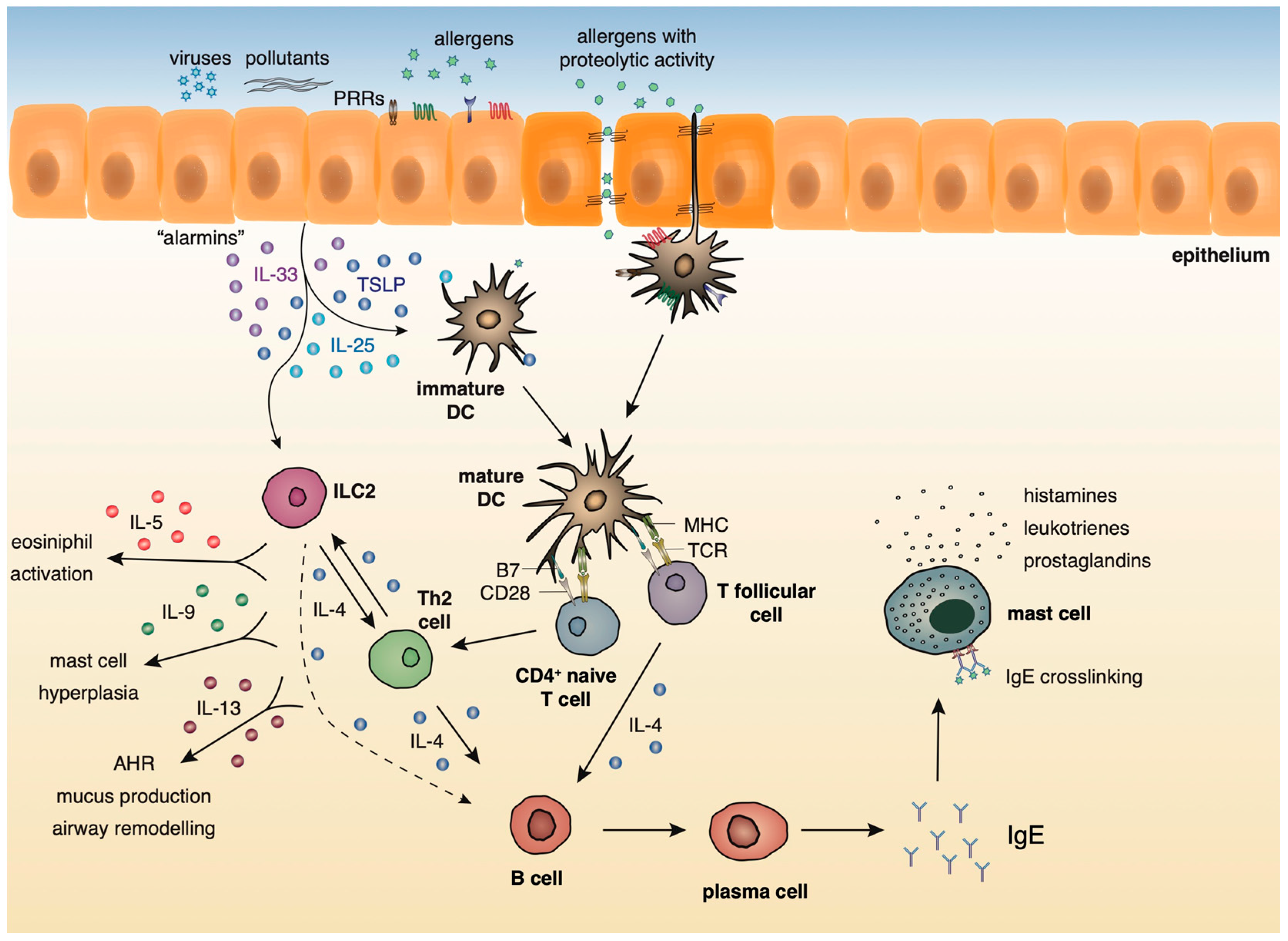
:1. Introduction
2. DC Subsets and Their Role in the Regulation of Allergic Asthma
2.1. Allergic Asthma
2.2. Innate and Adaptive Immune Responses in Lung Allergic Inflammation
2.3. Localization and Phenotype of DC Subsets in the Lung
2.4. The Role of Murine DCs in the Induction of Allergic Responses in Asthma
2.5. The Role of Human DC Subsets in Shaping Asthmatic Responses
2.6. The Role of Tolerogenic DCs in the Resolution of Allergic Airway Inflammation in the Airways
2.7. A Novel Subset of Tolerogenic DCs Confers Protection against Experimental Asthma
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHR | airway hyperresponsiveness |
| Bte | Blomia tropicalis mite |
| cDCs | conventional DCs |
| cDCs1 | cDCs type 1 |
| cDCs2 | cDCs type 2 |
| DCs | dendritic cells |
| Der p1 | Dermatophagoides pteronyssinus antigen P1 |
| FlaB | flagellin-B |
| HDM | house dust mite |
| MLNs | mediastinal lymph nodes |
| mo-DCs | monocyte-derived DCs |
| mDCs | myeloid DCs |
| Ova | ovalbumin |
| PB | peripheral blood |
| pDCs | plasmacytoid DCs |
| Th | T helper |
| TFH | T follicular helper |
| Treg | Regulatory T cell |
| TSLP | Thymic stromal lymphopoietin |
| WT | wild type |
References
- Schuijs, M.J.; Hammad, H.; Lambrecht, B.N. Professional and ‘Amateur’ Antigen-Presenting Cells In Type 2 Immunity. Trends Immunol. 2019, 40, 22–34. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Rizvi, Z.A.; Awasthi, A. Metabolic Checkpoints in Differentiation of Helper T Cells in Tissue Inflammation. Front. Immunol. 2019, 9, 3036. [Google Scholar] [CrossRef]
- Schülke, S. Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Gaurav, R.; Agrawal, D.K. Clinical view on the importance of dendritic cells in asthma. Expert Rev. Clin. Immunol. 2013, 10, 899–919. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Matsumoto, T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018, 9, 350. [Google Scholar] [CrossRef]
- Oertli, M.; Sundquist, M.; Hitzler, I.; Engler, D.B.; Arnold, I.C.; Reuter, S.; Maxeiner, J.; Hansson, M.; Taube, C.; Quiding-Järbrink, M.; et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J. Clin. Investig. 2012, 122, 1082–1096. [Google Scholar] [CrossRef] [Green Version]
- Hammad, H.; Kool, M.; Soullié, T.; Narumiya, S.; Trottein, F.; Hoogsteden, H.C.; Lambrecht, B.N. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 2007, 204, 357–367. [Google Scholar] [CrossRef]
- Semitekolou, M.; Morianos, I.; Banos, A.; Konstantopoulos, D.; Adamou-Tzani, M.; Sparwasser, T.; Xanthou, G. Dendritic cells conditioned by activin A-induced regulatory T cells exhibit enhanced tolerogenic properties and protect against experimental asthma. J. Allergy Clin. Immunol. 2018, 141, 671–684. [Google Scholar] [CrossRef] [Green Version]
- Theofani, E.; Semitekolou, M.; Morianos, I.; Samitas, K.; Xanthou, G. Targeting NLRP3 Inflammasome Activation in Severe Asthma. J. Clin. Med. 2019, 8, 1615. [Google Scholar] [CrossRef] [Green Version]
- Holgate, S.T.; Holloway, J.; Wilson, S.; Howarth, P.H.; Haitchi, H.M.; Babu, S.; Davies, D.E. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J. Allergy Clin. Immunol. 2006, 117, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Finotto, S. Resolution of allergic asthma. Semin. Immunopathol. 2019, 41, 665–674. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 induces IL-4, IL-5, and IL-13 pathologies and Th2-associated pathologies in vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type-2 associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-H.; Angkasekwinai, P.; Lu, N.; Voo, K.S.; Arima, K.; Hanabuchi, S.; Hippe, A.; Corrigan, C.J.; Dong, C.; Homey, B.; et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007, 204, 1837–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Fang, C.; Cousins, D.; Zhang, G.; Gu, S.; Gao, Z.; Shamji, B.; et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J. Immunol. 2008, 181, 2790–2798. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Comeau, M.R.; De Smedt, T.; Liggitt, H.D.; Dahl, M.E.; Lewis, D.B.; Gyarmati, D.; Aye, T.; Campbell, D.J.; Ziegler, S.F. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005, 6, 1047–1053. [Google Scholar] [CrossRef]
- Kearley, J.; Buckland, K.F.; Mathie, S.A.; Lloyd, C.M. Resolution of allergic inflammation and airway hyper-reactivity is dependent upon disruption of the T1/ ST2–IL-33 pathway. Am. J. Respir. Crit. Care. Med. 2009, 179, 772–781. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.D.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [Green Version]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Price, A.E.; Liang, H.E.; Sullivan, B.M.; Reinhardt, R.L.; Eisley, C.J.; Erle, D.J.; Locksley, R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 11489–11494. [Google Scholar] [CrossRef] [Green Version]
- Saenz, S.A.; Siracusa, M.C.; Perrigoue, J.G.; Spencer, S.P.; Urban, J.F.; Tocker, J.E.; Budelsky, A.L.; Kleinschek, M.A.; Kastelein, R.A.; Kambayashi, T.; et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 2010, 464, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.L.; McKenzie, A.N. Type-2 innate lymphoid cells in human allergic disease. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.E.; Doherty, T.A.; Baum, R.; Broide, D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J. Allergy Clin. Immunol. 2014, 133, 899–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewkowich, I.P.; Day, S.B.; Ledford, J.R.; Zhou, P.; Dienger, K.; Wills-Karp, M.; Page, K. Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation. Respir. Res. 2011, 12, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runswick, S.; Mitchell, T.; Davies, P.; Robinson, C.; Garrod, D.R. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007, 12, 834–842. [Google Scholar] [CrossRef]
- Sajjan, U.; Wang, Q.; Zhao, Y.; Gruenert, D.C.; Hershenson, M.B. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am. J. Respir. Crit. Care Med. 2008, 178, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Comstock, A.T.; Ganesan, S.; Chattoraj, A.; Faris, A.N.; Margolis, B.J.; Hershenson, M.B.; Sajjan, U.S. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J. Virol. 2011, 85, 6795–6808. [Google Scholar] [CrossRef] [Green Version]
- Schamberger, A.C.; Mise, N.; Jia, J.; Genoyer, E.; Yildirim, A.O.; Meiners, S.; Eickelberg, O. Cigarette smoke-induced disruption of bronchial epithelial tight junctions is prevented by transforming growth factor-beta. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Bayram, H.; Rusznak, C.; Khair, O.A.; Sapsford, R.J.; Abdelaziz, M.M. Effect of ozone and nitrogen dioxide on the permeability of bronchial epithelial cell cultures of non-asthmatic and asthmatic subjects. Clin. Exp. Allergy 2002, 32, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.D.; Blank, F.; Baum, O.; Gehr, P.; Rothen-Rutishauser, B.M. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part. Fibre. Toxicol. 2009, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vliet, S.J.; den Dunnen, J.; Gringhuis, S.I.; Geijtenbeek, T.B.; van Kooyk, Y. Innate signaling and regulation of Dendritic cell immunity. Curr. Opin. Immunol. 2007, 19, 435–440. [Google Scholar] [CrossRef]
- Vroling, A.B.; Fokkens, W.J.; van Drunen, C.M. How epithelial cells detect danger: Aiding the immune response. Allergy 2008, 63, 1110–1123. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, 73–80. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Hwang, Y.Y.; Scanlon, S.T.; Zaghouani, H.; Garbi, N.; Fallon, P.G.; McKenzie, A.N.J. Group 2 innate lymphoid cells license dendritic cells to potentiate memory T helper 2 cell responses. Nat. Immunol. 2016, 17, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Guilliams, M.; Dutertre, C.A.; Scott, C.L.; McGovern, N.; Sichien, D.; Chakarov, S.; Van Gassen, S.; Chen, J.; Poidinger, M.; De Prijck, S.; et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 2016, 45, 669–684. [Google Scholar] [CrossRef] [Green Version]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Sung, S.S.; Fu, S.M.; Rose, C.E.; Gaskin, F.; Ju, S.T.; Beaty, S.R. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expresSing Langerin and tight junction proteins. J. Immunol. 2006, 176, 2161–2172. [Google Scholar] [CrossRef] [Green Version]
- Von Garnier, C.; Filgueira, L.; Wikstrom, M.; Smith, M.; Thomas, J.A.; Strickland, D.H.; Holt, P.G.; Stumbles, P.A. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J. Immunol. 2005, 175, 1609–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, E.E.; Looney, M.R.; Bose, O.; Sen, D.; Sheppard, D.; Locksley, R.; Huang, X.; Krummel, M.F. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J. Exp. Med. 2012, 209, 1183–1199. [Google Scholar] [CrossRef] [PubMed]
- Asselin-Paturel, C.; Boonstra, A.; Dalod, M.; Durand, I.; Yessaad, N.; Dezutter-Dambuyant, C.; Vicari, A.; O’Garra, A.; Biron, C.; Brière, F.; et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2001, 2, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.A. The role of dendritic cells in asthma. J. Allergy Clin. Immunol. 2012, 129, 889–901. [Google Scholar] [CrossRef]
- Veres, T.Z.; Voedisch, S.; Spies, E.; Valtonen, J.; Prenzler, F.; Braun, A. Aeroallergen challenge promotes dendritic cell proliferation in the airways. J. Immunol. 2013, 190, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhan, Y.; Lew, A.M.; Naik, S.H.; Kershaw, M.H. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J. Immunol. 2007, 179, 7577–7584. [Google Scholar] [CrossRef] [Green Version]
- Greer, A.M.; Matthay, M.A.; Kukreja, J.; Bhakta, N.R.; Nguyen, C.P.; Wolters, P.J.; Woodruff, P.G.; Fahy, J.V.; Shin, J.S. Accumulation of BDCA1(+) dendritic cells in interstitial fibrotic lung diseases and Th2-high asthma. PLoS ONE 2014, 9, e99084. [Google Scholar] [CrossRef] [Green Version]
- Villani, A.-C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, 6335. [Google Scholar] [CrossRef] [Green Version]
- Calzetti, F.; Tamassia, N.; Micheletti, A.; Finotti, G.; Bianchetto-Aguilera, F.; Cassatella, M.A. Human dendritic cell subset 4 (DC4) correlates to a subset of CD14dim/-CD16++ monocytes. J. Allergy Clin. Immunol. 2018, 141, 2276–2279.e3. [Google Scholar] [CrossRef] [Green Version]
- Van Rijt, L.S.; Jung, S.; Kleinjan, A.; Vos, N.; Willart, M.; Duez, C.; Hoogsteden, H.C.; Lambrecht, B.N. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 2005, 201, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Vroman, H.; Hendriks, R.W.; Kool, M. Dendritic Cell Subsets in Asthma: Impaired Tolerance or Exaggerated Inflammation? Front. Immunol. 2017, 8, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.W.; Prabhu, N.; Betts, R.J.; Ge, M.Q.; Dai, X.; Hutchinson, P.E.; Lew, F.C.; Wong, K.L.; Hanson, B.J.; Macary, P.A.; et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J. Immunol. 2011, 187, 6011–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, H.; Free, M.E.; Whitehead, G.S.; Maruoka, S.; Wilson, R.H.; Nakano, K.; Cook, D.N. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal. Immunol. 2012, 5, 53–65. [Google Scholar] [CrossRef]
- Fear, V.S.; Lai, S.P.; Zosky, G.R.; Perks, K.L.; Gorman, S.; Blank, F.; von Garnier, C.; Stumbles, P.A.; Strickland, D.H. A pathogenic role for the integrin CD103 in experimental allergic airways disease. Physiol. Rep. 2016, 4, e13021. [Google Scholar] [CrossRef]
- Furuhashi, K.; Suda, T.; Hasegawa, H.; Suzuki, Y.; Hashimoto, D.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; Inui, I.; Shibata, K.; et al. Mouse lung CD103+ and CD11bhigh dendritic cells preferentially induce distinct CD4+ T-cell responses. Am. J. Respir. Cell Mol. Biol. 2012, 46, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Raymond, M.; Rubio, M.; Fortin, G.; Shalaby, K.H.; Hammad, H.; Lambrecht, B.N.; Sarfati, M. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. J. Allergy Clin. Immunol. 2009, 124, 1333–1342. [Google Scholar] [CrossRef]
- Norimoto, A.; Hirose, K.; Iwata, A.; Tamachi, T.; Yokota, M.; Takahashi, K.; Saijo, S.; Iwakura, Y.; Nakajima, H. Dectin-2 promotes house dust mite-induced T helper type 2 and type 17 cell differentiation and allergic airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2014, 51, 201–209. [Google Scholar] [CrossRef]
- Mishra, A.; Brown, A.L.; Yao, X.; Yang, S.; Park, S.J.; Liu, C.; Dagur, P.K.; McCoy, J.P.; Keeran, K.J.; Nugent, G.Z.; et al. Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase. Nat. Commun. 2015, 6, 6224. [Google Scholar] [CrossRef]
- Medoff, B.D.; Seung, E.; Hong, S.; Thomas, S.Y.; Sandall, B.P.; Duffield, J.S.; Kuperman, D.A.; Erle, D.J.; Luster, A.D. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J. Immunol. 2009, 182, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewkowich, I.P.; Lajoie, S.; Clark, J.R.; Herman, N.S.; Sproles, A.A.; Wills-Karp, M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS ONE 2008, 3, e3879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kool, M.; Geurtsvankessel, C.; Muskens, F.; Madeira, F.B.; van Nimwegen, M.; Kuipers, H.; Thielemans, K.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen-presenting potential of plasmacytoid DCs. J. Leukoc. Biol. 2011, 90, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, V.; Speak, A.O.; Kerzerho, J.; Szely, N.; Akbari, O. CD8alpha(+)beta(-) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 2012, 5, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Ishii, Y.; Hata-Suzuki, M.; Arai, R.; Chibana, K.; Takemasa, A.; Fukuda, T. Comparative analysis of circulating dendritic cell subsets in patients with atopic diseases and sarcoidosis. Respir. Res. 2013, 14, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dua, B.; Watson, R.M.; Gauvreau, G.M.; O’Byrne, P.M. Myeloid and plasmacytoid dendritic cells in induced sputum after allergen inhalation in subjects with asthma. J. Allergy Clin. Immunol. 2010, 126, 133–139. [Google Scholar] [CrossRef]
- Dua, B.; Tang, W.; Watson, R.; Gauvreau, G.; O’Byrne, P.M. Myeloid dendritic cells type 2 after allergen inhalation in asthmatic subjects. Clin. Exp. Allergy 2014, 44, 921–929. [Google Scholar] [CrossRef]
- El-Gammal, A.; Oliveria, J.P.; Howie, K.; Watson, R.; Mitchell, P.; Chen, R.; Baatjes, A.; Smith, S.; Al-Sajee, D.; Hawke, T.J.; et al. Allergen-induced changes in bone marrow and airway dendritic cells in subjects with asthma. Am. J. Respir. Crit. Care Med. 2016, 194, 169–177. [Google Scholar] [CrossRef]
- Sharquie, I.K.; Al-Ghouleh, A.; Fitton, P.; Clark, M.R.; Armour, K.L.; Sewell, H.F.; Shakib, F.; Ghaemmaghami, A.M. An investigation into IgE-facilitated allergen recognition and presentation by human dendritic cells. BMC Immunol. 2013, 14, 54. [Google Scholar] [CrossRef] [Green Version]
- Froidure, A.; Shen, C.; Gras, D.; Van Snick, J.; Chanez, P.; Pilette, C. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin-mediated induction of Th2 and Th9 responses. Allergy 2014, 69, 1068–1076. [Google Scholar] [CrossRef]
- Spears, M.; McSharry, C.; Donnelly, I.; Jolly, L.; Brannigan, M.; Thomson, J.; Lafferty, J.; Chaudhuri, R.; Shepherd, M.; Cameron, E.; et al. Peripheral blood dendritic cell subtypes are significantly elevated in subjects with asthma. Clin. Exp. Allergy 2011, 41, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.K.; Mistry, V.; Richardson, M.; Shelley, M.; Thornton, T.; Terry, S.; Barker, B.; Bafadhel, M.; Brightling, C. Toll-like receptor 9 dependent interferon-alpha release is impaired in severe asthma but is not associated with exacerbation frequency. Immunobiology 2015, 220, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Bratke, K.; Lommatzsch, M.; Julius, P.; Kuepper, M.; Kleine, H.D.; Luttmann, W.; Virchow, J.C. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax 2007, 62, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upham, J.W.; Zhang, G.; Rate, A.; Yerkovich, S.T.; Kusel, M.; Sly, P.D.; Holt, P.G. Plasmacytoid dendritic cells during infancy are inversely associated with childhood respiratory tract infections and wheezing. J. Allergy Clin. Immunol. 2009, 124, 707–713. [Google Scholar] [CrossRef]
- Hammad, H.; Charbonnier, A.S.; Duez, C.; Jacquet, A.; Stewart, G.A.; Tonnel, A.B.; Pestel, J. Th2 polarization by Der p 1—Pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood 2001, 98, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Geurts van Kessel, C.H.; Lambrecht, B.N. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008, 1, 442–450. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Khare, A.; Krishnamoorthy, N.; Oriss, T.B.; Fei, M.; Ray, P.; Ray, A. Cutting edge: Inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. J. Immunol. 2013, 191, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Bernatchez, E.; Gold, M.J.; Langlois, A.; Lemay, A.M.; Brassard, J.; Flamand, N.; Marsolais, D.; McNagny, K.M.; Blanchet, M.R. Pulmonary CD103 expression regulates airway inflammation in asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L816–L826. [Google Scholar] [CrossRef] [Green Version]
- Conejero, L.; Khouili, S.C.; Martínez-Cano, S.; Izquierdo, H.M.; Brandi, P.; Sancho, D. Lung CD103+ dendritic cells restrain allergic airway inflammation through IL-12 production. JCI Insight 2017, 2, 90420. [Google Scholar] [CrossRef] [Green Version]
- Engler, D.B.; Reuter, S.; van Wijck, Y.; Urban, S.; Kyburz, A.; Maxeiner, J.; Martin, H.; Yogev, N.; Waisman, A.; Gerhard, M.; et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc. Natl. Acad. Sci. USA 2014, 111, 11810–11815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koya, T.; Matsuda, H.; Takeda, K.; Matsubara, S.; Miyahara, N.; Balhorn, A.; Dakhama, A.; Gelfand, E.W. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J. Allergy Clin. Immunol. 2017, 119, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Dawicki, W.; Lu, M.; Nayyar, A.; Zhang, X.; Gordon, J.R. Regulatory dendritic cell expression of MHCII and IL-10 are jointly requisite for induction of tolerance in a murine model of OVA-asthma. Allergy 2013, 68, 1126–1135. [Google Scholar] [CrossRef]
- Fujita, S.; Yamashita, N.; Ishii, Y.; Sato, Y.; Sato, K.; Eizumi, K.; Fukaya, T.; Nozawa, R.; Takamoto, Y.; Yamashita, N.; et al. Regulatory dendritic cells protect against allergic airway inflammation in a murine asthmatic model. J. Allergy Clin. Immunol. 2008, 121, 95–104.e7. [Google Scholar] [CrossRef]
- Lu, M.; Dawicki, W.; Zhang, X.; Huang, H.; Nayyar, A.; Gordon, J.R. Therapeutic induction of tolerance by IL-10-differentiated dendritic cells in a mouse model of house dust mite-asthma. Allergy 2011, 66, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Dawicki, W.; Huang, H.; Lu, M.; Zhang, X.; Gordon, J.R. Induction of prolonged asthma tolerance by IL-10-differentiated dendritic cells: Differential impact on airway hyperresponsiveness and the Th2 immunoinflammatory response. J. Immunol. 2012, 189, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Aragão-França, L.S.; Rocha, V.C.J.; Cronemberger-Andrade, A.; Da Costa, F.H.B.; Vasconcelos, J.F.; Athanazio, D.A.; Silva, D.N.; Santos, E.S.; Meira, C.S.; Araujo, C.F.; et al. Tolerogenic Dendritic Cells Reduce Airway Inflammation in a Model of Dust Mite Triggered Allergic Inflammation. Allergy Asthma Immunol. Res. 2018, 10, 406–419. [Google Scholar] [CrossRef]
- Wong, T.H.; Chen, H.A.; Gau, R.J.; Yen, J.H.; Suen, J.L. Heme Oxygenase-1-Expressing Dendritic Cells Promote Foxp3+ Regulatory T Cell Differentiation and Induce Less Severe Airway Inflammation in Murine Models. PLoS ONE 2016, 11, e0168919. [Google Scholar] [CrossRef]
- Shim, J.U.; Lee, S.E.; Hwang, W.; Lee, C.; Park, J.W.; Sohn, J.H.; Nam, J.H.; Kim, Y.; Rhee, J.H.; Im, S.H.; et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J. Allergy Clin. Immunol. 2016, 137, 426–435. [Google Scholar] [CrossRef]
- Oriss, T.B.; Ostroukhova, M.; Seguin-Devaux, C.; Dixon-McCarthy, B.; Stolz, D.B.; Watkins, S.C.; Pillemer, B.; Ray, P.; Ray, A. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J. Immunol. 2005, 174, 854–863. [Google Scholar] [CrossRef] [Green Version]
- Lewkowich, I.P.; Herman, N.S.; Schleifer, K.W.; Dance, M.P.; Chen, B.L.; Dienger, K.M.; Sproles, A.A.; Shah, J.S.; Köhl, J.; Belkaid, Y.; et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005, 202, 1549–1561. [Google Scholar] [CrossRef]
- De Heer, H.J.; Hammad, H.; Soullié, T. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 2004, 200, 89–98. [Google Scholar] [CrossRef]
- Kool, M.; van Nimwegen, M.; Willart, M.A.; Muskens, F.; Boon, L.; Smit, J.J.; Coyle, A.; Clausen, B.J.; Hoogsteden, H.C.; Lambrecht, B.N.; et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J. Immunol. 2009, 183, 1074–1082. [Google Scholar] [CrossRef] [Green Version]
- Chairakaki, A.D.; Saridaki, M.I.; Pyrillou, K.; Mouratis, M.A.; Koltsida, O.; Walton, R.P.; Bartlett, N.W.; Stavropoulos, A.; Boon, L.; Rovina, N.; et al. Plasmacytoid dendritic cells drive acute asthma exacerbations. J. Allergy Clin. Immunol. 2018, 142, 542–556.e12. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.S.; Maizels, R.M. Alarming dendritic cells for Th2 induction. J. Exp. Med. 2008, 205, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willart, M.A.M.; Hammad, H. Alarming dendritic cells for allergic sensitization. Allergol. Int. 2010, 59, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Dahl, M.E.; Dabbagh, K.; Liggitt, D.; Kim, S.; Lewis, D.B. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat. Immunol. 2004, 5, 337–343. [Google Scholar] [CrossRef] [PubMed]
- D’Hulst, A.I.; Vermaelen, K.Y.; Brusselle, G.G.; Joos, G.F.; Pauwels, R.A. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur. Respir. J. 2005, 26, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Brewer, J.M.; Conacher, M.; Hunter, C.A.; Mohrs, M.; Brombacher, F.; Alexander, J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 1999, 163, 6448–6454. [Google Scholar]
- Ostroukhova, M.; Seguin-Devaux, C.; Oriss, T.B.; Dixon-McCarthy, B.; Yang, L.; Ameredes, B.T.; Corcoran, T.E.; Ray, A. Tolerance induced by inhaled antigen involves CD4 (+) T cells expressing membrane-bound TGF-beta and FOXP3. J. Clin. Investig. 2004, 114, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Gern, J.E. Barnyard microbes and childhood asthma. N. Engl. J. Med. 2011, 364, 769–770. [Google Scholar] [CrossRef]
- Rabinovitch, N.; Liu, A.H.; Zhang, L.; Rodes, C.E.; Foarde, K.; Dutton, S.J.; Murphy, J.R.; Gelfand, E.W. Importance of the personal endotoxin cloud in school-age children with asthma. J. Allergy Clin. Immunol. 2005, 116, 1053–1057. [Google Scholar] [CrossRef]
- Haspeslagh, E.; Heyndrickx, I.; Hammad, H.; Lambrecht, B.N. The hygiene hypothesis: Immunological mechanisms of airway tolerance. Curr. Opin. Immunol. 2018, 54, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Vanichsarn, C.; Nadeau, K.C. Impaired IL-10-dependent induction of tolerogenic dendritic cells by CD4+CD25hiCD127lo/- natural regulatory T cells in human allergic asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 823–833. [Google Scholar] [CrossRef]
- Semitekolou, M.; Alissafi, T.; Aggelakopoulou, M.; Kourepini, E.; Kariyawasam, H.H.; Kay, A.B.; Robinson, D.S.; Lloyd, C.M.; Panoutsakopoulou, V.; Xanthou, G. Activin-A induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. J. Exp. Med. 2009, 206, 1769–1785. [Google Scholar] [CrossRef] [Green Version]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Bellinghausen, I.; Brand, U.; Steinbrink, K.; Enk, A.H.; Knop, J.; Saloga, J. Inhibition of human allergic T-cell responses by IL-10-treated dendritic cells: Differences from hydrocortisone-treated dendritic cells. J. Allergy Clin. Immunol. 2001, 108, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Aguirre, A.; Guzmán, M.A.; González, R.; Catalán, D.; Acuña-Castillo, C.; Larrondo, M.; López, M.; Pesce, B.; Rolland, J.; et al. Tolerogenic dendritic cells derived from donors with natural rubber latex allergy modulate allergen-specific T-cell responses and IgE production. PLoS ONE 2014, 9, e85930. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morianos, I.; Semitekolou, M. Dendritic Cells: Critical Regulators of Allergic Asthma. Int. J. Mol. Sci. 2020, 21, 7930. https://doi.org/10.3390/ijms21217930
Morianos I, Semitekolou M. Dendritic Cells: Critical Regulators of Allergic Asthma. International Journal of Molecular Sciences. 2020; 21(21):7930. https://doi.org/10.3390/ijms21217930
Chicago/Turabian StyleMorianos, Ioannis, and Maria Semitekolou. 2020. "Dendritic Cells: Critical Regulators of Allergic Asthma" International Journal of Molecular Sciences 21, no. 21: 7930. https://doi.org/10.3390/ijms21217930
APA StyleMorianos, I., & Semitekolou, M. (2020). Dendritic Cells: Critical Regulators of Allergic Asthma. International Journal of Molecular Sciences, 21(21), 7930. https://doi.org/10.3390/ijms21217930



