Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway
Abstract
1. Introduction
2. Results
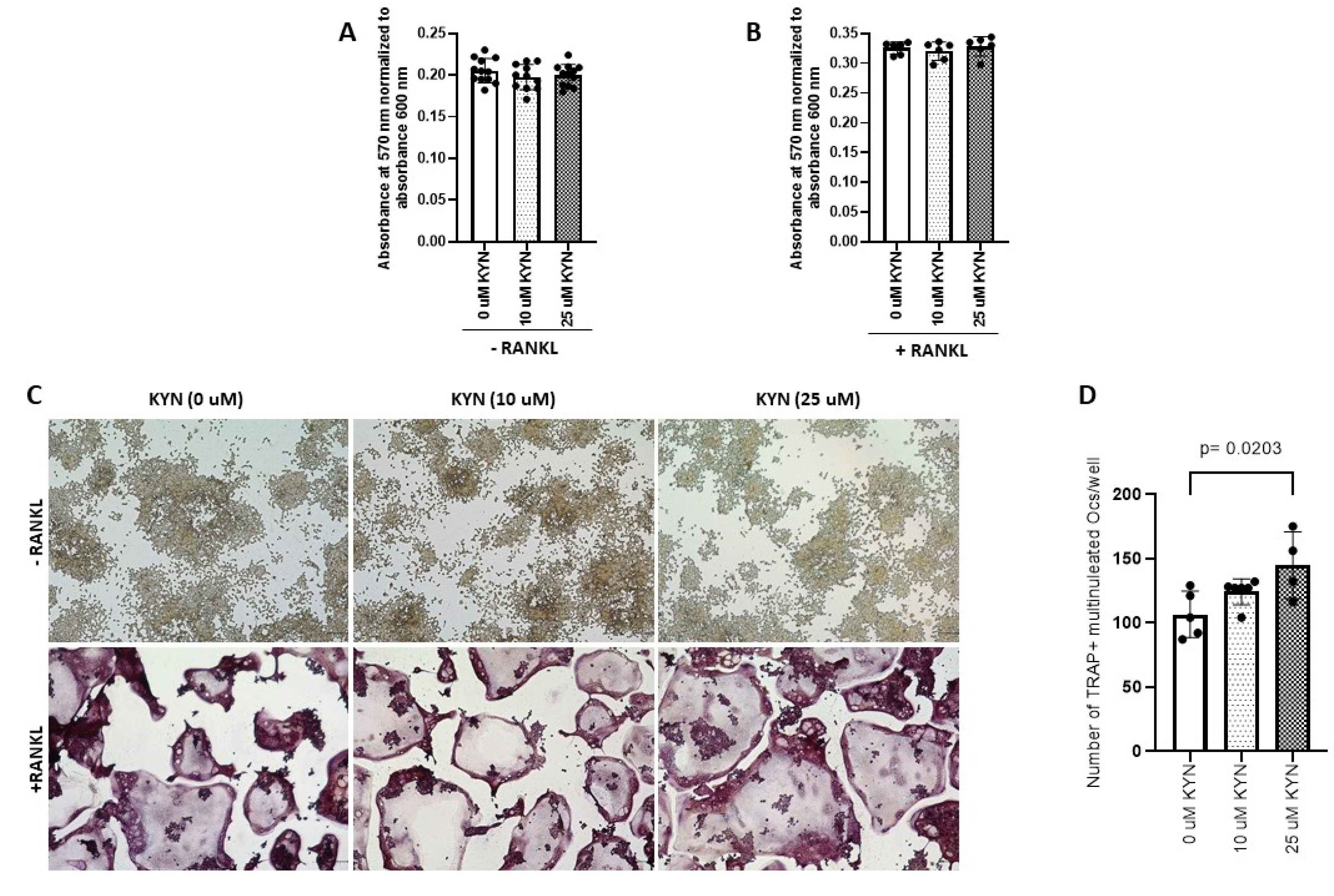
2.1. Kynurenine Treatment Enhances and Drives RANKL-Mediated Osteoclast Differentiation of Raw 264.7 Cells
2.2. Kynurenine Treatment Induces RANKL-Mediated Osteoclast Bone Resorption Activity of Raw 264.7 Cells
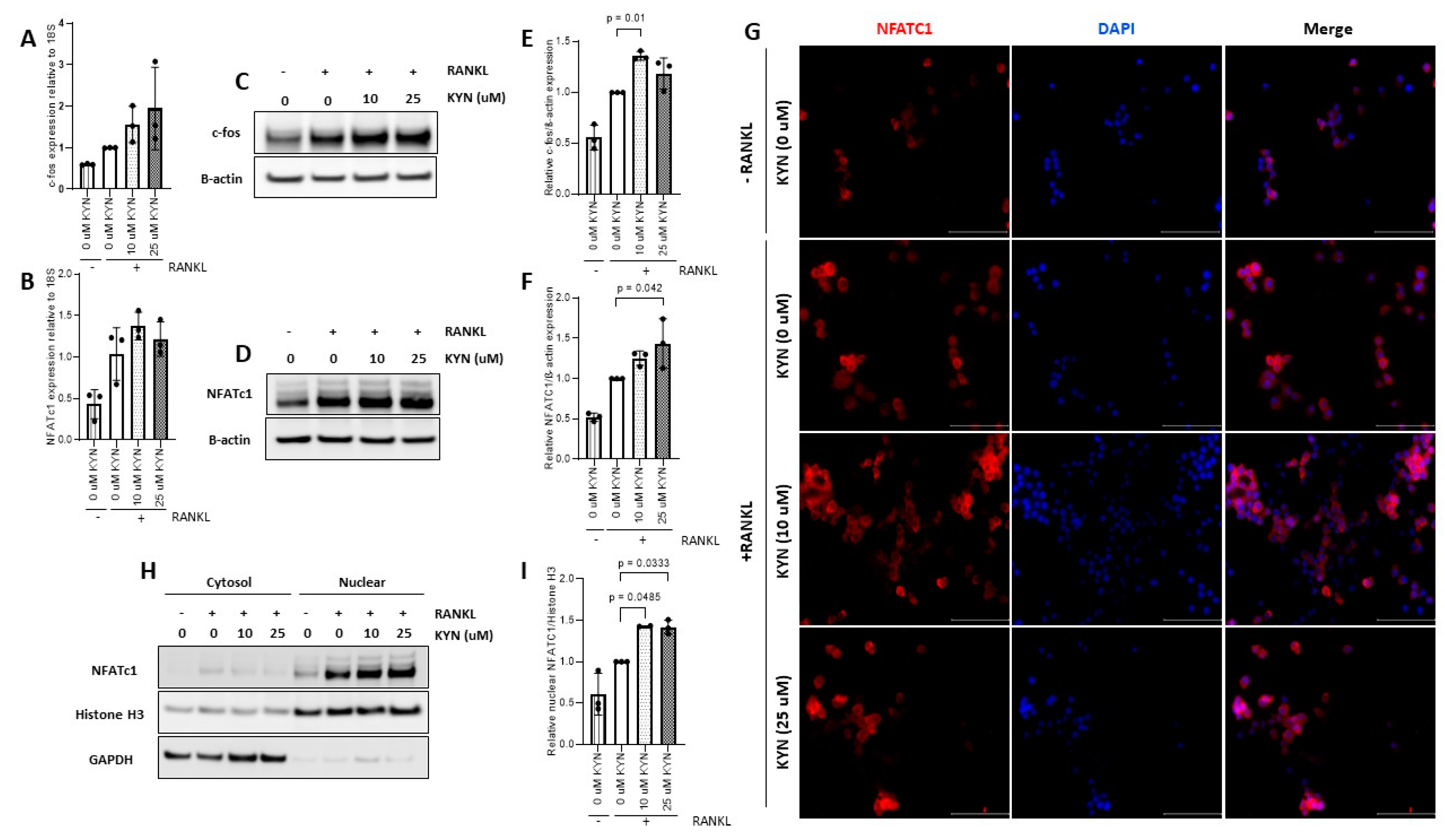
2.3. Kynurenine Treatment Upregulates the Expression of Osteoclast Differentiation Markers c-fos and NFATc1
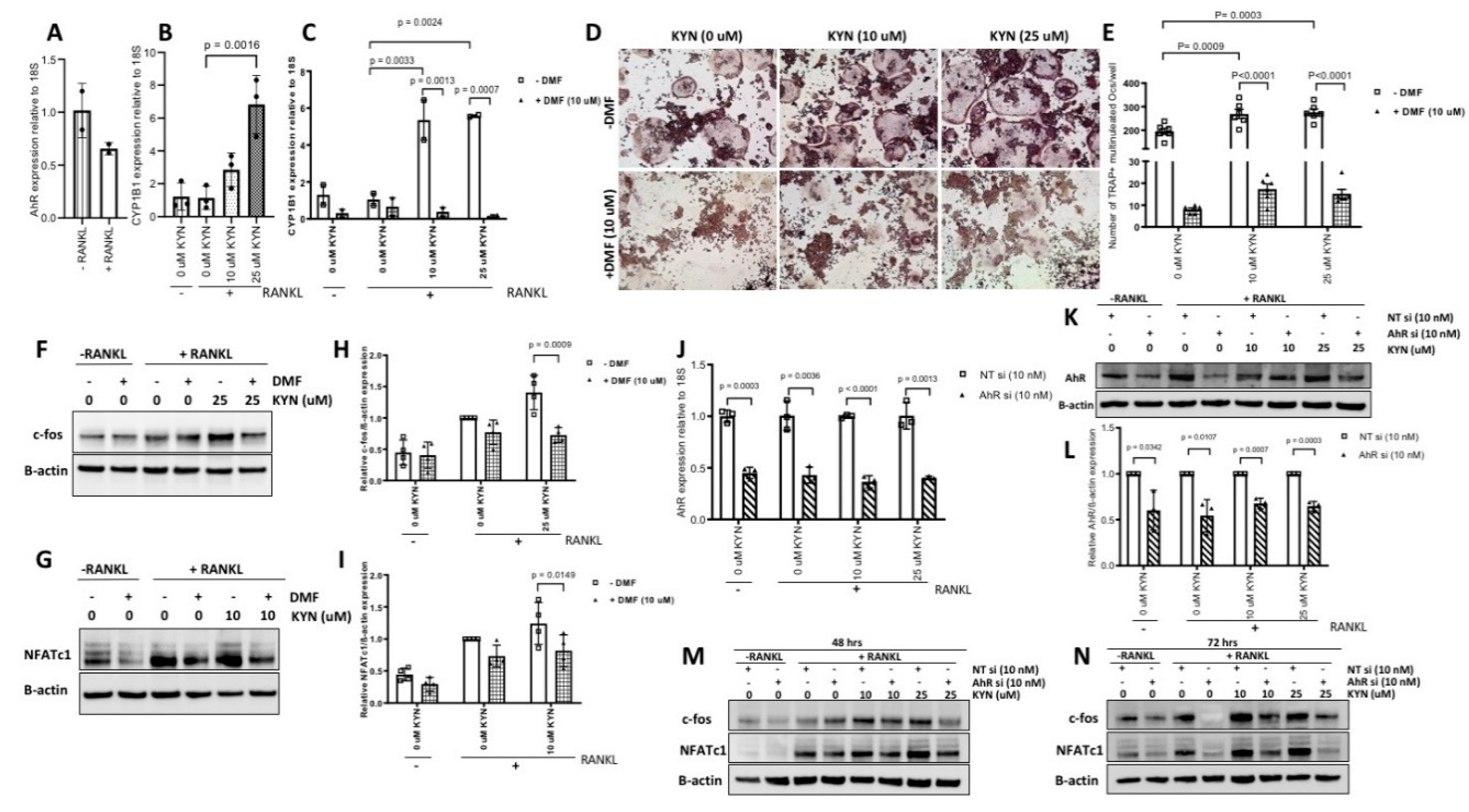
2.4. Blocking AhR Signaling Attenuates KYN/RANKL Induced Osteoclast Differentiation of Raw 264.7 Cells via Inhibiting c-fos and NFATc1 Expression
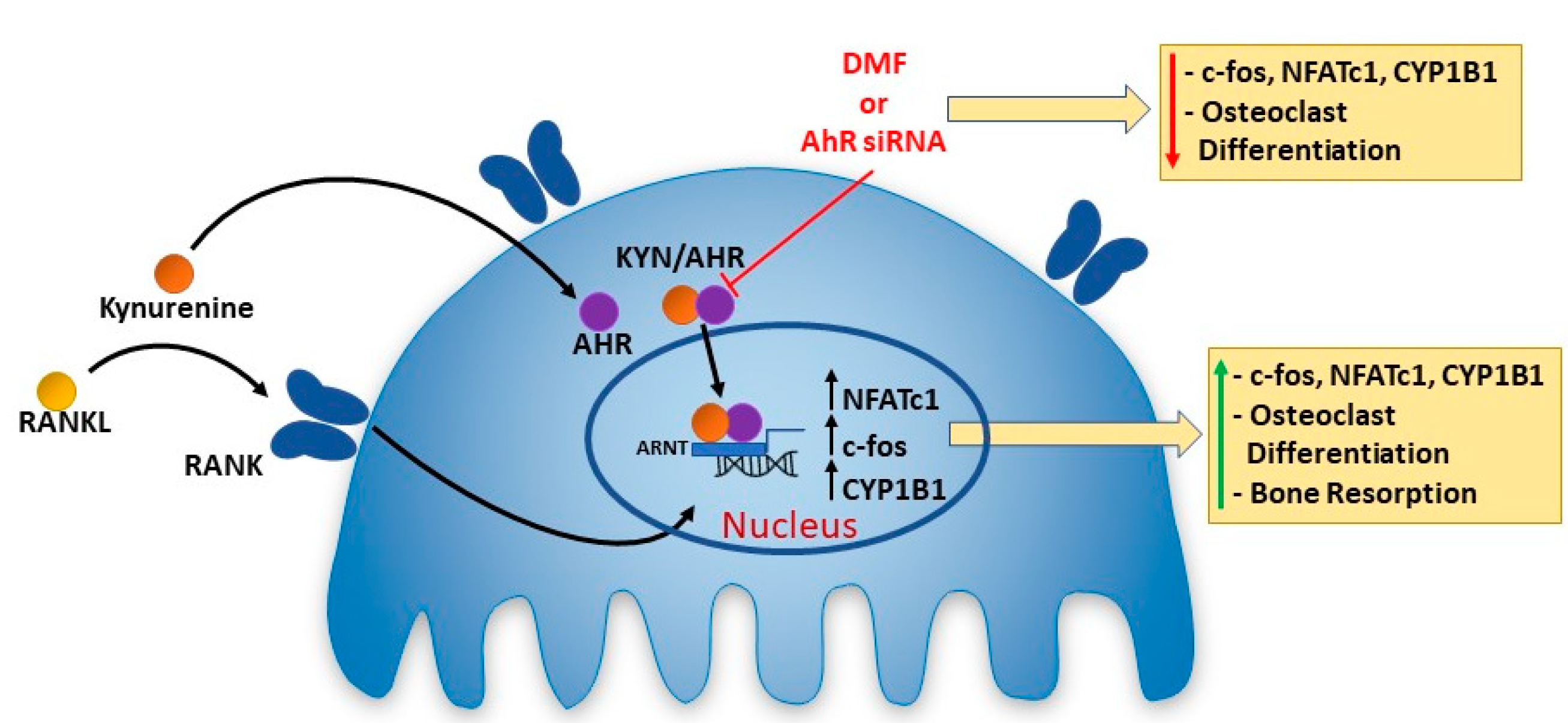
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Kynurenine (KYN) Doses and Cell Viability Assay
4.3. Osteoclast Differentiation and Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.4. Hydroxyapatite Resorption Assay
4.5. Western Blotting
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Immunofluorescence Microscopy
4.8. AhR Blocking
4.9. AhR Silencing
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| BaP | Benzo(a)pyrene |
| BMSCs | Bone marrow mesenchymal stem cells |
| Cyp1A/1B | cytochrome P450 1a/1b |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMF | 3′,4′-dimethoxyflavone |
| FBS | Heat-inactivated fetal bovine serum |
| IDO-1 or 2 | Indoleamine 2,3-dioxygenase-1 or 2 |
| KYN | kynurenine |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| siRNA | Small interfering RNA |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TDO | Tryptophan 2,3-dioxygenase |
| TRAP | Tartrate-resistant acid phosphatase |
| β-NF | β-naphthoflavone |
References
- Infante, A.; Rodriguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef]
- Hoffman, C.M.; Han, J.; Calvi, L.M. Impact of aging on bone, marrow and their interactions. Bone 2018, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ou, G.; Hamrick, M.; Hill, W.; Borke, J.; Wenger, K.; Chutkan, N.; Yu, J.; Mi, Q.-S.; Isales, C.M.; et al. Age-Related Changes in the Osteogenic Differentiation Potential of Mouse Bone Marrow Stromal Cells. J. Bone Miner. Res. 2008, 23, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.-L.; Zhou, S.; Eslami, B.; Shen, L.; LeBoff, M.S.; Glowacki, J. Effect of age on regulation of human osteoclast differentiation. J. Cell. Biochem. 2014, 115, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Wronski, T.J.; Iwaniec, U.; Phleger, L.; Kurimoto, P.; Boudignon, B.; Halloran, B.P. Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nowak, T.S.; Suyama, K.; Quearry, B.J.; Saito, M.; Crowley, J.S.; Markey, S.P.; Heyes, M.P. Kynurenine Pathway Enzymes in Brain: Responses to Ischemic Brain Injury Versus Systemic Immune Activation. J. Neurochem. 1993, 61, 2061–2070. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: Implications for aging and aging-associated psychiatric and medical disorders. J. Neural Transm. 2010, 118, 75–85. [Google Scholar] [CrossRef]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Grant, R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011, 278, 4425–4434. [Google Scholar] [CrossRef]
- Platten, M.; Weller, M.; Wick, W. Shaping the glioma immune microenvironment through tryptophan metabolism. CNS Oncol. 2012, 1, 99–106. [Google Scholar] [CrossRef]
- Refaey, M.E.; McGee-Lawrence, M.E.; Fulzele, S.; Kennedy, E.J.; Bollag, W.B.; Elsalanty, M.; Zhong, Q.; Ding, K.H.; Bendzunas, N.G.; Shi, X.M.; et al. Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Elmansi, A.M.; Hussein, K.A.; Herrero, S.M.; Periyasamy-Thandavan, S.; Aguilar-Pérez, A.; Kondrikova, G.; Kondrikov, D.; Eisa, N.H.; Pierce, J.L.; Kaiser, H.; et al. Age-related increase of kynurenine enhances miR29b-1-5p to decrease both CXCL12 signaling and the epigenetic enzyme Hdac3 in bone marrow stromal cells. Bone Rep. 2020, 12, 100270. [Google Scholar] [CrossRef]
- Kondrikov, D.; Elmansi, A.; Bragg, R.T.; Mobley, T.; Barrett, T.; Eisa, N.; Kondrikova, G.; Schoeinlein, P.; Aguilar-Perez, A.; Shi, X.M.; et al. Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp. Gerontol. 2020, 130, 110805. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hamrick, M.W.; Yoo, H.J.; Lee, S.H.; Kim, S.J.; Koh, J.M.; Isales, C.M. The Detrimental Effects of Kynurenine, a Tryptophan Metabolite, on Human Bone Metabolism. J. Clin. Endocrinol. Metab. 2019, 104, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef]
- Iqbal, J.; Sun, L.; Cao, J.; Yuen, T.; Lu, P.; Bab, I.; Leu, N.A.; Srinivasan, S.; Wagage, S.; Hunter, C.A.; et al. Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc. Natl. Acad. Sci. USA 2013, 110, 11115–11120. [Google Scholar] [CrossRef]
- Yu, T.Y.; Kondo, T.; Matsumoto, T.; Fujii-Kuriyama, Y.; Imai, Y. Aryl hydrocarbon receptor catabolic activity in bone metabolism is osteoclast dependent in vivo. Biochem. Biophys. Res. Commun. 2014, 450, 416–422. [Google Scholar] [CrossRef]
- Yu, T.-y.; Pang, W.-j.; Yang, G.-s. 3,3′-Diindolylmethane increases bone mass by suppressing osteoclastic bone resorption in mice. J. Pharmacol. Sci. 2015, 127, 75–82. [Google Scholar] [CrossRef]
- Voronov, I.; Heersche, J.N.; Casper, R.F.; Tenenbaum, H.C.; Manolson, M.F. Inhibition of osteoclast differentiation by polycyclic aryl hydrocarbons is dependent on cell density and RANKL concentration. Biochem. Pharm. 2005, 70, 300–307. [Google Scholar] [CrossRef]
- Jia, Y.; Tao, Y.; Lv, C.; Xia, Y.; Wei, Z.; Dai, Y. Tetrandrine enhances the ubiquitination and degradation of Syk through an AhR-c-src-c-Cbl pathway and consequently inhibits osteoclastogenesis and bone destruction in arthritis. Cell Death Dis. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jiang, L.; Wan, B.; Zhang, W.; Yao, L.; Che, T.; Gan, C.; Su, N.; He, J.; Huang, J.; et al. The role of aryl hydrocarbon receptor in bone remodeling. Prog. Biophys. Mol. Biol. 2018, 134, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.B.; Yang, D.; Ha, H.; Lee, J.H.; Kim, H.N.; Woo, E.R.; Lee, S.; Kim, H.H.; Lee, Z.H. Tanshinone IIA inhibits osteoclast differentiation through down-regulation of c-Fos and NFATc1. Exp. Mol. Med. 2006, 38, 256–264. [Google Scholar] [CrossRef]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Adv. Musculoskelet Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef]
- Boskey, A.L.; Coleman, R. Aging and bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef]
- Rodan, G.A. Bone homeostasis. Proc. Natl. Acad. Sci. USA 1998, 95, 13361–13362. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology (Bethesda) 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Bone Cell Communication Factors Provide a New Therapeutic Strategy for Osteoporosis. Chonnam Med. J. 2020, 56, 94–98. [Google Scholar] [CrossRef]
- Kalaska, B.; Pawlak, K.; Domaniewski, T.; Oksztulska-Kolanek, E.; Znorko, B.; Roszczenko, A.; Rogalska, J.; Brzoska, M.M.; Lipowicz, P.; Doroszko, M.; et al. Elevated Levels of Peripheral Kynurenine Decrease Bone Strength in Rats with Chronic Kidney Disease. Front. Physiol. 2017, 8, 836. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Grigoriadis, A.E.; Wang, Z.Q.; Cecchini, M.G.; Hofstetter, W.; Felix, R.; Fleisch, H.A.; Wagner, E.F. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994, 266, 443–448. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Ueki, Y.; Sulyanto, R.; Park, A.; Sigrist, K.S.; Sharma, S.M.; Ostrowski, M.C.; Olsen, B.R.; Glimcher, L.H. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Investig. 2008, 118, 3775–3789. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ovitt, C.; Grigoriadis, A.E.; Möhle-Steinlein, U.; Rüther, U.; Wagner, E.F. Bone and haematopoietic defects in mice lacking c-fos. Nature 1992, 360, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam Med. J. 2016, 52, 12–17. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef]
- Kamiya, Y.; Kikuchi, T.; Goto, H.; Okabe, I.; Takayanagi, Y.; Suzuki, Y.; Sawada, N.; Okabe, T.; Suzuki, Y.; Kondo, S.; et al. IL-35 and RANKL Synergistically Induce Osteoclastogenesis in RAW264 Mouse Monocytic Cells. Int. J. Mol. Sci. 2020, 21, 2069. [Google Scholar] [CrossRef]
- Choe, J.-Y.; Park, K.-Y.; Kim, S.-K. Monosodium Urate in the Presence of RANKL Promotes Osteoclast Formation through Activation of c-Jun N-Terminal Kinase. Mediat. Inflamm. 2015, 2015, 597512. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, C.; Li, X.; Yang, J.; Yu, T.; Zhang, R.; Zhang, T.; Saxena, D.; Snyder, M.; Wu, Y.; et al. Succinate and its G-protein-coupled receptor stimulates osteoclastogenesis. Nat. Commun. 2017, 8, 15621. [Google Scholar] [CrossRef]
- Yu, T.Y.; Pang, W.J.; Yang, G.S. Aryl hydrocarbon receptors in osteoclast lineage cells are a negative regulator of bone mass. PLoS ONE 2015, 10, e0117112. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Arakaki, R.; Mori, H.; Tsunematsu, T.; Kudo, Y.; Tanaka, E.; Ishimaru, N. The Nuclear Receptor AhR Controls Bone Homeostasis by Regulating Osteoclast Differentiation via the RANK/c-Fos Signaling Axis. J. Immunol. 2016, 197, 4639–4650. [Google Scholar] [CrossRef] [PubMed]
- Mulero-Navarro, S.; Fernandez-Salguero, P.M. New Trends in Aryl Hydrocarbon Receptor Biology. Front. Cell Dev. Biol. 2016, 4, 45. [Google Scholar] [CrossRef]
- Duan, R.; Porter, W.; Samudio, I.; Vyhlidal, C.; Kladde, M.; Safe, S. Transcriptional Activation of c-fos Protooncogene by 17β-Estradiol: Mechanism of Aryl Hydrocarbon Receptor-Mediated Inhibition. Mol. Endocrinol. 1999, 13, 1511–1521. [Google Scholar] [PubMed]
- Beischlag, T.V.; Luis Morales, J.; Hollingshead, B.D.; Perdew, G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Savas, U.; Alexander, D.L.; Jefcoate, C.R. Characterization of the mouse Cyp1B1 gene. Identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. J. Biol. Chem. 1998, 273, 5174–5183. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Shyu, J.F.; Lim, P.S.; Fang, T.C.; Lu, C.L.; Zheng, C.M.; Hou, Y.C.; Wu, C.C.; Lin, Y.F.; Lu, K.C. Concentration and Duration of Indoxyl Sulfate Exposure Affects Osteoclastogenesis by Regulating NFATc1 via Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 3486. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Arakaki, R.; Ishimaru, N. Crosstalk between Cytokine RANKL and AhR Signalling in Osteoclasts Controls Bone Homeostasis. J. Cytokine Biol. 2017, 2, 114. [Google Scholar] [CrossRef]
- Mejía-García, A.; González-Barbosa, E.; Martínez-Guzmán, C.; Torres-Ramos, M.A.; Rodríguez, M.S.; Guzmán-León, S.; Elizondo, G. Activation of AHR mediates the ubiquitination and proteasome degradation of c-Fos through the induction of Ubcm4 gene expression. Toxicology 2015, 337, 47–57. [Google Scholar] [CrossRef]
- Talari, N.K.; Panigrahi, M.K.; Madigubba, S.; Phanithi, P.B. Overexpression of aryl hydrocarbon receptor (AHR) signalling pathway in human meningioma. J. Neurooncol. 2018, 137, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.J.; Liu, J.; Coenraads, P.J.; Dong, L.; Zhao, L.J.; Ma, S.W.; Chen, X.; Zhang, C.M.; Ma, X.M.; Wei, W.G.; et al. Expression of AhR, CYP1A1, GSTA1, c-fos and TGF-alpha in skin lesions from dioxin-exposed humans with chloracne. Toxicol. Lett. 2008, 177, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017, 112, 248–263. [Google Scholar] [CrossRef]
- van der Goot, A.T.; Nollen, E.A.A. Tryptophan metabolism: Entering the field of aging and age-related pathologies. Trends Mol. Med. 2013, 19, 336–344. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hatabayashi, K.; Arita, M.; Yajima, N.; Takenaka, C.; Suzuki, T.; Takahashi, M.; Oshima, Y.; Hara, K.; Kagawa, K.; et al. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal. 2019, 12, eaaw3306. [Google Scholar] [CrossRef] [PubMed]

| Gene | Assay ID |
|---|---|
| 18S | Mm03928990_g1 |
| AhR | Mm00478932_m1 |
| c-fos | Mm00487425_m1 |
| NFATc1 | Mm01265944_m1 |
| CYP1B1 | Mm00487229_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisa, N.H.; Reddy, S.V.; Elmansi, A.M.; Kondrikova, G.; Kondrikov, D.; Shi, X.-M.; Novince, C.M.; Hamrick, M.W.; McGee-Lawrence, M.E.; Isales, C.M.; et al. Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway. Int. J. Mol. Sci. 2020, 21, 7931. https://doi.org/10.3390/ijms21217931
Eisa NH, Reddy SV, Elmansi AM, Kondrikova G, Kondrikov D, Shi X-M, Novince CM, Hamrick MW, McGee-Lawrence ME, Isales CM, et al. Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway. International Journal of Molecular Sciences. 2020; 21(21):7931. https://doi.org/10.3390/ijms21217931
Chicago/Turabian StyleEisa, Nada H., Sakamuri V. Reddy, Ahmed M. Elmansi, Galina Kondrikova, Dmitry Kondrikov, Xing-Ming Shi, Chad M. Novince, Mark W. Hamrick, Meghan E. McGee-Lawrence, Carlos M. Isales, and et al. 2020. "Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway" International Journal of Molecular Sciences 21, no. 21: 7931. https://doi.org/10.3390/ijms21217931
APA StyleEisa, N. H., Reddy, S. V., Elmansi, A. M., Kondrikova, G., Kondrikov, D., Shi, X.-M., Novince, C. M., Hamrick, M. W., McGee-Lawrence, M. E., Isales, C. M., Fulzele, S., & Hill, W. D. (2020). Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway. International Journal of Molecular Sciences, 21(21), 7931. https://doi.org/10.3390/ijms21217931





