The Role of Zinc in Male Fertility
Abstract
1. Introduction
2. Zinc and fertility
2.1. Regulation of Intracellular Zn2+ Concentrations
2.2. Involvement of Zn2+ in Sperm Motility
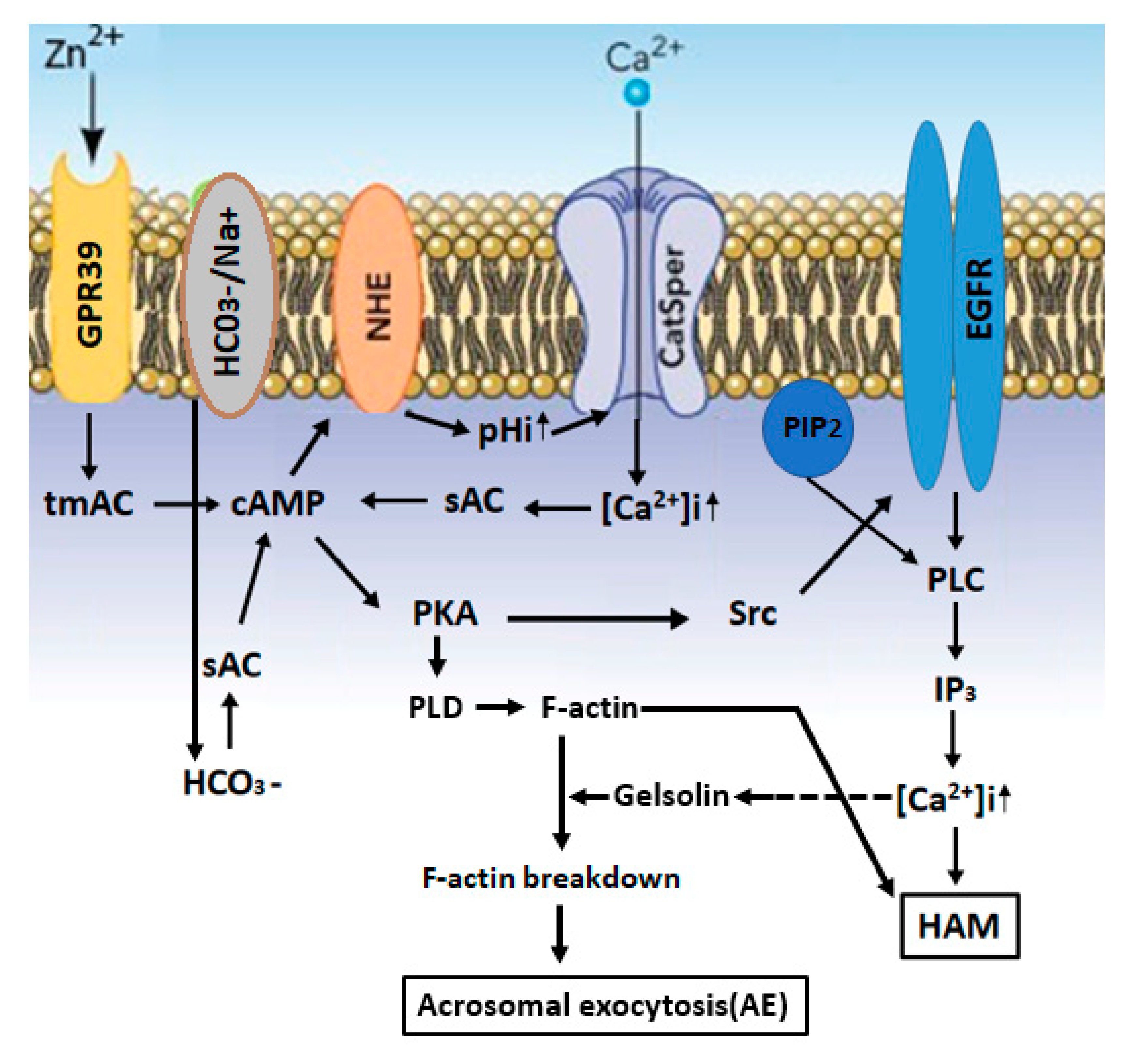
2.3. Zn2+ Mediates Sperm Capacitation and Acrosomal Exocytosis
2.4. Zinc in Assisted Reproductive Techniques
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | linear dichroism |
References
- Chvapil, M. New aspects in the biological role of zinc: A stabilizer of macromolecules and biological membranes. Life Sci. 1973, 13, 1041–1049. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Parashuramulu, S.; Nagalakshmi, D.; Rao, D.S.; Kumar, M.K.; Swain, P. Effect of Zinc supplementation on antioxidant status and immune response in buffalo calves. Anim. Nutr. Feed Technol. 2015, 15, 179–188. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef]
- Yan, M.; Hardin, K.; Ho, E. Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J. Nutr. Biochem. 2010, 21, 687–694. [Google Scholar] [CrossRef][Green Version]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc De-ficiency. In Nutritional Deficiency; Erkekoglu, P., Kocel-Gumusel, B., Eds.; Tech Open Science: Rijeka, Croatia, 2016; pp. 23–46. [Google Scholar]
- Wani, A.L.; Parveen, N.; Ansari, M.O.; Ahmad, M.F.; Ja-meel, S.; Shadab, G. Zinc: An element of extensive medical importance. Curr. Med. Res. Pract. 2017, 7, 90–98. [Google Scholar] [CrossRef]
- Wong, W.Y.; Flik, G.; Groenen, P.M.; Swinkels, D.W.; Thomas, C.M.; Copius-Peereboom, J.H.; Merkus, H.M.; Steegers-Theunissen, R.P. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod. Toxicol. 2001, 15, 131–136. [Google Scholar] [CrossRef]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Singh, A.; Prabha, V.; Singh, R.; Sharma, P. Escherichia coli attaches to human spermatozoa: Affecting sperm parameters. Arch. Appl. Sci. Res. 2011, 3, 618–623. [Google Scholar]
- Albert, A. Selective Toxicity: The Physico-Chemical Basis of Therapy, 6th ed.; Springer: Dordrecht, The Netherlands, 2012; p. 368. [Google Scholar]
- Vijayalakshmi, K.; Sivaraj, D. Enhanced antibacterial activity of Cr doped ZnO nanorods synthesized using microwave processing. RSC Adv. 2015, 5, 68461–68469. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Kjellberg, S.; Roomans, G.M.; Kvist, U. The human sperm nucleus takes up zinc at ejaculation. Int. J. Androl. 1986, 9, 77–80. [Google Scholar] [CrossRef]
- Bertrand, G.; Vladesco, M.R. Role of zinc in reproduction. Acad. Sci. 1921, 173, 176–179. [Google Scholar]
- Mankad, M.; Sathawara, N.G.; Doshi, H.; Saiyed, H.N.; Kumar, S. Seminal plasma zinc concentration and alpha-glucosidase activity with respect to semen quality. Biol. Trace Elem. Res. 2006, 110, 97–106. [Google Scholar] [CrossRef]
- Liu, D.Y.; Sie, B.S.; Liu, M.L.; Agresta, F.; Baker, H.W. Relationship between seminal plasma zinc concentration and spermatozoa-zona pellucida binding and the ZP-induced acrosome reaction in subfertile men. Asian J. Androl. 2009, 11, 499–507. [Google Scholar] [CrossRef]
- Ma, J.; Han, R.; Li, Y.; Cui, T.; Wang, S. The Mechanism of Zinc Sulfate in Improving Fertility in Obese Rats Analyzed by Sperm Proteomic Analysis. Biomed. Res. Int. 2020, 2020, 9876363. [Google Scholar] [CrossRef]
- Eggert-Kruse, W.; Zwick, E.M.; Batschulat, K.; Rohr, G.; Armbruster, F.P.; Petzoldt, D.; Strowitzki, T. Are zinc levels in seminal plasma associated with seminal leukocytes and other determinants of semen quality? Fertil. Steril. 2002, 77, 260–269. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chang, T.C.; Tseng, Y.J.; Lin, Y.L.; Huang, F.J.; Kung, F.T.; Chang, S.Y. Seminal plasma zinc levels and sperm motion characteristics in infertile samples. Chang. Gung. Med. J. 2000, 23, 260–266. [Google Scholar]
- Brito, M.; Figueroa, J.; Vera, J.C.; Cortés, P.; Hott, R.; Burzio, L.O. Phosphoproteins are structural components of bull sperm outer dense fiber. Gamete Res. 1986, 15, 327–336. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Hunt, C.D.; Johnson, P.E.; Herbel, J.; Mullen, L.K. Effects of dietary zinc depletion on seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. Am. J. Clin. Nutr. 1992, 56, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Alsalman, A.R.S.; Almashhedy, L.A.; Hadwan, M.H. Effect of Oral Zinc Supplementation on the Thiol Oxido-Reductive Index and Thiol-Related Enzymes in Seminal Plasma and Spermatozoa of Iraqi Asthenospermic Patients. Biol. Trace Elem. Res. 2018, 184, 340–349. [Google Scholar] [CrossRef]
- Nielsen, F.H. History of zinc in agriculture. Adv. Nutr. 2012, 3, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Verma, R.P.; Singh, L.P.; Varshney, V.P.; Dass, R.S. Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus × Bos taurus) bulls. Reprod. Nutr. Dev. 2006, 46, 663–675. [Google Scholar] [CrossRef]
- Hill, G.M.; Shannon, M.C. Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol. Trace Elem. Res. 2019, 188, 148–159. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Sutovsky, P. Porcine Cell-Free System to Study Mammalian Sperm Mitophagy. Methods Mol. Biol. 2019, 1854, 197–207. [Google Scholar] [PubMed]
- Roomans, G.M.; Lundevall, E.; Bjorndahl, L.; Kvist, U. Removal of zinc from subcellular regions of human spermatozoa by EDTA treatment studied by X-ray microanalysis. Int. J. Androl. 1982, 5, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Kvist, U. Importance of spermatozoal zinc as temporary inhibitor of sperm nuclear chromatin decondensation ability in man. Acta Physiol. Scand. 1980, 109, 79–84. [Google Scholar] [CrossRef]
- Kvist, U. Spermatozoal thiol-disulphide interaction: A possible event underlying physiological sperm nuclear chromatin decondensation. Acta Physiol. Scand. 1982, 115, 503–505. [Google Scholar] [CrossRef]
- Rodriguez, H.; Ohanian, C.; Bustos-Obregon, E. Nuclear chromatin decondensation of spermatozoa in vitro: A method for evaluating the fertilizing ability of ovine semen. Int. J. Androl. 1985, 8, 147–158. [Google Scholar] [CrossRef]
- Bin, B.H.; Seo, J.; Kim, S.T. Function, structure, and transport aspects of ZIP and ZnT zinc transporters in immune cells. J. Immunol. Res. 2018, 2018, 9365747. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Yuce, K. Zinc transporter proteins. Neurochem. Res. 2018, 43, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Fukada, T.; Hosaka, T.; Yamasaki, S.; Ohashi, W.; Hojyo, S.; Miyai, T.; Nishida, K.; Yokoyama, S.; Hirano, T. Biochemical characterization of human ZIP13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J. Biol. Chem. 2011, 286, 40255–40265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Nicholson, R.I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta 2003, 1611, 16–30. [Google Scholar] [CrossRef]
- Gaither, L.A.; Eide, D.J. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Fu, D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J. Biol. Chem. 2004, 279, 17173–17180. [Google Scholar] [CrossRef] [PubMed]
- L’Hernault, S.W.; Shakes, D.C.; Ward, S. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics 1988, 120, 435–452. [Google Scholar] [PubMed]
- Ward, S.; Miwa, J. Characterization of temperature-sensitive, fertilization-defective mutants of the nematode Caenorhabditis elegans. Genetics 1978, 88, 285–303. [Google Scholar]
- Muhlrad, P.J.; Clark, J.N.; Nasri, U.; Sullivan, N.G.; LaMunyon, C.W. SPE-8, a protein-tyrosine kinase, localizes to the spermatid cell membrane through interaction with other members of the SPE-8 group spermatid activation signaling pathway in C. elegans. BMC Genet. 2014, 15, 83. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Till, J.H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 2000, 69, 373–398. [Google Scholar] [CrossRef]
- Visconti, P.E.; Stewart-Savage, J.; Blasco, A.; Battaglia, L.; Miranda, P.; Kopf, G.S.; Tezon, J.G. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol. Reprod. 1999, 61, 76–84. [Google Scholar] [CrossRef]
- Geldziler, B.; Chatterjee, I.; Singson, A. The genetic and molecular analysis of spe-19, a gene required for sperm activation in Caenorhabditis elegans. Dev. Biol. 2005, 283, 424–436. [Google Scholar] [CrossRef]
- Nance, J.; Davis, E.B.; Ward, S. spe-29 encodes a small predicted membrane protein required for the initiation of sperm activation in Caenorhabditis elegans. Genetics 2000, 156, 1623–1633. [Google Scholar] [PubMed]
- Shakes, D.C.; Ward, S. Initiation of spermiogenesis in C. elegans: A pharmacological and genetic analysis. Dev. Biol. 1989, 134, 189–200. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Shang, Y.; Huang, P.; Miao, L. The micronutrient element zinc modulates sperm activation through the SPE-8 pathway in Caenorhabditis elegans. Development 2013, 140, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Muhlrad, P.J.; Ward, S. Spermiogenesis initiation in Caenorhabditis elegans involves a casein kinase 1 encoded by the spe-6 gene. Genetics 2002, 161, 143–155. [Google Scholar] [PubMed]
- Arduengo, P.M.; Appleberry, O.K.; Chuang, P.; L’Hernault, S.W. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J. Cell. Sci. 1998, 111, 3645–3654. [Google Scholar]
- Dietrich, N.; Schneider, D.L.; Kornfeld, K. A pathway for low zinc homeostasis that is conserved in animals and acts in parallel to the pathway for high zinc homeostasis. Nucleic Acids Res. 2017, 45, 11658–11672. [Google Scholar] [CrossRef]
- Shihan, M.; Chan, K.H.; Konrad, L.; Scheiner-Bobis, G. Non-classical testosterone signaling in spermatogenic GC-2 cells is mediated through ZIP9 interacting with Gnalpha11. Cell Signal 2015, 27, 2077–2086. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010, 16, 23–29. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Kvist, U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst. Biol. Reprod. Med. 2011, 57, 86–92. [Google Scholar] [CrossRef]
- Chu, D.S. Zinc: A small molecule with a big impact on sperm function. PLoS Biol. 2018, 16, e2006204. [Google Scholar] [CrossRef]
- Henkel, R.; Maass, G.; Schuppe, H.C.; Jung, A.; Schubert, J.; Schill, W.B. Molecular aspects of declining sperm motility in older men. Fertil. Steril. 2005, 84, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Boran, C.; Ozkan, K.U. The effect of zinc therapy on damaged testis in pre-pubertal rats. Pediatr. Surg. Int. 2004, 20, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Clapper, D.L.; Davis, J.A.; Lamothe, P.J.; Patton, C.; Epel, D. Involvement of zinc in the regulation of pHi, motility, and acrosome reactions in sea urchin sperm. J. Cell Biol. 1985, 100, 1817–1824. [Google Scholar] [CrossRef]
- Stoltenberg, M.; Sorensen, M.B.; Danscher, G.; Juhl, S.; Andreasen, A.; Ernst, E. Autometallographic demonstration of zinc ions in rat sperm cells. Mol. Hum. Reprod. 1997, 3, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, M.; Mori, H. Heavy metals and spermatozoan motility. I. Distribution of iron, zinc and copper in sea urchin spermatozoa. Exp. Cell Res. 1972, 70, 311–316. [Google Scholar] [CrossRef]
- Baccetti, B.; Pallini, V.; Burrini, A.G. The accessory fibers of the sperm tail. II. Their role in binding zinc in mammals and cephalopods. J. Ultrastruct. Res. 1976, 54, 261–275. [Google Scholar] [CrossRef]
- Calvin, H.I. Electrophoretic evidence for the identity of the major zinc-binding polypeptides in the rat sperm tail. Biol. Reprod. 1979, 21, 873–882. [Google Scholar] [CrossRef]
- Clermont, Y.; Oko, R.; Hermo, L. Immunocytochemical localization of proteins utilized in the formation of outer dense fibers and fibrous sheath in rat spermatids: An electron microscope study. Anat. Rec. 1990, 227, 447–457. [Google Scholar] [CrossRef]
- Henkel, R.; Baldauf, C.; Bittner, J.; Weidner, W.; Miska, W. Elimination of zinc from the flagella of spermatozoa during epididymal transit is important for motility. Reprod. Technol. 2001, 10, 280–285. [Google Scholar]
- Bolanca, I.; Obhodas, J.; Ljiljak, D.; Matjacic, L.; Kuna, K. Synergetic Effects of K, Ca, Cu and Zn in Human Semen in Relation to Parameters Indicative of Spontaneous Hyperactivation of Spermatozoa. PLoS ONE 2016, 11, e0152445. [Google Scholar] [CrossRef]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Allouche-Fitoussi, D.; Bakhshi, D.; Breitbart, H. Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn2+. Mol. Reprod. Dev. 2018, 85, 543–556. [Google Scholar] [CrossRef]
- Menezo, Y.; Pluntz, L.; Chouteau, J.; Gurgan, T.; Demirol, A.; Dalleac, A.; Benkhalifa, M. Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of Zn2+ transporters in human oocytes and cumulus cells. Reprod. Biomed. Online 2011, 22, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Granish, K.A.; Suarez, S.S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 2002, 250, 208–217. [Google Scholar] [CrossRef]
- Riffo, M.; Leiva, S.; Astudillo, J. Effect of zinc on human sperm motility and the acrosome reaction. Int. J. Androl. 1992, 15, 229–237. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Babcock, D.F.; Rufo, G.A.; Lardy, H.A. Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc. Natl. Acad. Sci. USA 1983, 80, 1327–1331. [Google Scholar] [CrossRef]
- Miller, M.R.; Kenny, S.J.; Mannowetz, N.; Mansell, S.A.; Wojcik, M.; Mendoza, S.; Zucker, R.S.; Xu, K.; Lishko, P.V. Asymmetrically positioned flagellar control units regulate human sperm rotation. Cell Rep. 2018, 24, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Chung, J.J.; Shim, S.H.; Everley, R.A.; Gygi, S.P.; Zhuang, X.; Clapham, D.E. Structurally distinct Ca2+ signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 2014, 157, 808–822. [Google Scholar] [CrossRef]
- Suarez, S.S.; Varosi, S.M.; Dai, X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc. Natl. Acad. Sci. USA 1993, 90, 4660–4664. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Laclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation in mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [PubMed]
- Navarrete, F.A.; Garcia-Vazquez, F.A.; Alvau, A.; Escoffier, J.; Krapf, D.; Sanchez-Cardenas, C.; Salicioni, A.M.; Darszon, A.; Visconti, P.E. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J. Cell Physiol. 2015, 230, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010, 588, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; King, S.M.; Quill, T.A.; Doolittle, L.K.; Garbers, D.L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 2003, 5, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Michailov, Y.; Ickowicz, D.; Breitbart, H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev. Biol. 2014, 396, 246–255. [Google Scholar] [CrossRef]
- Schneider, M.; Forster, H.; Boersma, A.; Seiler, A.; Wehnes, H.; Sinowatz, F.; Neumuller, C.; Deutsch, M.J.; Walch, A.; Hrabe de Angelis, M.; et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009, 23, 3233–3242. [Google Scholar] [CrossRef]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krahling, M.; Muller, A.; Kaupp, U.B.; Strunker, T. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef]
- Wertheimer, E.; Krapf, D.; de la Vega-Beltran, J.L.; Sanchez-Cardenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J. Biol. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef]
- Etkovitz, N.; Tirosh, Y.; Chazan, R.; Jaldety, Y.; Daniel, L.; Rubinstein, S.; Breitbart, H. Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev. Biol. 2009, 334, 447–457. [Google Scholar] [CrossRef]
- O’Brien, E.D.; Krapf, D.; Cabada, M.O.; Visconti, P.E.; Arranz, S.E. Transmembrane adenylyl cyclase regulates amphibian sperm motility through protein kinase A activation. Dev. Biol. 2011, 350, 80–88. [Google Scholar] [CrossRef]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 2005, 9, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Balbach, M.; Beckert, V.; Hansen, J.N.; Wachten, D. Shedding light on the role of cAMP in mammalian sperm physiology. Mol. Cell Endocrinol. 2018, 468, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.; Alvarez, L.; Balbach, M.; Strunker, T.; Hegemann, P.; Kaupp, U.B.; Wachten, D. Controlling fertilization and cAMP signaling in sperm by optogenetics. Elife 2015, 4, e05161. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.; Jikeli, J.F.; Wachten, D. How to control cyclic nucleotide signaling by light. Curr. Opin. Biotechnol. 2017, 48, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Jansen, V.; Jikeli, J.F.; Hamzeh, H.; Alvarez, L.; Dombrowski, M.; Balbach, M.; Strunker, T.; Seifert, R.; Kaupp, U.B.; et al. A novel biosensor to study cAMP dynamics in cilia and flagella. Elife 2016, 5, e14052. [Google Scholar] [CrossRef]
- Raju, D.N.; Hansen, J.N.; Rassmann, S.; Stuven, B.; Jikeli, J.F.; Strunker, T.; Korschen, H.G.; Moglich, A.; Wachten, D. Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains. Cells 2019, 8, 648. [Google Scholar] [CrossRef]
- Bajpai, M.; Fiedler, S.E.; Huang, Z.; Vijayaraghavan, S.; Olson, G.E.; Livera, G.; Conti, M.; Carr, D.W. AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol. Reprod. 2006, 74, 109–118. [Google Scholar] [CrossRef]
- Fisch, J.D.; Behr, B.; Conti, M. Enhancement of motility and acrosome reaction in human spermatozoa: Differential activation by type-specific phosphodiesterase inhibitors. Hum. Reprod. 1998, 13, 1248–1254. [Google Scholar] [CrossRef]
- Leclerc, P.; Kopf, G.S. Mouse sperm adenylyl cyclase: General properties and regulation by the zona pellucida. Biol. Reprod. 1995, 52, 1227–1233. [Google Scholar] [CrossRef][Green Version]
- Abdul-Rasheed, O.F. The relationship between seminal plasma zinc levels and high molecular weight zinc binding protein and sperm motility in Iraqi infertile men. Saudi Med. J. 2009, 30, 485–489. [Google Scholar] [PubMed]
- Narasimhaiah, M.; Arunachalam, A.; Sellappan, S.; Mayasula, V.K.; Guvvala, P.R.; Ghosh, S.K.; Chandra, V.; Ghosh, J.; Kumar, H. Organic zinc and copper supplementation on antioxidant protective mechanism and their correlation with sperm functional characteristics in goats. Reprod. Domest. Anim. 2018, 53, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Shahar, S.; Wiser, A.; Ickowicz, D.; Lubart, R.; Shulman, A.; Breitbart, H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum. Reprod. 2011, 26, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Beconi, M.; Beorlegui, N. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia 1997, 29, 269–275. [Google Scholar] [CrossRef]
- Sikka, S.C. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001, 8, 851–862. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Yoshida, K.; Yoshiike, T.M.; Iwamoto, T.; Gagnon, C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J. Androl. 2001, 22, 672–679. [Google Scholar]
- Kerns, K.; Zigo, M.; Sutovsky, P. Zinc: A Necessary Ion for Mammalian Sperm Fertilization Competency. Int. J. Mol. Sci. 2018, 19, 4097. [Google Scholar] [CrossRef]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Que, E.L.; Duncan, F.E.; Bayer, A.R.; Philips, S.J.; Roth, E.W.; Bleher, R.; Gleber, S.C.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr. Biol. (Camb) 2017, 9, 135–144. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Cubilla, M.; Moreno, A.; Molino, M.V.; Bahamondes, L.; Giojalas, L.C. Sperm chemorepulsion, a supplementary mechanism to regulate fertilization. Hum. Reprod. 2017, 32, 1560–1573. [Google Scholar] [CrossRef]
- Stephenson, J.L.; Brackett, B.G. Influences of zinc on fertilisation and development of bovine oocytes in vitro. Zygote 1999, 7, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Beek, J.; Nauwynck, H.; Maes, D.; Van Soom, A. Inhibitors of zinc-dependent metalloproteases hinder sperm passage through the cumulus oophorus during porcine fertilization in vitro. Reproduction 2012, 144, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Warinrak, C.; Wu, J.T.; Hsu, W.L.; Liao, J.W.; Chang, S.C.; Cheng, F.P. Expression of matrix metalloproteinases (MMP-2, MMP-9) and their inhibitors (TIMP-1, TIMP-2) in canine testis, epididymis and semen. Reprod. Domest. Anim. 2015, 50, 48–57. [Google Scholar] [CrossRef]
- Buchman-Shaked, O.; Kraiem, Z.; Gonen, Y.; Goldman, S. Presence of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinase in human sperm. J. Androl. 2002, 23, 702–708. [Google Scholar]
- Ferrer, M.; Rodriguez, H.; Zara, L.; Yu, Y.; Xu, W.; Oko, R. MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell Tissue Res. 2012, 349, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Azriel-Tamir, H.; Sharir, H.; Schwartz, B.; Hershfinkel, M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 2004, 279, 51804–51816. [Google Scholar] [CrossRef]
- Besser, L.; Chorin, E.; Sekler, I.; Silverman, W.F.; Atkin, S.; Russell, J.T.; Hershfinkel, M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 2009, 29, 2890–2901. [Google Scholar] [CrossRef]
- Chorin, E.; Vinograd, O.; Fleidervish, I.; Gilad, D.; Herrmann, S.; Sekler, I.; Aizenman, E.; Hershfinkel, M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 2011, 31, 12916–12926. [Google Scholar] [CrossRef]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef]
- Ho, H.C.; Suarez, S.S. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol. Reprod. 2001, 65, 1606–1615. [Google Scholar] [CrossRef]
- Wiser, A.; Sachar, S.; Ghetler, Y.; Shulman, A.; Breitbart, H. Assessment of sperm hyperactivated motility and acrosome reaction can discriminate the use of spermatozoa for conventional in vitro fertilisation or intracytoplasmic sperm injection: Preliminary results. Andrologia 2014, 46, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Shabtay, O.; Breitbart, H. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol. 2016, 415, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Huta, Y.; Nitzan, Y.; Breitbart, H. Ezrin protects bovine spermatozoa from spontaneous acrosome reaction. Theriogenology 2020, 151, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tsirulnikov, E.; Huta, Y.; Breitbart, H. PKA and PI3K activities during capacitation protect sperm from undergoing spontaneous acrosome reaction. Theriogenology 2019, 128, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, F.; Zitranski, N.; Borth, H.; Buech, T.; Gudermann, T.; Boekhoff, I. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J. Cell Sci. 2009, 122, 4547–4557. [Google Scholar] [CrossRef]
- Kobayashi, T.; Miyazaki, T.; Natori, M.; Nozawa, S. Protective role of superoxide dismutase in human sperm motility: Superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum. Reprod. 1991, 6, 987–991. [Google Scholar] [CrossRef]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef]
- Nallella, K.P.; Sharma, R.K.; Allamaneni, S.S.; Aziz, N.; Agarwal, A. Cryopreservation of human spermatozoa: Comparison of two cryopreservation methods and three cryoprotectants. Fertil. Steril. 2004, 82, 913–918. [Google Scholar] [CrossRef]
- Critser, J.K.; Huse-Benda, A.R.; Aaker, D.V.; Arneson, B.W.; Ball, G.D. Cryopreservation of human spermatozoa. III. The effect of cryoprotectants on motility. Fertil. Steril. 1988, 50, 314–320. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; McLachlan, R.I. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009, 32, 46–56. [Google Scholar] [CrossRef]
- Zribi, N.; Feki Chakroun, N.; El Euch, H.; Gargouri, J.; Bahloul, A.; Ammar Keskes, L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; McClure, N.; Lewis, S.E. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K. Vitamin E and oxidative stress. Free Radic. Biol. Med. 1991, 11, 215–232. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 1999, 14, 505–512. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Xie, Y.; Wang, Z.; Wu, R.; Cai, J.; Luo, X.; Huang, S.; You, L. Zinc protects sperm from being damaged by reactive oxygen species in assisted reproduction techniques. Reprod. Biomed. Online 2015, 30, 334–339. [Google Scholar] [CrossRef]
- Berkovitz, A.; Allouche-Fitoussi, D.; Izhakov, D.; Breitbart, H. Cryopreservation of human sperm in the presence of Zn2+ increases the motility rate. J. Obs. Gynecol. Investig. 2018, 1, 6–12. [Google Scholar]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Blazak, W.F.; Overstreet, J.W. Instability of nuclear chromatin in the ejaculated spermatozoa of fertile men. J. Reprod. Fertil. 1982, 65, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kotdawala, A.P.; Kumar, S.; Salian, S.R.; Thankachan, P.; Govindraj, K.; Kumar, P.; Kalthur, G.; Adiga, S.K. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J. Assist. Reprod. Genet. 2012, 29, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Bettger, W.J.; O’Dell, B.L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981, 28, 1425–1438. [Google Scholar] [CrossRef]
- Kendall, N.R.; McMullen, S.; Green, A.; Rodway, R.G. The effect of a zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim. Reprod. Sci. 2000, 62, 277–283. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhou, X.; Cao, Y.; Li, C. Preventive effects of supplemental dietary zinc on heat-induced damage in the epididymis of boars. J. Therm. Biol. 2017, 64, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA 2020, 323, 35–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. https://doi.org/10.3390/ijms21207796
Allouche-Fitoussi D, Breitbart H. The Role of Zinc in Male Fertility. International Journal of Molecular Sciences. 2020; 21(20):7796. https://doi.org/10.3390/ijms21207796
Chicago/Turabian StyleAllouche-Fitoussi, Deborah, and Haim Breitbart. 2020. "The Role of Zinc in Male Fertility" International Journal of Molecular Sciences 21, no. 20: 7796. https://doi.org/10.3390/ijms21207796
APA StyleAllouche-Fitoussi, D., & Breitbart, H. (2020). The Role of Zinc in Male Fertility. International Journal of Molecular Sciences, 21(20), 7796. https://doi.org/10.3390/ijms21207796




