Abstract
Several studies proposed the importance of zinc ion in male fertility. Here, we describe the properties, roles and cellular mechanisms of action of Zn2+ in spermatozoa, focusing on its involvement in sperm motility, capacitation and acrosomal exocytosis, three functions that are crucial for successful fertilization. The impact of zinc supplementation on assisted fertilization techniques is also described. The impact of zinc on sperm motility has been investigated in many vertebrate and invertebrate species. It has been reported that Zn2+ in human seminal plasma decreases sperm motility and that Zn2+ removal enhances motility. Reduction in the intracellular concentration of Zn2+ during epididymal transit allows the development of progressive motility and the subsequent hyper activated motility during sperm capacitation. Extracellular Zn2+ affects intracellular signaling pathways through its interaction with the Zn2+ sensing receptor (ZnR), also named GPR39. This receptor was found in the sperm tail and the acrosome, suggesting the possible involvement of Zn2+ in sperm motility and acrosomal exocytosis. Our studies showed that Zn2+ stimulates bovine sperm acrosomal exocytosis, as well as human sperm hyper-activated motility, were both mediated by GPR39. Zn2+ binds and activates GPR39, which activates the trans-membrane-adenylyl-cyclase (tmAC) to catalyze cAMP production. The NHE (Na+/H+-exchanger) is activated by cAMP, leading in increased pHi and activation of the sperm-specific Ca2+ channel CatSper, resulting in an increase in [Ca2+]i, which, together with HCO3−, activates the soluble adenylyl-cyclase (sAC). The increase in [cAMP]i activates protein kinase A (PKA), followed by activation of the Src-epidermal growth factor receptor-Pphospholipase C (Src-EGFR-PLC) cascade, resulting in inositol-triphosphate (IP3) production, which mobilizes Ca2+ from the acrosome, causing a further increase in [Ca2+]i and the development of hyper-activated motility. PKA also activates phospholipase D1 (PLD1), leading to F-actin formation during capacitation. Prior to the acrosomal exocytosis, PLC induces phosphadidylinositol-4,5-bisphosphate (PIP2) hydrolysis, leading to the release of the actin-severing protein gelsolin to the cytosol, which is activated by Ca2+, resulting in F-actin breakdown and the occurrence of acrosomal exocytosis.
1. Introduction
Zinc is an essential element with a wide range of biological functions. More than 200 Zn-metalloenzymes are regulated by Zn2+. It plays an essential role in the organization of DNA, RNA and proteins, as well as in the stability of cell membranes and cell division [1]. We know today that zinc is important for health. It is present in several organs like the kidney, skin, eyes, lung, brain, heart and pancreas at relatively high concentrations. It has been demonstrated that zinc ion assists immune function, helps cells to grow and proliferate in a healthy way and preserves prostate and sexual health. Zn2+ is required in diverse mechanisms, like the DNA replication, RNA transcription, differentiation, proliferation and activation of immune cells in many organs of the body [2,3,4]. In addition, zinc impacts on genes expressions have been reported. The structure of zinc finger domains found in transcription factors and regulating genes activity are also modulated by zinc [5]. Zinc ions also help to keep appropriated thyroid functions by creating “thyroid releasing hormones” in the brain that impact metabolism and development in the body. Low levels of zinc may affect the good production of these hormones, as well as testosterone levels [6].
2. Zinc and fertility
It has also been shown that zinc plays a significant role in the male reproductive system [7,8,9,10]. It was reported that fertile groups had higher levels of zinc in comparison to infertile groups. The level of Zn2+ in fertile men was 14.08± 2.01 and, in the infertile group, was 10.32 ± 2.98 (mg/100 mL) [11]. A Zn2+ lack can be linked with a diminution in testis volume and testicular weight and failure of spermatogenesis [12]. The presence of bacterial contamination in male and female reproductive tracts may negatively affect sperm function [13]. We know also that zinc has antibacterial activity: Zinc oxide nanoparticles kill both Gram-positive and Gram-negative bacteria and are also effective against spores, which are high temperature and high pressure-resistant [14,15]. The prostatic fluid of healthy men with high zinc affects mammalian spermatozoa after ejaculation [16]. The presence of zinc in semen was first described in 1921 [17]. Since then, many studies were performed to understand the role of zinc in male reproduction. The concentration of seminal zinc is associated with sperm count [18,19], and zinc-deficient nutrition causes a low quality of sperm and male infertility [11]. Rats treated with zinc show an increase in sperm count, sperm motility and testosterone levels and improved testicular structure and spermatogenesis abnormalities caused by obesity [20]. However, other studies claim that there is no significant association between zinc and sperm quality [21,22]. Zinc is found in spermatozoa and in the seminal fluid, where its concentration is higher than in all other body fluids. In semen, Zn2+ is secreted mainly from the prostate [23]. Zinc ion has been linked with key events in the acquisition of fertilization ability by spermatozoa, including motility, capacitation and acrosomal exocytosis. To enable fertilization, sperm must reside in the female reproductive tract for several hours, during which time, they undergo a series of biochemical and motility changes collectively called capacitation, allowing the spermatozoon to interact with the oocyte, undergo acrosomal exocytosis, and, finally, penetrate the egg. Defects in sperm quantity, quality or motility account for up to 50% of infertility cases and may affect approximately 7% of all men [24]. About 25% of infertility cases in humans are defined as “unexplained infertility”, and in many cases, successful fertilization in these men can be achieved by the technique of intracytoplasmic sperm injection (ICSI). On the other hand, in a non-negligible fraction of these unexplained cases, despite normal sperm quantity, morphology and motility, no egg penetration/fertilization occurs. Thus, it is possible that a significant proportion of unexplained infertility is, in fact, caused by spermatozoon failure to perform proper capacitation. It was shown that zinc deficiency is correlated with a decrease in male fertility [25,26], and the presence of zinc in the diets of humans [27] and domestic animals [28,29,30] is required for the achievement of an optimal fertility rate. The addition of Zn2+ to semen extenders before freezing reduces reactive oxygen species (ROS) [31]; however, excess Zn2+ can act as a pro-oxidant, leading to mitochondrial oxidative stress [32]. Structurally, the sperm mitochondrial sheath [33,34] and sperm chromatin [35,36] are stabilized by zinc bridges. The nuclear chromatin of mammalian sperm undergoes high condensation during the latter stages of spermatogenesis. This process is accomplished by a replacement of histones by the more basic amino acids arginine and cysteine-rich protamines. Zinc plays an essential role in nuclear chromatin decondensation after fertilization [37]. Relatively high concentrations of zinc inhibit chromatin decondensation in ram sperm, and the reverse situation was observed by chelating this Zn2+ [38]. Thus, the decrease in sperm Zn2+ during the epididymal transit is essential for the development of progressive motility, as well as chromatin decondensation, two necessary events for successful fertilization.
In this review, we will focus on the effect of Zn2+ on sperm motility, capacitation and acrosomal exocytosis, including the mechanisms of action and the impact of zinc supplementation on assisted fertilization techniques.
2.1. Regulation of Intracellular Zn2+ Concentrations
Two types of Zn2+ transporters are conserved in mammals, SLC39s/ZIPs and SLC30s/ZnTs, which transport Zn2+ in opposite directions through cellular and intracellular membranes (rev. by [39]). ZIPs increase the zinc concentration in the cytosol. For this, the ZIPs carry the zinc from extracellular and intracellular compartments to the cytosol. ZnTs reduce the concentration of zinc in the cytosol. For this, ZnTs carry the zinc from the cytosol to extracellular and intracellular compartments. After being transported to the cell, 50% of zinc is found in the cytoplasm, 30–40% in the nucleus and 10% in the plasma and organelle membranes (rev. by [40]). ZIP and ZnT have two major conformations, the inward-open and outward-open conformations (rev. by [39]). Based on sequence analyses, ZIP family members contain eight transmembrane domains as membrane transport proteins, with a cytoplasmic region between TM3 and TM4 [41,42]. The ZIP2 activity was significantly stimulated by HCO3—a treatment with high affinity to the first zinc and the next cadmium, suggesting a Zn2+/ HCO3-symporter mechanism [43]. ZnTs belong to a superfamily of cation diffusion facilitators observed in a wide range of taxa, including plants, bacteria, and fungi [38]. In mammals, at least 10 ZnT members have been identified [44,45]. They are predicted to have six trans-membrane domains (TMDs), except for ZnT5, which has additional TMDs, and their N- and C-termini face the cytoplasm, unlike those of ZIPs [44,45]. In ZnTs, zinc efflux occurs via a Zn2+/H+ antiporter [46]. This transporter mediates Zn2+ efflux to the outside of cells or to the luminal side of the intracellular compartment. At the same time, H+ ions are transported into the cytoplasm. Therefore, this transporter requires a proton motive force to mediate the Zn2+ efflux.
Investigations in Caenorhabditis elegans identified several genes that resulted in phenotypes of defective spermatogenesis (spe) and fertilization when mutated [47,48]. For example, the spe-8gene, which encodes a protein tyrosine kinase, is involved in protein tyrosine phosphorylation [49,50], a known process that occurs in sperm capacitation [51]. Several proteins function with spe-8-mediating signaling pathways, which promote motility [52,53]. It has been suggested that Zn2+ may initiate the spe-8 signaling cascade, leading to sperm activation [54,55].
Zipt-7.1 is a transmembrane protein localized within intracellular organelles [56,57] and, together with spe-8, regulates the release of Zn2+ from internal stores. The released Zn2+ in the cytoplasm activates zinc-regulated proteins that develop motility. Studies of zinc transporters revealed that the deletion of zipt-7.1 causes sterility [58]. ZIP9 serves as a Zn2+ transporter associated with the G-protein Gnα11, which mediates testosterone signaling in murine spermatogenic cells [59]. During ejaculation, Zn2+ is transported into the nucleus [16], which is important for chromatin stabilization [60,61]. Thus, Zn2+ acts as a second messenger that modulates sperm functions, including motility and capacitation. This suggests that intracellular Zn2+ levels should be well-controlled by zinc transporters localized at intracellular membranes and in the cell plasma membrane, which import Zn2+ from the external environment [62].
2.2. Involvement of Zn2+ in Sperm Motility
The impact of zinc on sperm motility has been investigated in many vertebrate and invertebrate species. It has been reported that Zn2+ in human seminal plasma decreases sperm motility [63] and that Zn2+ removal, by binding to a protein named semenogelin, enhances motility [64]. However, in sea urchin, a treatment with ethylenediamine tetra acetic acid (EDTA), a bivalent metal ion chelator, inhibits sperm motility, an effect that was reversed by the addition of Zn2+ [65]. In C. elegans, the zinc released within cells acts as a messenger in a signaling pathway to promote mobility acquisition [62]. Thus, extracellular Zn2+ affects sperm motility, but whether its effect is inhibitory or stimulatory seems to be species- and concentration-dependent, whereby relatively low Zn2+ concentrations stimulate motility, whereas high Zn2+ inhibits sperm motility.
Furthermore, it has been shown that Zn2+ is present in sperm mitochondria and along the flagella [66,67] and is predominantly localized in the outer dense fibers (ODF) [68,69]. The binding of Zn2+ to cysteine-sulfhydryl of ODF during spermatogenesis [70] protects it from premature oxidation [71]. Reduction in the intracellular concentration of Zn2+ during epididymal transit enables sulfhydryl oxidation and stiffening of the ODF to allow the development of progressive motility [71] and the subsequent hyper-activated motility (HAM) during sperm capacitation [72]. Hyper-activated motility is a form of sperm motility developed during sperm capacitation at the site of fertilization. This movement is characterized by an intensive and asymmetric beating of the middle and principal pieces of the flagella, which engender a strong driving force to infiltrate the extracellular matrix of oocytes [73]. It has been demonstrated that low concentrations of Zn2+ (5–10 µM) stimulate hyper-activated motility in human sperm under capacitation conditions, whereas, at 30-µM Zn2+, no stimulation was observed [74]. We also know that relatively high concentrations of Zn2+ (in the mM range) in the semen are inhibitory to sperm function, whereas, in the female reproductive tract, the concentration of Zn2+ is much lower (1.0–1.5 µM) [75]. This low concentration of zinc allows the occurrence of normal sperm capacitation, including hyper-activated motility, leading to a physiological acrosome reaction and successful fertilization.
Motility initiation after sperm ejaculation, and the development of hyper-activated motility during sperm capacitation, are both dependent on intracellular alkalization [76]. Excessive Zn2+ concentration (0.1 mM) inhibits sperm motility [77]. High concentrations of Zn2+ (0.2 mM), which compromise capacitation and hyper-activated motility, also inhibit the voltage-gated H+ channel Hv1, localized in the sperm tail and responsible for sperm cytoplasmic alkalization [78,79] and the regulation of human sperm tail rotation and hyper-activated motility [80]. The cytoplasmic alkalization leads to the activation of the sperm-specific Ca2+ channel CatSper [81], which is localized in the flagellum and mediates the development of capacitation-dependent hyper-activated motility [82] (see Figure 1). It has been shown that the CatSper inhibitor negatively impacts the stimulatory effect of Zn2+ on human sperm HAM, indicating that CatSper mediates Zn2+-stimulated hyper-activated motility [74]. Hyper-activated motility develops during sperm capacitation [83], a process that depends on protein kinase A (PKA) activity [84]. CatSper-null sperm show normal PKA activity [82,85], indicating that PKA activity is not dependent on CatSper. We found that CatSper inhibition by NNC-55-0396(1S,2S-2-(2-[N-((3-benzimidazole-2-yl)propyl)-N-methyamino]ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtylcyclopropanecarboxylate-dihydrochloride hydrate) resulted in PKA inhibition, indicating that PKA activity depends on CatSper activity [74]. This possible contradiction could be answered as follows: Ca2+ influx via CatSper causes membrane depolarization, leading to the activation of voltage-gated H+ channel Hv1, which is the predominant alkalization system in human sperm [86]. Thus, the inhibition of CatSper by NNC-55-0396 will cause hyperpolarization, leading to the inhibition of Hv1 and preventing intracellular alkalization. The increase of intracellular pH will shift the equilibrium of the reaction: CO2+H2O-→HCO3−+H+ to the left, resulting in decreasing the concentration of bicarbonate and a reduction in sAC/PKA activities. CatSper-null sperm was produced in the mouse, in which intracellular alkalization occurs by Na+/H+ exchanger (NHE) and Hv1 does not exist. NHE-null sperm is not motile, and motility could be restored by artificial alkalization of the cytosol [87]. Since sAC activity is defected in NHE-null mice, it was suggested that lower intracellular pH in these cells is responsible for the motility defect [87], and it is possible that intracellular alkalization might activate sAC. Thus, the inhibition of Ca2+ influx in CatSper-null sperm would not affect intracellular alkalization, allowing the activation of sAC/PKA. We suggested that Zn2+ activates the PKA-Src-EGFR cascade, which is CatSper-dependent [74] (see Figure 1). Since CatSper activation is required for hyperactivation and, ultimately, for male fertility [82], it is not unexpected that zinc would be important for preparing the spermatozoa for hyperactivation and successful fertilization.
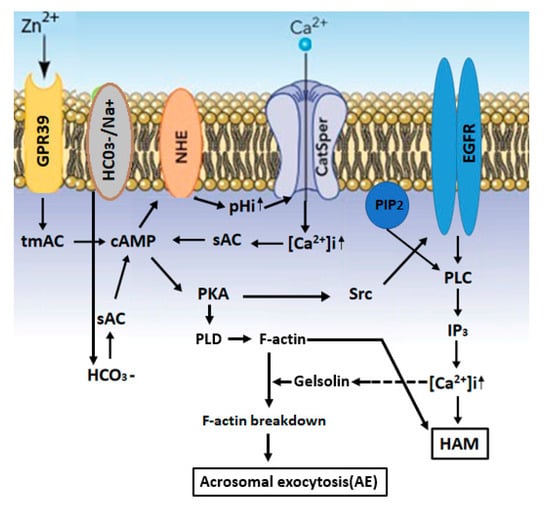
Figure 1.
A model describing the mechanisms that mediate the stimulation of hyper-activated motility (HAM) and acrosomal exocytosis (AE) by Zn2+: Zn2+ binds and activates GPR39, which activates the tmAC to catalyze cAMP production. The NHE (Na+/H+-exchanger) is activated by cAMP, leading to increased pHi and the activation of CatSper, resulting in an increase in [Ca2+]i, which, together with HCO3−, activates sAC. The increase in [cAMP]i causes PKA activation, followed by activation of the Src-epidermal growth factor receptor-Pphospholipase C (Src-EGFR-PLC) cascade, resulting in inositol-triphosphate (IP3) production, which mobilizes Ca2+ from the acrosome, causing a further increase in [Ca2+]i and the development of hyper-activated motility. PKA also activates PLD1 leading to F-actin formation during capacitation. Prior to the AE, PLC induces phosphadidylinositol-4,5-bisphosphate (PIP2) hydrolysis, leading to the release of the actin-severing protein gelsolin to the cytosol, which is activated by Ca2+, resulting in F-actin breakdown and acrosomal exocytosis (AE).
2.3. Zn2+ Mediates Sperm Capacitation and Acrosomal Exocytosis
Extracellular Zn2+ affects intracellular signaling pathways through its interaction with the Zn2+-sensing receptor (ZnR), also named GPR39 [65]. This receptor was found in the sperm tail and the acrosome [74,88,89], suggesting the possible involvement of Zn2+ in sperm motility and acrosomal exocytosis. Our studies showed that Zn2+ stimulates bovine sperm acrosomal exocytosis [88], as well as human sperm hyper-activated motility [74], both mediated by GPR39 (see Figure 1). The GPR39 receptors belong to the GPCR family, known to activate trans-membrane adenylyl-cyclase (tmAC). The function of tmAC in mammalian sperm is still controversial [90]. As mentioned above, tmAC is regulated by the G-protein, and the stimulatory G-protein (Gs) is, so far, localized in the sperm head [91], suggesting its possible involvement in the acrosome reaction but probably not in sperm motility. A previous study from our laboratory showed the involvement of two GPCRs in bovine sperm, angiotensin II-receptor (AngII-R) and lysophosphatidic acid-receptor, in sperm capacitation [92]. Motility and PKA activation in amphibian sperm are also stimulated by tmAC [93]. Human sperm treated with 5-µM Zn2+ show a 40% increase in intracellular cAMP levels, which is a necessary event in the capacitation process [88]. Higher concentrations of zinc (20–30 µM) caused a much smaller increase in cAMP cellular levels [74]. These effects of zinc concentrations of cAMP levels are well-correlated with the stimulation of HAM, with a high increase in HAM with 5-µM zinc and lower effect with 20–30-µM zinc [74]. It has been suggested that Zn2+ mediates the activity of the two adenylyl-cyclase(AC) isoforms, the soluble AC (sAC), activated by bicarbonate, as well as the trans-membrane AC (tmAC), activated by GPCR, leading to an increase of intracellular cAMP (see Figure 1). Interestingly, the addition of 8Br-cAMP (a membrane-permeable cAMP analog) to human sperm revealed a significant stimulation of hyper-activated motility at relatively low concentrations (10–15 µM), and this stimulatory effect was reduced at 20 µM and disappeared at 30-µM 8Br-cAMP [74]. The similarity of the dose response effect on the hyper-activated motility between zinc and cAMP clearly suggests that zinc mediates the hyper-activated motility by increasing the intracellular cAMP levels. Surprisingly, the stimulatory effect of extracellularly administered 8Br-cAMP on HAM was inhibited by sAC but not by tmAC inhibitors [74], conditions under which the cellular levels of cAMP should not be affected, since 8Br-cAMP was added to the cells. We can explain this result by suggesting that the cAMP supplied to the cells may be excluded from cellular locations at which sAC provides cAMP for HAM. Interestingly, attempts to bypass the need for sAC activity by providing cAMP did not restore fertilization competence of sAC-null sperm [94]. Several studies suggested that cAMP signaling in sperm is compartmentalized to the head and three regions of the tail: the midpiece, principal piece and endpiece [95,96,97,98]. This compartmentalization might be the basis for different cAMP responses of the tail waveform in the proximal and distal regions of the flagella upon bicarbonate (an activator of sAC) induction [99]. The cAMP microdomains are formed due to the distinct flagellar distribution of at least two phosphodiesterase (PDE) isoforms [100], PDE4 and PDE1, which regulate sperm capacitation and motility [84,101,102].
It has been shown that the in vitro addition of high concentrations of Zn2+ to bovine [88] and human [74] sperm could lead to the inhibition of several capacitation processes and reduced fertility rate [103]. Zinc has antioxidant activity and may decrease the levels of reactive oxygen species (ROS) [104,105]. It was shown that ROS production is essential for sperm capacitation [106,107]; however, relatively high levels of ROS can harm sperm functions [108]. Thus, low zinc concentrations might be beneficial in reducing excessive levels of ROS, whereas high zinc might decrease ROS to a level that is inhibitory to sperm capacitation [74,109]. The relatively low concentrations of Zn2+ (1.0–1.5µM) [75] in the female reproductive tract allows the occurrence of sperm capacitation and the acrosome reaction, leading to fertilization. It has been proposed [110] that zona pellucida (ZP) proteinases implicated in endowing the acrosome reacted spermatozoon with the ability to penetrate the ZP are negatively regulated by Zn2+. Sperm can induce Zn2+ release from the oocyte cortex [111,112], leading to proteinase inhibition; as a result, sperm that are still bound to the ZP became decapacitated, and polyspermy was prevented. It was also suggested that Zn2+ inhibits sperm chemoattraction to the egg induced by oocyte-secreted progesterone in human, mouse and rabbit sperm [113], and the addition of Zn2+ (~0.1 mM) to bovine in vitro fertilization (IVF) medium reduces the fertilization rate [114]. Additionally, blockers of Zn2+-dependent metalloproteases inhibit sperm passage via the cumulus oophorus in porcine IVF [115].
Matrix metalloproteinases (MMPs) are part of the Zn2+-dependent endopeptidase family. The isoform MMP2 is localized to the acrosomal region and tail, and MMP9 is expressed in the tail of human sperm [116,117]. MMP2, together with the proteinase acrosin, are localized to the inner acrosomal membrane, suggesting the possibility of their cooperation in oocyte penetration [118]. We have shown that MMP is involved in the transactivation of the epidermal growth factor receptor (EGFR) by activating GPCRs during sperm capacitation [92].
Low concentrations of Zn2+ (in the µM range) increase the in vitro capacitation efficiency [74,88] by activating several proteins during this process, including the PKA, Src, EGFR and phosphatidylinositol-3-kinase (PI3K) [119,120,121,122], leading to intracellular Ca2+ mobilization and acrosomal exocytosis (see Figure 1). We suggested the following mechanism that regulates human sperm hyper-activated motility: Zn2+ stimulates HAM via CatSper-dependent activation of the adenylyl-cyclase (AC)/cAMP/PKA/Src/EGFR and phospholipase C (PLC) cascade [74] (see Figure 1). In bovine sperm, Zn2+ activates the EGFR during capacitation, which is mediated by the activation of tmAC, PKA and Src [88]. The addition of Zn2+ to capacitated bovine sperm further stimulates the EGFR and the downstream effectors PI3K, PLC and protein kinase C (PKC), leading to acrosomal exocytosis [88] (see Figure 1). Under physiological conditions, inositol-3-phosphate receptor (IP3R) localized in the outer acrosomal membrane and in the redundant nuclear envelope (RNE) are activated by IP3 generated from the hydrolysis of phosphadidyl-inositol-4,5-bisphosphate (PIP2) by PLC, inducing the release of Ca2+ from the acrosome and RNE and promoting the development of HAM [123]. Thus, this cascade can be initiated by Zn2+-activated GPR39, leading to PLC activation, as described above.
It is known that the occurrence of spontaneous acrosomal reaction (sAR) before the sperm reaches the proximity of the egg reduces the fertilization rate [124]. Sperm contain several mechanisms that protect it from undergoing sAR [125,126,127,128]. We showed that actin polymerization during sperm capacitation is crucial for preventing sAR [125] and found that the addition of 5-10µM Zn2+ to bovine sperm increases actin polymerization and decreases the sAR rate (unpublished). The integrity of the sperm acrosome is likely to be defective in sperm with a high rate of sAR. Accordingly, a dietary supplementation of Zn2+ to goats increases sperm acrosome integrity [104], supporting the role of Zn2+ in preventing sAR.
2.4. Zinc in Assisted Reproductive Techniques
The effectiveness of assisted reproductive methods has been improved over the last decade. The cryopreservation of sperm under liquid nitrogen is now used in assisted reproduction centers to conserve sperm cells for extended periods of time. However, freezing and thawing processes damage the fertilizing capacity of the sperm due to osmotic effects and oxidative stress. Under cryopreservation, sperm is deprived of the seminal plasma, which contains antioxidants and vitamins that act against free radicals and, thereby, protect the sperm cells [129,130].
Cryopreservation affects sperm cells in many ways: by diminishing the fertilization capacity, reducing motility, altering morphology (such as coiled tails), reducing viability [131], damaging the cell membrane [132] and causing DNA fragmentation [133,134] and loss of mitochondrial function [135].
The addition of antioxidants, such as zinc, in the freezing medium to improve the fertility capacity and preserve the sperm from oxidative damage is becoming increasingly widespread [136,137]. Studies revealed that, after incubation with hydrogen peroxide, the DNA fragmentation percentage in spermatozoa was increased, and the effect was reversed by zinc supplementation to the medium [138].
Recent studies demonstrated the beneficial effects of zinc addition to human ejaculate before cryopreservation on sperm viability and motility after thawing [138,139]. The freezing of human sperm in the presence of 50-µM zinc revealed a 26–184% increase in the number of motile sperm after thawing and a 130% increase in the percentage of progressive motility compared to a control without zinc [139]. Moreover when cells were frozen, thawed and refrozen in the presence of zinc, a considerable increase in motility was observed [139] relative to the first thaw. Moreover, zinc has been reported to preserve genomic integrity [140], chromosomal stability [141,142] and to protect the sperm membrane [143,144] and cell morphology during cryopreservation.
Zinc oxide nanoparticles (ZnONPs), initially developed for drug delivery in cancer research [145], were applied to study sperm preservation during cryopreservation. The ZnONPs seemed to prevent DNA damage and stabilize sperm chromatin [146]. These protective effects were reported to be linked to the creation of a protective layer of ZnONPs around the sperm cell, preventing lipid peroxidation at the membrane [146].
Zinc-deficient nutrition causes a low quality of sperm and male infertility [11], and the presence of zinc in the diets of humans [27] and domestic animals [28,29,30,147] is required for the achievement of an optimal fertility rate. Nevertheless, recent clinical studies investigated the effect of dietary supplements containing zinc and showed that zinc supplementation does not appear to improve pregnancy rates, sperm counts or sperm function [148]. The addition of zinc in the micromolar range to an in vitro fertilization medium can contribute to improve sperm motility and capacitation, which will increase the fertilization rate.
3. Conclusions
An adequate Zn2+ concentration in the seminal plasma is needed for normal sperm function and fertilization; however, highly the toxic content of Zn2+ may have a negative effect on sperm quality. Although many studies prove the association between seminal plasma Zn2+ concentration and sperm physiology, it certainly cannot be said that seminal Zn2+ deficiency causes infertility. The addition of zinc in the micromolar range to a sperm medium in vitro can contribute to the amelioration of sperm motility and capacitation to fertilize the oocyte. Zinc supplementation should be considered as a good player for the improvement of in vitro-assisted reproduction procedures, although Zn2+ dietary supplementation has not already been proved to ameliorate pregnancy rate in humans. Altogether, the currently available data revealed the importance of zinc ions for male fertility, which could be important to improve human reproductive health and the reproductive efficiency in agriculturally important livestock species. Further studies in fertile and infertile men are required to prove this claim, and the determination of the seminal Zn2+ level in these men will help to solve this question.
Author Contributions
The two authors equally contributed in writing this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | linear dichroism |
References
- Chvapil, M. New aspects in the biological role of zinc: A stabilizer of macromolecules and biological membranes. Life Sci. 1973, 13, 1041–1049. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Parashuramulu, S.; Nagalakshmi, D.; Rao, D.S.; Kumar, M.K.; Swain, P. Effect of Zinc supplementation on antioxidant status and immune response in buffalo calves. Anim. Nutr. Feed Technol. 2015, 15, 179–188. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef]
- Yan, M.; Hardin, K.; Ho, E. Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J. Nutr. Biochem. 2010, 21, 687–694. [Google Scholar] [CrossRef][Green Version]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc De-ficiency. In Nutritional Deficiency; Erkekoglu, P., Kocel-Gumusel, B., Eds.; Tech Open Science: Rijeka, Croatia, 2016; pp. 23–46. [Google Scholar]
- Wani, A.L.; Parveen, N.; Ansari, M.O.; Ahmad, M.F.; Ja-meel, S.; Shadab, G. Zinc: An element of extensive medical importance. Curr. Med. Res. Pract. 2017, 7, 90–98. [Google Scholar] [CrossRef]
- Wong, W.Y.; Flik, G.; Groenen, P.M.; Swinkels, D.W.; Thomas, C.M.; Copius-Peereboom, J.H.; Merkus, H.M.; Steegers-Theunissen, R.P. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod. Toxicol. 2001, 15, 131–136. [Google Scholar] [CrossRef]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Singh, A.; Prabha, V.; Singh, R.; Sharma, P. Escherichia coli attaches to human spermatozoa: Affecting sperm parameters. Arch. Appl. Sci. Res. 2011, 3, 618–623. [Google Scholar]
- Albert, A. Selective Toxicity: The Physico-Chemical Basis of Therapy, 6th ed.; Springer: Dordrecht, The Netherlands, 2012; p. 368. [Google Scholar]
- Vijayalakshmi, K.; Sivaraj, D. Enhanced antibacterial activity of Cr doped ZnO nanorods synthesized using microwave processing. RSC Adv. 2015, 5, 68461–68469. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Kjellberg, S.; Roomans, G.M.; Kvist, U. The human sperm nucleus takes up zinc at ejaculation. Int. J. Androl. 1986, 9, 77–80. [Google Scholar] [CrossRef]
- Bertrand, G.; Vladesco, M.R. Role of zinc in reproduction. Acad. Sci. 1921, 173, 176–179. [Google Scholar]
- Mankad, M.; Sathawara, N.G.; Doshi, H.; Saiyed, H.N.; Kumar, S. Seminal plasma zinc concentration and alpha-glucosidase activity with respect to semen quality. Biol. Trace Elem. Res. 2006, 110, 97–106. [Google Scholar] [CrossRef]
- Liu, D.Y.; Sie, B.S.; Liu, M.L.; Agresta, F.; Baker, H.W. Relationship between seminal plasma zinc concentration and spermatozoa-zona pellucida binding and the ZP-induced acrosome reaction in subfertile men. Asian J. Androl. 2009, 11, 499–507. [Google Scholar] [CrossRef]
- Ma, J.; Han, R.; Li, Y.; Cui, T.; Wang, S. The Mechanism of Zinc Sulfate in Improving Fertility in Obese Rats Analyzed by Sperm Proteomic Analysis. Biomed. Res. Int. 2020, 2020, 9876363. [Google Scholar] [CrossRef]
- Eggert-Kruse, W.; Zwick, E.M.; Batschulat, K.; Rohr, G.; Armbruster, F.P.; Petzoldt, D.; Strowitzki, T. Are zinc levels in seminal plasma associated with seminal leukocytes and other determinants of semen quality? Fertil. Steril. 2002, 77, 260–269. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chang, T.C.; Tseng, Y.J.; Lin, Y.L.; Huang, F.J.; Kung, F.T.; Chang, S.Y. Seminal plasma zinc levels and sperm motion characteristics in infertile samples. Chang. Gung. Med. J. 2000, 23, 260–266. [Google Scholar]
- Brito, M.; Figueroa, J.; Vera, J.C.; Cortés, P.; Hott, R.; Burzio, L.O. Phosphoproteins are structural components of bull sperm outer dense fiber. Gamete Res. 1986, 15, 327–336. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Hunt, C.D.; Johnson, P.E.; Herbel, J.; Mullen, L.K. Effects of dietary zinc depletion on seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. Am. J. Clin. Nutr. 1992, 56, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Alsalman, A.R.S.; Almashhedy, L.A.; Hadwan, M.H. Effect of Oral Zinc Supplementation on the Thiol Oxido-Reductive Index and Thiol-Related Enzymes in Seminal Plasma and Spermatozoa of Iraqi Asthenospermic Patients. Biol. Trace Elem. Res. 2018, 184, 340–349. [Google Scholar] [CrossRef]
- Nielsen, F.H. History of zinc in agriculture. Adv. Nutr. 2012, 3, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Verma, R.P.; Singh, L.P.; Varshney, V.P.; Dass, R.S. Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus × Bos taurus) bulls. Reprod. Nutr. Dev. 2006, 46, 663–675. [Google Scholar] [CrossRef]
- Hill, G.M.; Shannon, M.C. Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol. Trace Elem. Res. 2019, 188, 148–159. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Sutovsky, P. Porcine Cell-Free System to Study Mammalian Sperm Mitophagy. Methods Mol. Biol. 2019, 1854, 197–207. [Google Scholar] [PubMed]
- Roomans, G.M.; Lundevall, E.; Bjorndahl, L.; Kvist, U. Removal of zinc from subcellular regions of human spermatozoa by EDTA treatment studied by X-ray microanalysis. Int. J. Androl. 1982, 5, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Kvist, U. Importance of spermatozoal zinc as temporary inhibitor of sperm nuclear chromatin decondensation ability in man. Acta Physiol. Scand. 1980, 109, 79–84. [Google Scholar] [CrossRef]
- Kvist, U. Spermatozoal thiol-disulphide interaction: A possible event underlying physiological sperm nuclear chromatin decondensation. Acta Physiol. Scand. 1982, 115, 503–505. [Google Scholar] [CrossRef]
- Rodriguez, H.; Ohanian, C.; Bustos-Obregon, E. Nuclear chromatin decondensation of spermatozoa in vitro: A method for evaluating the fertilizing ability of ovine semen. Int. J. Androl. 1985, 8, 147–158. [Google Scholar] [CrossRef]
- Bin, B.H.; Seo, J.; Kim, S.T. Function, structure, and transport aspects of ZIP and ZnT zinc transporters in immune cells. J. Immunol. Res. 2018, 2018, 9365747. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Yuce, K. Zinc transporter proteins. Neurochem. Res. 2018, 43, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Fukada, T.; Hosaka, T.; Yamasaki, S.; Ohashi, W.; Hojyo, S.; Miyai, T.; Nishida, K.; Yokoyama, S.; Hirano, T. Biochemical characterization of human ZIP13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J. Biol. Chem. 2011, 286, 40255–40265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Nicholson, R.I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta 2003, 1611, 16–30. [Google Scholar] [CrossRef]
- Gaither, L.A.; Eide, D.J. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Fu, D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J. Biol. Chem. 2004, 279, 17173–17180. [Google Scholar] [CrossRef] [PubMed]
- L’Hernault, S.W.; Shakes, D.C.; Ward, S. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics 1988, 120, 435–452. [Google Scholar] [PubMed]
- Ward, S.; Miwa, J. Characterization of temperature-sensitive, fertilization-defective mutants of the nematode Caenorhabditis elegans. Genetics 1978, 88, 285–303. [Google Scholar]
- Muhlrad, P.J.; Clark, J.N.; Nasri, U.; Sullivan, N.G.; LaMunyon, C.W. SPE-8, a protein-tyrosine kinase, localizes to the spermatid cell membrane through interaction with other members of the SPE-8 group spermatid activation signaling pathway in C. elegans. BMC Genet. 2014, 15, 83. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Till, J.H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 2000, 69, 373–398. [Google Scholar] [CrossRef]
- Visconti, P.E.; Stewart-Savage, J.; Blasco, A.; Battaglia, L.; Miranda, P.; Kopf, G.S.; Tezon, J.G. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol. Reprod. 1999, 61, 76–84. [Google Scholar] [CrossRef]
- Geldziler, B.; Chatterjee, I.; Singson, A. The genetic and molecular analysis of spe-19, a gene required for sperm activation in Caenorhabditis elegans. Dev. Biol. 2005, 283, 424–436. [Google Scholar] [CrossRef]
- Nance, J.; Davis, E.B.; Ward, S. spe-29 encodes a small predicted membrane protein required for the initiation of sperm activation in Caenorhabditis elegans. Genetics 2000, 156, 1623–1633. [Google Scholar] [PubMed]
- Shakes, D.C.; Ward, S. Initiation of spermiogenesis in C. elegans: A pharmacological and genetic analysis. Dev. Biol. 1989, 134, 189–200. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Shang, Y.; Huang, P.; Miao, L. The micronutrient element zinc modulates sperm activation through the SPE-8 pathway in Caenorhabditis elegans. Development 2013, 140, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Muhlrad, P.J.; Ward, S. Spermiogenesis initiation in Caenorhabditis elegans involves a casein kinase 1 encoded by the spe-6 gene. Genetics 2002, 161, 143–155. [Google Scholar] [PubMed]
- Arduengo, P.M.; Appleberry, O.K.; Chuang, P.; L’Hernault, S.W. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J. Cell. Sci. 1998, 111, 3645–3654. [Google Scholar]
- Dietrich, N.; Schneider, D.L.; Kornfeld, K. A pathway for low zinc homeostasis that is conserved in animals and acts in parallel to the pathway for high zinc homeostasis. Nucleic Acids Res. 2017, 45, 11658–11672. [Google Scholar] [CrossRef]
- Shihan, M.; Chan, K.H.; Konrad, L.; Scheiner-Bobis, G. Non-classical testosterone signaling in spermatogenic GC-2 cells is mediated through ZIP9 interacting with Gnalpha11. Cell Signal 2015, 27, 2077–2086. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010, 16, 23–29. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Kvist, U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst. Biol. Reprod. Med. 2011, 57, 86–92. [Google Scholar] [CrossRef]
- Chu, D.S. Zinc: A small molecule with a big impact on sperm function. PLoS Biol. 2018, 16, e2006204. [Google Scholar] [CrossRef]
- Henkel, R.; Maass, G.; Schuppe, H.C.; Jung, A.; Schubert, J.; Schill, W.B. Molecular aspects of declining sperm motility in older men. Fertil. Steril. 2005, 84, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Boran, C.; Ozkan, K.U. The effect of zinc therapy on damaged testis in pre-pubertal rats. Pediatr. Surg. Int. 2004, 20, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Clapper, D.L.; Davis, J.A.; Lamothe, P.J.; Patton, C.; Epel, D. Involvement of zinc in the regulation of pHi, motility, and acrosome reactions in sea urchin sperm. J. Cell Biol. 1985, 100, 1817–1824. [Google Scholar] [CrossRef]
- Stoltenberg, M.; Sorensen, M.B.; Danscher, G.; Juhl, S.; Andreasen, A.; Ernst, E. Autometallographic demonstration of zinc ions in rat sperm cells. Mol. Hum. Reprod. 1997, 3, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, M.; Mori, H. Heavy metals and spermatozoan motility. I. Distribution of iron, zinc and copper in sea urchin spermatozoa. Exp. Cell Res. 1972, 70, 311–316. [Google Scholar] [CrossRef]
- Baccetti, B.; Pallini, V.; Burrini, A.G. The accessory fibers of the sperm tail. II. Their role in binding zinc in mammals and cephalopods. J. Ultrastruct. Res. 1976, 54, 261–275. [Google Scholar] [CrossRef]
- Calvin, H.I. Electrophoretic evidence for the identity of the major zinc-binding polypeptides in the rat sperm tail. Biol. Reprod. 1979, 21, 873–882. [Google Scholar] [CrossRef]
- Clermont, Y.; Oko, R.; Hermo, L. Immunocytochemical localization of proteins utilized in the formation of outer dense fibers and fibrous sheath in rat spermatids: An electron microscope study. Anat. Rec. 1990, 227, 447–457. [Google Scholar] [CrossRef]
- Henkel, R.; Baldauf, C.; Bittner, J.; Weidner, W.; Miska, W. Elimination of zinc from the flagella of spermatozoa during epididymal transit is important for motility. Reprod. Technol. 2001, 10, 280–285. [Google Scholar]
- Bolanca, I.; Obhodas, J.; Ljiljak, D.; Matjacic, L.; Kuna, K. Synergetic Effects of K, Ca, Cu and Zn in Human Semen in Relation to Parameters Indicative of Spontaneous Hyperactivation of Spermatozoa. PLoS ONE 2016, 11, e0152445. [Google Scholar] [CrossRef]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Allouche-Fitoussi, D.; Bakhshi, D.; Breitbart, H. Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn2+. Mol. Reprod. Dev. 2018, 85, 543–556. [Google Scholar] [CrossRef]
- Menezo, Y.; Pluntz, L.; Chouteau, J.; Gurgan, T.; Demirol, A.; Dalleac, A.; Benkhalifa, M. Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of Zn2+ transporters in human oocytes and cumulus cells. Reprod. Biomed. Online 2011, 22, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Granish, K.A.; Suarez, S.S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 2002, 250, 208–217. [Google Scholar] [CrossRef]
- Riffo, M.; Leiva, S.; Astudillo, J. Effect of zinc on human sperm motility and the acrosome reaction. Int. J. Androl. 1992, 15, 229–237. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Babcock, D.F.; Rufo, G.A.; Lardy, H.A. Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc. Natl. Acad. Sci. USA 1983, 80, 1327–1331. [Google Scholar] [CrossRef]
- Miller, M.R.; Kenny, S.J.; Mannowetz, N.; Mansell, S.A.; Wojcik, M.; Mendoza, S.; Zucker, R.S.; Xu, K.; Lishko, P.V. Asymmetrically positioned flagellar control units regulate human sperm rotation. Cell Rep. 2018, 24, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Chung, J.J.; Shim, S.H.; Everley, R.A.; Gygi, S.P.; Zhuang, X.; Clapham, D.E. Structurally distinct Ca2+ signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 2014, 157, 808–822. [Google Scholar] [CrossRef]
- Suarez, S.S.; Varosi, S.M.; Dai, X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc. Natl. Acad. Sci. USA 1993, 90, 4660–4664. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Laclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation in mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [PubMed]
- Navarrete, F.A.; Garcia-Vazquez, F.A.; Alvau, A.; Escoffier, J.; Krapf, D.; Sanchez-Cardenas, C.; Salicioni, A.M.; Darszon, A.; Visconti, P.E. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J. Cell Physiol. 2015, 230, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010, 588, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; King, S.M.; Quill, T.A.; Doolittle, L.K.; Garbers, D.L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 2003, 5, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Michailov, Y.; Ickowicz, D.; Breitbart, H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev. Biol. 2014, 396, 246–255. [Google Scholar] [CrossRef]
- Schneider, M.; Forster, H.; Boersma, A.; Seiler, A.; Wehnes, H.; Sinowatz, F.; Neumuller, C.; Deutsch, M.J.; Walch, A.; Hrabe de Angelis, M.; et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009, 23, 3233–3242. [Google Scholar] [CrossRef]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krahling, M.; Muller, A.; Kaupp, U.B.; Strunker, T. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef]
- Wertheimer, E.; Krapf, D.; de la Vega-Beltran, J.L.; Sanchez-Cardenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J. Biol. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef]
- Etkovitz, N.; Tirosh, Y.; Chazan, R.; Jaldety, Y.; Daniel, L.; Rubinstein, S.; Breitbart, H. Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev. Biol. 2009, 334, 447–457. [Google Scholar] [CrossRef]
- O’Brien, E.D.; Krapf, D.; Cabada, M.O.; Visconti, P.E.; Arranz, S.E. Transmembrane adenylyl cyclase regulates amphibian sperm motility through protein kinase A activation. Dev. Biol. 2011, 350, 80–88. [Google Scholar] [CrossRef]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 2005, 9, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Balbach, M.; Beckert, V.; Hansen, J.N.; Wachten, D. Shedding light on the role of cAMP in mammalian sperm physiology. Mol. Cell Endocrinol. 2018, 468, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.; Alvarez, L.; Balbach, M.; Strunker, T.; Hegemann, P.; Kaupp, U.B.; Wachten, D. Controlling fertilization and cAMP signaling in sperm by optogenetics. Elife 2015, 4, e05161. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.; Jikeli, J.F.; Wachten, D. How to control cyclic nucleotide signaling by light. Curr. Opin. Biotechnol. 2017, 48, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Jansen, V.; Jikeli, J.F.; Hamzeh, H.; Alvarez, L.; Dombrowski, M.; Balbach, M.; Strunker, T.; Seifert, R.; Kaupp, U.B.; et al. A novel biosensor to study cAMP dynamics in cilia and flagella. Elife 2016, 5, e14052. [Google Scholar] [CrossRef]
- Raju, D.N.; Hansen, J.N.; Rassmann, S.; Stuven, B.; Jikeli, J.F.; Strunker, T.; Korschen, H.G.; Moglich, A.; Wachten, D. Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains. Cells 2019, 8, 648. [Google Scholar] [CrossRef]
- Bajpai, M.; Fiedler, S.E.; Huang, Z.; Vijayaraghavan, S.; Olson, G.E.; Livera, G.; Conti, M.; Carr, D.W. AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol. Reprod. 2006, 74, 109–118. [Google Scholar] [CrossRef]
- Fisch, J.D.; Behr, B.; Conti, M. Enhancement of motility and acrosome reaction in human spermatozoa: Differential activation by type-specific phosphodiesterase inhibitors. Hum. Reprod. 1998, 13, 1248–1254. [Google Scholar] [CrossRef]
- Leclerc, P.; Kopf, G.S. Mouse sperm adenylyl cyclase: General properties and regulation by the zona pellucida. Biol. Reprod. 1995, 52, 1227–1233. [Google Scholar] [CrossRef][Green Version]
- Abdul-Rasheed, O.F. The relationship between seminal plasma zinc levels and high molecular weight zinc binding protein and sperm motility in Iraqi infertile men. Saudi Med. J. 2009, 30, 485–489. [Google Scholar] [PubMed]
- Narasimhaiah, M.; Arunachalam, A.; Sellappan, S.; Mayasula, V.K.; Guvvala, P.R.; Ghosh, S.K.; Chandra, V.; Ghosh, J.; Kumar, H. Organic zinc and copper supplementation on antioxidant protective mechanism and their correlation with sperm functional characteristics in goats. Reprod. Domest. Anim. 2018, 53, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Shahar, S.; Wiser, A.; Ickowicz, D.; Lubart, R.; Shulman, A.; Breitbart, H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum. Reprod. 2011, 26, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Beconi, M.; Beorlegui, N. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia 1997, 29, 269–275. [Google Scholar] [CrossRef]
- Sikka, S.C. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001, 8, 851–862. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Yoshida, K.; Yoshiike, T.M.; Iwamoto, T.; Gagnon, C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J. Androl. 2001, 22, 672–679. [Google Scholar]
- Kerns, K.; Zigo, M.; Sutovsky, P. Zinc: A Necessary Ion for Mammalian Sperm Fertilization Competency. Int. J. Mol. Sci. 2018, 19, 4097. [Google Scholar] [CrossRef]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Que, E.L.; Duncan, F.E.; Bayer, A.R.; Philips, S.J.; Roth, E.W.; Bleher, R.; Gleber, S.C.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr. Biol. (Camb) 2017, 9, 135–144. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Cubilla, M.; Moreno, A.; Molino, M.V.; Bahamondes, L.; Giojalas, L.C. Sperm chemorepulsion, a supplementary mechanism to regulate fertilization. Hum. Reprod. 2017, 32, 1560–1573. [Google Scholar] [CrossRef]
- Stephenson, J.L.; Brackett, B.G. Influences of zinc on fertilisation and development of bovine oocytes in vitro. Zygote 1999, 7, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Beek, J.; Nauwynck, H.; Maes, D.; Van Soom, A. Inhibitors of zinc-dependent metalloproteases hinder sperm passage through the cumulus oophorus during porcine fertilization in vitro. Reproduction 2012, 144, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Warinrak, C.; Wu, J.T.; Hsu, W.L.; Liao, J.W.; Chang, S.C.; Cheng, F.P. Expression of matrix metalloproteinases (MMP-2, MMP-9) and their inhibitors (TIMP-1, TIMP-2) in canine testis, epididymis and semen. Reprod. Domest. Anim. 2015, 50, 48–57. [Google Scholar] [CrossRef]
- Buchman-Shaked, O.; Kraiem, Z.; Gonen, Y.; Goldman, S. Presence of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinase in human sperm. J. Androl. 2002, 23, 702–708. [Google Scholar]
- Ferrer, M.; Rodriguez, H.; Zara, L.; Yu, Y.; Xu, W.; Oko, R. MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell Tissue Res. 2012, 349, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Azriel-Tamir, H.; Sharir, H.; Schwartz, B.; Hershfinkel, M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 2004, 279, 51804–51816. [Google Scholar] [CrossRef]
- Besser, L.; Chorin, E.; Sekler, I.; Silverman, W.F.; Atkin, S.; Russell, J.T.; Hershfinkel, M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 2009, 29, 2890–2901. [Google Scholar] [CrossRef]
- Chorin, E.; Vinograd, O.; Fleidervish, I.; Gilad, D.; Herrmann, S.; Sekler, I.; Aizenman, E.; Hershfinkel, M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 2011, 31, 12916–12926. [Google Scholar] [CrossRef]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef]
- Ho, H.C.; Suarez, S.S. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol. Reprod. 2001, 65, 1606–1615. [Google Scholar] [CrossRef]
- Wiser, A.; Sachar, S.; Ghetler, Y.; Shulman, A.; Breitbart, H. Assessment of sperm hyperactivated motility and acrosome reaction can discriminate the use of spermatozoa for conventional in vitro fertilisation or intracytoplasmic sperm injection: Preliminary results. Andrologia 2014, 46, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Shabtay, O.; Breitbart, H. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol. 2016, 415, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Huta, Y.; Nitzan, Y.; Breitbart, H. Ezrin protects bovine spermatozoa from spontaneous acrosome reaction. Theriogenology 2020, 151, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tsirulnikov, E.; Huta, Y.; Breitbart, H. PKA and PI3K activities during capacitation protect sperm from undergoing spontaneous acrosome reaction. Theriogenology 2019, 128, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, F.; Zitranski, N.; Borth, H.; Buech, T.; Gudermann, T.; Boekhoff, I. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J. Cell Sci. 2009, 122, 4547–4557. [Google Scholar] [CrossRef]
- Kobayashi, T.; Miyazaki, T.; Natori, M.; Nozawa, S. Protective role of superoxide dismutase in human sperm motility: Superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum. Reprod. 1991, 6, 987–991. [Google Scholar] [CrossRef]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef]
- Nallella, K.P.; Sharma, R.K.; Allamaneni, S.S.; Aziz, N.; Agarwal, A. Cryopreservation of human spermatozoa: Comparison of two cryopreservation methods and three cryoprotectants. Fertil. Steril. 2004, 82, 913–918. [Google Scholar] [CrossRef]
- Critser, J.K.; Huse-Benda, A.R.; Aaker, D.V.; Arneson, B.W.; Ball, G.D. Cryopreservation of human spermatozoa. III. The effect of cryoprotectants on motility. Fertil. Steril. 1988, 50, 314–320. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; McLachlan, R.I. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009, 32, 46–56. [Google Scholar] [CrossRef]
- Zribi, N.; Feki Chakroun, N.; El Euch, H.; Gargouri, J.; Bahloul, A.; Ammar Keskes, L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; McClure, N.; Lewis, S.E. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K. Vitamin E and oxidative stress. Free Radic. Biol. Med. 1991, 11, 215–232. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 1999, 14, 505–512. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Xie, Y.; Wang, Z.; Wu, R.; Cai, J.; Luo, X.; Huang, S.; You, L. Zinc protects sperm from being damaged by reactive oxygen species in assisted reproduction techniques. Reprod. Biomed. Online 2015, 30, 334–339. [Google Scholar] [CrossRef]
- Berkovitz, A.; Allouche-Fitoussi, D.; Izhakov, D.; Breitbart, H. Cryopreservation of human sperm in the presence of Zn2+ increases the motility rate. J. Obs. Gynecol. Investig. 2018, 1, 6–12. [Google Scholar]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Blazak, W.F.; Overstreet, J.W. Instability of nuclear chromatin in the ejaculated spermatozoa of fertile men. J. Reprod. Fertil. 1982, 65, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kotdawala, A.P.; Kumar, S.; Salian, S.R.; Thankachan, P.; Govindraj, K.; Kumar, P.; Kalthur, G.; Adiga, S.K. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J. Assist. Reprod. Genet. 2012, 29, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Bettger, W.J.; O’Dell, B.L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981, 28, 1425–1438. [Google Scholar] [CrossRef]
- Kendall, N.R.; McMullen, S.; Green, A.; Rodway, R.G. The effect of a zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim. Reprod. Sci. 2000, 62, 277–283. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhou, X.; Cao, Y.; Li, C. Preventive effects of supplemental dietary zinc on heat-induced damage in the epididymis of boars. J. Therm. Biol. 2017, 64, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA 2020, 323, 35–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).