Brucella Genomics: Macro and Micro Evolution
Abstract
1. Introduction
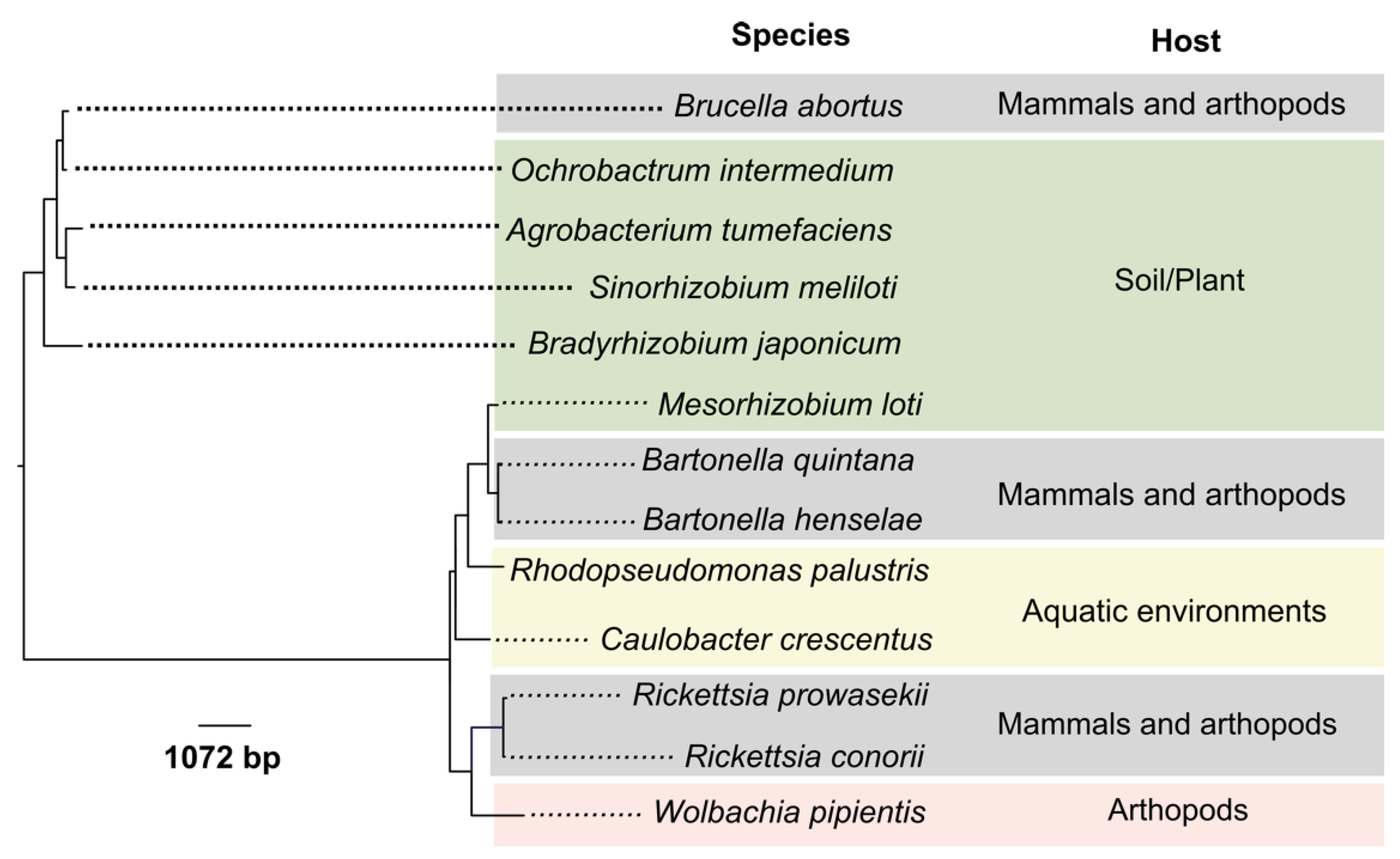
2. Genome Reduction in Cell-Associated Alphaproteobacteria
3. Brucella Genome Macroevolution Includes Gene Loss
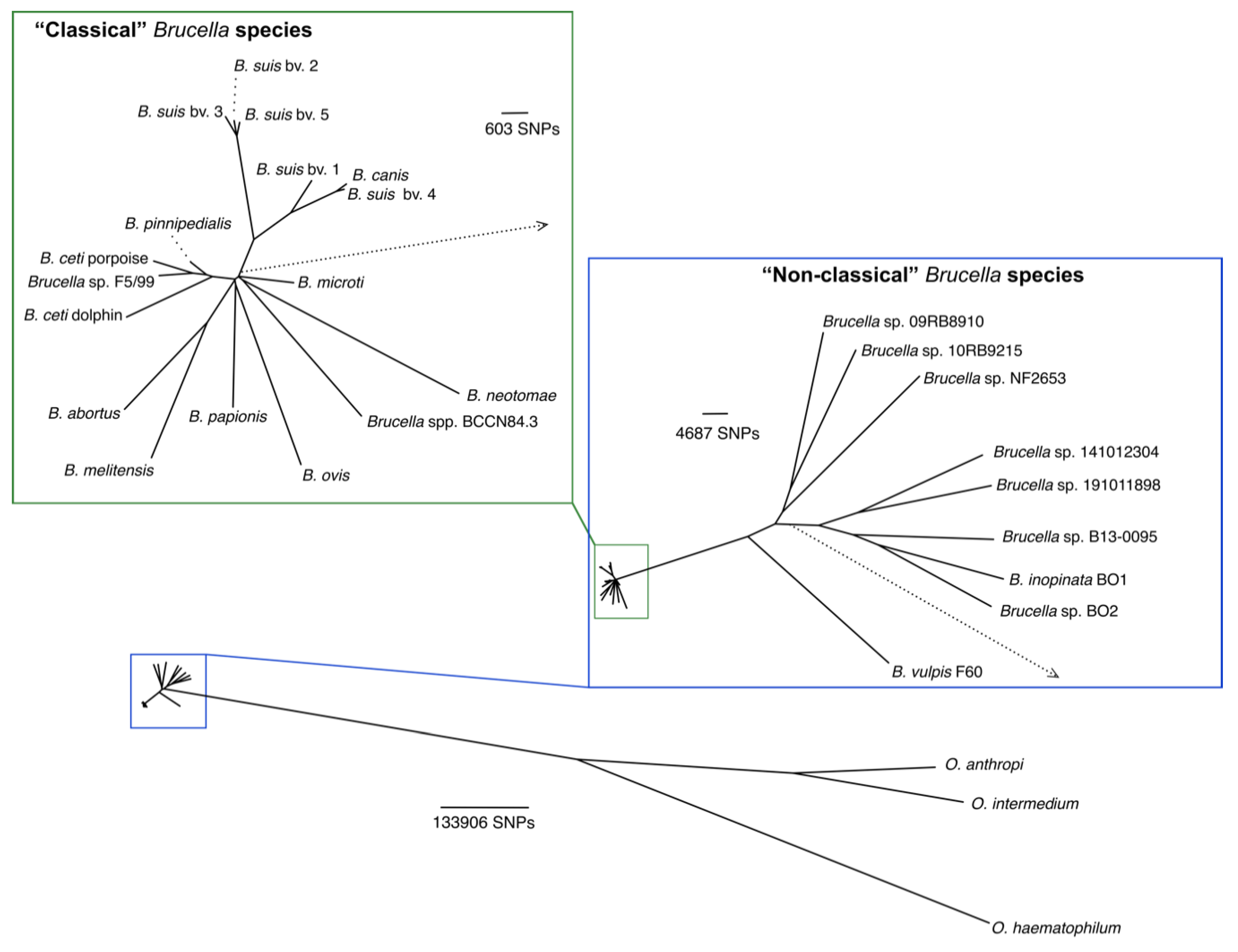
4. Expanding the Number of Brucella Species
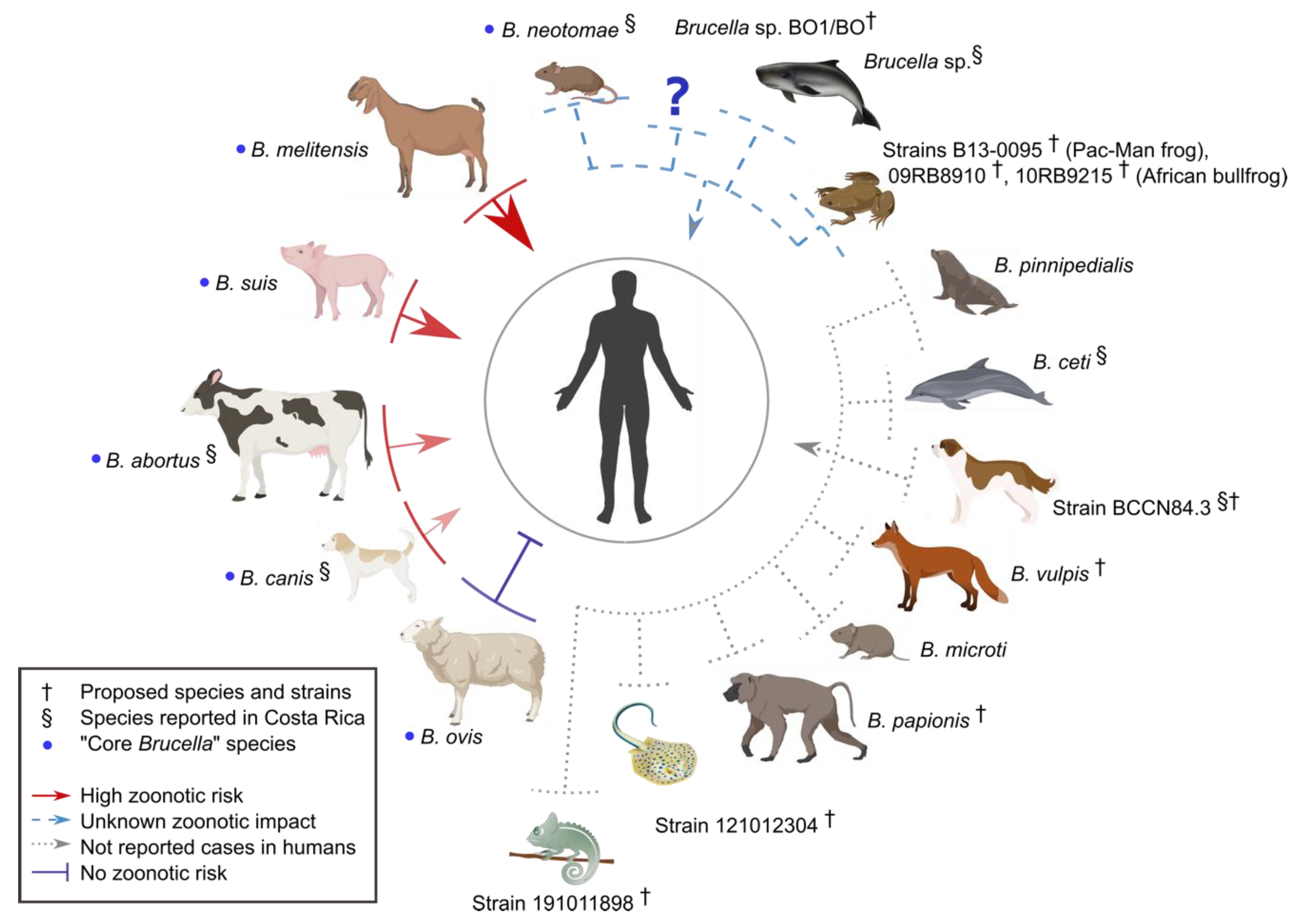
5. Brucella Speciation Bottleneck Through the Domestication of the Preferred Host
6. Brucella Host Distribution from Different Geographic Areas
7. Brucella Speciation and Host Preference: Small Genetic Differences Matter
7.1. Variable Number of Tandem Repeats
7.2. SNPs Barcode Patterns and Allelic Variation
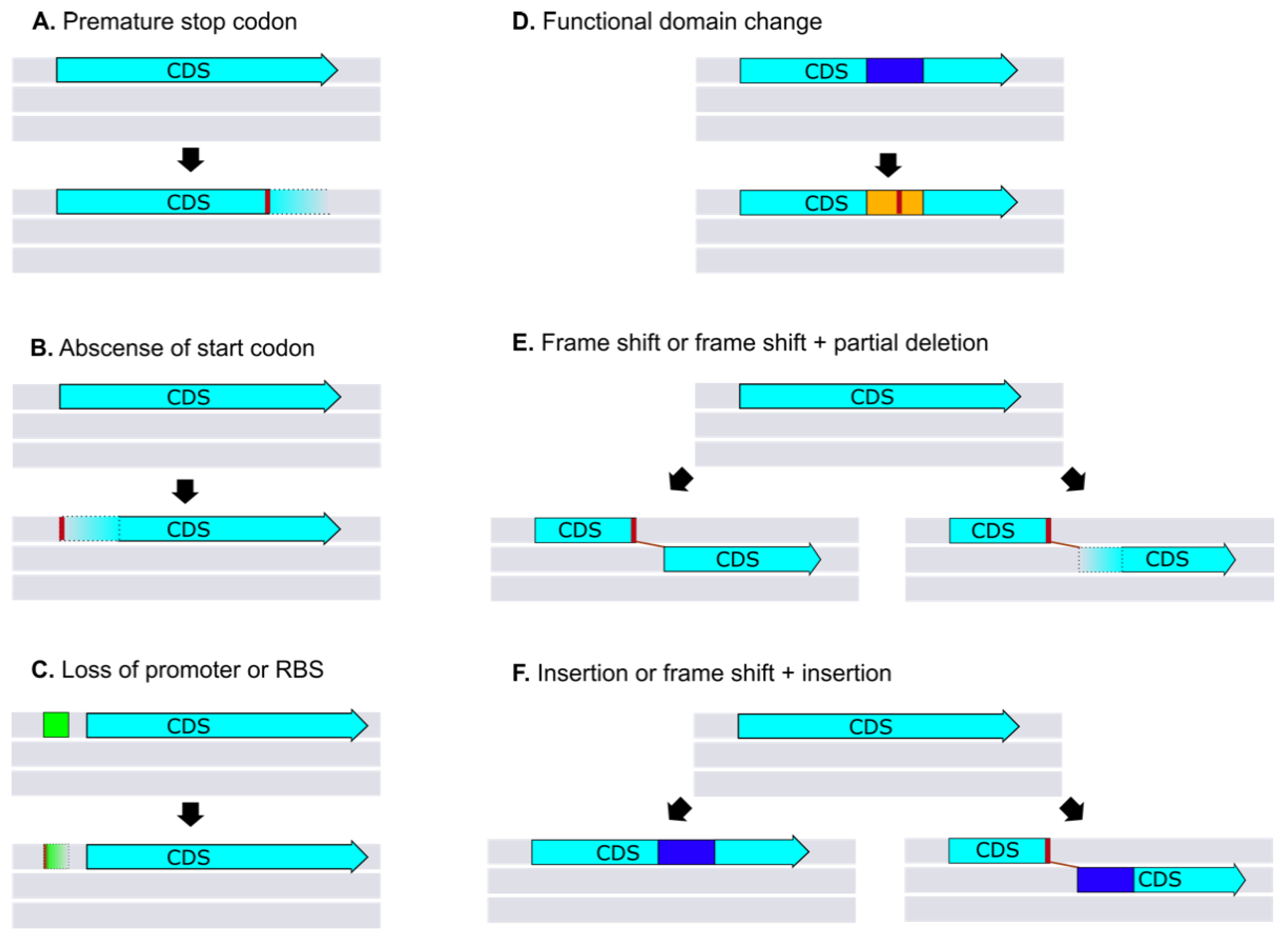
7.3. Pseudogenes
7.4. Genetic Mobile Elements
7.5. Genomic Islands and Anomalous Regions Modifications and Distribution
8. Microevolution of Brucella in the Hosts
9. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDS | Coding sequences |
| GIs | Genomic islands |
| IS | Insertion sequences |
| MGEs | Mobile genetic elements |
| MLST | Multi-locus sequence type |
| MLVA | Multiple loci variable number of tandem repeats |
| NAO | North Atlantic Ocean |
| PFGE | Pulse-field gel electrophoresis |
| SARs | shared anomalous region |
| SNPs | Single nucleotide polymorphisms |
| ST | Sequence type (ST) |
| TR | Tandem repeats |
| VNTR | Variable number of tandem repeats |
| WGS | Whole-genome sequencing |
References
- Stackebrandt, E.; Murray, R.G.E.; Truper, H.G. Proteobacteria classis nov., a Name for the Phylogenetic Taxon That Includes the “Purple Bacteria and Their Relatives”. Int. J. Syst. Bacteriol. 1988, 38, 321–325. [Google Scholar] [CrossRef]
- Moreno, E. In search of a bacterial species definition. Rev. Biol. Trop. 1997, 45, 753–771. [Google Scholar]
- Moreno, E. Genome evolution within the alpha Proteobacteria: Why do some bacteria not possess plasmids and others exhibit more than one different chromosome? Fems Microbiol. Rev. 1998, 22, 255–275. [Google Scholar] [CrossRef]
- Ettema, T.J.G.; Andersson, S.G.E. The α-proteobacteria: The Darwin finches of the bacterial world. Biol. Lett. 2009, 5, 429–432. [Google Scholar] [CrossRef]
- Leclercq, S.; Cloeckaert, A.; Zygmunt, M. Taxonomic organization of the family Brucellaceae based on a phylogenomic approach. Front. Microbiol. 2019, 10, 3083. [Google Scholar] [CrossRef]
- Michaux, S.; Paillisson, J.; Carles-Nurit, M.J.; Bourg, G.; Allardet-Servent, A.; Ramuz, M. Presence of two independent chromosomes in the Brucella melitensis 16M genome. J. Bacteriol. 1993, 175, 701–705. [Google Scholar] [CrossRef]
- Jumas-Bilak, E.; Michaux-Charachon, S.; Bourg, G.; O’Callaghan, D.; Ramuz, M. Differences in chromosome number and genome rearrangements in the genus Brucella. Mol. Microbiol. 1998, 27, 99–106. [Google Scholar] [CrossRef]
- Whatmore, A.M.; Perrett, L.L.; MacMillan, A.P. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 2007, 7, 34. [Google Scholar] [CrossRef]
- Bohlin, J.; Snipen, L.; Cloeckaert, A.; Lagesen, K.; Ussery, D.; Kristoffersen, A.B.; Godfroid, J. Genomic comparisons of Brucella spp. and closely related bacteria using base compositional and proteome based methods. Bmc Evol. Biol. 2010, 10, 249. [Google Scholar] [CrossRef]
- Whatmore, A.M. Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. Infect. Genet. Evol. 2009, 9, 1168–1184. [Google Scholar] [CrossRef]
- Tsolis, R.M. Comparative genome analysis of the alpha -proteobacteria: Relationships between plant and animal pathogens and host specificity. Proc. Natl. Acad. Sci. USA 2002, 99, 12503–12505. [Google Scholar] [CrossRef]
- Moreno, E. Retrospective and prospective perspectives on zoonotic brucellosis. Front. Microbiol. 2014, 5, 1–18. [Google Scholar] [CrossRef]
- Atluri, V.L.; Xavier, M.N.; de Jong, M.F.; den Hartigh, A.B.; Tsolis, R.M. Interactions of the Human Pathogenic Brucella Species with Their Hosts. Annu. Rev. Microbiol. 2011, 65, 523–541. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.P.; Sobral, B.W.; Dickerman, A.W. A robust species tree for the Alphaproteobacteria. J. Bacteriol. 2007, 189, 4578–4586. [Google Scholar] [CrossRef] [PubMed]
- Barquero-Calvo, E.; Conde-Alvarez, R.; Chacón-Díaz, C.; Quesada-Lobo, L.; Martirosyan, A.; Guzmán-Verri, C.; Iriarte, M.; Mancek-Keber, M.; Jerala, R.; Pierre, J.P.; et al. The Differential Interaction of Brucella and Ochrobactrum with Innate Immunity Reveals Traits Related to the Evolution of Stealthy Pathogens. PLoS ONE 2009, 4, e5893. [Google Scholar] [CrossRef]
- Lebuhn, M.; Achouak, W.; Schloter, M.; Berge, O.; Meier, H.; Barakat, M.; Hartmann, A.; Heulin, T. Taxonomic characterization of Ochrobactrum sp. isolates from soil samples and wheat roots, and description of Ochrobactrum tritici sp. nov. and Ochrobactrum grignonense sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 2207–2223. [Google Scholar] [CrossRef]
- Michaux-Charachon, S.; Bourg, G.; Jumas-Bilak, E.; Guigue-Talet, P.; Allardet-Servent, A.; O’Callaghan, D.; Ramuz, M. Genome structure and phylogeny in the genus Brucella. J. Bacteriol. 1997, 179, 3244–3249. [Google Scholar] [CrossRef]
- Jensen, A.E.; Cheville, N.F.; Thoen, C.O.; MacMillan, A.P.; Miller, W.G. Genomic fingerprinting and development of a dendrogram for Brucella spp. isolated from seals, porpoises, and dolphins. J. Vet. Diagn. Investig. 1999, 11, 152–157. [Google Scholar] [CrossRef]
- Whatmore, A.M.; Shankster, S.J.; Perrett, L.L.; Murphy, T.J.; Brew, S.D.; Thirlwall, R.E.; Cutler, S.J.; MacMillan, A.P. Identification and characterization of variable-number tandem-repeat markers for typing of Brucella spp. J. Clin. Microbiol. 2006, 44, 1982–1993. [Google Scholar] [CrossRef]
- Le Flèche, P.; Jacques, I.; Grayon, M.; Al Dahouk, S.; Bouchon, P.; Denoeud, F.; Nöckler, K.; Neubauer, H.; Guilloteau, L.A.; Vergnaud, G. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Whatmore, A.M.; Koylass, M.S.; Muchowski, J.; Edwards-Smallbone, J.; Gopaul, K.K.; Perrett, L.L. Extended multilocus sequence analysis to describe the global population structure of the genus Brucella: Phylogeography and relationship to biovars. Front. Microbiol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.M.; Mick, V.; Sacchini, L.; Janowicz, A.; de Miguel, M.J.; Cherfa, M.A.; Nevado, C.R.; Girault, G.; Andrés-Barranco, S.; Jay, M.; et al. Phylogeography and epidemiology of Brucella suis biovar 2 in wildlife and domestic swine. Vet. Microbiol. 2019, 233, 68–77. [Google Scholar] [CrossRef]
- O’Callaghan, D.; Whatmore, A.M. Brucella genomics as we enter the multi-genome era. Brief. Funct. Genom. 2011, 10, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Ancora, M.; Marcacci, M.; Orsini, M.; Zilli, K.; Di Giannatale, E.; Garofolo, G.; Cammà, C. Complete Genome Sequence of a Brucella ceti ST26 Strain Isolated from a Striped Dolphin (Stenella coeruleoalba) on the Coast of Italy. Genome Announc. 2014, 2, 9–10. [Google Scholar] [CrossRef]
- Duvnjak, S.; Špic, S.; Kušar, D.; Papic, B.; Reil, I.; Zdelar-Tuk, M.; Pavlinec, Z.; Duras, M.; Gomerčić, T.; Hendriksen, R.S.; et al. Whole-genome sequence of the first sequence type 27 brucella ceti strain isolated from european waters. Genome Announc. 2017, 5, 1–2. [Google Scholar] [CrossRef]
- Garofolo, G.; Di Giannatale, E.; Platone, I.; Zilli, K.; Sacchini, L.; Abass, A.; Ancora, M.; Cammà, C.; Di Donato, G.; De Massis, F.; et al. Origins and global context of Brucella abortus in Italy. BMC Microbiol. 2017, 17, 1–7. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Baker, K.S.; Ruiz-Villalobos, N.; Hernández-Mora, G.; Barquero-Calvo, E.; González-Barrientos, R.; Castillo-Zeledón, A.; Jiménez-Rojas, C.; Chacón-Díaz, C.; Cloeckaert, A.; et al. Brucella genetic variability in wildlife marine mammals populations relates to host preference and ocean distribution. Genome Biol. Evol. 2017, 9, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Batut, J.; Andersson, S.G.E.; O’Callaghan, D. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat. Rev. Microbiol. 2004, 2, 933–945. [Google Scholar] [CrossRef]
- Nierman, W.C.; Feldblyum, T.V.; Laub, M.T.; Paulsen, I.T.; Nelson, K.E.; Eisen, J.; Heidelberg, J.F.; Alley, M.R.K.; Ohta, N.; Maddock, J.R.; et al. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 2001, 98, 4136–4141. [Google Scholar] [CrossRef]
- Kaneko, T.; Maita, H.; Hirakawa, H.; Uchiike, N.; Minamisawa, K.; Watanabe, A.; Sato, S. Complete genome sequence of the soybean symbiont Bradyrhizobium japonicum strain USDA6 T. Genes 2011, 2, 763–787. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Seshadri, R.; Nelson, K.E.; Eisen, J.A.; Heidelberg, J.F.; Read, T.D.; Dodson, R.J.; Umayam, L.; Brinkac, L.M.; Beanan, M.J.; et al. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 2002, 99, 13148–13153. [Google Scholar] [CrossRef] [PubMed]
- Alsmark, C.M.; Frank, A.C.; Karlberg, E.O.; Legault, B.-A.; Ardell, D.H.; Cänback, B.; Eriksson, A.-S.; Näslund, A.K.; Handley, S.A.; Huvet, M.; et al. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc. Natl. Acad. Sci. USA 2004, 101, 9716–9721. [Google Scholar] [CrossRef]
- Ogata, H.; Audic, S.; Renesto-Audiffren, P.; Fournier, P.-E.; Barbe, V.; Samson, D.; Roux, V.; Cossart, P.; Weissenbach, J.; Claverie, J.-M.; et al. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Scince 2001, 293, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sun, L.V.; Vamathevan, J.; Riegler, M.; Deboy, R.; Brownlie, J.C.; McGraw, E.A.; Martin, W.; Esser, C.; Ahmadinejad, N.; et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004, 2, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cano, D.J.; Reyes-Prieto, M.; Martínez-Romero, E.; Partida-Martínez, L.P.; Latorre, A.; Moya, A.; Delaye, L. Evolution of small prokaryotic genomes. Front. Microbiol. 2015, 5, 742. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.G.R.; Charlesworth, J.; Miller, E.L.; Casey, M.J.; Lloyd, C.T.; Gottschalk, M.; Tucker, D.; Welch, J.J.; Weinert, L.A. Genome reduction is associated with bacterial pathogenicity across different scales of temporal and ecological divergence. bioRxiv 2020. [Google Scholar] [CrossRef]
- Blanc, G.; Ogata, H.; Robert, C.; Audic, S.; Suhre, K.; Vestris, G.; Claverie, J.-M.; Raoult, D. Reductive genome evolution from the mother of Rickettsia. Plos Genet. 2007, 3, e14. [Google Scholar] [CrossRef]
- Nakayama, K.; Yamashita, A.; Kurokawa, K.; Morimoto, T.; Ogawa, M.; Fukuhara, M.; Urakami, H.; Ohnishi, M.; Uchiyama, I.; Ogura, Y.; et al. The Whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. Dna Res. 2008, 15, 85–99. [Google Scholar] [CrossRef]
- Darby, A.C.; Cho, N.H.; Fuxelius, H.H.; Westberg, J.; Andersson, S.G. Intracellular pathogens go extreme: Genome evolution in the Rickettsiales. Trends Genet. 2007, 23, 511–520. [Google Scholar] [CrossRef]
- Esser, C.; Martin, W.; Dagan, T. The origin of mitochondria in light of a fluid prokaryotic chromosome model. Biol. Lett. 2007, 3, 180–184. [Google Scholar] [CrossRef][Green Version]
- Sela, I.; Wolf, Y.I.; Koonin, E.V. Theory of prokaryotic genome evolution. Proc. Natl. Acad. Sci. USA 2016, 113, 11399–11407. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, R.M.; Seshadri, R.; Santos, R.L.; Sangari, F.J.; García Lobo, J.M.; de Jong, M.F.; Ren, Q.; Myers, G.; Brinkac, L.M.; Nelson, W.C.; et al. Genome degradation in Brucella ovis corresponds with narrowing of its host range and tissue tropism. PLoS ONE 2009, 4, e5519. [Google Scholar] [CrossRef] [PubMed]
- Wattam, A.R.; Foster, J.T.; Mane, S.P.; Beckstrom-Sternberg, S.M.; Beckstrom-Sternberg, J.M.; Dickerman, A.W.; Keim, P.; Pearson, T.; Shukla, M.; Ward, D.V.; et al. Comparative phylogenomics and evolution of the brucellae reveal a path to virulence. J. Bacteriol. 2014, 196, 920–930. [Google Scholar] [CrossRef]
- Eisenberg, T.; Riße, K.; Schauerte, N.; Geiger, C.; Blom, J.; Scholz, H. Isolation of a novel “atypical” Brucella strain from a bluespotted ribbontail ray (Taeniura lymma). Antonie Van Leeuwenhoek 2017, 110, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, M.; Moreno, E.; Stackebrandt, E. Nucleotide sequence of the 16S rRNA from Brucella abortus. Nucleic Acids Res. 1989, 17, 1765. [Google Scholar] [CrossRef][Green Version]
- Moreno, E.; Stackebrandt, E.; Dorsch, M.; Wolters, J.; Busch, M.; Mayer, H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J. Bacteriol. 1990, 172, 3569–3576. [Google Scholar] [CrossRef]
- Kettaneh, A.; Poilane, I.; Fain, O.; Thomas, M.; Herrmann, J.; Hocqueloux, L. Septic Shock Caused by Ochrobactrum anthropi in an Otherwise Healthy Host. J. Clin. Microbiol. 2003, 41, 1339–1341. [Google Scholar] [CrossRef]
- Chain, P.S.G.; Lang, D.M.; Comerci, D.J.; Malfatti, S.A.; Vergez, L.M.; Shin, M.; Ugalde, R.A.; Garcia, E.; Tolmasky, M.E. Genome of Ochrobactrum anthropi ATCC 49188 T, a Versatile Opportunistic Pathogen and Symbiont of Several Eukaryotic Hosts. J. Bacteriol. 2011, 193, 4274–4275. [Google Scholar] [CrossRef]
- Aujoulat, F.; Romano-Bertrand, S.; Masnou, A.; Marchandin, H.; Jumas-Bilak, E. Niches, population structure and genome reduction in Ochrobactrum intermedium: Clues to technology-driven emergence of pathogens. PLoS ONE 2014, 9, e83376. [Google Scholar] [CrossRef] [PubMed]
- Jumas-Bilak, E.; Michaux-Charachon, S.; Bourg, G.; Ramuz, M.; Allardet-servent, A. Unconventional Genomic Organization in the Alpha Subgroup of the Proteobacteria. J. Bacteriol. 1998, 180, 2749–2755. [Google Scholar] [CrossRef]
- Hammerl, J.A.; Göllner, C.; Al Dahouk, S.; Nöckler, K.; Reetz, J.; Hertwig, S. Analysis of the first temperate broad host range Brucellaphage (BiPBO1) isolated from B. inopinata. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Plommet, M. Prevention of Brucellosis in the Mediterranean countries; CIHEAM Publishers: Wageningen, The Netherlands, 1992; pp. 198–218. [Google Scholar]
- Chain, P.S.G.; Comerci, D.J.; Tolmasky, M.E.; Larimer, F.W.; Malfatti, S.A.; Vergez, L.M.; Aguero, F.; Land, M.L.; Ugalde, R.A.; Garcia, E. Whole-genome analyses of speciation events in pathogenic brucellae. Infect. Immun. 2005, 73, 8353–8361. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Ochman, H.; Moran, N. Deletional bias and the evolution of bacterial genomes. Trends Genet. 2001, 17, 589–596. [Google Scholar] [CrossRef]
- Lynch, M. Streamlining and simplification of microbial genome architecture. Annu Rev. Microbiol. 2006, 60, 327–349. [Google Scholar] [CrossRef]
- Frank, A.C.; Alsmark, C.M.; Thollesson, M.; Andersson, S.G.E. Functional divergence and horizontal transfer of type IV secretion systems. Mol. Biol. Evol. 2005, 22, 1325–1336. [Google Scholar] [CrossRef]
- Barquero-Calvo, E.; Chaves-Olarte, E.; Weiss, D.S.; Guzmán-Verri, C.; Chacón-Díaz, C.; Rucavado, A.; Moriyón, I.; Moreno, E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE 2007, 2. [Google Scholar] [CrossRef]
- Moreno, E.; Cloeckaert, A.; Moriyón, I. Brucella evolution and taxonomy. Vet. Microbiol. 2002, 90, 209–227. [Google Scholar] [CrossRef]
- Rajashekara, G.; Glasner, J.D.; Glover, D.A.; Splitter, G.A. Comparative Whole-Genome Hybridization Reveals Genomic Islands in Brucella Species. J. Bacteriol. 2004, 186, 5040–5051. [Google Scholar] [CrossRef]
- Mancilla, M. The Brucella genomic islands. In Brucella: Molecular Microbiology and Genomics; López-Goñi, I., O’Callaghan, D., Eds.; Caister Academic Press: Wymondham, UK, 2012; pp. 36–57. ISBN 978-1-904455-93-6. [Google Scholar]
- Al Dahouk, S.; Köhler, S.; Occhialini, A.; Jiménez de Bagüés, M.P.; Hammerl, J.A.; Eisenberg, T.; Vergnaud, G.; Cloeckaert, A.; Zygmunt, M.S.; Whatmore, A.M.; et al. Brucella spp. of amphibians comprise genomically diverse motile strains competent for replication in macrophages and survival in mammalian hosts. Sci. Rep. 2017, 7, 44420. [Google Scholar] [CrossRef]
- Soler-Lloréns, P.F.; Quance, C.R.; Lawhon, S.D.; Stuber, T.P.; Edwards, J.F.; Ficht, T.A.; Robbe-Austerman, S.; O’Callaghan, D.; Keriel, A. A Brucella spp. Isolate from a Pac-Man Frog (Ceratophrys ornata) Reveals Characteristics Departing from Classical Brucellae. Front. Cell. Infect. Microbiol. 2016, 6, 116. [Google Scholar] [CrossRef]
- Eisenberg, T.; Hamann, H.-P.; Kaim, U.; Schlez, K.; Seeger, H.; Schauerte, N.; Melzer, F.; Tomaso, H.; Scholz, H.C.; Koylass, M.S.; et al. Isolation of potentially novel Brucella spp. from frogs. Appl. Env. Microbiol. 2012, 78, 3753–3755. [Google Scholar] [CrossRef]
- Fischer, D.; Lorenz, N.; Heuser, W.; Kämpfer, P.; Scholz, H.; Lierz, M. Abscesses associated with a Brucella inopinata-like bacterium in a big-eyed tree frog (Leptopelis vermiculatus). J. Zoo Wildl. Med. 2012, 45, 625–628. [Google Scholar] [CrossRef]
- Sobral, B.W.; Wattam, A.R. Comparative Genomics and Phuylogenomics of Brucella. In Brucella Molecular Microbiology and Genomics; Lopez-Goñi, I., O’Callaghan, D., Eds.; Caister Academic Press: Great, Britain, 2012; pp. 13–36. ISBN 978-1-904455-93-6. [Google Scholar]
- Eisenberg, T.; Schlez, K.; Fawzy, A.; Völker, I.; Hechinger, S.; Curić, M.; Schauerte, N.; Geiger, C.; Blom, J.; Scholz, H.C. Expanding the host range: Infection of a reptilian host (Furcifer pardalis) by an atypical Brucella strain. Antonie Van LeeuwenhoekInt. J. Gen. Mol. Microbiol. 2020, 113, 1531–1537. [Google Scholar] [CrossRef]
- El-Sayed, A.; Awad, W. Brucellosis: Evolution and expected comeback. Int. J. Vet. Sci. Med. 2018, 6, S31–S35. [Google Scholar] [CrossRef]
- Foster, J.T.; Beckstrom-Sternberg, S.M.; Pearson, T.; Beckstrom-Sternberg, J.S.; Chain, P.S.G.; Roberto, F.F.; Hnath, J.; Brettin, T.; Keim, P. Whole-genome-based phylogeny and divergence of the genus Brucella. J. Bacteriol. 2009, 191, 2864–2870. [Google Scholar] [CrossRef]
- Moreno, E.; Moriyón, I. The Genus Brucella. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer Link: Berlin/Heidelberg, Germany, 2006; pp. 315–456. ISBN 978-0-387-30745-9. [Google Scholar]
- Nielsen, O.; Nielsen, K.; Stewart, R.E. Serologic evidence of Brucella spp. exposure in Atlantic walruses (Odobenus rosmarus rosmarus) and ringed seals (Phoca hispida) of Arctic Canada. Arctic 1996, 49, 383–386. [Google Scholar] [CrossRef][Green Version]
- Nymo, I.H.; Tryland, M.; Godfroid, J. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Vet. Res. 2011, 42, 93. [Google Scholar] [CrossRef]
- Hernández-Mora, G.; Palacios-Alfaro, J.; González-Barrientos, R. Wildlife reservoirs of brucellosis: Brucella in aquatic environments and brucellosis serology. Rev. Sci. Tech. Off. Int. Epiz. 2013, 32, 89–103. [Google Scholar] [CrossRef]
- McClelland, M.; Sanderson, K.E.; Clifton, S.W.; Latreille, P.; Porwollik, S.; Sabo, A.; Meyer, R.; Bieri, T.; Ozersky, P.; McLellan, M.; et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 2004, 36, 1268–1274. [Google Scholar] [CrossRef]
- Thomson, N.R.; Clayton, D.J.; Windhorst, D.; Vernikos, G.; Davidson, S.; Churcher, C.; Quail, M.A.; Stevens, M.; Jones, M.A.; Watson, M.; et al. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008, 18, 1624–1637. [Google Scholar] [CrossRef]
- Holt, K.E.; Thomson, N.R.; Wain, J.; Langridge, G.C.; Hasan, R.; Bhutta, Z.A.; Quail, M.A.; Norbertczak, H.; Walker, D.; Simmonds, M.; et al. Pseudogene accumulation in the evolutionary histories of Salmonella enterica serovars Paratyphi A and Typhi. Bmc Genom. 2009, 10, 1–12. [Google Scholar] [CrossRef]
- Kuijpers, L.M.F.; Le Hello, S.; Fawal, N.; Fabre, L.; Tourdjman, M.; Dufour, M.; Sar, D.; Kham, C.; Phe, T.; Vlieghe, E.; et al. Genomic analysis of Salmonella enterica serotype Paratyphi A during an outbreak in Cambodia, 2013–2015. Microb. Genom. 2016, 2, e000092. [Google Scholar] [CrossRef]
- Guzmán-Verri, C.; Suárez-Esquivel, M.; Ruíz-Villalobos, N.; Michel, S.; Gonnet, M.; Campos, E.; Víquez-Ruiz, E.; Chacón-Díaz, C.; Conde-Alvarez, R.; Moriyón, I.; et al. Genetic and phenotypic characterization of the etiological agent of canine orchiepididymitis smooth Brucella sp. BCCN84.3. Front. Vet. Sci. 2019, 6, 175. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Ruiz-Villalobos, N.; Jiménez-Rojas, C.; Barquero-Calvo, E.; Chacón-Díaz, C.; Víquez-Ruiz, E.; Rojas-Campos, N.; Baker, K.S.; Oviedo-Sánchez, G.; Amuy, E.; et al. Brucella neotomae Infection in Humans, Costa Rica. Emerg. Infect. Dis. J. 2017, 23, 997. [Google Scholar] [CrossRef]
- Villalobos-Vindas, J.M.; Amuy, E.; Barquero-Calvo, E.; Rojas, N.; Chacón-Díaz, C.; Chaves-Olarte, E.; Guzman-Verri, C.; Moreno, E. Brucellosis caused by the wood rat pathogen Brucella neotomae: Two case reports. J. Med. Case Rep. 2017, 11, 1–4. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Brown, D.A.; Kirby, J.E. Brucella neotomae Recapitulates Attributes of Zoonotic Human Disease in a Murine Infection Model. Infect. Immun. 2018, 87, e00255-e18. [Google Scholar] [CrossRef]
- Waldrop, S.G.; Sriranganathan, N. Intracellular invasion and survival of Brucella neotomae, another possible zoonotic Brucella species. PLoS ONE 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Sequeira, A.; Campos, E.; Mendoza, L.; San-Román, M.; Moreno, E. Identificación de especies y biotipos de Brucella aisladas en Costa Rica. Turrialba 1984, 34, 525–526. [Google Scholar]
- Scholz, H.; Nöckler, K.; Göllner, C.; Bahn, P.; Cloeckaert, A.; Maquart, M.; Zygmunt, M.; Whatmore, A.; Pfeffer, M.; Huber, B.; et al. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 2010, 60, 801–808. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Ruiz-Villalobos, N.; Hernández-Mora, G.; González-Barrientos, R.; Palacios-Alfaro, J.D.; Barquero-Calvo, E.; Chaves-Olarte, E.; Thomson, N.; Moreno, E.; Guzmán Verri, C. Brucella Sequence Type 27 Isolated from Dwarf Sperm Whale (Kogia sima) stranded in the Costa Rican Pacifi Coast. Access Microbioly 2019, 1, 911. [Google Scholar]
- Whatmore, A.M.; Dawson, C.E.; Groussaud, P.; Koylass, M.S.; King, A.C.; Shankster, S.J.; Sohn, A.H.; Probert, W.S.; McDonald, W.L. Marine mammal Brucella genotype associated with zoonotic infection. Emerg. Infect. Dis. 2008, 14, 517–518. [Google Scholar] [CrossRef]
- Cook, I.; Campbell, R.; Barrow, G. Brucellosis in North Queensland rodents. Aust. Vet. J. 1966, 42, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Davis, D. Brucellosis in wildlife. In Animal brucellosis; Nielsen, K., Duncan, J., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 321–334. [Google Scholar]
- Tiller, R.V.; Gee, J.E.; Frace, M.A.; Taylor, T.K.; Setubal, J.C.; Hoffmaster, A.R.; De, B.K. Characterization of novel Brucella strains originating from wild native rodent species in North Queensland, Australia. Appl. Env. Microbiol. 2010, 76, 5837–5845. [Google Scholar] [CrossRef]
- Hume, D.A.; Whitelaw, C.B.A.; Archibald, A.L. The future of animal production: Improving productivity and sustainability. J. Agric. Sci. 2011, 149, 9–16. [Google Scholar] [CrossRef]
- Glass, E.J. The molecular pathways underlying host resistance and tolerance to pathogens. Front. Genet. 2012, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Fuller, D.Q. The Evolution of Animal Domestication. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 115–136. [Google Scholar] [CrossRef]
- Marshall, F.B.; Dobney, K.; Denham, T.; Capriles, J.M. Evaluating the roles of directed breeding and gene flow in animal domestication. Proc. Natl. Acad. Sci. USA 2014, 111, 6153–6158. [Google Scholar] [CrossRef]
- Read, A.; Baigent, S.; Powers, C.; Kgosana, L.; Blackwell, L.; Al, E. Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLoS Biol. 2015, 13, e1002198. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, M.M.; Araki, H.; Karp, D.S.; Poveda, K.; Whitehead, S.R. The eco-evolutionary impacts of domestication and agricultural practices on wild species. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160033. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Welch, J.J.; Suchard, M.A.; Lemey, P.; Rambaut, A.; Fitzgerald, J.R. Molecular dating of human-to-bovid host jumps by Staphylococcus aureus reveals an association with the spread of domestication. Biol. Lett. 2012, 8, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Wattam, A.R.; Williams, K.P.; Snyder, E.E.; Almeida, N.F.; Shukla, M.; Dickerman, A.W.; Crasta, O.R.; Kenyon, R.; Lu, J.; Shallom, J.M.; et al. Analysis of ten Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifestyle. J. Bacteriol. 2009, 191, 3569–3579. [Google Scholar] [CrossRef]
- Foster, J.T.; Price, L.B.; Beckstrom-Sternberg, S.M.; Pearson, T.; Brown, W.D.; Kiesling, D.M.; Allen, C.A.; Liu, C.M.; Beckstrom-Sternberg, J.; Roberto, F.F.; et al. Genotyping of Brucella species using clade specific SNPs. BMC Microbiol. 2012, 12, 110. [Google Scholar] [CrossRef]
- Vergnaud, G.; Hauck, Y.; Christiany, D.; Daoud, B.; Pourcel, C.; Jacques, I.; Cloeckaert, A.; Zygmunt, M.S. Genotypic expansion within the population structure of classical Brucella species revealed by MLVA16 typing of 1404 Brucella isolates from different animal and geographic origins, 1974–2006. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Groussaud, P.; Shankster, S.J.; Koylass, M.S.; Whatmore, A.M. Molecular typing divides marine mammal strains of Brucella into at least three groups with distinct host preferences. J. Med. Microbiol. 2007, 56, 1512–1518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maquart, M.; Le Flèche, P.; Foster, G.; Tryland, M.; Ramisse, F.; Djønne, B.; Al Dahouk, S.; Jacques, I.; Neubauer, H.; Walravens, K.; et al. MLVA-16 typing of 295 marine mammal Brucella isolates from different animal and geographic origins identifies 7 major groups within Brucella ceti and Brucella pinnipedialis. Bmc Microbiol. 2009, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Isidoro-Ayza, M.; Ruiz-Villalobos, N.; Pérez, L.; Guzmán-Verri, C.; Muñoz, P.M.; Alegre, F.; Barberán, M.; Chacón-Díaz, C.; Chaves-Olarte, E.; González-Barrientos, R.; et al. Brucella ceti infection in dolphins from the Western Mediterranean sea. Bmc Vet. Res. 2014, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.E.; Perrett, L.L.; Young, E.J.; Davison, N.J.; Monies, R.J. Isolation of Brucella species from a bottlenosed dolphin (Tursiops truncatus). Vet. Rec. 2006, 158, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.K.; Guttman, D.S.; Fitzegerald, J.R. Population genomics of bacterial host adaptation. Nat. Rev. Genet. 2018, 19, 1. [Google Scholar] [CrossRef]
- Godfroid, J.; Cloeckert, A.; Liautard, J.P.; Kohler, S.; Fretin, D.; Walravens, K.; Garin-Bastuji, B.; Letesson, J.-J. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 2005, 36, 313–326. [Google Scholar] [CrossRef]
- Godfroid, J.; Garin-Bastuji, B.; Saegerman, C.; Blasco, J.M. Brucellosis in terrestrial wildlife. Rev. Sci. Technol. 2013, 32, 27–42. [Google Scholar] [CrossRef]
- Lord, V.R.; Cherwonogrodzky, J.W.; Marcano, M.J.; Melendez, G. Serological and bacteriological study of swine brucellosis. J. Clin. Microbiol. 1997, 35, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Poester, F.P.; Gonçalves, V.S.P.; Lage, A.P. Brucellosis in Brazil. Vet. Microbiol. 2002, 90, 55–62. [Google Scholar] [CrossRef]
- Cvetnić, Z.; Špičić, S.; Tončić, J.; Majnarić, D.; Benić, M.; Albert, D.; Thiébaud, M.; Garin-Bastuji, B. Brucella suis infection in domestic pigs and wild boar in Croatia. Rev. Sci. Tech. Off. Int. Epiz. 2009, 28, 1057–1067. [Google Scholar] [CrossRef]
- Becker, H.N.; Belden, R.C.; Breault, T.; Burridge, M.J.; Frankenberger, W.; Nicoletti, P. Brucellosis in feral swine in Florida. J. Am.Vet. Med. Assoc. 1978, 173, 1181–1182. [Google Scholar] [PubMed]
- Cornell, W.D.; Chengappa, M.M.; Stuart, L.A.; Maddux, R.L.; Hail, R.I. Brucella suis biovar 3 infection in a Kentucky swine herd. J. Vet. Diagn. Investig. 1989, 1, 20–21. [Google Scholar] [CrossRef]
- Robson, J.M.; Harrison, M.W.; Wood, R.N.; Tilse, M.H.; McKay, A.B.; Brodribb, T.R. Brucellosis: Re- emergence and changing epidemiology in Queensland. Med. J. Aust. 1993, 159, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Deqiu, S.; Donglou, X.; Jiming, Y. Epidemiology and control of brucellosis in China. Vet. Microbiol. 2002, 90, 165–182. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Tenreiro, R.; da Sá, M.I.C.; Dias, R. Evolution and genome specialization of Brucella suis biovar 2 Iberian lineages. BMC Genom. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Mailles, A.; Ogielska, M.; Kemiche, F.; Garin-Bastuji, B.; Al, E. Brucella suis biovar 2 infection in humans in France: Emerging infection or better recognition? Epidemiol. Infect. 2017, 145, 2711–2716. [Google Scholar] [CrossRef]
- Forbes, L.B. Isolates of Brucella suis biovar 4 from animals and humans in Canada, 1982–1990. Can. Vet. J. 1991, 32, 686–688. [Google Scholar]
- Olsen, S.; Tatum, F. Swine brucellosis: Current perspectives. Vet. Med. Res. Rep. 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Hernández-Mora, G.; Ruiz-Villalobos, N.; Barquero-Calvo, E.; Rojas-Campos, N.; Ladner, J.; Oviedo-Sánchez, G.; Chacón-Díaz, C.; Chaves-Olarte, E.; Foster, J.T.; et al. Persistence of Brucella abortus lineages revealed by genomic characterization and phylodynamic analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008235. [Google Scholar]
- Dorneles, E.M.S.; Santana, J.A.; Alves, T.M.; Pauletti, R.B.; Mol, J.P.D.S.; Heinemann, M.B.; Lage, A.P. Genetic stability of Brucella abortus isolates from an outbreak by multiple-locus variable-number tandem repeat analysis (MLVA16). BMC Microbiol. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mora, G.; Ruiz-Villalobos, N.; Bonilla-Montoya, R.; Romero-Zúniga, J.J.; Jiménez-Arias, J.; González-Barrientos, R.; Barquero-Calvo, E.; Chacón-Díaz, C.; Rojas, N.; Chaves-Olarte, E.; et al. Epidemiology of bovine brucellosis in Costa Rica: Lessons learned from failures in the control of the disease. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.P.; Ruiz-Villalobos, N.; Suárez-Esquivel, M.; Thomson, N.R.; Marcellino, R.; Víquez-Ruiz, E.; Robles, C.A.; Guzmán-Verri, C. Molecular characterization of Brucella ovis in Argentina. Vet. Microbiol. 2020, 245, 108703. [Google Scholar] [CrossRef]
- Lan, R.; Reeves, P.R. Intraspecies variation in bacterial genomes: The need for a species genome concept. Trends Microbiol. 2000, 8, 396–401. [Google Scholar] [CrossRef]
- Wion, D.; Casadesús, J. N6-methyl-adenine: An epigenetic signal for DNA–protein interactions. Nat. Rev. Microbiol. 2006, 4, 183–192. [Google Scholar] [CrossRef]
- Robertson, G.; Reisenauer, A.; Wright, R.; Jensen, R.; Jensen, A.; Shapiro, L.; Roop II, R.M. The Brucella abortus CcrM DNA Methyltransferase Is Essential for Viability, and Its Overexpression Attenuates Intracellular Replication in Murine Macrophages. J. Bacteriol. 2000, 182, 3482–3489. [Google Scholar] [CrossRef]
- Zhou, K.; Aertsen, A.; Michiels, C.W. The role of variable DNA tandem repeats in bacterial adaptation. Fems Microbiol. Rev. 2014, 38, 119–141. [Google Scholar] [CrossRef]
- Press, M.O.; Queitsch, C. Variability in a short tandem repeat mediates complex epistatic interactions in Arabidopsis thaliana. Genetics 2017, 205, 455–464. [Google Scholar] [CrossRef]
- Gerner-Smidt, P.; Hyytiä-Trees, E.; Rota, P.A. Molecular Epidemiology. In Manual of Clinical Microbiology; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.M., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 100–123. [Google Scholar]
- Deka, R.; Shriver, M.D.; Yu, L.; Ferrell, R.; Chakraborty, R. Intra- and inter-population diversity at short tandem repeat loci in diverse populations of the world. Electrophoresis 1995, 16, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Bricker, B.J.; Ewalt, D.R.; Halling, S.M. Brucella “HOOF-Prints”: Strain typing by multi-locus analysis of variable number tandem repeats (VNTRs). BMC Microbiol. 2003, 13, 15. [Google Scholar]
- Higgins, J.; Stuber, T.; Quance, C.; Edwards, W.H.; Tiller, R.V.; Linfield, T.; Rhyan, J.; Berte, A.; Harris, B. Molecular epidemiology of Brucella abortus isolates from Cattle, Elk, and Bison in the United States, 1998 to 2011. Appl. Env. Microbiol. 2012, 78, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Breadon, E.; Byrne, A.; Mallon, T.; Skuce, R.; Groussaud, P.; Dainty, A.; Graham, J.; Jones, K.; Pollock, L.; et al. Molecular epidemiology of Brucella abortus in Northern Ireland-1991 to 2012. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef]
- Shevtsov, A.; Ramanculov, E.; Shevtsova, E.; Kairzhanova, A.; Tarlykov, P.; Filipenko, M.; Dymova, M.; Abisheva, G.; Jailbekova, A.; Kamalova, D.; et al. Genetic diversity of Brucella abortus and Brucella melitensis in Kazakhstan using MLVA-16. Infect. Genet. Evol. 2015, 34, 173–180. [Google Scholar] [CrossRef]
- Hernández-Mora, G.; Bonilla-Montoya, R.; Barrantes-Granados, O.; Esquivel-Suárez, A.; Montero-Caballero, D.; González-Barrientos, R.; Fallas-Monge, Z.; Palacios-Alfaro, J.D.; Baldi, M.; Campos, E.; et al. Brucellosis in mammals of Costa Rica: An epidemiological survey. PLoS ONE 2017, 12, e0182644. [Google Scholar] [CrossRef]
- Al Dahouk, S.; Le Flèche, P.; Nockler, K.; Jacques, I.; Grayon, M.; Scholz, H.C.; Tomaso, H.; Vergnaud, G.; Neubauer, H. Evaluation of Brucella MLVA typing for human brucellosis. J. Microbiol. Methods 2007, 69, 137–145. [Google Scholar] [CrossRef]
- Foster, J.T.; Walker, F.M.; Rannals, B.D.; Hammad Hussain, M.; Drees, K.P.; Tiller, R.V.; Hoffmaster, A.R.; Al-Rawahi, A.; Keim, P.; Saqib, M. African lineage Brucella melitensis Isolates from Omani livestock. Front. Microbiol. 2018, 8, 2702. [Google Scholar] [CrossRef] [PubMed]
- Georgi, E.; Walter, M.C.; Pfalzgraf, M.T.; Northoff, B.H.; Holdt, L.M.; Scholz, H.C.; Zoeller, L.; Zange, S.; Antwerpen, M.H. Whole genome sequencing of Brucella melitensis isolated from 57 patients in Germany reveals high diversity in strains from Middle East. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Janowicz, A.; De Massis, F.; Ancora, M.; Cammà, C.; Patavino, C.; Battisti, A.; Prior, K.; Harmsen, D.; Scholz, H.; Zilli, K.; et al. Core Genome Multilocus Sequence Typing and Single Nucleotide Polymorphism Analysis in the Epidemiology of Brucella melitensis Infections. J. Clin. Microbiol. 2018, 56, e00517–e00518. [Google Scholar] [CrossRef]
- Brito, P.H.; Edwards, S.V. Multilocus phylogeography and phylogenetics using sequence-based markers. Genetica 2009, 135, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Halling, S.M.; Peterson-burch, B.D.; Betsy, J.; Zuerner, R.L.; Qing, Z.; Li, L.; Alt, D.P.; Olsen, S.C.; Bricker, B.J.; Kapur, V. Completion of the Genome Sequence of Brucella abortus and Comparison to the Highly Similar Genomes of Brucella melitensis and Brucella suis. J. Bacteriol. 2005, 187, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Borriello, G.; Russo, V.; Paradiso, R.; Riccardi, M.G.; Criscuolo, D.; Verde, G.; Marasco, R.; Pedone, P.V.; Galiero, G.; Baglivo, I. Different impacts of mucr binding to the babr and virb promoters on gene expression in Brucella abortus 2308. Biomolecules 2020, 10, 788. [Google Scholar] [CrossRef]
- Viana, D.; Comos, M.; Mcadam, P.R.; Ward, M.J.; Selva, L.; Guinane, M.; González-muñoz, B.M.; Tristan, A.; Foster, S.J.; Ross, J. A single natural nucleotide mutation alters bacterial pathogen host-tropism. Nat. Genet. 2015, 47, 361–366. [Google Scholar] [CrossRef]
- Hammarlöf, D.L.; Kröger, C.; Owen, S.V.; Canals, R.; Lacharme-Lora, L.; Wenner, N.; Schager, A.E.; Wells, T.J.; Henderson, I.R.; Wigley, P.; et al. Role of a single noncoding nucleotide in the evolution of an epidemic African clade of Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, E2614–E2623. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, M.; Girault, G.; Keriel, A.; Ponsart, C.; O’Callaghan, D.; Mick, V. Comparative genomics and in vitro infection of field clonal isolates of Brucella melitensis biovar 3 did not identify signature of host Adaptation. Front. Microbiol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Maiden, M.C.J.; Jansen Van Rensburg, M.J.; Bray, J.E.; Earle, S.G.; Ford, S.A.; Jolley, K.A.; McCarthy, N.D. MLST revisited: The gene-by-gene approach to bacterial genomics. Nat. Rev. Microbiol. 2013, 11, 728–736. [Google Scholar] [CrossRef]
- Maiden, M.C.J.; Bygraves, J.A.; Feil, E.; Morelli, G.; Russell, J.E.; Urwin, R.; Zhang, Q.; Zhou, J.; Zurth, K.; Caugant, D.A.; et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 1998, 95, 3140–3145. [Google Scholar] [CrossRef]
- Goodhead, I.; Darby, A.C. Taking the pseudo out of pseudogenes. Curr. Opin. Microbiol. 2015, 23, 102–109. [Google Scholar] [CrossRef]
- Dagan, T.; Blekhman, R.; Graur, D. The “domino theory” of gene death: Gradual and mass gene extinction events in three lineages of obligate symbiotic bacterial pathogens. Mol. Biol. Evol. 2006, 23, 310–316. [Google Scholar] [CrossRef]
- Sasidharan, R.; Gerstein, M. Genomics: Protein fossils live on as RNA. Nature 2008, 453, 729–731. [Google Scholar] [CrossRef]
- Tutar, Y. Pseudogenes. Comp. Funct. Genom. 2012, 2012, 424526. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Mayer, H.; Moriyon, I. Characterization of a native polysaccharide hapten from Brucella melitensis. Infect. Immun. 1987, 55, 2850–2853. [Google Scholar] [CrossRef] [PubMed]
- Scholz, H.C.; Mu, K.; Shilton, C.; Benedict, S. The Change of a Medically Important Genus: Worldwide Occurrence of Genetically Diverse Novel Brucella Species in Exotic Frogs. PLoS ONE 2016, 11, e0168872. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. Fems Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Houdt, R. Van. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef]
- Halling, S.M.; Tatum, F.M.; Bricker, B.J. Sequence and characterization of an insertion sequence, IS711 from Brucella ovis. Gene 1993, 133, 123–127. [Google Scholar] [CrossRef]
- Halling, S.M.; Zuerner, R.L. Evidence for lateral transfer to Brucellae: Characterization of a locus with a Tn-like element (Tn2020). Biochim. Biophys. Acta Gene Struct. Expr. 2002, 1574, 109–116. [Google Scholar] [CrossRef]
- Sriranganathan, N.; Seleem, M.N.; Olsen, S.C.; Samartino, L.E.; Whatmore, A.M.; Bricker, B.; O’Callaghan, D.; Halling, S.M.; Crasta, O.R.; Wattam, R.A.; et al. Brucella. In Genome Mapping and Genomics in Animal-Associated Microbes; Nene, V., Kole, C., Eds.; Springer: Heidelberg, Germany, 2009; pp. 15–16. ISBN 978-3-540-74040-7. [Google Scholar]
- Ocampo-Sosa, A.A.; García-Lobo, J.M. Demonstration of IS711 transposition in Brucella ovis and Brucella pinnipedialis. BMC Microbiol. 2008, 8, 1–10. [Google Scholar] [CrossRef]
- Bounaadja, L.; Albert, D.; Chénais, B.; Hénault, S.; Zygmunt, M.S.; Poliak, S.; Garin-Bastuji, B. Real-time PCR for identification of Brucella spp.: A comparative study of IS711, bcsp31 and per target genes. Vet. Microbiol. 2009, 137, 156–164. [Google Scholar] [CrossRef]
- Bricker, B.J. PCR as a diagnostic tool for brucellosis. Vet. Microbiol. 2002, 90, 435–446. [Google Scholar] [CrossRef]
- Cloeckaert, A.; Bernardet, N.; Koylass, M.S.; Whatmore, A.M.; Zygmunt, M.S. Novel IS711 chromosomal location useful for identification of marine mammal Brucella genotype ST27, which is associated with zoonotic infection. J. Clin. Microbiol. 2011, 49, 3954–3959. [Google Scholar] [CrossRef]
- Mancilla, M.; Ulloa, M.; Lápez-Gõi, I.; Moriyán, I.; María Zárraga, A. Identification of new IS711 insertion sites in Brucella abortus field isolates. BMC Microbiol. 2011, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Bricker, B.J.; Ewalt, D.R.; MacMillan, A.P.; Foster, G.; Brew, S. Molecular characterization of Brucella strains isolated from marine mammals. J. Clin. Microbiol. 2000, 38, 1258–1262. [Google Scholar] [CrossRef]
- Zygmunt, M.S.; Maquart, M.; Bernardet, N.; Doublet, B.; Cloeckaert, A. Novel IS711-specific chromosomal locations useful for identification and classification of marine mammal Brucella strains. J. Clin. Microbiol. 2010, 48, 3765–3769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suárez-Esquivel, M.; Ruiz-Villalobos, N.; Castillo-Zeledón, A.; Jiménez-Rojas, C.; Roop II, R.M.; Comerci, D.J.; Barquero-Calvo, E.; Chacón-Díaz, C.; Caswell, C.C.; Baker, K.S.; et al. Brucella abortus Strain 2308 Wisconsin Genome: Importance of the Definition of Reference Strains. Front. Microbiol. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Łoś, M.; Węgrzyn, G. Pseudolysogeny. Adv. Virus Res. 2012, 82, 339–349. [Google Scholar]
- Hacker, J.; Kaper, J.B. Pathogenicity Islands and the Evolution of Microbes. Annu. Rev. Microbiol. 2008, 54, 641–679. [Google Scholar] [CrossRef]
- Vernikos, G.S.; Parkhill, J. Resolving the structural features of genomic islands: A machine learning approach. Genome Res. 2008, 18, 331–342. [Google Scholar] [CrossRef]
- Darmon, E.; Leach, D.R.F. Bacterial Genome Instability. Microbiol. Mol. Biol. Rev. 2014, 78, 1–39. [Google Scholar] [CrossRef]
- Pérez-Lago, L.; Comas, I.; Navarro, Y.; González-Candelas, F.; Herranz, M.; Bouza, E.; García-De-Viedma, D. Whole Genome Sequencing Analysis of Intrapatient Microevolution in Mycobacterium tuberculosis: Potential Impact on the Inference of Tuberculosis Transmission. J. Infect. Dis. 2014, 209, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gyuranecz, M.; Rannals, B.D.; Allen, C.A.; Jánosi, S.; Keim, P.S.; Foster, J.T. Within-host evolution of Brucella canis during a canine brucellosis outbreak in a kennel. BMC Vet. Res. 2013, 9, 76. [Google Scholar] [CrossRef]
- Viana, M.V.C.; Wattam, A.R.; Govil Batra, D.; Boisvert, S.; Brettin, T.S.; Frace, M.; Xia, F.; Azevedo, V.; Tiller, R.; Hoffmaster, A.R. Genome Sequences of Two Brucella suis Strains Isolated from the Same Patient, 8 Years Apart. Genome Announc. 2017, 5, e01687-e16. [Google Scholar] [CrossRef]
- Ke, Y.; Yuan, X.; Wang, Y.; Bai, Y.; Xu, J.; Song, H.; Huang, L.; Chen, Z. Genome sequences of Brucella melitensis 16M and its two derivatives 16M1w and 16M13w, which evolved in vivo. J. Bacteriol. 2012, 194, 5489. [Google Scholar] [CrossRef]
- Ewald, P.W. Evolution of virulence. Infect. Dis. Clin. N. Am. 2004, 18, 1–15. [Google Scholar] [CrossRef]
- Rouzic, N.; Desmier, L.; Cariou, M.; Gay, E.; Foster, J.; Williamson, C.; Schmitt, F.; Le Henaff, M.; Le Coz, A.; Lorléac’h, A.; et al. First case of brucellosis caused by an amphibian-type Brucella. Clin Infect. Dis 2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Esquivel, M.; Chaves-Olarte, E.; Moreno, E.; Guzmán-Verri, C. Brucella Genomics: Macro and Micro Evolution. Int. J. Mol. Sci. 2020, 21, 7749. https://doi.org/10.3390/ijms21207749
Suárez-Esquivel M, Chaves-Olarte E, Moreno E, Guzmán-Verri C. Brucella Genomics: Macro and Micro Evolution. International Journal of Molecular Sciences. 2020; 21(20):7749. https://doi.org/10.3390/ijms21207749
Chicago/Turabian StyleSuárez-Esquivel, Marcela, Esteban Chaves-Olarte, Edgardo Moreno, and Caterina Guzmán-Verri. 2020. "Brucella Genomics: Macro and Micro Evolution" International Journal of Molecular Sciences 21, no. 20: 7749. https://doi.org/10.3390/ijms21207749
APA StyleSuárez-Esquivel, M., Chaves-Olarte, E., Moreno, E., & Guzmán-Verri, C. (2020). Brucella Genomics: Macro and Micro Evolution. International Journal of Molecular Sciences, 21(20), 7749. https://doi.org/10.3390/ijms21207749





