TRAP1 Regulates Wnt/β-Catenin Pathway through LRP5/6 Receptors Expression Modulation
Abstract
:1. Introduction
2. Results
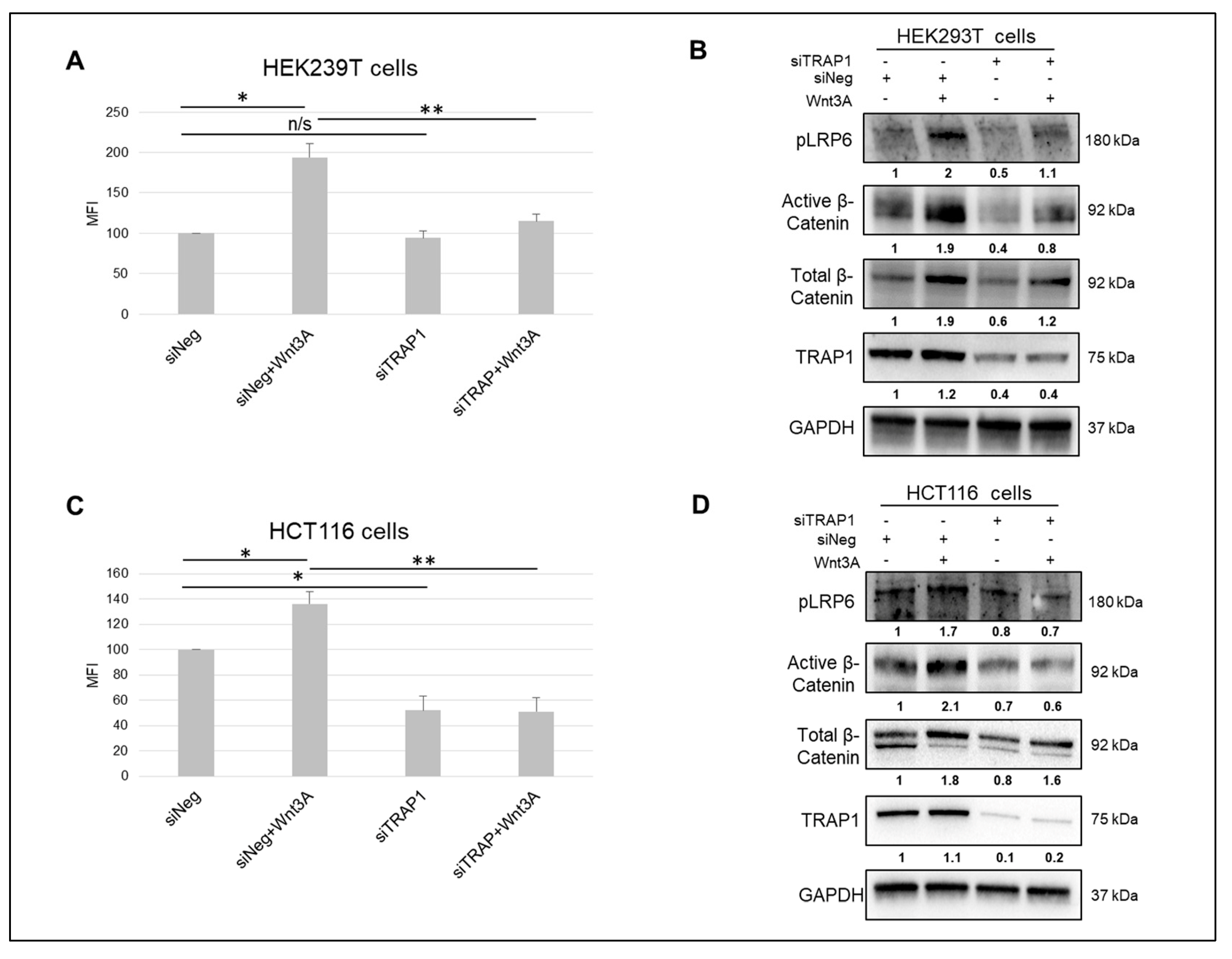
2.1. TRAP1 Modulates the Activity of Wnt/β-Catenin Pathway
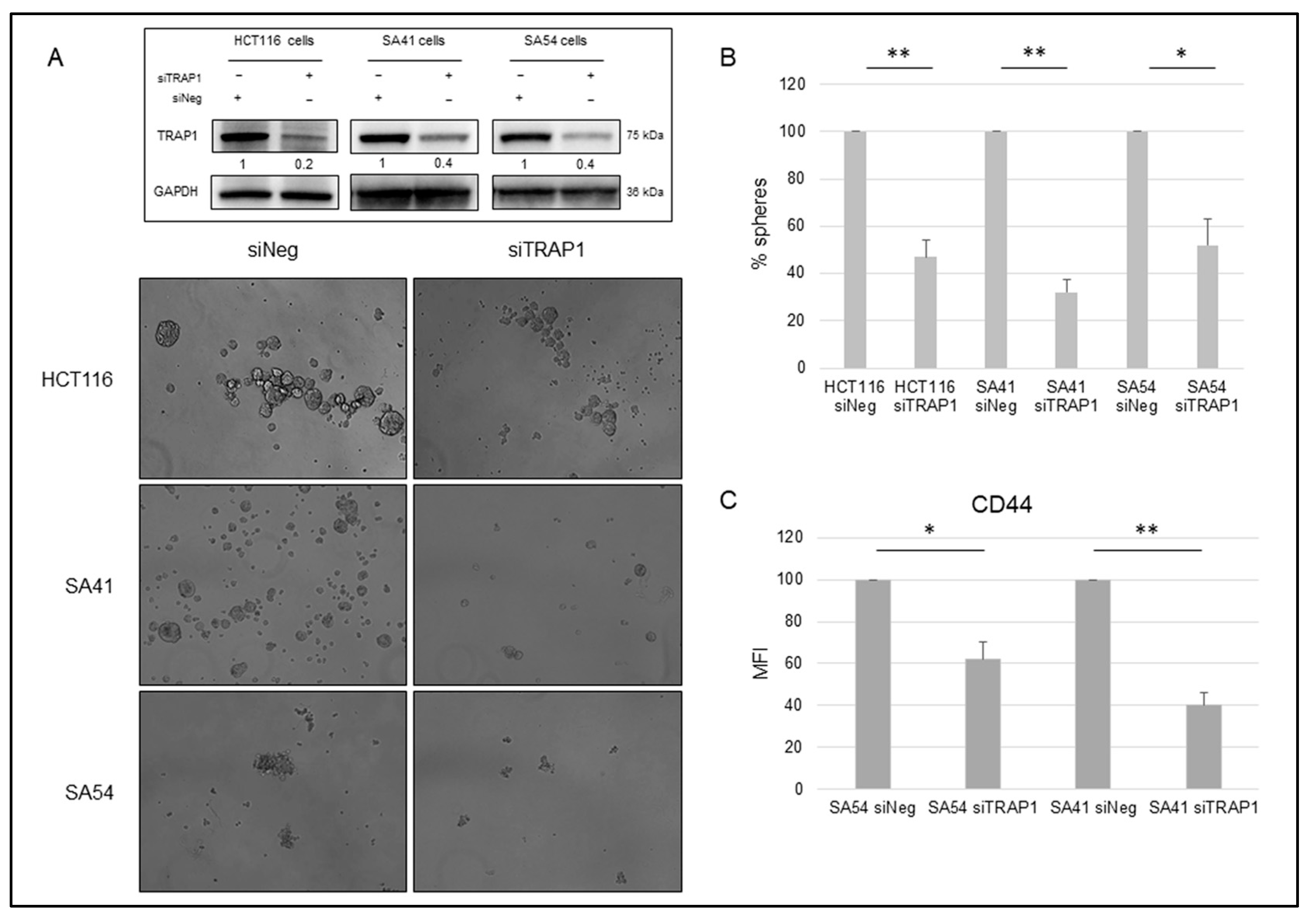
2.2. TRAP1 Enhances the Sphere-Forming Ability of Colon Carcinoma and Cancer Stem Cells
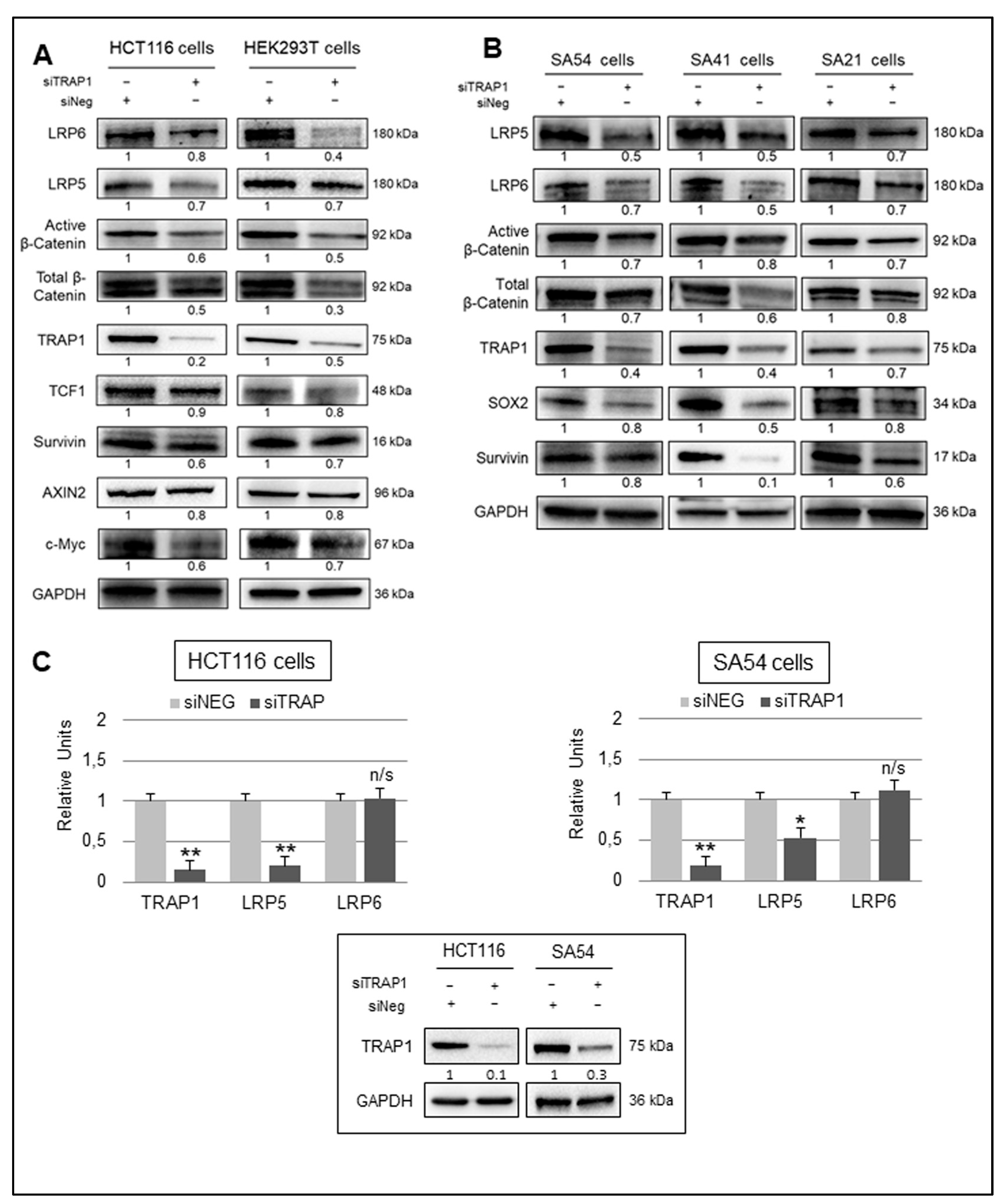
2.3. TRAP1 Regulation of Wnt/β-Catenin Pathway Activation Occurs through LRP5/6 Receptors
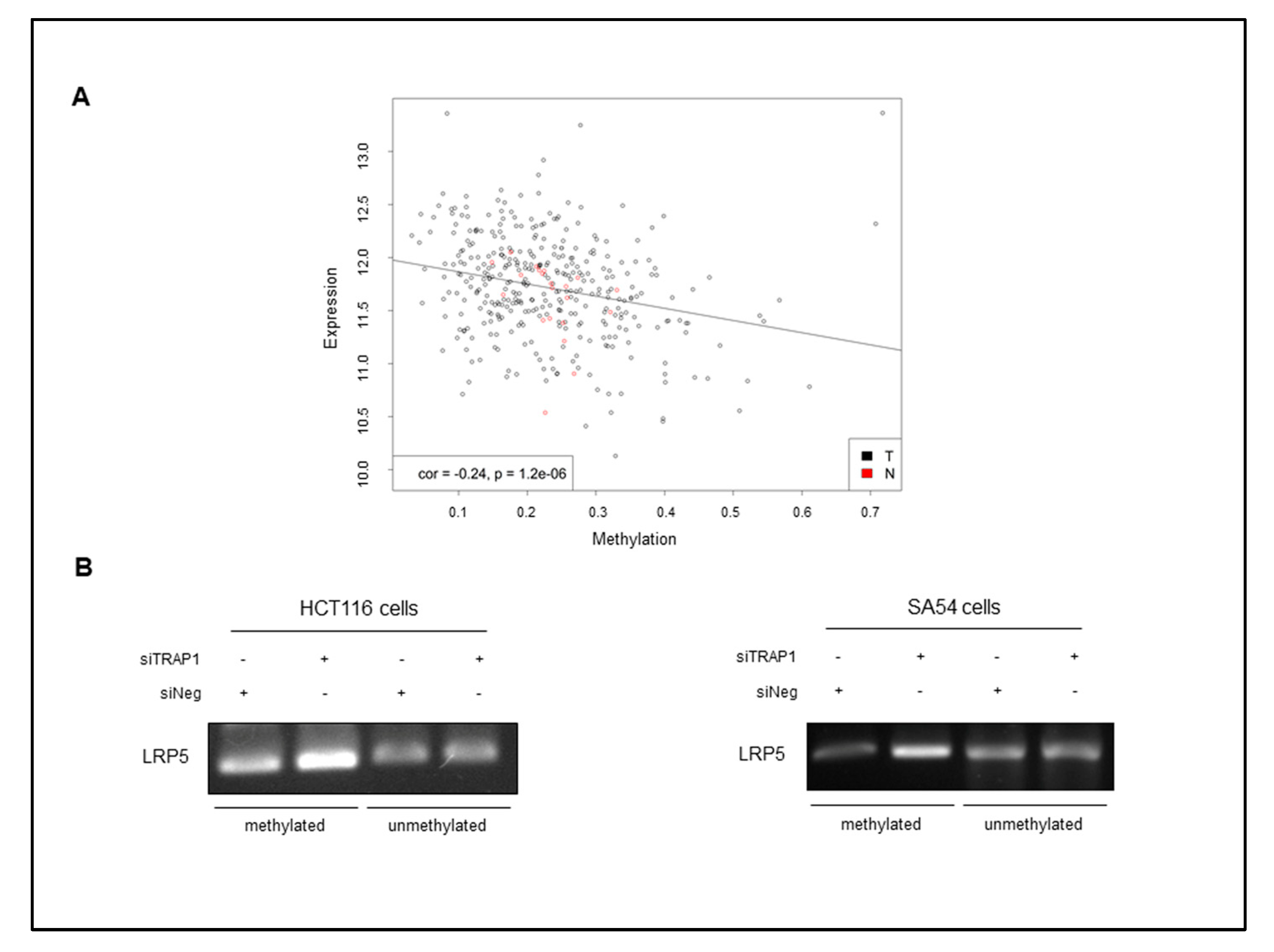
2.4. TRAP1 Regulates LRP5/6 Expression through a Double Distinct Molecular Mechanism
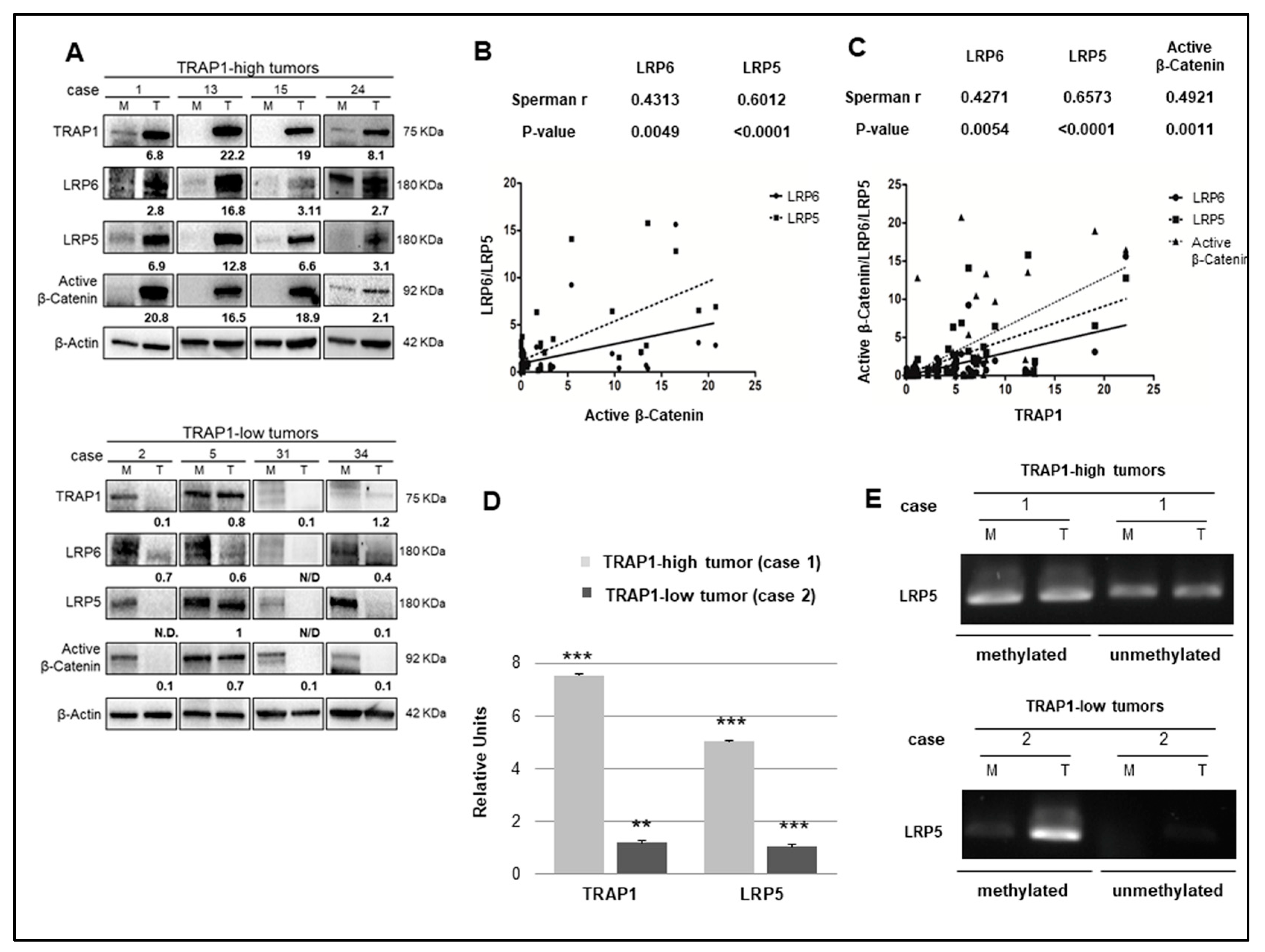
2.5. TRAP1 Is Co-Expressed with Active β-Catenin and LRP5/6 in Human CRCs
3. Discussion
4. Materials and Methods
4.1. Cell Line and Patients-Derived Spheroids
4.2. Transfection Procedures
4.3. Immunoblot Analysis
4.4. RNA Extraction and Real-Time RT-PCR
4.5. Methylation Specific PCR (MS-PCR)
4.6. Flow Cytometry
4.7. Sphere-Forming Assay
4.8. Tumor Specimens and TCGA Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Allophycocyanin |
| CRC | colorectal carcinoma |
| CSC | cancer stem cell |
| FBS | fetal bovine serum |
| FZD | Frizzled |
| LRP | low-density lipoprotein receptor-related protein |
| MFI | mean fluorescence intensity |
| PBS | phosphate-buffered saline |
| PE | Phycoerythrin |
| TRAP1 | TNF receptor-associated protein 1 |
References
- Ng, L.F.; Kaur, P.; Bunnag, N.; Suresh, J.; Sung, I.C.H.; Tan, Q.H.; Gruber, J.; Tolwinski, N.S. WNT Signaling in Disease. Cells 2019, 8, 826. [Google Scholar] [CrossRef] [Green Version]
- Tortelote, G.G.; Reis, R.R.; de Almeida Mendes, F.; Abreu, J.G. Complexity of the Wnt/β-catenin pathway: Searching for an activation model. Cell. Signal. 2017, 40, 30–43. [Google Scholar] [CrossRef]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front. Immunol. 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Veeman, M.T.; Axelrod, J.D.; Moon, R.T. A second canon: Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 2003, 5, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Seyed Forootan, F.; Nasr Esfahani, M.H.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Giles, R.H.; Van Es, J.H.; Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta-Rev. Cancer 2003, 1653, 1–24. [Google Scholar] [CrossRef]
- Maddalena, F.; Sisinni, L.; Lettini, G.; Condelli, V.; Matassa, D.S.; Piscazzi, A.; Amoroso, M.R.; La Torre, G.; Esposito, F.; Landriscina, M. Resistance to paclitxel in breast carcinoma cells requires a quality control of mitochondrial antiapoptotic proteins by TRAP1. Mol. Oncol. 2013, 7, 895–906. [Google Scholar] [CrossRef]
- Sisinni, L.; Maddalena, F.; Lettini, G.; Condelli, V.; Matassa, D.S.; Esposito, F.; Landriscina, M. TRAP1 role in endoplasmic reticulum stress protection favors resistance to anthracyclins in breast carcinoma cells. Int. J. Oncol. 2014, 44, 573–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condelli, V.; Maddalena, F.; Sisinni, L.; Lettini, G.; Matassa, D.S.; Piscazzi, A.; Palladino, G.; Amoroso, M.R.; Esposito, F.; Landriscina, M. Targeting TRAP1 as a downstream effector of BRAF cytoprotective pathway: A novel strategy for human BRAF-driven colorectal carcinoma. Oncotarget 2015, 6, 22298–22309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoroso, M.R.; Matassa, D.S.; Agliarulo, I.; Avolio, R.; Lu, H.; Sisinni, L.; Lettini, G.; Gabra, H.; Landriscina, M.; Esposito, F. TRAP1 downregulation in human ovarian cancer enhances invasion and epithelial–mesenchymal transition. Cell Death Dis. 2016, 7, e2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisinni, L.; Maddalena, F.; Condelli, V.; Pannone, G.; Simeon, V.; Li Bergolis, V.; Lopes, E.; Piscazzi, A.; Matassa, D.S.; Mazzoccoli, C.; et al. TRAP1 controls cell cycle G2–M transition through the regulation of CDK1 and MAD2 expression/ubiquitination. J. Pathol. 2017, 243, 123–134. [Google Scholar] [CrossRef]
- Maddalena, F.; Simeon, V.; Vita, G.; Bochicchio, A.; Possidente, L.; Sisinni, L.; Lettini, G.; Condelli, V.; Matassa, D.S.; Li Bergolis, V.; et al. TRAP1 protein signature predicts outcome in human metastatic colorectal carcinoma. Oncotarget 2017, 8, 21229–21240. [Google Scholar] [CrossRef]
- Lettini, G.; Maddalena, F.; Sisinni, L.; Condelli, V.; Matassa, D.S.; Costi, M.P.; Simoni, D.; Esposito, F.; Lettini, G.; Maddalena, F.; et al. Targets TRAP1: A viable therapeutic target for future cancer treatments? Expert Opin. Ther. Targets 2017, 21, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Pak, M.G.; Koh, H.J.; Roh, M.S. Clinicopathologic significance of TRAP1 expression in colorectal cancer: A large scale study of human colorectal adenocarcinoma tissues. Diagn. Pathol. 2017, 12, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Lettini, G.; Sisinni, L.; Condelli, V.; Matassa, D.S.; Simeon, V.; Maddalena, F.; Gemei, M.; Lopes, E.; Vita, G.; Del Vecchio, L.; et al. TRAP1 regulates stemness through Wnt/β-catenin pathway in human colorectal carcinoma. Cell Death Differ. 2016, 23, 1792–1803. [Google Scholar] [CrossRef]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, L.; De Sousa, E.; Melo, F.; Van Der Heijden, M.; Cameron, K.; De Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- Planutis, K.; Planutiene, M.; Holcombe, R.F. A novel signaling pathway regulates colon cancer angiogenesis through Norrin. Sci. Rep. 2014, 4, 5630. [Google Scholar] [CrossRef] [Green Version]
- Raisch, J.; Côté-Biron, A.; Rivard, N. A role for the WNT co-receptor LRP6 in pathogenesis and therapy of epithelial cancers. Cancers 2019, 11, 1162. [Google Scholar] [CrossRef] [Green Version]
- Condelli, V.; Piscazzi, A.; Sisinni, L.; Matassa, D.S.; Maddalena, F.; Lettini, G.; Simeon, V.; Palladino, G.; Amoroso, M.R.; Trino, S.; et al. TRAP1 is involved in BRAF regulation and downstream attenuation of ERK phosphorylation and cell-cycle progression: A novel target for BRAF-mutated colorectal tumors. Cancer Res. 2014, 74, 6693–6704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landriscina, M.; Laudiero, G.; Maddalena, F.; Amoroso, M.R.; Piscazzi, A.; Cozzolino, F.; Monti, M.; Garbi, C.; Fersini, A.; Pucci, P.; et al. Mitochondrial chaperone trap1 and the calcium binding protein sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010, 70, 6577–6586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoroso, M.R.; Matassa, D.S.; Laudiero, G.; Egorova, A.V.; Polishchuk, R.S.; Maddalena, F.; Piscazzi, A.; Paladino, S.; Sarnataro, D.; Garbi, C.; et al. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell Death Differ. 2012, 19, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, O.; Fisch, K.M.; Wineinger, N.E.; Akagi, R.; Saito, M.; Sasho, T.; Su, A.I.; Lotz, M.K. Increased DNA Methylation and Reduced Expression of Transcription Factors in Human Osteoarthritis Cartilage. Arthritis Rheumatol. 2016, 68, 1876–1886. [Google Scholar] [CrossRef]
- Neumeyer, S.; Popanda, O.; Edelmann, D.; Butterbach, K.; Toth, C.; Roth, W.; Bläker, H.; Jiang, R.; Herpel, E.; Jäkel, C.; et al. Genome-wide DNA methylation differences according to oestrogen receptor beta status in colorectal cancer. Epigenetics 2019, 14, 477–493. [Google Scholar] [CrossRef]
- Kabakov, A.; Yakimova, A.; Matchuk, O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells 2020, 9, 892. [Google Scholar] [CrossRef] [Green Version]
- Lettini, G.; Lepore, S.; Crispo, F.; Sisinni, L.; Esposito, F.; Landriscina, M. Heat shock proteins in cancer stem cell maintenance: A potential therapeutic target? Histol. Histopathol. 2020, 35, 25–37. [Google Scholar]
- Pietrafesa, M.; Maddalena, F.; Possidente, L.; Condelli, V.; Zoppoli, P.; Li Bergolis, V.; Rodriquenz, M.G.; Aieta, M.; Vita, G.; Esposito, F.; et al. Gene copy number and post-transductional mechanisms regulate TRAP1 expression in human colorectal carcinomas. Int. J. Mol. Sci. 2019, 21, 145. [Google Scholar] [CrossRef] [Green Version]
- Amoroso, M.R.; Matassa, D.S.; Sisinni, L.; Lettini, G.; Landriscina, M.; Esposito, F. TRAP1 revisited: Novel localizations and functions of a “next-generation” biomarker (Review). Int. J. Oncol. 2014, 45, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masgras, I.; Sanchez-Martin, C.; Colombo, G.; Rasola, A. The chaperone TRAP1 as a modulator of the mitochondrial adaptations in cancer cells. Front. Oncol. 2017, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avolio, R.; Matassa, D.S.; Criscuolo, D.; Landriscina, M.; Esposito, F. Modulation of mitochondrial metabolic reprogramming and oxidative stress to overcome Chemoresistance in Cancer. Biomolecules 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The Mitochondrial Chaperone TRAP1 Promotes Neoplastic Growth by Inhibiting Succinate Dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Tsutsumi, S.; Muhlebach, G.; Sourbier, C.; Lee, M.J.; Lee, S.; Vartholomaiou, E.; Tatokoro, M.; Beebe, K.; Miyajima, N.; et al. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc. Natl. Acad. Sci. USA 2013, 110, E1604–E1612. [Google Scholar] [CrossRef] [Green Version]
- Condelli, V.; Crispo, F.; Pietrafesa, M.; Lettini, G.; Matassa, D.S.; Esposito, F.; Landriscina, M.; Maddalena, F. HSP90 Molecular Chaperone, Metabolic Rewiring and Epigenetics: Impact on Tumor Progression and Perspective for Anticancer Therapy. Cells 2019, 8, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispo, F.; Condelli, V.; Lepore, S.; Notarangelo, T.; Sgambato, A.; Esposito, F.; Maddalena, F.; Landriscina, M. Metabolic Dysregulations and Epigenetics: A Bidirectional Interplay that Drives Tumor Progression. Cells 2019, 8, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, J.-L.; Jeon, Y.-K.; Park, J.-G. Methylation-specific PCR. In Epigenetics Protocols-Methods in Molecular Biology; Tollefsbol, T., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 791, pp. 23–32. [Google Scholar]
- Huang, Z.; Bassil, C.F.; Murphy, S.K. Methylation-Specific PCR. In Ovarian Cancer. Methods in Molecular Biology; Malek, A., Tchernitsa, O., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 1049, pp. 75–82. [Google Scholar]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 669–673. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 10 September 2020).

| Patients | 41 | |
| Age (years) | ||
| Median | 69 | |
| Range | 36–89 | |
| Sex | n° | |
| Female | 16 | |
| Male | 25 | |
| Tumor stage | n° | (%) |
| T1 | 2 | 5 |
| T2 | 3 | 7 |
| T3 | 30 | 73 |
| T4 | 6 | 15 |
| N0 | 18 | 44 |
| N1 | 14 | 34 |
| N2 | 9 | 22 |
| M0 | 32 | 78 |
| M1 | 9 | 22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettini, G.; Condelli, V.; Pietrafesa, M.; Crispo, F.; Zoppoli, P.; Maddalena, F.; Laurenzana, I.; Sgambato, A.; Esposito, F.; Landriscina, M. TRAP1 Regulates Wnt/β-Catenin Pathway through LRP5/6 Receptors Expression Modulation. Int. J. Mol. Sci. 2020, 21, 7526. https://doi.org/10.3390/ijms21207526
Lettini G, Condelli V, Pietrafesa M, Crispo F, Zoppoli P, Maddalena F, Laurenzana I, Sgambato A, Esposito F, Landriscina M. TRAP1 Regulates Wnt/β-Catenin Pathway through LRP5/6 Receptors Expression Modulation. International Journal of Molecular Sciences. 2020; 21(20):7526. https://doi.org/10.3390/ijms21207526
Chicago/Turabian StyleLettini, Giacomo, Valentina Condelli, Michele Pietrafesa, Fabiana Crispo, Pietro Zoppoli, Francesca Maddalena, Ilaria Laurenzana, Alessandro Sgambato, Franca Esposito, and Matteo Landriscina. 2020. "TRAP1 Regulates Wnt/β-Catenin Pathway through LRP5/6 Receptors Expression Modulation" International Journal of Molecular Sciences 21, no. 20: 7526. https://doi.org/10.3390/ijms21207526
APA StyleLettini, G., Condelli, V., Pietrafesa, M., Crispo, F., Zoppoli, P., Maddalena, F., Laurenzana, I., Sgambato, A., Esposito, F., & Landriscina, M. (2020). TRAP1 Regulates Wnt/β-Catenin Pathway through LRP5/6 Receptors Expression Modulation. International Journal of Molecular Sciences, 21(20), 7526. https://doi.org/10.3390/ijms21207526





