Sequenced Combinations of Cisplatin and Selected Phytochemicals towards Overcoming Drug Resistance in Ovarian Tumour Models
Abstract
1. Introduction
2. Results
2.1. Growth-Inhibitory Effect of Single Drugs
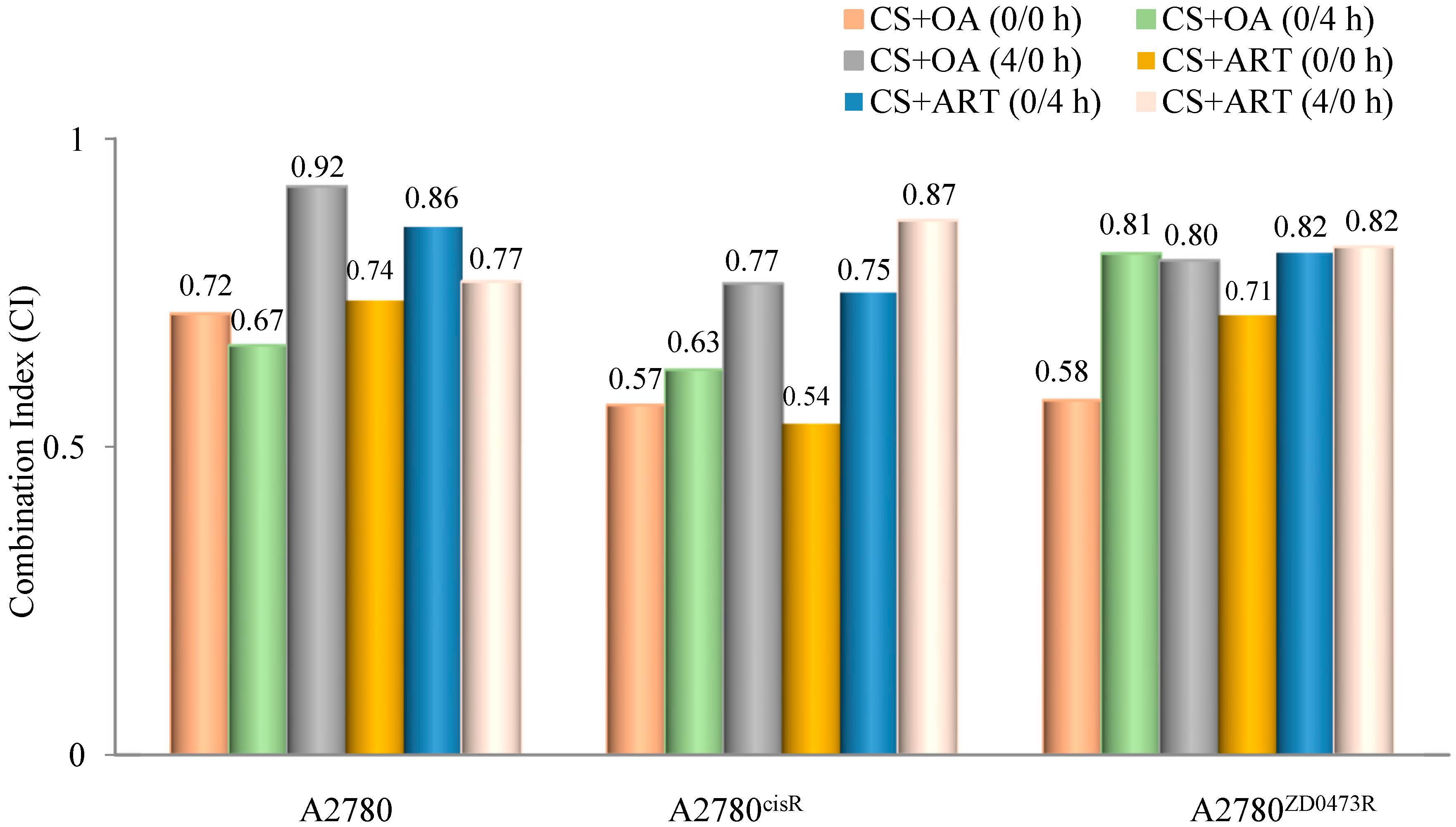
2.2. Combination Studies
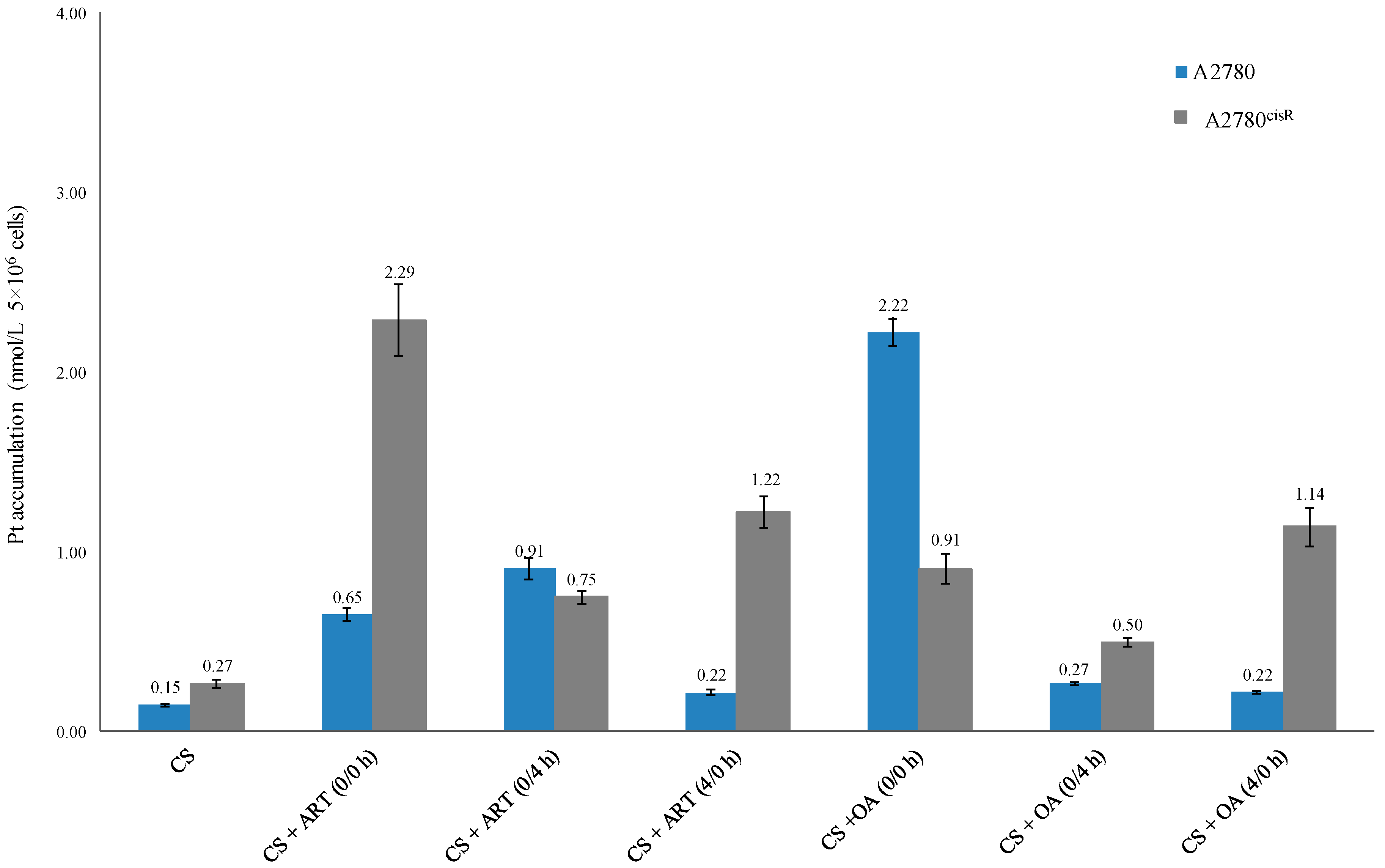
2.3. Cellular Accumulation of Platinum and Platinum–DNA Binding Level
2.3.1. Cellular Accumulation of Platinum
2.3.2. Platinum-DNA Binding Level
3. Discussion
3.1. Cytotoxicity of Single Drug
3.2. Combination Indices
3.3. Cellular Accumulation of Platinum
3.4. Platinum–DNA Binding Level
3.5. In Proteomics Study Involving 2-D Gel Electrophoresis
4. Materials and Methods
4.1. Cell Culture and Subculture
4.2. Drugs Preparation
4.3. Single-Drug Treatments
4.4. Combination Studies
4.5. Platinum Cellular Accumulation and Platinum DNA Binding Studies
4.6. Cellular Accumulation
4.7. Platinum–DNA Binding
4.8. Proteomic Studies
4.9. Sample Preparation, Treatments, Pellet Collection and Protein Quantification
4.10. Two-Dimensional Gel Electrophoresis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nowak, M.; Glowacka, E.; Kielbik, M.; Kulig, A.; Sulowska, Z.; Klink, M. Secretion of cytokines and heat shock protein (HspA1A) by ovarian cancer cells depending on the tumor type and stage of disease. Cytokine 2017, 89, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Hacker, N.F.; Rao, A. Surgery for advanced epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Peiretti, M. Quality control in ovarian cancer surgery. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006, 66, 3347–3350. [Google Scholar] [CrossRef]
- Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol. Res. 2016, 106, 27–36. [Google Scholar] [CrossRef]
- Ohmichi, M.; Hayakawa, J.; Tasaka, K.; Kurachi, H.; Murata, Y. Mechanisms of platinum drug resistance. Trends Pharmacol. Sci. 2005, 26, 113–116. [Google Scholar] [CrossRef]
- Apps, M.G.; Choi, E.H.; Wheate, N.J. The state-of-play and future of platinum drugs. Endocr. Relat. Cancer 2015, 22, R219–R233. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef]
- Zhang, R.W. Artemisinin (Qinghaosu), Nobel Prize, anti-malaria, and beyond. Chin. J. Nat. Med. 2016, 14, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J.; Cambra, H.M.; Desrosiers, M.R.; Rassias, D.; Towler, M.J. Chapter 5—Artemisinin the Nobel Molecule: From Plant to Patient. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 52, pp. 193–229. [Google Scholar]
- Gong, X.M.; Zhang, Q.; Torossian, A.; Cao, J.P.; Fu, S. Selective radiosensitization of human cervical cancer cells and normal cells by artemisinin through the abrogation of radiation-induced G2 block. Int. J. Gynecol. Cancer 2012, 22, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- UniPortKB. Protein Knowledgebase. Available online: http://www.uniprot.org/uniprot/ (accessed on 28 December 2015).
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.P.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Firestone, G.L.; Sundar, S.N. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev. Mol. Med. 2009, 11, e32. [Google Scholar] [CrossRef]
- Corte-Rodríguez, M.; Espina, M.; Sierra, L.M.; Blanco, E.; Ames, T.; Montes-Bayón, M.; Sanz-Medel, A. Quantitative evaluation of cellular uptake, DNA incorporation and adduct formation in cisplatin sensitive and resistant cell lines: Comparison of different Pt-containing drugs. Biochem. Pharmacol. 2015, 98, 69–77. [Google Scholar] [CrossRef]
- Muenyi, C.S.; Pinhas, A.R.; Fan, T.W.; Brock, G.N.; Helm, C.W.; States, J.C.J.T.S. Sodium arsenite±hyperthermia sensitizes p53-expressing human ovarian cancer cells to cisplatin by modulating platinum-DNA damage responses. Toxicol. Sci. 2012, 127, 139–149. [Google Scholar] [CrossRef]
- Amable, L.; Smith, S.; Stephan, C.; Shelton, C. New Research Evaluating Cisplatin Uptake in Ovarian Cancer Cells by Single Cell ICP-MS; Perkin Elmer Application Note; Perkin Elmer: Waltham, MA, USA, 2017. [Google Scholar]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Wexselblatt, E.; Yavin, E.; Gibson, D. Cellular interactions of platinum drugs. Inorg. Chim. Acta 2012, 393, 75–83. [Google Scholar] [CrossRef]
- Song, I.S.; Savaraj, N.; Siddik, Z.H.; Liu, P.; Wei, Y.; Wu, C.J.; Kuo, M.T. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol. Cancer Ther. 2004, 3, 1543–1549. [Google Scholar]
- Mazumder, M.E.H. Studies on New Tumour Active Palladium Complexes Targeted to Overcome Resistance in Ovarian Cancer. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2013. [Google Scholar]
- Gamberi, T.; Massai, L.; Magherini, F.; Landini, I.; Fiaschi, T.; Scaletti, F.; Gabbiani, C.; Bianchi, L.; Bini, L.; Nobili, S.; et al. Proteomic analysis of A2780/S ovarian cancer cell response to the cytotoxic organogold(III) compound Aubipyc. J. Proteom. 2014, 103, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Chen, Y.; Yang, Y.J.; Yang, L.; Yu, M.; Zhao, J.; Wu, J.J.; Huang, F.; Liu, W.; Ding, Z.T. Involvement of mortalin/GRP75/mthsp70 in the mitochondrial impairments induced by A53T mutant α-synuclein. Brain Res. 2015, 1604, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, H.; Jiang, Y.; Zuo, J.; Liu, W. Inhibition of mortalin expression reverses cisplatin resistance and attenuates growth of ovarian cancer cells. Cancer Lett. 2013, 336, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Nessa, M.U. Studies on Combinations between Platinum Drugs and Phytochemicals in Ovarian Tumour Models. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2013. [Google Scholar]
- Al-Eisawi, Z. Combinations between Platinum Drugs and Bortezomib and Changes in Nature of Administration in Ovarian Tumour Models. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2013. [Google Scholar]
- Alamro, A.A.S. Studies on Combination between Tumour Active Compounds in Ovarian Tumour Models. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2015. [Google Scholar]
- Li, Y.; Chen, X.; Shi, M.; Wang, H.; Cao, W.; Wang, X.; Li, C.; Feng, W. Proteomic-based identification of Apg-2 as a therapeutic target for chronic myeloid leukemia. Cell. Signal. 2013, 25, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhao, G.; Lin, T.; Tang, S.; Xu, G.; Hu, S.; Bi, Q.; Guo, C.; Sun, L.; Han, S. A peptide derived from phage display library exhibits anti-tumor activity by targeting GRP78 in gastric cancer multidrug resistance cells. Cancer Lett. 2013, 339, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef]
- Yang, L.; Yang, S.; Liu, J.; Wang, X.; Ji, J.; Cao, Y.; Lu, K.; Wang, J.; Gao, Y. Expression of GRP78 predicts taxane-based therapeutic resistance and recurrence of human gastric cancer. Exp. Mol. Pathol. 2014, 96, 235–241. [Google Scholar] [CrossRef]
- Lauber, K.; Brix, N.; Ernst, A.; Hennel, R.; Krombach, J.; Anders, H.; Belka, C. Targeting the heat shock response in combination with radiotherapy: Sensitizing cancer cells to irradiation-induced cell death and heating up their immunogenicity. Cancer Lett. 2015, 368, 209–229. [Google Scholar] [CrossRef]
- Partridge, J.R.; Lavery, L.A.; Elnatan, D.; Naber, N.; Cooke, R.; Agard, D.A. A novel N-terminal extension in mitochondrial TRAP1 serves as a thermal regulator of chaperone activity. eLife 2015, 3, e03487. [Google Scholar] [CrossRef]
- Subbarao Sreedhar, A.; Kalmár, É.; Csermely, P.; Shen, Y.F. Hsp90 isoforms: Functions, expression and clinical importance. Fed. Eur. Biochem. Soc. Lett. 2004, 562, 11–15. [Google Scholar] [CrossRef]
- Elstrand, M.B.; Stavnes, H.T.; Tropé, C.G.; Davidson, B. Heat shock protein 90 is a putative therapeutic target in patients with recurrent advanced-stage ovarian carcinoma with serous effusions. Hum. Pathol. 2012, 43, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Dejeans, N.; Glorieux, C.; Guenin, S.; Beck, R.; Sid, B.; Rousseau, R.; Bisig, B.; Delvenne, P.; Calderon, P.B.; Verrax, J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: Implications for tumor recurrence. Free Radic. Biol. Med. 2012, 52, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Fatima, N.; Xiao, Z.; Stauffer, S.; Smythers, G.; Greenwald, P.; Ali, I.U. Proteomic profiling identifies cyclooxygenase-2-independent global proteomic changes by celecoxib in colorectal cancer cells. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1598–1606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.; Kim, S.S. Current implications of cyclophilins in human cancers. J. Exp. Clin. Cancer Res. 2010, 29, 97. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.R.; Isakov, N. Insights into peptidyl-prolyl cis–trans isomerase structure and function in immunocytes. Immunol. Lett. 2015, 163, 120–131. [Google Scholar] [CrossRef]
- Pinto, R.D.; Moreira, A.R.; Pereira, P.J.; dos Santos, N.M. Two thioredoxin-superfamily members from sea bass (Dicentrarchus labrax, L.): Characterization of PDI (PDIA1) and ERp57 (PDIA3). Fish Shellfish Immunol. 2013, 35, 1163–1175. [Google Scholar] [CrossRef]
- Kullmann, M.; Kalayda, G.V.; Hellwig, M.; Kotz, S.; Hilger, R.A.; Metzger, S.; Jaehde, U. Assessing the contribution of the two protein disulfide isomerases PDIA1 and PDIA3 to cisplatin resistance. J. Inorg. Biochem. 2015, 153, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Caorsi, C.; Niccolai, E.; Capello, M.; Vallone, R.; Chattaragada, M.S.; Alushi, B.; Castiglione, A.; Ciccone, G.; Mautino, A.; Cassoni, P. Protein disulfide isomerase A3–specific Th1 effector cells infiltrate colon cancer tissue of patients with circulating anti–protein disulfide isomerase A3 autoantibodies. Transl. Res. 2016, 171, 17–28. [Google Scholar] [CrossRef]
- Andreu, C.I.; Woehlbier, U.; Torres, M.; Hetz, C. Protein disulfide isomerases in neurodegeneration: From disease mechanisms to biomedical applications. Fed. Eur. Biochem. Soc. Lett. 2012, 586, 2826–2834. [Google Scholar] [CrossRef]
- Trnková, L.; Ricci, D.; Grillo, C.; Colotti, G.; Altieri, F. Green tea catechins can bind and modify ERp57/PDIA3 activity. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 2671–2682. [Google Scholar] [CrossRef]
- Lee, E.; Lee, D.H. Emerging roles of protein disulfide isomerase in cancer. BMB Rep. 2017, 50, 401. [Google Scholar] [CrossRef] [PubMed]
- Leys, C.M.; Nomura, S.; LaFleur, B.J.; Ferrone, S.; Kaminishi, M.; Montgomery, E.; Goldenring, J.R. Expression and prognostic significance of prothymosin-α and ERp57 in human gastric cancer. Surgery 2007, 141, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wu, H.; Lei, Y.; Zhang, H.; Liu, R.; Zhao, Y.; Chen, X.; Zeng, D.; Tong, A.; Chen, L. Quantitative proteomics identification of phosphoglycerate mutase 1 as a novel therapeutic target in hepatocellular carcinoma. Mol. Cancer 2010, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Wang, F.; Wang, Y.; He, S.; Jing, Y.; Wu, X.; Zhang, H. Lactate dehydrogenase B is critical for hyperactive mTOR-mediated tumorigenesis. Cancer Res. 2011, 71, 13–18. [Google Scholar] [CrossRef]
- Huang, S.K.; Darfler, M.M.; Nicholl, M.B.; You, J.; Bemis, K.G.; Tegeler, T.J.; Wang, M.; Wery, J.P.; Chong, K.K.; Nguyen, L. LC/MS-based quantitative proteomic analysis of paraffin-embedded archival melanomas reveals potential proteomic biomarkers associated with metastasis. PLoS ONE 2009, 4, e4430. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wu, C.C.; Chang, K.P.; Yu, J.S.; Chang, Y.C.; Liao, P.C. Cell secretome analysis using hollow fiber culture system leads to the discovery of CLIC1 protein as a novel plasma marker for nasopharyngeal carcinoma. J. Proteome Res. 2009, 8, 5465–5474. [Google Scholar] [CrossRef]
- Ding, S.J.; Li, Y.; Shao, X.X.; Zhou, H.; Zeng, R.; Tang, Z.Y.; Xia, Q.C. Proteome analysis of hepatocellular carcinoma cell strains, MHCC97-H and MHCC97-L, with different metastasis potentials. Proteomics 2004, 4, 982–994. [Google Scholar] [CrossRef]
- Wang, J.W.; Peng, S.Y.; Li, J.T.; Wang, Y.; Zhang, Z.P.; Cheng, Y.; Cheng, D.Q.; Weng, W.H.; Wu, X.S.; Fei, X.Z. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009, 281, 71–81. [Google Scholar] [CrossRef]
- Lamb, R.; Harrison, H.; Hulit, J.; Smith, D.L.; Lisanti, M.P.; Sotgia, F. Mitochondria as new therapeutic targets for eradicating cancer stem cells: Quantitative proteomics and functional validation via MCT1/2 inhibition. Oncotarget 2014, 5, 11029–11037. [Google Scholar] [CrossRef]
- Onda, M.; Emi, M.; Yoshida, A.; Miyamoto, S.; Akaishi, J.; Asaka, S.; Mizutani, K.; Shimizu, K.; Nagahama, M.; Ito, K. Comprehensive gene expression profiling of anaplastic thyroid cancers with cDNA microarray of 25 344 genes. Endocr. Relat. Cancer 2004, 11, 843–854. [Google Scholar] [CrossRef]
- Duffy, M.J. Predictive markers in breast and other cancers: A review. Clin. Chem. 2005, 51, 494–503. [Google Scholar] [CrossRef]
- Wang, J.; Tai, L.S.; Tzang, C.H.; Fong, W.F.; Guan, X.Y.; Yang, M. 1p31, 7q21 and 18q21 chromosomal aberrations and candidate genes in acquired vinblastine resistance of human cervical carcinoma KB cells. Oncol. Rep. 2008, 19, 1155–1164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofmann, W.K.; de Vos, S.; Elashoff, D.; Gschaidmeier, H.; Hoelzer, D.; Koeffler, H.P.; Ottmann, O.G. Relation between resistance of Philadelphia-chromosome-positive acute lymphoblastic leukaemia to the tyrosine kinase inhibitor STI571 and gene-expression profiles: A gene-expression study. Lancet 2002, 359, 481–486. [Google Scholar] [CrossRef]
- Soltysova, A.; Breza, J.; Takacova, M.; Feruszova, J.; Hudecova, S.; Novotna, B.; Rozborilova, E.; Pastorekova, S.; Kadasi, L.; Krizanova, O. Deregulation of energetic metabolism in the clear cell renal cell carcinoma: A multiple pathway analysis based on microarray profiling. Int. J. Oncol. 2015, 47, 287–295. [Google Scholar] [CrossRef]
- Yusenko, M.V.; Ruppert, T.; Kovacs, G. Analysis of differentially expressed mitochondrial proteins in chromophobe renal cell carcinomas and renal oncocytomas by 2-D gel electrophoresis. Int. J. Biol. Sci. 2010, 6, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gharib, T.G.; Huang, C.C.; Thomas, D.G.; Shedden, K.A.; Taylor, J.M.; Kardia, S.L.; Misek, D.E.; Giordano, T.J.; Iannettoni, M.D. Proteomic analysis of lung adenocarcinoma identification of a highly expressed set of proteins in tumors. Clin. Cancer Res. 2002, 8, 2298–2305. [Google Scholar]
- Zhang, X.Z.; Xiao, Z.F.; Li, C.; Xiao, Z.Q.; Yang, F.; Li, D.J.; Li, M.Y.; Li, F.; Chen, Z.C. Triosephosphate isomerase and peroxiredoxin 6, two novel serum markers for human lung squamous cell carcinoma. Cancer Sci. 2009, 100, 2396–2401. [Google Scholar] [CrossRef] [PubMed]
- Altenberg, B.A.; Greulich, K. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 2004, 84, 1014–1020. [Google Scholar] [CrossRef]
- Di Michele, M.; Marcone, S.; Cicchillitti, L.; Della Corte, A.; Ferlini, C.; Scambia, G.; Donati, M.B.; Rotilio, D. Glycoproteomics of paclitaxel resistance in human epithelial ovarian cancer cell lines: Towards the identification of putative biomarkers. J. Proteom. 2010, 73, 879–898. [Google Scholar] [CrossRef]
- Jiang, X.; Chang, H.; Zhou, Y. Expression, purification and preliminary crystallographic studies of human glutamate oxaloacetate transaminase 1 (GOT1). Protein Expr. Purif. 2015, 113, 102–106. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, Q.; Zhou, Y.; Fu, Z.; Tan, L.; Ye, X.; Zeng, B.; Gao, W.; Zhou, J.; Liu, Y. Metabolic phenotypes in pancreatic cancer. PLoS ONE 2015, 10, e0115153. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, G. Mutant KRAS associated malic enzyme 1 expression is a predictive marker for radiation therapy response in non-small cell lung cancer. Radiat. Oncol. 2015, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Takács-Vellai, K. The metastasis suppressor Nm23 as a modulator of Ras/ERK signaling. J. Mol. Signal. 2014, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Liu, G.; Zhang, C.H.; Lu, C.H.; Xiong, S.; Zhang, M.Y.; Liu, Q.Y.; Ge, F.; He, Q.Y.; Kitazato, K.; et al. Nm23-H1 regulates the proliferation and differentiation of the human chronic myeloid leukemia K562 cell line: A functional proteomics study. Life Sci. 2009, 84, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Guangqi, H.; Guoli, H. The association of the expression of MTA1, nm23H1 with the invasion, metastasis of ovarian carcinoma. Chin. Med. Sci. J. 2003, 18, 87–92. [Google Scholar] [PubMed]
- Liu, S.; Sun, Y.; Tian, D.; He, Y.; Zeng, L.; He, Y.; Ling, C.; Sun, S. Downregulated NM23-H1 expression is associated with intracranial invasion of nasopharyngeal carcinoma. Br. J. Cancer 2008, 98, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.F.; Sun, L.H.; Zhang, R.; Zhang, Y.; Luo, Y.X.; Zheng, W.; Zhang, Z.Q.; Chen, H.Z.; Liu, D.P. Suppression of Mic60 compromises mitochondrial transcription and oxidative phosphorylation. Sci. Rep. 2015, 5, 7990. [Google Scholar] [CrossRef]
- Liu, Z.; Marquez, M.; Nilsson, S.; Holmberg, A.R. Comparison of protein expression in two prostate cancer cell-lines, LNCaP and DU145, after treatment with somatostatin. Oncol. Rep. 2009, 22, 1451–1458. [Google Scholar] [CrossRef][Green Version]
- Wong, T.S.; Liu, X.B.; Chung-Wai Ho, A.; Po-Wing Yuen, A.; Wai-Man Ng, R.; Ignace Wei, W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int. J. Cancer 2008, 123, 251–257. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Liu, H.; Xu, X.; He, S.; Tang, J.; Huang, Y.; Miao, X.; Wu, Y.; Wang, Q. Pyruvate kinase isoform M2 (PKM2) participates in multiple myeloma cell proliferation, adhesion and chemoresistance. Leuk. Res. 2015, 39, 1428–1436. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, X.; Ma, J.; Peng, H.; Wang, F.; Zha, X.; Wang, Y.; Jing, Y.; Yang, H.; Chen, R. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc. Natl. Acad. Sci. USA 2011, 108, 4129–4134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Li, C.; Xiao, Z.Q. Proteomics for identifying mechanisms and biomarkers of drug resistance in cancer. J. Proteom. 2011, 74, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Balibrea, E.; Plasencia, C.; Ginés, A.; Martinez-Cardús, A.; Musulén, E.; Aguilera, R.; Manzano, J.L.; Neamati, N.; Abad, A. A proteomic approach links decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer and in human cell lines. Mol. Cancer Ther. 2009, 8, 771–778. [Google Scholar] [CrossRef]
- Desai, S.; Ding, M.; Wang, B.; Lu, Z.; Zhao, Q.; Shaw, K.; Yung, W.; Weinstein, J.N.; Tan, M.; Yao, J. Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers. Oncotarget 2014, 5, 8202–8210. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Jun, D.Y.; Dennis, T.; Kim, Y.H. Characterization of human phosphoserine aminotransferase involved in the phosphorylated pathway of L-serine biosynthesis. Biochem. J. 2003, 373, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Vié, N.; Copois, V.; Bascoul-Mollevi, C.; Denis, V.; Bec, N.; Robert, B.; Fraslon, C.; Conseiller, E.; Molina, F.; Larroque, C. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol. Cancer 2008, 7, 14. [Google Scholar] [CrossRef]
- Toyama, A.; Suzuki, A.; Shimada, T.; Aoki, C.; Aoki, Y.; Umino, Y.; Nakamura, Y.; Aoki, D.; Sato, T.A. Proteomic characterization of ovarian cancers identifying annexin-A4, phosphoserine aminotransferase, cellular retinoic acid-binding protein 2, and serpin B5 as histology-specific biomarkers. Cancer Sci. 2012, 103, 747–755. [Google Scholar] [CrossRef]
- Arif, T.; Krelin, Y.; Shoshan-Barmatz, V. Reducing VDAC1 expression induces a non-apoptotic role for pro-apoptotic proteins in cancer cell differentiation. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1228–1242. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Ben-Hail, D.; Admoni, L.; Krelin, Y.; Tripathi, S.S. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2547–2575. [Google Scholar] [CrossRef]
- Capello, M.; Ferri-Borgogno, S.; Cappello, P.; Novelli, F. Alpha-Enolase: A promising therapeutic and diagnostic tumor target. Fed. Eur. Biochem. Soc. J. 2011, 278, 1064–1074. [Google Scholar] [CrossRef]
- Capello, M.; Caorsi, C.; Bogantes Hernandez, P.J.; Dametto, E.; Bertinetto, F.E.; Magistroni, P.; Rendine, S.; Amoroso, A.; Novelli, F. Phosphorylated alpha-enolase induces autoantibodies in HLA-DR8 pancreatic cancer patients and triggers HLA-DR8 restricted T-cell activation. Immunol. Lett. 2015, 167, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.E.; Shumaker, D.K.; Ridge, K.M. The role of vimentin intermediate filaments in the progression of lung cancer. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fontana, S.; Alessandro, R.; Barranca, M.; Giordano, M.; Corrado, C.; Zanella-Cleon, I.; Becchi, M.; Kohn, E.C.; De Leo, G. Comparative proteome profiling and functional analysis of chronic myelogenous leukemia cell lines. J. Proteome Res. 2007, 6, 4330–4342. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.L.; Chu, S.C.; Yang, S.S.; Li, M.C.; Lai, J.C.; Yang, S.F.; Chiou, H.L.; Hsieh, Y.S. The aberrant expression of cytosolic carbonic anhydrase and its clinical significance in human non-small cell lung cancer. Cancer Lett. 2002, 188, 199–205. [Google Scholar] [CrossRef]
- Kuo, W.H.; Chiang, W.L.; Yang, S.F.; Yeh, K.T.; Yeh, C.M.; Hsieh, Y.S.; Chu, S.C. The differential expression of cytosolic carbonic anhydrase in human hepatocellular carcinoma. Life Sci. 2003, 73, 2211–2223. [Google Scholar] [CrossRef]
- Goldmann, W.H.; Auernheimer, V.; Thievessen, I.; Fabry, B. Vinculin, cell mechanics and tumour cell invasion. Cell Biol. Int. 2013, 37, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.X.; Xu, J.D.; Liu, X.L.; Xu, J.W.; Wang, W.J.; Li, Q.Q.; Chen, Q.; Xu, Z.D.; Liu, X.P. RACK1: A superior independent predictor for poor clinical outcome in breast cancer. Int. J. Cancer 2010, 127, 1172–1179. [Google Scholar] [CrossRef]
- Kuramitsu, Y.; Wang, Y.; Okada, F.; Baron, B.; Tokuda, K.; Kitagawa, T.; Akada, J.; Nakamura, K. Malignant progressive tumor cell clone exhibits significant up-regulation of cofilin-2 and 27-kDa modified form of cofilin-1 compared to regressive clone. Anticancer Res. 2013, 33, 3661–3665. [Google Scholar]
- Yan, H.; Yang, K.; Xiao, H.; Zou, Y.J.; Zhang, W.B.; Liu, H.Y. Over-expression of cofilin-1 and phosphoglycerate kinase 1 in astrocytomas involved in pathogenesis of radioresistance. CNS Neurosci. Ther. 2012, 18, 729–736. [Google Scholar] [CrossRef]
- Tariq, M.; Ito, A.; Ishfaq, M.; Bradshaw, E.; Yoshida, M. Eukaryotic translation initiation factor 5A (eIF5A) is essential for HIF-1α activation in hypoxia. Biochem. Biophys. Res. Commun. 2016, 470, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Deng, Y.Z.; Zhao, J.S.; Ji, X.D.; Shi, J.; Feng, Y.X.; Li, G.; Li, J.J.; Zhu, D.; Koeffler, H.P. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J. Biol. Chem. 2012, 287, 7845–7858. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.B.; Hershey, J.W. The translation factor eIF5A and human cancer. Biochim. Biophys. Acta Gene Regul. Mech. 2015, 1849, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Scuoppo, C.; Miething, C.; Lindqvist, L.; Reyes, J.; Ruse, C.; Appelmann, I.; Yoon, S.; Krasnitz, A.; Teruya-Feldstein, J.; Pappin, D. A tumour suppressor network relying on the polyamine-hypusine axis. Nature 2012, 487, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Aoyagi, S.; Nanchi, I.; Nakatsuka, S.I.; Hirata, E.; Shibata, S.; Fukuda, M.; Yamamoto, Y.; Fukuda, I.; Tatsumi, N. Overexpression of eukaryotic elongation factor eEF2 in gastrointestinal cancers and its involvement in G2/M progression in the cell cycle. Int. J. Oncol. 2009, 34, 1181. [Google Scholar]
- Hamrita, B.; Nasr, H.B.; Hammann, P.; Kuhn, L.; Guillier, C.L.; Chaieb, A.; Khairi, H.; Chahed, K. An elongation factor-like protein (EF-Tu) elicits a humoral response in infiltrating ductal breast carcinomas: An immunoproteomics investigation. Clin. Biochem. 2011, 44, 1097–1104. [Google Scholar] [CrossRef]
- Tomaino, B.; Cappello, P.; Capello, M.; Fredolini, C.; Ponzetto, A.; Novarino, A.; Ciuffreda, L.; Bertetto, O.; De Angelis, C.; Gaia, E. Autoantibody signature in human ductal pancreatic adenocarcinoma. J. Proteome Res. 2007, 6, 4025–4031. [Google Scholar] [CrossRef]
- Srisomsap, C.; Sawangareetrakul, P.; Subhasitanont, P.; Panichakul, T.; Keeratichamroen, S.; Lirdprapamongkol, K.; Chokchaichamnankit, D.; Sirisinha, S.; Svasti, J. Proteomic analysis of cholangiocarcinoma cell line. Proteomics 2004, 4, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Ateeq, B.; Sharma, H.; Datta, P.; Gupta, S.D.; Bal, S.; Kumar, A.; Singh, N. Molecular profiling of genes in squamous cell lung carcinoma in Asian Indians. Life Sci. 2008, 82, 772–779. [Google Scholar] [CrossRef]
- Ryningen, A.; Ersvær, E.; Øyan, A.M.; Kalland, K.H.; Vintermyr, O.K.; Gjertsen, B.T.; Bruserud, Ø. Stress-induced in vitro apoptosis of native human acute myelogenous leukemia (AML) cells shows a wide variation between patients and is associated with low BCL-2: Bax ratio and low levels of heat shock protein 70 and 90. Leuk. Res. 2006, 30, 1531–1540. [Google Scholar] [CrossRef]
- Ogawa, K.; Utsunomiya, T.; Mimori, K.; Tanaka, Y.; Tanaka, F.; Inoue, H.; Murayama, S.; Mori, M. Clinical significance of elongation factor-1 delta mRNA expression in oesophageal carcinoma. Br. J. Cancer 2004, 91, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Eugene, C.Y.; Donohoe, S.; Pan, S.; Eng, J.; Cooke, K.; Crispin, D.A.; Lane, Z.; Goodlett, D.R.; Bronner, M.P. Pancreatic cancer proteome: The proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology 2005, 129, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Zhang, J.; Yang, S.; Soh, U.J.; Buschdorf, J.P.; Zhou, Y.T.; Yang, D.; Low, B.C. The SAM domain of the RhoGAP DLC1 binds EF1A1 to regulate cell migration. J. Cell Sci. 2009, 122, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.; MacKay, C.; Alnabulsi, A.; MacKay, M.; Telfer, C.; Melvin, W.T.; Murray, G.I. The roles of heterogeneous nuclear ribonucleoproteins in tumour development and progression. Biochim. Biophys. Acta Rev. Cancer 2006, 1765, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Jean-Philippe, J.; Paz, S.; Caputi, M. hnRNP A1: The Swiss army knife of gene expression. Int. J. Mol. Sci. 2013, 14, 18999–19024. [Google Scholar] [CrossRef] [PubMed]
- Pino, I.; Pio, R.; Toledo, G.; Zabalegui, N.; Vicent, S.; Rey, N.; Lozano, M.D.; Torre, W.; Garcia-Foncillas, J.; Montuenga, L.M. Altered patterns of expression of members of the heterogeneous nuclear ribonucleoprotein (hnRNP) family in lung cancer. Lung Cancer 2003, 41, 131–143. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Cai, L.; Zhu, J.; Chen, M.; Chen, J.; Li, Z.H.; Liu, X.D.; Wang, S.G.; Bie, P.; Jiang, P. Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis 2011, 32, 1419–1426. [Google Scholar] [CrossRef]
- Yan-Sanders, Y.; Hammons, G.J.; Lyn-Cook, B.D. Increased expression of heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNP) in pancreatic tissue from smokers and pancreatic tumor cells. Cancer Lett. 2002, 183, 215–220. [Google Scholar] [CrossRef]
- Zhang, D.; Tai, L.K.; Wong, L.L.; Chiu, L.L.; Sethi, S.K.; Koay, E.S. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in her-2/neu-positive breast cancer. Mol. Cell. Proteom. 2005, 4, 1686–1696. [Google Scholar] [CrossRef]
- Duan, Z.; Lamendola, D.; Yusuf, R.; Penson, R.; Preffer, F.; Seiden, M. Overexpression of human phosphoglycerate kinase 1 (PGK1) induces a multidrug resistance phenotype. Anticancer Res. 2001, 22, 1933–1941. [Google Scholar]
- Hwang, T.L.; Liang, Y.; Chien, K.Y.; Yu, J.S. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics 2006, 6, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Unwin, R.D.; Craven, R.A.; Harnden, P.; Hanrahan, S.; Totty, N.; Knowles, M.; Eardley, I.; Selby, P.J.; Banks, R.E. Proteomic changes in renal cancer and co-ordinate demonstration of both the glycolytic and mitochondrial aspects of the Warburg effect. Proteomics 2003, 3, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Ye, H.; Dai, L.; Liu, M.; Liu, X.; Chai, Y.; Shao, Q.; Li, Y.; Lei, N.; Peng, B. Peroxiredoxin 1 is a tumor-associated antigen in esophageal squamous cell carcinoma. Oncol. Rep. 2013, 30, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Yanai, T.; Sakai, H. Overexpression of Peroxiredoxin 6 Protects Neoplastic Cells against Apoptosis in Canine Haemangiosarcoma. J. Comp. Pathol. 2016, 155, 29–39. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Lee, H.P.; Lee, D.H.; Jo, M.; Shin, D.H.; Yoon, D.Y.; Han, S.B.; Hong, J.T. PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. Free Radic. Biol. Med. 2014, 69, 367–376. [Google Scholar] [CrossRef]
- Sherbet, G.V.; Cajone, F. Stathmin in cell proliferation and cancer progression. Cancer Genom. Proteom. 2005, 2, 227–238. [Google Scholar]
- Fang, L.; Min, L.; Lin, Y.; Ping, G.; Rui, W.; Ying, Z.; Xi, W.; Ting, H.; Li, L.; Ke, D. Downregulation of stathmin expression is mediated directly by Egr1 and associated with p53 activity in lung cancer cell line A549. Cell. Signal. 2010, 22, 166–173. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, J.Y.; Moon, J.H.; Kim, K.B.; Kim, T.S.; Hong, S.J.; Cheon, Y.P.; Pak, J.H.; Seo, S.B. Transcriptional induction of minichromosome maintenance protein 7 (Mcm7) in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory–secretory products. Mol. Biochem. Parasitol. 2010, 173, 10–16. [Google Scholar] [CrossRef]
- Luo, J.H. Oncogenic activity of MCM7 transforming cluster. World J. Clin. Oncol. 2011, 2, 120. [Google Scholar] [CrossRef]
- Milton, R.H.; Abeti, R.; Averaimo, S.; DeBiasi, S.; Vitellaro, L.; Jiang, L.; Curmi, P.M.; Breit, S.N.; Duchen, M.R.; Mazzanti, M. CLIC1 function is required for β-amyloid-induced generation of reactive oxygen species by microglia. J. Neurosci. 2008, 28, 11488–11499. [Google Scholar] [CrossRef]
- Barrès, V.; Ouellet, V.; Lafontaine, J.; Tonin, P.N.; Provencher, D.M.; Mes-Masson, A.M. An essential role for Ran GTPase in epithelial ovarian cancer cell survival. Mol. Cancer 2010, 9, 272. [Google Scholar] [CrossRef]
- Caputo, E.; Wang, E.; Valentino, A.; Crispi, S.; De Giorgi, V.; Fico, A.; Ficili, B.; Capone, M.; Anniciello, A.; Cavalcanti, E. Ran signaling in melanoma: Implications for the development of alternative therapeutic strategies. Cancer Lett. 2015, 357, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Meyerkord, C.L.; Du, Y.; Khuri, F.R.; Fu, H. 14-3-3 proteins as potential therapeutic targets. Semin. Cell Dev. Biol. 2011, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Klemm, C.; Dommisch, H.; Göke, F.; Kreppel, M.; Jepsen, S.; Rolf, F.; Dommisch, K.; Perner, S.; Standop, J. Expression profiles for 14-3-3 zeta and CCL20 in pancreatic cancer and chronic pancreatitis. Pathol. Res. Pract. 2014, 210, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, H.; He, H.; Ying, W.; Liu, X.; Dai, Z.; Yin, J.; Mao, N.; Qian, X.; Pan, L. Quantitative proteomic analysis of mitochondria from human ovarian cancer cells and their paclitaxel-resistant sublines. Cancer Sci. 2015, 106, 1075–1083. [Google Scholar] [CrossRef]
- Niemantsverdriet, M.; Wagner, K.; Visser, M.; Backendorf, C. Cellular functions of 14-3-3ζ in apoptosis and cell adhesion emphasize its oncogenic character. Oncogene 2008, 27, 1315–1319. [Google Scholar] [CrossRef]
- Livinskaya, V.A.; Barlev, N.A.; Nikiforov, A.A. Immunoaffinity purification of the functional 20S proteasome from human cells via transient overexpression of specific proteasome subunits. Protein Expr. Purif. 2014, 97, 37–43. [Google Scholar] [CrossRef]
- Moghanibashi, M.; Jazii, F.R.; Soheili, Z.S.; Zare, M.; Karkhane, A.; Parivar, K.; Mohamadynejad, P. Proteomics of a new esophageal cancer cell line established from Persian patient. Gene 2012, 500, 124–133. [Google Scholar] [CrossRef]
- Hu, X.T.; Chen, W.; Wang, D.; Shi, Q.L.; Zhang, F.B.; Liao, Y.Q.; Jin, M.; He, C. The proteasome subunit PSMA7 located on the 20q13 amplicon is overexpressed and associated with liver metastasis in colorectal cancer. Oncol. Rep. 2008, 19, 441–446. [Google Scholar] [CrossRef][Green Version]
- Langdon, S.P. Cancer Cell Culture: Methods and Protocols. In Methods in Molecular Biology; Illustrated Edition; Langdon, S.P., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2004; pp. 133–138. [Google Scholar]
- Holford, J.; Beale, P.; Boxall, F.; Sharp, S.; Kelland, L. Mechanisms of drug resistance to the platinum complex ZD0473 in ovarian cancer cell lines. Eur. J. Cancer 2000, 36, 1984–1990. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- GENOMED. Purifying gDNA from Mammalian Cells. Available online: http://manualzz.com/doc/7146492/cst-genomic-dna-purification-kits----tissues (accessed on 2 June 2015).
- Holford, J.; Sharp, S.; Murrer, B.; Abrams, M.; Kelland, L. In vitro circumvention of cisplatin resistance by the novel sterically hindered platinum complex AMD473. Br. J. Cancer 1998, 77, 366. [Google Scholar] [CrossRef] [PubMed]
- Maloney, A.; Clarke, P.A.; Naaby-Hansen, S.; Stein, R.; Koopman, J.O.; Akpan, A.; Yang, A.; Zvelebil, M.; Cramer, R.; Stimson, L. Gene and protein expression profiling of human ovarian cancer cells treated with the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2007, 67, 3239–3253. [Google Scholar] [CrossRef] [PubMed]
- Wood, R. Bio-Rad Protein Assay. Available online: http://www.bio-rad.com/webroot/web/pdf/lsr/literature/LIT33.pdf (accessed on 2 June 2015).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bio-Rad. ReadyPrep 2-D Starter Kit. Available online: http://www.bio-rad.com/webroot/web/pdf/lsr/literature/4110009A.pdf (accessed on 2 June 2015).
- Bio-Rad. 2-D Electrophoresis Workflow How-to Guide. Available online: http://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_2651.pdf (accessed on 2 June 2015).
- Bio-Rad. Criterion Dodeca Cell. Available online: http://www.bio-rad.com/webroot/web/pdf/lsr/literature/4006197a.pdf (accessed on 2 June 2015).
- Bio-Rad. ChemiDoc XRS+Systems with Image Lab Software User Guid. Available online: http://www.bio-rad.com/webroot/web/pdf/lsr/literature/10017218.pdf (accessed on 2 June 2015).
- Bio-Rad. Melanie 3 User Manual. Available online: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/4000151A.pdf (accessed on 2 June 2015).
- Winnik, W.M.; DeKroon, R.M.; Jeong, J.S.; Mocanu, M.; Robinette, J.B.; Osorio, C.; Dicheva, N.N.; Hamlett, E.; Alzate, O. Analysis of proteins using DIGE and MALDI mass spectrometry. In Difference Gel Electrophoresis (DIGE) Methods and Protocols; Humana Press: Tortowa, NJ, USA, 2012; Volume 854, pp. 47–66. [Google Scholar]
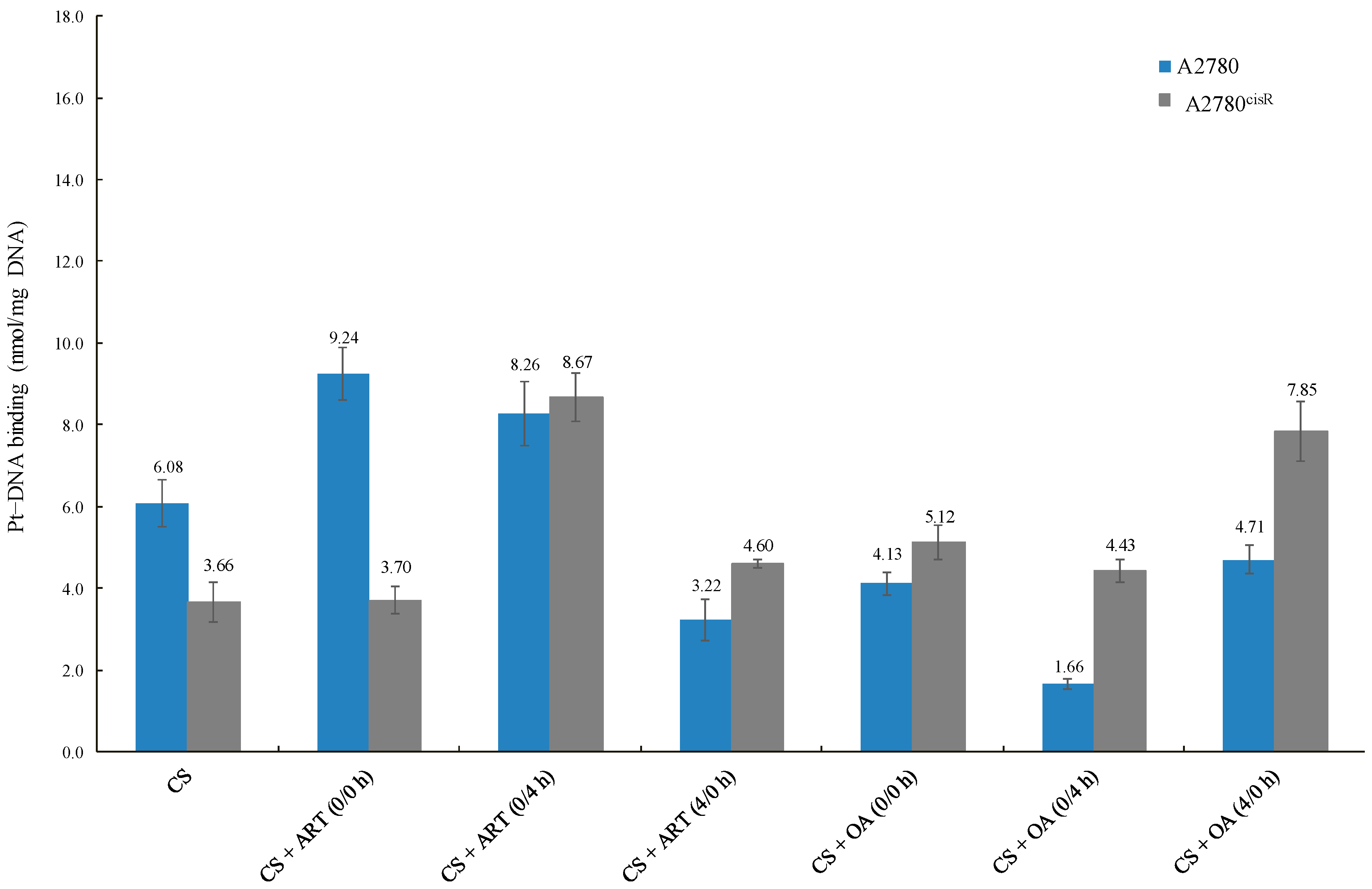
| IC50 (μM) and RF Values | |||||
|---|---|---|---|---|---|
| Drug | A2780 | A2780cisR | *RF | A2780ZD0473R | RF |
| CS | 0.66 ± 0.08 | 6.44 ± 0.11 | 9.75.63 | 8.37± 0.06 | 12.6 |
| ART | 16.78 ± 0.06 | 26.8 ± 0.13 | 1.60 | 36.36 ± 0.09 | 2.16 |
| OA | 34.0 ± 0.31 | 26.15 ± 0.28 | 0.76 | 10.85 ± 0.05 | 0.3 |
| CI Value at | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Sequence (h) | Molar Ratio | ED50 | ED75 | ED90 | Dm | m | r |
| CS | N/A | N/A | N/A | 1.16 | 0.88 | 0.99 | ||
| OA | N/A | N/A | N/A | 37.19 | 0.64 | 1.00 | ||
| CS + OA | 0/0 | 1:51.52 | 0.72 | 0.50 | 0.37 | 0.53 | 1.06 | 0.99 |
| CS + OA | 0/4 | 0.68 | 0.59 | 0.54 | 0.49 | 0.86 | 0.97 | |
| CS + OA | 4/0 | 0.92 | 1.07 | 1.28 | 0.69 | 0.72 | 0.98 | |
| ART | N/A | N/A | N/A | 26.5 | 0.55 | 0.99 | ||
| CS + ART | 0/0 | 1:25.42 | 0.74 | 0.55 | 0.47 | 0.40 | 0.85 | 1.00 |
| CS + ART | 0/4 | 0.86 | 1.05 | 1.46 | 0.47 | 0.62 | 0.99 | |
| CS + ART | 4/0 | 0.77 | 0.55 | 0.60 | 0.32 | 0.72 | 0.99 | |
| CI Values at | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Sequence (h) | Molar Ratio | ED50 | ED75 | ED90 | Dm | m | r |
| CS | N/A | N/A | N/A | 8.03 | 0.63 | 0.99 | ||
| OA | N/A | N/A | N/A | 56.44 | 0.40 | 1.00 | ||
| CS + OA | 0/0 | 1:4.05 | 0.57 | 0.49 | 0.50 | 2.88 | 0.61 | 1.00 |
| CS + OA | 0/4 | 0.63 | 0.42 | 0.32 | 3.15 | 0.72 | 1.00 | |
| CS + OA | 4/0 | 0.77 | 0.53 | 0.43 | 3.85 | 0.70 | 0.99 | |
| ART | N/A | N/A | N/A | 77.82 | 0.49 | 1.00 | ||
| CS + ART | 0/0 | 1:4.16 | 0.54 | 0.88 | 1.51 | 2.98 | 0.48 | 1.00 |
| CS + ART | 0/4 | 0.75 | 0.67 | 0.64 | 4.17 | 0.65 | 1.00 | |
| CS + ART | 4/0 | 0.87 | 0.80 | 0.78 | 4.82 | 0.64 | 1.00 | |
| CI Values at | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Sequence (h) | Molar Ratio | ED50 | ED75 | ED90 | Dm | m | r |
| CS | N/A | N/A | N/A | 11.35 | 0.83 | 0.98 | ||
| OA | N/A | N/A | N/A | 29.43 | 0.43 | 0.96 | ||
| CS + OA | 0/0 | 1:1.3 | 0.58 | 0.61 | 0.86 | 3.55 | 0.61 | 0.99 |
| CS + OA | 0/4 | 0.81 | 0.56 | 0.52 | 5.00 | 0.80 | 0.99 | |
| CS + OA | 4/0 | 0.80 | 0.71 | 0.74 | 5.53 | 0.73 | 0.99 | |
| ART | N/A | N/A | N/A | 17.19 | 0.57 | 0.99 | ||
| CS + ART | 0/0 | 1:4.35 | 0.71 | 0.80 | 0.95 | 1.46 | 0.56 | 1.00 |
| CS + ART | 0/4 | 0.82 | 0.91 | 1.36 | 1.71 | 0.53 | 0.99 | |
| CS + ART | 4/0 | 0.82 | 0.85 | 1.09 | 1.89 | 0.58 | 1.00 | |
| * Platinum Accumulation | ||||
|---|---|---|---|---|
| A2780 | A2780cisR | |||
| DRUG | Value | Fold Change | Value | Fold Change |
| CS | 0.15 ± 0.01 | - | 0.27 ± 0.02 | - |
| CS + ART (0/0 h) | 0.65 ± 0.04 | 4.33 | 2.29 ± 0.20 | 8.48 |
| CS + ART (0/4 h) | 0.91 ± 0.06 | 6.06 | 0.75 ± 0.04 | 2.77 |
| CS + ART (4/0 h) | 0.22 ± 0.02 | 1.46 | 1.22 ± 0.09 | 4.51 |
| CS + OA (0/0 h) | 2.22 ± 0.08 | 14.8 | 0.91 ± 0.08 | 3.33 |
| CS + OA (0/4 h) | 0.27 ± 0.01 | 1.8 | 0.50 ± 0.02 | 1.85 |
| CS + OA (4/0 h) | 0.22 ± 0.01 | 1.46 | 1.14 ± 0.11 | 4.22 |
| * Platinum–DNA Binding Level | ||||
|---|---|---|---|---|
| A2780 | A2780cisR | |||
| DRUG | Value | Fold Change | Value | Fold Change |
| CS | 6.08 ± 0.57 | - | 3.66 ± 0.49 | - |
| CS + ART (0/0 h) | 9.24 ± 0.64 | 1.52 | 3.70 ± 0.33 | 1.01 |
| CS + ART (0/4 h) | 8.26 ± 0.78 | 1.36 | 8.67 ± 0.59 | 2.37 |
| CS + ART (4/0 h) | 3.22 ± 0.49 | 0.53 | 4.60 ± 0.11 | 1.26 |
| CS + OA (0/0 h) | 4.13 ± 0.28 | 0.68 | 5.12 ± 0.43 | 1.40 |
| CS + OA (0/4 h) | 1.66 ± 0.11 | 0.27 | 4.43 ± 0.29 | 1.21 |
| CS + OA (4/0 h) | 4.71 ± 0.36 | 0.77 | 7.85 ± 0.73 | 2.14 |
| Match ID | Expression in A2780cisR/A2780 | CS + ART (4/0 h) | CS + OA (0/4 h) | CS + ART (0/0 h) | CS + OA (0/0 h) | Match ID | Expression in A2780cisR/A2780 | CS + ART (4/0 h) | CS + OA (0/4 h) | CS + ART (0/0 h) | CS + OA (0/0 h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UR | PR | FUR | 48 | ND | OR | |||||
| 3 | DR | PR | PR | PR | 51 | DR | OR | PR | PR | PR | |
| 4 | DR | PR | PR | 52 | ND | PR | PR | OR | |||
| 5 | DR | PR | PR | PR | 54 | DR | OR | OR | OR | OR | |
| 7 | DR | OR | 55 | DR | PR | PR | |||||
| 8 | ND | PR | PR | OR | OR | 62 | DR | ||||
| 11 | ND | OR | PR | 63 | DR | PR | |||||
| 12 | ND | PR | PR | 66 | ND | ||||||
| 13 | DR | PR | OR | OR | 68 | UR | |||||
| 14 | NC | OR | OR | 69 | DR | OR | OR | ||||
| 15 | UR | OR | OR | 70 | ND | OR | |||||
| 16 | UR | PR | PR | PR | 74 | ND | OR | OR | |||
| 17 | ND | OR | PR | OR | OR | 76 | UR | ||||
| 19 | DR | OR | PR | OR | OR | 78 | ND | ||||
| 20 | ND | OR | OR | 82 | ND | ||||||
| 21 | UR | FUR | 85 | ND | |||||||
| 25 | ND | OR | PR | 88 | ND | ||||||
| 27 | ND | PR | OR | PR | 89 | ND | FR | OR | |||
| 29 | ND | OR | 94 | ND | OR | OR | OR | OR | |||
| 31 | ND | PR | OR | 95 | ND | OR | OR | ||||
| 32 | ND | PR | OR | FR | 96 | ND | OR | OR | |||
| 33 | ND | PR | OR | 97 | ND | OR | OR | ||||
| 34 | ND | OR | OR | 98 | ND | ||||||
| 35 | DR | PR | OR | 102 | ND | ||||||
| 36 | UR | OR | OR | PR | 103 | DR | OR | ||||
| 39 | ND | 105 | ND | ||||||||
| 40 | DR | 106 | ND | OR | |||||||
| 42 | ND | OR | OR | 108 | ND | ||||||
| 43 | ND | 111 | ND | OR | |||||||
| 44 | ND | OR | 116 | UR | PR | PR | |||||
| 45 | DR | PR | FDR | FDR | FDR | 119 | ND | OR | |||
| 46 | DR | 120 | ND | OR | |||||||
| 47 | UR | OR | OR | OR | OR | 122 | ND |
| Match ID | Expression in A2780cisR/A2780 | CS + ART (4/0 h) | CS + OA (0/4h) | CS + ART (0/0 h) | CS + OA (0/0 h) |
|---|---|---|---|---|---|
| 8 | UR | ||||
| 9 | UR | OR | PR | PR | |
| 11 | DR | OR | OR | OR | |
| 12 | UR | PR | OR | PR | |
| 13 | UR | PR | OR | FR | |
| 16 | ND | ||||
| 18 | ND | OR | PR | ||
| 19 | DR | OR | OR | OR | |
| 22 | UR | PR | PR | PR | FUR |
| 25 | UR | PR | |||
| 26 | UR | FUR | FUR | ||
| 28 | DR | OR | OR | ||
| 29 | ND | FUR | FUR | ||
| 30 | UR | OR | OR | PR | |
| 32 | UR | FUR | PR | FUR | FUR |
| 41 | UR | PR | PR | ||
| 44 | DR | PR | OR | ||
| 46 | UR | PR | PR | ||
| 47 | UR | OR | |||
| 50 | UR | ||||
| 56 | UR | ||||
| 57 | UR | ||||
| 58 | UR | PR | PR | ||
| 59 | DR | ||||
| 61 | UR | PR | |||
| 62 | UR | FUR | |||
| 63 | UR | PR | PR | ||
| 69 | DR | OR |
| Mach ID | Protein ID | Full Name | Mascot Search Results | Location | References |
|---|---|---|---|---|---|
| 8 | ATP5H O75947 | ATP synthase subunit d, mitochondrial | Mass: 18480 Mascot score: 128 Coverage: 57% pI: 5.21 MS: 11 MSMS: 3 | Mitochondrion | [15] http://www.matrixscience.com |
| 9 | RAN P62826 | GTP-binding nuclear protein Ran | Mass: 24408 Mascot score: 132 Coverage: 39% pI: 7.01 MS: 11 MSMS:1 | Nucleus | [15] http://www.matrixscience.com |
| 12 | PSA7 O14818 | Proteasome subunit alpha type-7 | Mass: 27870 Mascot score: 85 Coverage: 39% pI: 8.60 MS: 10 MSMS: 1 | Cytoplasm | [15] http://www.matrixscience.com |
| 13 | TPI P60174 | Triosephosphate isomerase | Mass: 30772 Mascot score: 340 Coverage: 49% pI: 5.65 MS: 20 MSMS:3 | [15] http://www.matrixscience.com | |
| 16 | VDAC1 P21796 | Voltage-dependent anion-selective channel protein 1 | Mass: 30754 Mascot score: 368 Coverage: 51% pI: 8.62 MS: 12 MSMS: 3 | Mitochondrion outer membrane | [15] http://www.matrixscience.com |
| 18 | hnRNPA2/B1 P22626 | Heterogeneous nuclear ribonucleoproteins A2/B1 | Mass: 37407 Mascot score: 258 Coverage: 48% pI: 8.97 MS: 21 MSMS: 6 | Nucleus, nucleoplasm | [15] http://www.matrixscience.com |
| 22 | PGK1 P00558 | Phosphoglycerate kinase 1 | Mass: 44586 Mascot score: 145 Coverage: 48% pI: 8.30 MS: 22 MSMS: 1 | Cytoplasm | [15] http://www.matrixscience.com |
| 25 | GOT1 P17174 | Glutamate oxaloacetate transaminase 1 | Mass: 46219 Mascot score: 331 Coverage: 66% pI: 6.52 MS: 27 MSMS: 6 | Cytoplasm | [15] http://www.matrixscience.com |
| 30 | EF1A1 P68104 | Elongation factor 1-alpha 1 | Mass: 50109 Mascot score: 135 Coverage: 25% PI: 9.10 MS: 13 MSMS: 2 | Cytoplasm | [15] http://www.matrixscience.com |
| 32 | ACTG P63261 | Actin, cytoplasmic 2 | Mass: 41766 Mascot score: 598 Coverage: 55% pI: 5.31 MS:26 MSMS:5 | Cytoplasm, cytoskeleton | [15] http://www.matrixscience.com |
| 41 | KPYM P14618 | Pyruvate kinase PKM | Mass: 57900 Mascot score: 188 Coverage: 33% pI: 7.96 MS: 23 MSMS: 3 | Cytoplasm. | [15] http://www.matrixscience.com |
| 44 | P4HB P07237 | Prolyl 4-hydroxylase subunit beta | Mass: 57081 Mascot score: 433 Coverage: 50% PI: 4.76 MS: 29 MSMS: 6 | Endoplasmic reticulum lumen | [15] http://www.matrixscience.com |
| 57 | APG2 P34932 | Shock 70-related protein APG-2 | Mass: 94271 Mascot score: 125 Coverage: 25% pI: 5.11 MS: 22 MSMS:3 | Cytoplasm (Probable) | [15] http://www.matrixscience.com |
| 58 | VINC P18206 | Vinculin | Mass: 123722 Mascot score: 226 Coverage: 34% pI: 5.50 MS: 40 MSMS:4 | Cytoplasm, cytoskeleton | [15] http://www.matrixscience.com |
| 63 | EF2 P13639 | Elongation factor 2 | Mass: 95277 Mascot score: 425 Coverage: 27% pI: 6.41 MS: 39 MSMS:7 | Cytoplasm | [15] http://www.matrixscience.com |
| 69 | CLIC1 O00299 | Chloride intracellular channel protein 1 | Mass: 26906 Mascot score: 158 Coverage: 47% pI: 5.09 MS: 11 MSMS: 3 | Nucleus | [15] http://www.matrixscience.com |
| Match ID | A2780cisR/A2780 | Protein ID |
|---|---|---|
| Stress and Chaperones | ||
| 3 | DR | CYPA |
| 32 | ND | TCPB |
| 35 | DR | ERp57 |
| 39 | ND | TCPH |
| 45 | DR | HSP7C |
| 46 | DR | mortalin |
| 47 | UP | BIP |
| 51 | DR | HSP90B |
| 54 | DR | GRP94 |
| 88 | ND | Hop |
| 105 | ND | TCPA |
| Metabolism and Biosynthetic Processes | ||
| 11 | ND | PGAM1 |
| 17/31 | ND | ENOA |
| 19 | DR | LDHB |
| 33 | ND | SERA |
| 55 | DR | IMMT |
| 63 | DR | NM23 |
| 97 | ND | ATPA |
| 95 | ND | PSAT |
| Cytoskeletal Proteins | ||
| 5 | DR | P18 |
| 36/96 | UR | VIME |
| 94 | ND | GBLP |
| 108 | ND | CAH2 |
| Initiation and Elongation | ||
| 25 | ND | EFTU |
| 4 | DR | EIF5A1 |
| 27 | ND | EF1G |
| 52 | ND | EF2 |
| mRNA Processing Proteins | ||
| 15 | UR | hnRNPA1 |
| 16/76 | UR | hnRNP A2/B1 |
| Detoxification and Drug Resistance | ||
| 8 | ND | PRDX1 |
| 66 | ND | PRDX6 |
| Cell Cycle Regulation and Cell Proliferation | ||
| 7 | DR | Op18 |
| 89 | ND | MCM7 |
| Signal Transduction and Cell Cycle | ||
| 13 | DR | 1433Z |
| Protein Synthesis and Degradation | ||
| 69 | DR | PSA3 |
| Match ID | A2780cisR/A2780 | Protein ID |
|---|---|---|
| Stress and Chaperones | ||
| 44 | DR | P4HB |
| 57 | UR | APG2 |
| Metabolism and Biosynthetic Processes | ||
| 13 | UR | TPI |
| 8 | UR | ATP5H |
| 25 | UR | GOT1 |
| 16 | UR | VDAC1 |
| 41 | UR | KPYM |
| Cytoskeletal Proteins | ||
| 32 | UR | ACTG |
| 58 | UR | VINC |
| Initiation and Elongation | ||
| 30 | UR | EF1A1 |
| 63 | UR | EF2 |
| mRNA Processing Proteins | ||
| 18 | ND | hnRNP A2/B1 |
| Detoxification and Drug Resistance | ||
| 22 | UR | PGK1 |
| Cell Cycle Regulation and Cell Proliferation | ||
| 69 | DR | CLIC1 |
| Signal Transduction and Cell Cycle | ||
| 9 | UR | RAN |
| Protein Synthesis and Degradation | ||
| 12 | UR | PSA7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althurwi, S.I.; Yu, J.Q.; Beale, P.; Huq, F. Sequenced Combinations of Cisplatin and Selected Phytochemicals towards Overcoming Drug Resistance in Ovarian Tumour Models. Int. J. Mol. Sci. 2020, 21, 7500. https://doi.org/10.3390/ijms21207500
Althurwi SI, Yu JQ, Beale P, Huq F. Sequenced Combinations of Cisplatin and Selected Phytochemicals towards Overcoming Drug Resistance in Ovarian Tumour Models. International Journal of Molecular Sciences. 2020; 21(20):7500. https://doi.org/10.3390/ijms21207500
Chicago/Turabian StyleAlthurwi, Safiah Ibrahim, Jun Q. Yu, Philip Beale, and Fazlul Huq. 2020. "Sequenced Combinations of Cisplatin and Selected Phytochemicals towards Overcoming Drug Resistance in Ovarian Tumour Models" International Journal of Molecular Sciences 21, no. 20: 7500. https://doi.org/10.3390/ijms21207500
APA StyleAlthurwi, S. I., Yu, J. Q., Beale, P., & Huq, F. (2020). Sequenced Combinations of Cisplatin and Selected Phytochemicals towards Overcoming Drug Resistance in Ovarian Tumour Models. International Journal of Molecular Sciences, 21(20), 7500. https://doi.org/10.3390/ijms21207500





