Advances of Zinc Signaling Studies in Prostate Cancer
Abstract
1. Introduction
2. Zinc Levels in Prostate Tissues and Sera of PCa Patients
3. Biological Functions of Zinc in PCa
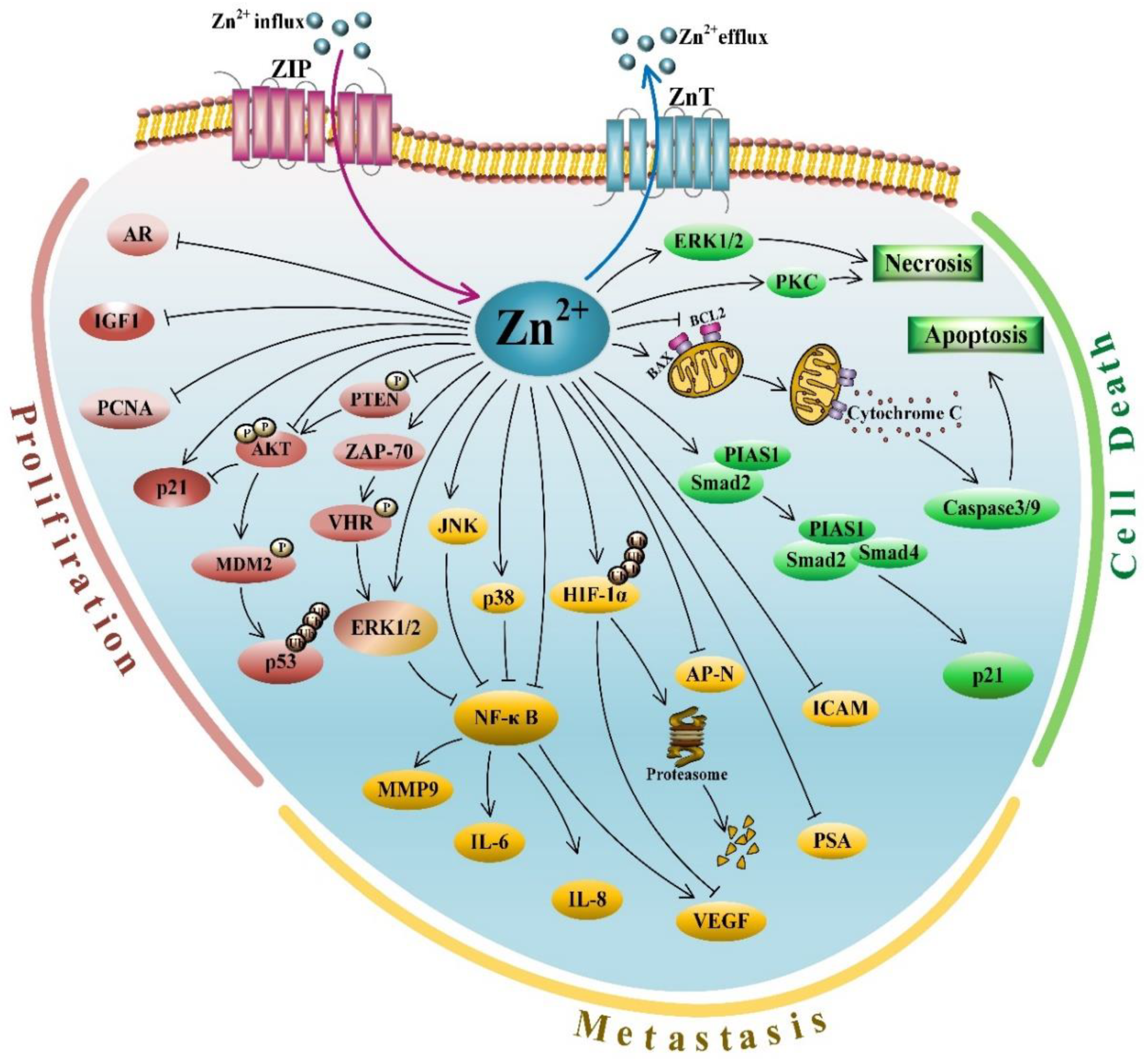
3.1. Zinc and Its Anti-Proliferative Activities
3.2. Zinc and Its Role in Cell Death
3.3. Zinc and Its Anti-Metastasis Effects
4. Zinc-Associated Compounds and Their Functions in PCa
4.1. Zinc Transporters
4.2. Zinc Finger-Containing Transcription Factors
5. Clinical Applications of Zinc Signaling in PCa
6. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectrophotometry |
| AP-N | Aminopeptidase N |
| AR | Androgen receptor |
| BAPTA | 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid |
| BAX | BCL2-associated X protein |
| BCL2 | B cell leukemia/lymphoma 2 |
| bFGF | Basic fibroblast growth factor |
| CD164 | CD164 antigen, sialomucin |
| DHT | Dihydrotestosterone |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transformation |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| FBL | Fibrillarin |
| HDAC1 | Histone deacetylase 1 |
| HIF-1α | Hypoxia inducible factor-1α |
| ICAM-1 | Intercellular adhesion molecule 1 |
| iCEST | Ion chemical exchange saturation transfer |
| IGF-1 | Insulin like growth factor 1 |
| IGFBP-3 | Insulin like growth factor binding protein 3 |
| IHC | Immunohistochemistry |
| IL-1 | Interleukin 1 |
| JNK | c-Jun N-terminal kinase |
| MAPKs | Mitogen activated protein kinases |
| MDM2 | Murine double minute 2 |
| MMP-9 | Matrix metallopeptidase 9 |
| MRI | Magnetic resonance imaging |
| mTOR | Mechanistic target of rapamycin kinase |
| NCoR | Nuclear receptor corepressor 1 |
| NF-κB | Nuclear factor kappa B |
| PCa | Prostate cancer |
| PCNA | Proliferating cell nuclear antigen |
| PIAS1 | Protein inhibitor of activated STAT 1 |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PLZF | Promyelocytic leukemia zinc finger |
| PrEC | Prostatic epithelial cell |
| PSA | Prostate-specific antigen |
| PTEN | Phosphatase and tensin homolog |
| RREB-1 | Ras responsive element binding protein 1 |
| SP1 | Specificity protein 1 |
| TF-BAPTA | 5,5′,6,6′-tetrafluoro-BAPTA |
| TFs | Transcription factors |
| TGFβ | Transforming growth factor β |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor α |
| TPEN | N,N,N′,N′-tetrakis(2-pyridylmethyl)-ethylenediamine |
| TRAMP | Transgenic adenocarcinoma of the mouse prostate |
| Treg | Regulatory T cell |
| UTR | Untranslated regions |
| VEGF | Vascular endothelial growth factor |
| VHR | Vaccinia H1-related phosphatase |
| ZAP-70 | Zeta chain-associated protein-70 |
| ZFs | Zinc fingers |
| ZIPs | ZRT- and Irt-like proteins |
| ZnTs | Zinc transporters |
| ZP | Zn-pyrithione |
| ZPP1 | Zinpyr (ZP) family of zinc probes |
References
- Connor, M.J.; Shah, T.T.; Horan, G.; Bevan, C.L.; Winkler, M.; Ahmed, H.U. Cytoreductive treatment strategies for de novo metastatic prostate cancer. Nat. Rev. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Farashi, S.; Kryza, T.; Clements, J.; Batra, J. Post-GWAS in prostate cancer: From genetic association to biological contribution. Nat. Rev. Cancer 2019, 19, 46–59. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Huland, H.; Graefen, M. Changing Trends in Surgical Management of Prostate Cancer: The End of Overtreatment? Eur. Urol. 2015, 68, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Simmons, L.H. Prevention of Prostate Cancer Morbidity and Mortality: Primary Prevention and Early Detection. Med. Clin. N. Am. 2017, 101, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.V.; Kattan, M.W. Prostate cancer: Personalized risk—Stratified screening or abandoning it altogether? Nat. Rev. Clin. Oncol. 2016, 13, 140–142. [Google Scholar] [CrossRef]
- Eidelman, E.; Twum-Ampofo, J.; Ansari, J.; Siddiqui, M.M. The Metabolic Phenotype of Prostate Cancer. Front. Oncol. 2017, 7, 131. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Jani, A.B.; Hellman, S. Early prostate cancer: Clinical decision-making. Lancet 2003, 361, 1045–1053. [Google Scholar] [CrossRef]
- Martel, C.L.; Gumerlock, P.H.; Meyers, F.J.; Lara, P.N. Current strategies in the management of hormone refractory prostate cancer. Cancer Treat. Rev. 2003, 29, 171–187. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Malik, S.; Manne, U.; Mishra, M. Prostate cancer health disparities: An immuno-biological perspective. Cancer Lett. 2018, 414, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Geler, M.; Urquiza-Salvat, N.; Cozar, J.M.; Robles-Fernandez, I.; Rivas, A.; Martinez-Gonzalez, L.J.; Ocana-Peinado, F.M.; Lorente, J.A.; Alvarez-Cubero, M.J. The influence of nutritional factors on prostate cancer incidence and aggressiveness. Aging Male 2018, 21, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Beaver, L.M.; Williams, D.E.; Dashwood, R.H. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv. Nutr. 2011, 2, 497–510. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef]
- Yu, H.N.; Shen, S.R.; Yin, J.J. Effects of metal ions, catechins, and their interactions on prostate cancer. Crit. Rev. Food Sci. Nutr. 2007, 47, 711–719. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Kambe, T.; Matsunaga, M.; Takeda, T.A. Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway. Int. J. Mol. Sci. 2017, 18, 2179. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, Q.; Su, Y.; Sun, W.; Huang, Q.; Wei, W. Zinc and its regulators in pancreas. Inflammopharmacology 2019, 27, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Kolenko, V.; Teper, E.; Kutikov, A.; Uzzo, R. Zinc and zinc transporters in prostate carcinogenesis. Nat. Rev. Urol. 2013, 10, 219–226. [Google Scholar] [CrossRef]
- Portbury, S.D.; Adlard, P.A. Zinc Signal in Brain Diseases. Int. J. Mol. Sci. 2017, 18, 2506. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Ames, B.N. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed]
- Ziliotto, S.; Ogle, O.; Taylor, K.M. Targeting Zinc(II) Signalling to Prevent Cancer. Met. Ions Life Sci. 2018, 18. [Google Scholar] [CrossRef]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Pan, Z.; Choi, S.; Ouadid-Ahidouch, H.; Yang, J.M.; Beattie, J.H.; Korichneva, I. Zinc transporters and dysregulated channels in cancers. Front. Biosci. 2017, 22, 623–643. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef]
- Wakwe, V.C.; Odum, E.P.; Amadi, C. The impact of plasma zinc status on the severity of prostate cancer disease. Investig. Clin. Urol. 2019, 60, 162–168. [Google Scholar] [CrossRef]
- Aydin, A.; Arsova-Sarafinovska, Z.; Sayal, A.; Eken, A.; Erdem, O.; Erten, K.; Ozgok, Y.; Dimovski, A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin. Biochem. 2006, 39, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Christudoss, P.; Selvakumar, R.; Fleming, J.J.; Gopalakrishnan, G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J. Urol. 2011, 27, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Darago, A.; Sapota, A.; Matych, J.; Nasiadek, M.; Skrzypinska-Gawrysiak, M.; Kilanowicz, A. The correlation between zinc and insulin-like growth factor 1 (IGF-1), its binding protein (IGFBP-3) and prostate-specific antigen (PSA) in prostate cancer. Clin. Chem. Lab. Med. 2011, 49, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Kaba, M.; Pirincci, N.; Yuksel, M.B.; Gecit, I.; Gunes, M.; Ozveren, H.; Eren, H.; Demir, H. Serum levels of trace elements in patients with prostate cancer. Asian Pac. J. Cancer Prev. 2014, 15, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, Q.; Hu, X.; Dong, X.; Wang, L.; Liu, Q.; Long, Z.; Li, L. Comparative study of serum zinc concentrations in benign and malignant prostate disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 25778. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Zinc is decreased in prostate cancer: An established relationship of prostate cancer! J. Biol. Inorg. Chem. 2011, 16, 3–8. [Google Scholar] [CrossRef]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 2005, 4, 32. [Google Scholar] [CrossRef]
- Clavijo Jordan, M.V.; Lo, S.T.; Chen, S.; Preihs, C.; Chirayil, S.; Zhang, S.; Kapur, P.; Li, W.H.; De Leon-Rodriguez, L.M.; Lubag, A.J.; et al. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc. Natl. Acad. Sci. USA 2016, 113, E5464–E5471. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, Z.; Chu, C.; Zhang, J.; Song, X.; Walczak, P.; Bulte, J.W.M. Development of Zinc-Specific iCEST MRI as an Imaging Biomarker for Prostate Cancer. Angew. Chem. Int. Ed. Engl. 2019, 58, 15512–15517. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.X.; Han, B.; Shaw, D.G.; Nimni, M. Zinc as a possible preventive and therapeutic agent in pancreatic, prostate, and breast cancer. Eur. J. Cancer Prev. 2016, 25, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Pitschmann, A.; Ahmad, N. Resveratrol-zinc combination for prostate cancer management. Cell Cycle 2014, 13, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.C.; Anderle, P.; Burzle, M.; Suzuki, Y.; Freeman, M.R.; Hediger, M.A.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Asp. Med. 2013, 34, 735–741. [Google Scholar] [CrossRef]
- Zaichick, V.; Sviridova, T.V.; Zaichick, S.V. Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int. Urol. Nephrol. 1997, 29, 565–574. [Google Scholar] [CrossRef]
- Costello, L.C.; Feng, P.; Milon, B.; Tan, M.; Frankin, R.B. Role of zinc in the pathogenesis and treatment of prostate cancer: Critical issues to resolve. Prostate Cancer Prostatic Dis. 2004, 7, 111–117. [Google Scholar] [CrossRef]
- Takatani-Nakase, T. Zinc Transporters and the Progression of Breast Cancers. Biol. Pharm. Bull. 2018, 41, 1517–1522. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, Z.; Chen, Z.; Yang, L.; Ma, M.; Lu, S.; Wang, C.; Teng, C.; Nie, Y. Zinc chelator TPEN induces pancreatic cancer cell death through causing oxidative stress and inhibiting cell autophagy. J. Cell. Physiol. 2019, 234, 20648–20661. [Google Scholar] [CrossRef]
- Ninsontia, C.; Phiboonchaiyanan, P.P.; Kiratipaiboon, C.; Chanvorachote, P. Zinc suppresses stem cell properties of lung cancer cells through protein kinase C-mediated beta-catenin degradation. Am. J. Physiol. Cell Physiol. 2017, 312, C487–C499. [Google Scholar] [CrossRef]
- Hosui, A.; Kimura, E.; Abe, S.; Tanimoto, T.; Onishi, K.; Kusumoto, Y.; Sueyoshi, Y.; Matsumoto, K.; Hirao, M.; Yamada, T.; et al. Long-Term Zinc Supplementation Improves Liver Function and Decreases the Risk of Developing Hepatocellular Carcinoma. Nutrients 2018, 10, 1955. [Google Scholar] [CrossRef]
- Mawson, C.A.; Fischer, M.I. The occurrence of zinc in the human prostate gland. Can. J. Med. Sci. 1952, 30, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate 1998, 35, 285–296. [Google Scholar] [CrossRef]
- Platz, E.A.; Helzlsouer, K.J. Selenium, zinc, and prostate cancer. Epidemiol. Rev. 2001, 23, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ogunlewe, J.O.; Osegbe, D.N. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer 1989, 63, 1388–1392. [Google Scholar] [CrossRef]
- Zaichick, V.Y.; Sviridova, T.V.; Zaichick, S.V. Zinc concentration in human prostatic fluid: Normal, chronic prostatitis, adenoma and cancer. Int. Urol. Nephrol. 1996, 28, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Kanak, M.A.; Kajdacsy-Balla, A.; Pestaner, J.P.; Bagasra, O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods 2010, 52, 316–321. [Google Scholar] [CrossRef]
- Györkey, F.; Min, K.W.; Huff, J.A.; Györkey, P. Zinc and magnesium in human prostate gland: Normal, hyperplastic, and neoplastic. Cancer Res. 1967, 27, 1348–1353. [Google Scholar]
- Ghosh, S.K.; Kim, P.; Zhang, X.A.; Yun, S.H.; Moore, A.; Lippard, S.J.; Medarova, Z. A novel imaging approach for early detection of prostate cancer based on endogenous zinc sensing. Cancer Res. 2010, 70, 6119–6127. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, L.; Li, J.; Li, Y.; Wang, H.L.; Ji, G.Y.; Kuwahara, M.; Zhao, X.J. Measurement of serum zinc improves prostate cancer detection efficiency in patients with PSA levels between 4 ng/mL and 10 ng/mL. Asian J. Androl. 2005, 7, 323–328. [Google Scholar] [CrossRef]
- Onyema-iloh, B.O.; Meludu, S.C.; Iloh, E.; Nnodim, J.; Onyegbule, O.; Mykembata, B. Biochemical changes in some trace elements, antioxidant vitamins and their therapeutic importance in prostate cancer patients. Asian J. Med. Sci. 2015, 6, 95–97. [Google Scholar] [CrossRef]
- Chen, G.; Wu, L.; Wu, Y.; Zhu, J. Content change of zinc and cadmium in serum of patients with prostate cancer and its clinical significance. J. Clin. Urol. 2015, 30, 439–441. [Google Scholar] [CrossRef]
- Feustel, A.; Wennrich, R.; Schmidt, B. Serum-Zn-levels in prostatic cancer. Urol. Res. 1989, 17, 41–42. [Google Scholar] [CrossRef]
- Park, S.Y.; Wilkens, L.R.; Morris, J.S.; Henderson, B.E.; Kolonel, L.N. Serum zinc and prostate cancer risk in a nested case-control study: The multiethnic cohort. Prostate 2013, 73, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, K.; Marciniak, W.; Muszynska, M.; Baszuk, P.; Gupta, S.; Jaworska-Bieniek, K.; Sukiennicki, G.; Durda, K.; Gromowski, T.; Prajzendanc, K.; et al. Association of zinc level and polymorphism in MMP-7 gene with prostate cancer in Polish population. PLoS ONE 2018, 13, e0201065. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Johnes, A.; Fragmann, C. The content of serum zinc concentration in prostate disease. Int. J. Surg. 1977, 4, 225–227. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Sui, G. YY1 Is an Inducer of Cancer Metastasis. Crit. Rev. Oncog. 2017, 22, 1–11. [Google Scholar] [CrossRef]
- Liang, J.Y.; Liu, Y.Y.; Zou, J.; Franklin, R.B.; Costello, L.C.; Feng, P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate 1999, 40, 200–207. [Google Scholar] [CrossRef]
- Yan, M.; Hardin, K.; Ho, E. Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J. Nutr. Biochem. 2010, 21, 687–694. [Google Scholar] [CrossRef][Green Version]
- Banudevi, S.; Senthilkumar, K.; Sharmila, G.; Arunkumar, R.; Vijayababu, M.R.; Arunakaran, J. Effect of zinc on regulation of insulin-like growth factor signaling in human androgen-independent prostate cancer cells. Clin. Chim. Acta 2010, 411, 172–178. [Google Scholar] [CrossRef]
- Uzzo, R.G.; Crispen, P.L.; Golovine, K.; Makhov, P.; Horwitz, E.M.; Kolenko, V.M. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: Implications for prostate cancer progression. Carcinogenesis 2006, 27, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.F.; Abubakar, S. High intracellular Zn2+ ions modulate the VHR, ZAP-70 and ERK activities of LNCaP prostate cancer cells. Cell. Mol. Biol. Lett. 2008, 13, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Han, C.T.; Schoene, N.W.; Lei, K.Y. Influence of zinc deficiency on Akt-Mdm2-p53 and Akt-p21 signaling axes in normal and malignant human prostate cells. Am. J. Physiol. Cell Physiol. 2009, 297, C1188–C1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Tang, X.; Guo, R.; Li, J.; Chen, Y.Y.; Guo, H.; Su, J.; Sun, L.; Liu, Y. Zinc enhances chemosensitivity to paclitaxel in PC3 prostate cancer cells. Oncol. Rep. 2018, 40, 2269–2277. [Google Scholar] [CrossRef]
- To, P.K.; Do, M.H.; Cho, Y.S.; Kwon, S.Y.; Kim, M.S.; Jung, C. Zinc Inhibits Expression of Androgen Receptor to Suppress Growth of Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3062. [Google Scholar] [CrossRef]
- Hacioglu, C.; Kacar, S.; Kar, F.; Kanbak, G.; Sahinturk, V. Concentration-Dependent Effects of Zinc Sulfate on DU-145 Human Prostate Cancer Cell Line: Oxidative, Apoptotic, Inflammatory, and Morphological Analyzes. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Wong, P.F.; Abubakar, S. Comparative transcriptional study of the effects of high intracellular zinc on prostate carcinoma cells. Oncol. Rep. 2010, 23, 1501–1516. [Google Scholar] [CrossRef]
- Tikkanen, R.; Nikolic-Paterson, D.J. Mitogen-Activated Protein Kinases: Functions in Signal Transduction and Human Diseases. Int. J. Mol. Sci. 2019, 20, 4844. [Google Scholar] [CrossRef]
- Meister, M.; Tomasovic, A.; Banning, A.; Tikkanen, R. Mitogen-Activated Protein (MAP) Kinase Scaffolding Proteins: A Recount. Int. J. Mol. Sci. 2013, 14, 4854–4884. [Google Scholar] [CrossRef]
- Banudevi, S.; Elumalai, P.; Arunkumar, R.; Senthilkumar, K.; Gunadharini, D.N.; Sharmila, G.; Arunakaran, J. Chemopreventive effects of zinc on prostate carcinogenesis induced by N-methyl-N-nitrosourea and testosterone in adult male Sprague-Dawley rats. J. Cancer Res. Clin. Oncol. 2011, 137, 677–686. [Google Scholar] [CrossRef]
- Shah, M.R.; Kriedt, C.L.; Lents, N.H.; Hoyer, M.K.; Jamaluddin, N.; Klein, C.; Baldassare, J. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J. Exp. Clin. Cancer Res. 2009, 28, 84. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Mukhtar, H.; Beck, F.W.; Adhami, V.M.; Siddiqui, I.A.; Din, M.; Hafeez, B.B.; Kucuk, O. Dietary zinc and prostate cancer in the TRAMP mouse model. J. Med. Food 2010, 13, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, B.; Liu, Y.; Zhong, L.; Tang, Y.; Guo, H.; Jiang, T.; Wang, L.; Li, Y.; Cai, L. Murine double minute 2 siRNA and wild-type p53 gene therapy interact positively with zinc on prostate tumours in vitro and in vivo. Eur. J. Cancer 2014, 50, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.Y.; Jing, R.; Smalley, K.J.; Wang, Z.X.; Taccioli, C.; Fan, S.; Chen, H.; Alder, H.; Huebner, K.; Farber, J.L.; et al. Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats. Proc. Natl. Acad. Sci. USA 2018, 115, E11091–E11100. [Google Scholar] [CrossRef]
- Song, Y.; Ho, E. Zinc and prostatic cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 640–645. [Google Scholar] [CrossRef]
- Feng, P.; Li, T.L.; Guan, Z.X.; Franklin, R.B.; Costello, L.C. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate 2002, 52, 311–318. [Google Scholar] [CrossRef]
- Feng, P.; Li, T.; Guan, Z.; Franklin, R.B.; Costello, L.C. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol. Cancer 2008, 7, 25. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, Y.S.; Cho, H.J.; Lee, J.Y.; Kim, J.C.; Hwang, T.K.; Kim, S.W. Antiproliferative effects of zinc-citrate compound on hormone refractory prostate cancer. Chin. J. Cancer Res. 2012, 24, 124–129. [Google Scholar] [CrossRef][Green Version]
- Feng, P.; Li, T.L.; Guan, Z.X.; Franklin, R.B.; Costello, L.C. Effect of zinc on prostatic tumorigenicity in nude mice. Ann. N. Y. Acad. Sci. 2003, 1010, 316–320. [Google Scholar] [CrossRef]
- Ku, J.H.; Seo, S.Y.; Kwak, C.; Kim, H.H. The role of survivin and Bcl-2 in zinc-induced apoptosis in prostate cancer cells. Urol. Oncol. 2012, 30, 562–568. [Google Scholar] [CrossRef]
- Yang, N.; Zhao, B.; Rasul, A.; Qin, H.; Li, J.; Li, X. PIAS1-modulated Smad2/4 complex activation is involved in zinc-induced cancer cell apoptosis. Cell Death Dis. 2013, 4, e811. [Google Scholar] [CrossRef] [PubMed]
- Carraway, R.E.; Dobner, P.R. Zinc pyrithione induces ERK- and PKC-dependent necrosis distinct from TPEN-induced apoptosis in prostate cancer cells. Biochim. Biophys. Acta 2012, 1823, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. The Coming Decade of Cell Death Research: Five Riddles. Cell 2019, 177, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; Conner, C.M.; Rosenblatt, J.; Montell, D.J. Unconventional Ways to Live and Die: Cell Death and Survival in Development, Homeostasis, and Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 311–332. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.R.; Dong, L.; Xu, M.R.; Zhang, L.; Ding, W.P.; Zhang, J.Q.; Lin, J.; Zhang, Y.J.; Qiu, B.S.; et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 2019, 11, 11789–11807. [Google Scholar] [CrossRef]
- Kang, W.; Hong, S.H.; Lee, H.M.; Kim, N.Y.; Lim, Y.C.; Le le, T.M.; Lim, B.; Kim, H.C.; Kim, T.Y.; Ashida, H.; et al. Structural and biochemical basis for the inhibition of cell death by APIP, a methionine salvage enzyme. Proc. Natl. Acad. Sci. USA 2014, 111, E54–E61. [Google Scholar] [CrossRef]
- Palmer, L.D.; Jordan, A.T.; Maloney, K.N.; Farrow, M.A.; Gutierrez, D.B.; Gant-Branum, R.; Burns, W.J.; Romer, C.E.; Tsui, T.; Allen, J.L.; et al. Zinc intoxication induces ferroptosis in A549 human lung cells. Metallomics 2019, 11, 982–993. [Google Scholar] [CrossRef]
- Jensen, A.R.; Adams, Y.; Hviid, L. Cerebral Plasmodium falciparum malaria: The role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it. Immunol. Rev. 2019. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhai, C.; Qiu, W.; Li, D.; Yi, Z.; Wang, L.; Tang, J.; Qian, M.; Luo, J.; et al. Maslinic acid potentiates the anti-tumor activity of tumor necrosis factor alpha by inhibiting NF-kappaB signaling pathway. Mol. Cancer 2010, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Xu, W. The structure and main functions of aminopeptidase N. Curr. Med. Chem. 2007, 14, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Adhikari, N.; Jha, T. Design of Aminopeptidase N Inhibitors as Anti-cancer Agents. J. Med. Chem. 2018, 61, 6468–6490. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Usui, S.; Sugimura, Y.; Yoshida, S.; Hioki, T.; Tatematsu, M.; Yamamoto, H.; Hirano, K. Aminopeptidase N regulated by zinc in human prostate participates in tumor cell invasion. Int. J. Cancer 2001, 92, 49–54. [Google Scholar] [CrossRef]
- Ishii, K.; Otsuka, T.; Iguchi, K.; Usui, S.; Yamamoto, H.; Sugimura, Y.; Yoshikawa, K.; Hayward, S.W.; Hirano, K. Evidence that the prostate-specific antigen (PSA)/Zn2+ axis may play a role in human prostate cancer cell invasion. Cancer Lett. 2004, 207, 79–87. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Golovine, K.; Uzzo, R.G.; Makhov, P.; Crispen, P.L.; Kunkle, D.; Kolenko, V.M. Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL-6 and IL-8 in prostate cancer cells via NF-kappaB-dependent pathway. Prostate 2008, 68, 1443–1449. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Pantisano, V.; Puca, R.; Porru, M.; Aiello, A.; Grasselli, A.; Leonetti, C.; Safran, M.; Rechavi, G.; Givol, D.; et al. Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS ONE 2010, 5, e15048. [Google Scholar] [CrossRef]
- Brahimi-Horn, C.; Berra, E.; Pouysségur, J. Hypoxia: The tumor’s gateway to progression along the angiogenic pathway. Trends Cell Biol. 2001, 11, S32–S36. [Google Scholar] [CrossRef]
- Kerkar, S.P.; Restifo, N.P. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012, 72, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichon, T.; Kulach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ok Lee, S.; Liang, L.; Huang, C.K.; Li, L.; Wen, S.; Chang, C. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene 2014, 33, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Mertens, C.; Meier, J.K.; Fuhrmann, D.C.; Brune, B.; Jung, M. Strategies to Interfere with Tumor Metabolism through the Interplay of Innate and Adaptive Immunity. Cells 2019, 8, 445. [Google Scholar] [CrossRef]
- Meng, W.; Hao, Y.; He, C.; Li, L.; Zhu, G. Exosome-orchestrated hypoxic tumor microenvironment. Mol. Cancer 2019, 18, 57. [Google Scholar] [CrossRef]
- Talbot, L.J.; Bhattacharya, S.D.; Kuo, P.C. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int. J. Biochem. Mol. Biol. 2012, 3, 117–136. [Google Scholar]
- Bao, B.; Thakur, A.; Li, Y.; Ahmad, A.; Azmi, A.S.; Banerjee, S.; Kong, D.; Ali, S.; Lum, L.G.; Sarkar, F.H. The immunological contribution of NF-kappaB within the tumor microenvironment: A potential protective role of zinc as an anti-tumor agent. Biochim. Biophys. Acta 2012, 1825, 160–172. [Google Scholar] [CrossRef]
- Ferrandino, F.; Grazioli, P.; Bellavia, D.; Campese, A.F.; Screpanti, I.; Felli, M.P. Notch and NF-kappaB: Coach and Players of Regulatory T-Cell Response in Cancer. Front. Immunol. 2018, 9, 2165. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Bao, G.W.; Singh, T.; Ali, S.; Sarkar, F.H. Intracellular free zinc up-regulates IFN-gamma and T-bet essential for Th1 differentiation in Con-A stimulated HUT-78 cells. Biochem. Biophys. Res. Commun. 2011, 407, 703–707. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Grabowski, S.M.; Kaplan, J.; Mathog, R.H. Zinc deficiency: Changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc. Assoc. Am. Physicians 1997, 109, 68–77. [Google Scholar] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of Zinc-Exporters Expression in Prostate Cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef]
- Desouki, M.M.; Geradts, J.; Milon, B.; Franklin, R.B.; Costello, L.C. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer 2007, 6, 37. [Google Scholar] [CrossRef]
- Chen, Q.G.; Zhang, Z.; Yang, Q.; Shan, G.Y.; Yu, X.Y.; Kong, C.Z. The role of zinc transporter ZIP4 in prostate carcinoma. Urol. Oncol. 2012, 30, 906–911. [Google Scholar] [CrossRef]
- Franklin, R.B.; Ma, J.; Zou, J.; Guan, Z.; Kukoyi, B.I.; Feng, P.; Costello, L.C. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J. Inorg. Biochem. 2003, 96, 435–442. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.P.; Zhang, Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: A possible role in prostate cancer progression. Cancer Cell Int. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Golovine, K.; Makhov, P.; Uzzo, R.G.; Shaw, T.; Kunkle, D.; Kolenko, V.M. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin. Cancer Res. 2008, 14, 5376–5384. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Milon, B.C.; Desouki, M.M.; Costello, L.C.; Franklin, R.B. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1). Prostate 2011, 71, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Mihelich, B.L.; Khramtsova, E.A.; Arva, N.; Vaishnav, A.; Johnson, D.N.; Giangreco, A.A.; Martens-Uzunova, E.; Bagasra, O.; Kajdacsy-Balla, A.; Nonn, L. miR-183-96-182 cluster is overexpressed in prostate tissue and regulates zinc homeostasis in prostate cells. J. Biol. Chem. 2011, 286, 44503–44511. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Findley, S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [CrossRef]
- Hasumi, M.; Suzuki, K.; Matsui, H.; Koike, H.; Ito, K.; Yamanaka, H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003, 200, 187–195. [Google Scholar] [CrossRef]
- Beck, F.W.; Prasad, A.S.; Butler, C.E.; Sakr, W.A.; Kucuk, O.; Sarkar, F.H. Differential expression of hZnT-4 in human prostate tissues. Prostate 2004, 58, 374–381. [Google Scholar] [CrossRef]
- Henshall, S.M.; Afar, D.E.; Rasiah, K.K.; Horvath, L.G.; Gish, K.; Caras, I.; Ramakrishnan, V.; Wong, M.; Jeffry, U.; Kench, J.G.; et al. Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 2003, 22, 6005–6012. [Google Scholar] [CrossRef]
- Tepaamorndech, S.; Huang, L.; Kirschke, C.P. A null-mutation in the Znt7 gene accelerates prostate tumor formation in a transgenic adenocarcinoma mouse prostate model. Cancer Lett. 2011, 308, 33–42. [Google Scholar] [CrossRef]
- Baumgart, S.J.; Nevedomskaya, E.; Haendler, B. Dysregulated Transcriptional Control in Prostate Cancer. Int. J. Mol. Sci. 2019. [Google Scholar] [CrossRef]
- Ulz, P.; Perakis, S.; Zhou, Q.; Moser, T.; Belic, J.; Lazzeri, I.; Wolfler, A.; Zebisch, A.; Gerger, A.; Pristauz, G.; et al. Inference of transcription factor binding from cell-free DNA enables tumor subtype prediction and early detection. Nat. Commun. 2019, 10, 4666. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.I.; Young, R.A. Transcriptional regulation and its misregulation in disease. Cell 2013, 152, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Mackeh, R.; Marr, A.K.; Fadda, A.; Kino, T. C2H2-Type Zinc Finger Proteins: Evolutionarily Old and New Partners of the Nuclear Hormone Receptors. Nucl. Recept. Signal. 2018, 15, 1550762918801071. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lim, C. Factors controlling the reactivity of zinc finger cores. J. Am. Chem. Soc. 2011, 133, 8691–8703. [Google Scholar] [CrossRef]
- Klug, A. The discovery of zinc fingers and their development for practical applications in gene regulation and genome manipulation. Q. Rev. Biophys. 2010, 43, 1–21. [Google Scholar] [CrossRef]
- Iuchi, S. Three classes of C2H2 zinc finger proteins. Cell. Mol. Life Sci. 2001, 58, 625–635. [Google Scholar] [CrossRef]
- Collins, T.; Stone, J.R.; Williams, A.J. All in the family: The BTB/POZ, KRAB, and SCAN domains. Mol. Cell. Biol. 2001, 21, 3609–3615. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Mackenzie, G.G. Zinc, oxidant-triggered cell signaling, and human health. Mol. Asp. Med. 2005, 26, 245–255. [Google Scholar] [CrossRef]
- Kroncke, K.D. Zinc finger proteins as molecular targets for nitric oxide-mediated gene regulation. Antioxid. Redox Signal. 2001, 3, 565–575. [Google Scholar] [CrossRef]
- Orlov, A.P.; Orlova, M.A.; Trofimova, T.P.; Kalmykov, S.N.; Kuznetsov, D.A. The role of zinc and its compounds in leukemia. J. Biol. Inorg. Chem. 2018, 23, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, B.; Lehvaslaiho, H.; Beitel, L.K.; Lumbroso, R.; Pinsky, L.; Trifiro, M. The Androgen Receptor Gene Mutations Database. Nucleic Acids Res. 1998, 26, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, P.L.; Jivan, A.; Dollins, D.E.; Claessens, F.; Gewirth, D.T. Structural basis of androgen receptor binding to selective androgen response elements. Proc. Natl. Acad. Sci. USA 2004, 101, 4758–4763. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Sadi, M.V.; Walsh, P.C.; Barrack, E.R. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer 1991, 67, 3057–3064. [Google Scholar] [CrossRef]
- Chodak, G.W.; Kranc, D.M.; Puy, L.A.; Takeda, H.; Johnson, K.; Chang, C. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J. Urol. 1992, 147, 798–803. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef]
- Cao, S.; Zhan, Y.; Dong, Y. Emerging data on androgen receptor splice variants in prostate cancer. Endocr. Relat. Cancer 2016, 23, T199–T210. [Google Scholar] [CrossRef]
- Elshan, N.; Rettig, M.B.; Jung, M.E. Molecules targeting the androgen receptor (AR) signaling axis beyond the AR-Ligand binding domain. Med. Res. Rev. 2019, 39, 910–960. [Google Scholar] [CrossRef]
- Azoitei, A.; Merseburger, A.S.; Godau, B.; Hoda, M.R.; Schmid, E.; Cronauer, M.V. C-terminally truncated constitutively active androgen receptor variants and their biologic and clinical significance in castration-resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2017, 166, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; Foye, A.; Kothari, V.; Perry, M.D.; Bailey, A.M.; et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell 2018, 174, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, A.; Sharp, A.; Welti, J.C.; Neeb, A.; Raj, G.V.; Luo, J.; Plymate, S.R.; de Bono, J.S. Alternative splicing in prostate cancer. Nat. Rev. Clin. Oncol. 2018, 15, 663–675. [Google Scholar] [CrossRef]
- Lee, D.K.; Chang, C. Endocrine mechanisms of disease: Expression and degradation of androgen receptor: Mechanism and clinical implication. J. Clin. Endocrinol. Metab. 2003, 88, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bravo, V.; Pippa, R.; Song, W.M.; Carceles-Cordon, M.; Dominguez-Andres, A.; Fujiwara, N.; Woo, J.; Koh, A.P.; Ertel, A.; Lokareddy, R.K.; et al. Nuclear Pores Promote Lethal Prostate Cancer by Increasing POM121-Driven E2F1, MYC, and AR Nuclear Import. Cell 2018, 174, 1200–1215. [Google Scholar] [CrossRef] [PubMed]
- Mowszowicz, I.; Lee, H.J.; Chen, H.T.; Mestayer, C.; Portois, M.C.; Cabrol, S.; Mauvais-Jarvis, P.; Chang, C. A point mutation in the second zinc finger of the DNA-binding domain of the androgen receptor gene causes complete androgen insensitivity in two siblings with receptor-positive androgen resistance. Mol. Endocrinol. 1993, 7, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Sankpal, U.T.; Goodison, S.; Abdelrahim, M.; Basha, R. Targeting Sp1 transcription factors in prostate cancer therapy. Med. Chem. 2011, 7, 518–525. [Google Scholar] [CrossRef]
- Berg, J.M. Sp1 and the subfamily of zinc finger proteins with guanine-rich binding sites. Proc. Natl. Acad. Sci. USA 1992, 89, 11109–11110. [Google Scholar] [CrossRef]
- Kaczynski, J.; Cook, T.; Urrutia, R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003, 4, 206. [Google Scholar] [CrossRef]
- Deng, X.; Shao, G.; Zhang, H.T.; Li, C.; Zhang, D.; Cheng, L.; Elzey, B.D.; Pili, R.; Ratliff, T.L.; Huang, J.; et al. Protein arginine methyltransferase 5 functions as an epigenetic activator of the androgen receptor to promote prostate cancer cell growth. Oncogene 2017, 36, 1223–1231. [Google Scholar] [CrossRef]
- Shin, T.; Sumiyoshi, H.; Matsuo, N.; Satoh, F.; Nomura, Y.; Mimata, H.; Yoshioka, H. Sp1 and Sp3 transcription factors upregulate the proximal promoter of the human prostate-specific antigen gene in prostate cancer cells. Arch. Biochem. Biophys. 2005, 435, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Faber, P.W.; van Rooij, H.C.; van der Korput, H.A.; Baarends, W.M.; Brinkmann, A.O.; Grootegoed, J.A.; Trapman, J. Characterization of the human androgen receptor transcription unit. J. Biol. Chem. 1991, 266, 10743–10749. [Google Scholar] [PubMed]
- Barna, M.; Merghoub, T.; Costoya, J.A.; Ruggero, D.; Branford, M.; Bergia, A.; Samori, B.; Pandolfi, P.P. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev. Cell 2002, 3, 499–510. [Google Scholar] [CrossRef]
- Ono, R.; Masuya, M.; Nakajima, H.; Enomoto, Y.; Miyata, E.; Nakamura, A.; Ishii, S.; Suzuki, K.; Shibata-Minoshima, F.; Katayama, N.; et al. Plzf drives MLL-fusion-mediated leukemogenesis specifically in long-term hematopoietic stem cells. Blood 2013, 122, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; David, G.; Wong, C.W.; Dejean, A.; Privalsky, M.L. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor alpha (RARalpha) and PLZF-RARalpha oncoproteins associated with acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 1997, 94, 9028–9033. [Google Scholar] [CrossRef]
- David, G.; Alland, L.; Hong, S.H.; Wong, C.W.; DePinho, R.A.; Dejean, A. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene 1998, 16, 2549–2556. [Google Scholar] [CrossRef]
- Jin, Y.; Nenseth, H.Z.; Saatcioglu, F. Role of PLZF as a tumor suppressor in prostate cancer. Oncotarget 2017, 8, 71317–71324. [Google Scholar] [CrossRef]
- Hoatlin, M.E.; Zhi, Y.; Ball, H.; Silvey, K.; Melnick, A.; Stone, S.; Arai, S.; Hawe, N.; Owen, G.; Zelent, A.; et al. A novel BTB/POZ transcriptional repressor protein interacts with the Fanconi anemia group C protein and PLZF. Blood 1999, 94, 3737–3747. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, S.; Zhou, W.; Li, J.; Liu, C.; Xuan, H.; Yan, J.; Zheng, L.; Zhou, L.; Yu, J.; et al. PLZF mediates the PTEN/AKT/FOXO3a signaling in suppression of prostate tumorigenesis. PLoS ONE 2013, 8, e77922. [Google Scholar] [CrossRef][Green Version]
- Xiao, G.Q.; Unger, P.; Yang, Q.; Kinoshita, Y.; Singh, K.; McMahon, L.; Nastiuk, K.; Sha, K.; Krolewski, J.; Burstein, D. Loss of PLZF expression in prostate cancer by immunohistochemistry correlates with tumor aggressiveness and metastasis. PLoS ONE 2015, 10, e0121318. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Botta, G.; Gao, S.; Li, T.; Van Allen, E.M.; Treacy, D.J.; Cai, C.; He, H.H.; Sweeney, C.J.; Brown, M.; et al. PLZF, a tumor suppressor genetically lost in metastatic castration-resistant prostate cancer, is a mediator of resistance to androgen deprivation therapy. Cancer Res. 2015, 75, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qu, S.; Tesikova, M.; Wang, L.; Kristian, A.; Maelandsmo, G.M.; Kong, H.; Zhang, T.; Jeronimo, C.; Teixeira, M.R.; et al. Molecular circuit involving KLK4 integrates androgen and mTOR signaling in prostate cancer. Proc. Natl. Acad. Sci. USA 2013, 110, E2572–E2581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, Z. Identification and characterization of PLZF as a prostatic androgen-responsive gene. Prostate 2004, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, T.; Kinugasa, Y.; Shiraishi, K.; Nanba, D.; Nakashiro, K.; Tanji, N.; Yokoyama, M.; Higashiyama, S. PLZF regulates Pbx1 transcription and Pbx1-HoxC8 complex leads to androgen-independent prostate cancer proliferation. Prostate 2006, 66, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Gallus, S.; Foschi, R.; Negri, E.; Talamini, R.; Franceschi, S.; Montella, M.; Ramazzotti, V.; Tavani, A.; Dal Maso, L.; La Vecchia, C. Dietary zinc and prostate cancer risk: A case-control study from Italy. Eur. Urol. 2007, 52, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef]
- Banudevi, S.; Elumalai, P.; Sharmila, G.; Arunkumar, R.; Senthilkumar, K.; Arunakaran, J. Protective effect of zinc on N-methyl-N-nitrosourea and testosterone-induced prostatic intraepithelial neoplasia in the dorsolateral prostate of Sprague Dawley rats. Exp. Biol. Med. 2011, 236, 1012–1021. [Google Scholar] [CrossRef]
- Fitzsimons, N.J.; Sun, L.; Moul, J.W. Medical technologies for the diagnosis of prostate cancer. Expert Rev. Med. Devices 2007, 4, 227–239. [Google Scholar] [CrossRef]
- Hoeks, C.M.; Hambrock, T.; Yakar, D.; Hulsbergen-van de Kaa, C.A.; Feuth, T.; Witjes, J.A.; Futterer, J.J.; Barentsz, J.O. Transition zone prostate cancer: Detection and localization with 3-T multiparametric MR imaging. Radiology 2013, 266, 207–217. [Google Scholar] [CrossRef]
- Costa, D.N.; Pedrosa, I.; Donato, F., Jr.; Roehrborn, C.G.; Rofsky, N.M. MR Imaging-Transrectal US Fusion for Targeted Prostate Biopsies: Implications for Diagnosis and Clinical Management. Radiographics 2015, 35, 696–708. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Prostatic fluid electrolyte composition for the screening of prostate cancer: A potential solution to a major problem. Prostate Cancer Prostatic Dis. 2009, 12, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cortesi, M.; Chechik, R.; Breskin, A.; Vartsky, D.; Ramon, J.; Raviv, G.; Volkov, A.; Fridman, E. Evaluating the cancer detection and grading potential of prostatic-zinc imaging: A simulation study. Phys. Med. Biol. 2009, 54, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lin, Z.H.; Yang, N.; Li, Y.; Su, X.G. A novel carboxymethyl chitosan-quantum dot-based intracellular probe for Zn2+ ion sensing in prostate cancer cells. Acta Biomater. 2014, 10, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wan, X.; Du, C.; Wang, H.; Zhou, J.; Wang, Z. A novel fluorescent probe for the early detection of prostate cancer based on endogenous zinc sensing. Prostate 2019, 79, 1378–1385. [Google Scholar] [CrossRef]
- Zhang, X.A.; Hayes, D.; Smith, S.J.; Friedle, S.; Lippard, S.J. New strategy for quantifying biological zinc by a modified zinpyr fluorescence sensor. J. Am. Chem. Soc. 2008, 130, 15788–15789. [Google Scholar] [CrossRef][Green Version]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Zhang, H.; Christensen, C.L.; Dries, R.; Oser, M.G.; Deng, J.; Diskin, B.; Li, F.; Pan, Y.; Zhang, X.; Yin, Y.; et al. CDK7 Inhibition Potentiates Genome Instability Triggering Anti-Tumor Immunity in Small Cell Lung Cancer. Cancer Cell 2019. [Google Scholar] [CrossRef]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- Bryant, G.; Wang, L.; Mulholland, D.J. Overcoming Oncogenic Mediated Tumor Immunity in Prostate Cancer. Int. J. Mol. Sci. 2017, 18, 1542. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447s–463s. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; O’Connor, K.S.; Suppiah, V.; Ahlenstiel, C.L.E.; Obeid, S.; Cook, K.M.; Cunningham, A.; Douglas, M.W.; Hogg, P.J.; Booth, D.; et al. Zinc is a potent and specific inhibitor of IFN-lambda3 signalling. Nat. Commun. 2017, 8, 15245. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Miyai, T.; Hojyo, S.; Ikawa, T.; Kawamura, M.; Irie, T.; Ogura, H.; Hijikata, A.; Bin, B.H.; Yasuda, T.; Kitamura, H.; et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl. Acad. Sci. USA 2014, 111, 11780–11785. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Morikawa, H.; Kamon, H.; Iguchi, M.; Hojyo, S.; Fukada, T.; Yamashita, S.; Kaisho, T.; Akira, S.; Murakami, M.; et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat. Immunol. 2006, 7, 971–977. [Google Scholar] [CrossRef]
- Yu, M.; Lee, W.W.; Tomar, D.; Pryshchep, S.; Czesnikiewicz-Guzik, M.; Lamar, D.L.; Li, G.; Singh, K.; Tian, L.; Weyand, C.M.; et al. Regulation of T cell receptor signaling by activation-induced zinc influx. J. Exp. Med. 2011, 208, 775–785. [Google Scholar] [CrossRef]
- Anzilotti, C.; Swan, D.J.; Boisson, B.; Deobagkar-Lele, M.; Oliveira, C.; Chabosseau, P.; Engelhardt, K.R.; Xu, X.; Chen, R.; Alvarez, L.; et al. An essential role for the Zn(2+) transporter ZIP7 in B cell development. Nat. Immunol. 2019, 20, 350–361. [Google Scholar] [CrossRef]
- Hojyo, S.; Miyai, T.; Fujishiro, H.; Kawamura, M.; Yasuda, T.; Hijikata, A.; Bin, B.H.; Irie, T.; Tanaka, J.; Atsumi, T.; et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl. Acad. Sci. USA 2014, 111, 11786–11791. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef]
- Rosenkranz, E.; Hilgers, R.D.; Uciechowski, P.; Petersen, A.; Plumakers, B.; Rink, L. Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. Eur. J. Nutr. 2017, 56, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.B.; Dao, M.C.; Hamer, D.H.; Kandel, R.; Brandeis, G.; Wu, D.; Dallal, G.E.; Jacques, P.F.; Schreiber, R.; Kong, E.; et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2016, 103, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Kucuk, O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, A.K.; Sharp, A.; Ellis, L.; Jones, J.; Kaochar, S.; Larman, H.B.; Quigley, D.A.; Ye, H.; Simons, J.W.; Pienta, K.J.; et al. Prostate cancer research: The next generation; report from the 2019 Coffey-Holden Prostate Cancer Academy Meeting. Prostate 2020, 80, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Yunger, S.; Bar El, A.; Zeltzer, L.A.; Fridman, E.; Raviv, G.; Laufer, M.; Schachter, J.; Markel, G.; Itzhaki, O.; Besser, M.J. Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy. Oncoimmunology 2019, 8, e1672494. [Google Scholar] [CrossRef] [PubMed]
| PCa/Control (Serum Zinc Level, µg/dL) | PCa/Control (Number) | Testing Assay | Cohort Sources/Populations | Age Medians or Ranges (PCa/Control) | References |
|---|---|---|---|---|---|
| 61.60 ± 19.75/ 99.59 ± 29.23 | 220/220 | AAS | Nigeria | 69.73 ± 6.32/ 68.97 ± 5.76 | Wakwe et al. 2019 [30] |
| 576 ± 102/ 711 ± 164 | 25/24 | AAS | Turkey | 67.5 ± 8.8/ 65.0 ± 6.0 | Aydin et al. 2006 [31] |
| 63.40 ± 6.40/ 86.50 ± 15.20 | 18/20 | AAS | India | 55−85/ 30−50 | Christudoss et al. 2011 [32] |
| 4.66 ± 2.22/ 19.26 ± 3.26 | 30/32 | AAS | Turkey | 65.4 ± 4.2/ 62.8 ± 5.8 | Kaba et al. 2014 [34] |
| 8300 ± 213/ 9780 ± 257 | 42/101 | AAS | China | 70.1 ± 1.32/ 67.8 ± 0.85 | Li et al. 2005 [59] |
| 147.75 ± 42.05/ 168.78 ± 59.80 | 50/50 | AAS | Nigeria | 50−70/ 50−70 | Onyema-iloh et al. 2015 [60] |
| 63.92 ± 19.10/ 103.61 ± 32.43 | 85/90 | AAS | China | 64.7 ± 9.2/ 65.9 ± 8.4 | Chen et al. 2015 [61] |
| 91.55 ± 12.42/ 90.89 ± 12.42 | 50/10 | AAS | German | 68.6/65.9 | Feustel et al. 1989 [62] |
| 94.09 ± 20.40/ 93.9 ± 17.60 | 392/783 | AAS | America | 69.1 ± 7.1/ 68.9 ± 7.2 | Park et al. 2013 [63] |
| 89.89 ± 1.20/ 85.66 ± 1.31 | 197/197 | AAS | Poland | 72/72 | Białkowska et al. 2018 [64] |
| 112.93 ± 18.10/ 98.12 ± 8.24 | 40/28 | AAS | China | ND/ND | Yao et al. 1977 [65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Stovall, D.B.; Wang, W.; Sui, G. Advances of Zinc Signaling Studies in Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 667. https://doi.org/10.3390/ijms21020667
Li D, Stovall DB, Wang W, Sui G. Advances of Zinc Signaling Studies in Prostate Cancer. International Journal of Molecular Sciences. 2020; 21(2):667. https://doi.org/10.3390/ijms21020667
Chicago/Turabian StyleLi, Dangdang, Daniel B. Stovall, Wenmeng Wang, and Guangchao Sui. 2020. "Advances of Zinc Signaling Studies in Prostate Cancer" International Journal of Molecular Sciences 21, no. 2: 667. https://doi.org/10.3390/ijms21020667
APA StyleLi, D., Stovall, D. B., Wang, W., & Sui, G. (2020). Advances of Zinc Signaling Studies in Prostate Cancer. International Journal of Molecular Sciences, 21(2), 667. https://doi.org/10.3390/ijms21020667




