The Effects of TiO2 Nanoparticles on Cisplatin Cytotoxicity in Cancer Cell Lines
Abstract
1. Introduction
2. Results
2.1. IC50 of Cisplatin in HepG2 and A431 Cells
2.2. The Effect of TiO2 PEG NPs on Cisplatin Cytotoxicity in HepG2 and A431 Cell Lines
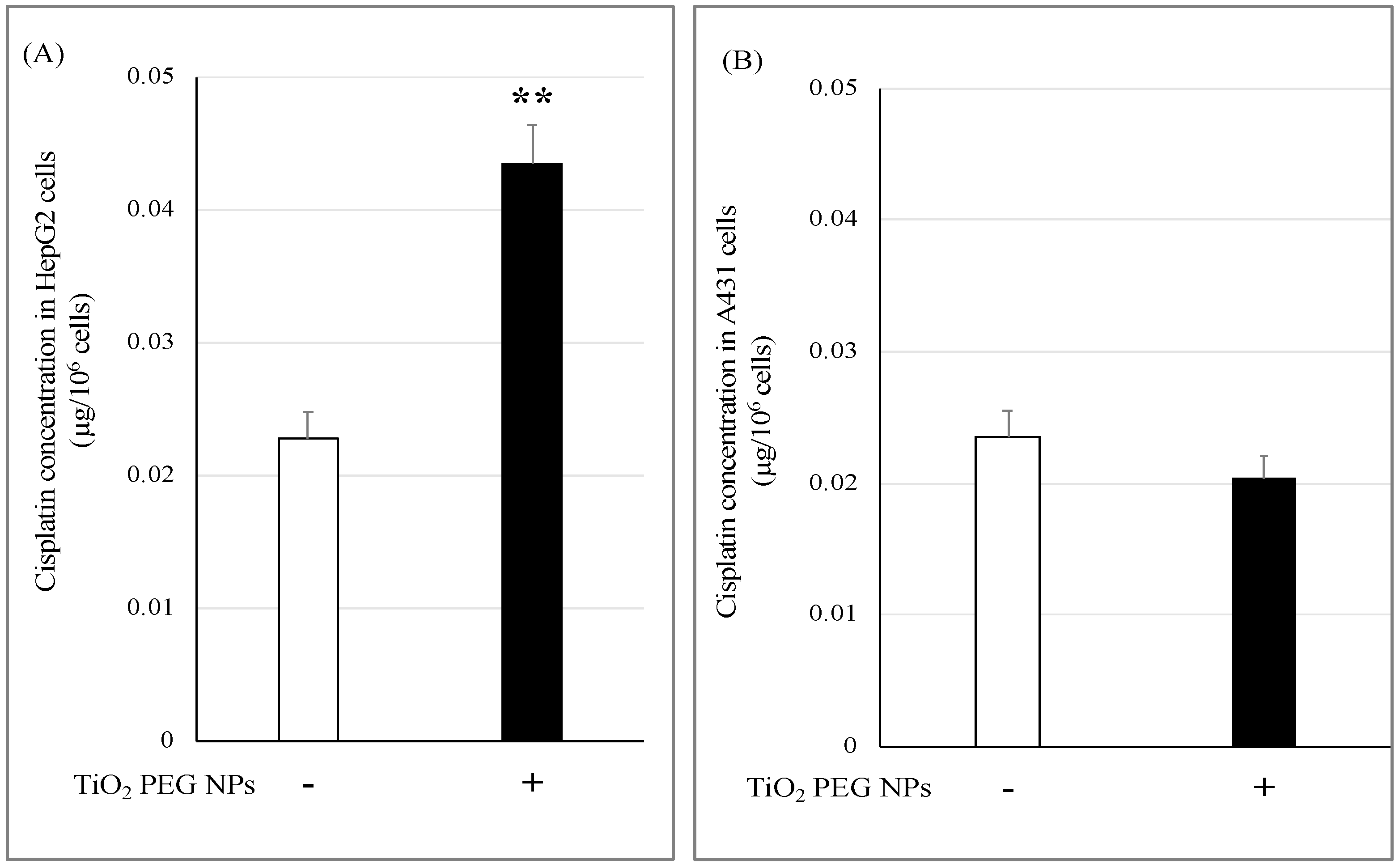
2.3. TiO2 PEG NPs Enhance Cisplatin Uptake by HepG2 Cells
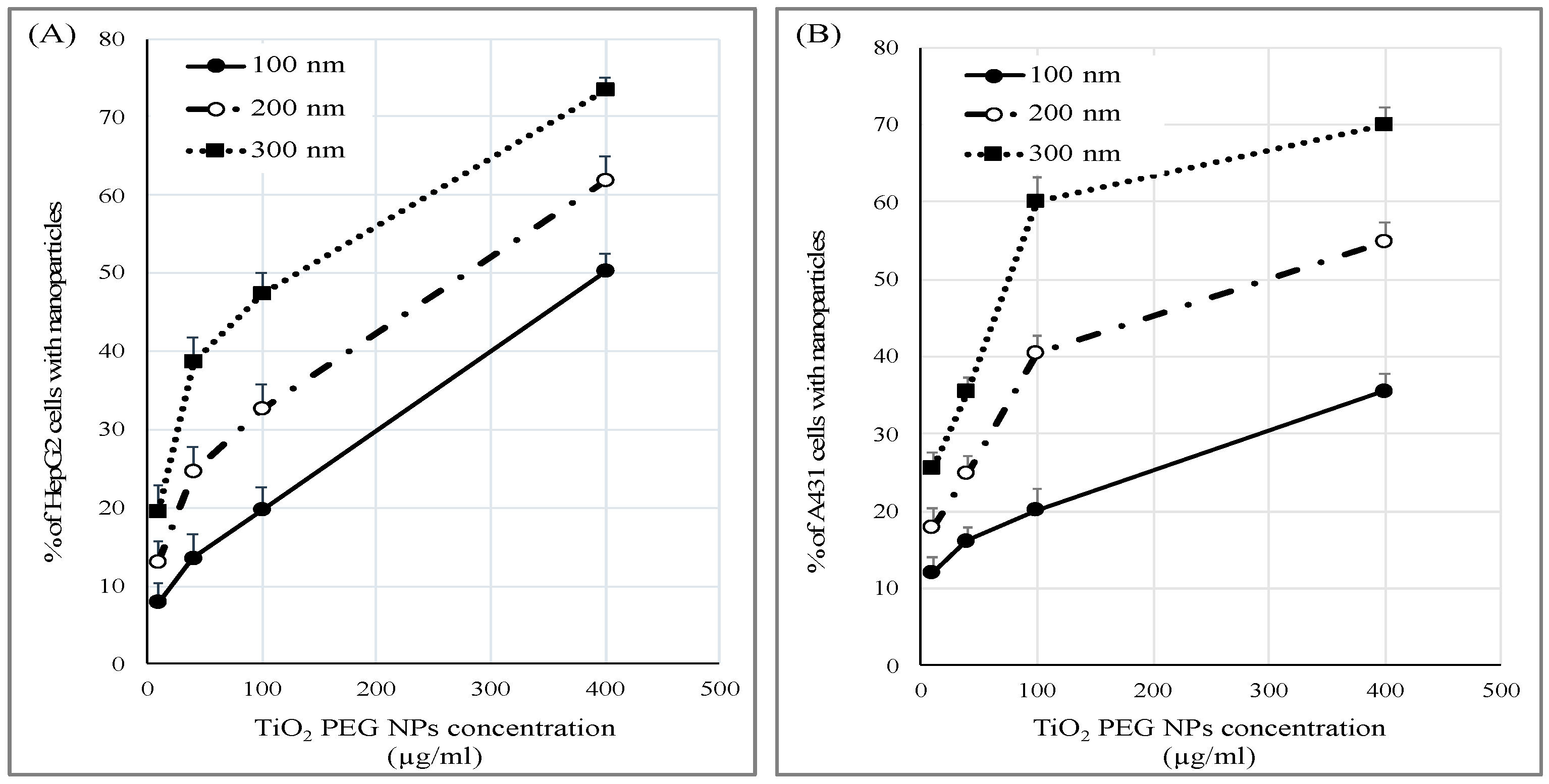
2.4. Cellular Uptake of TiO2 PEG NPs
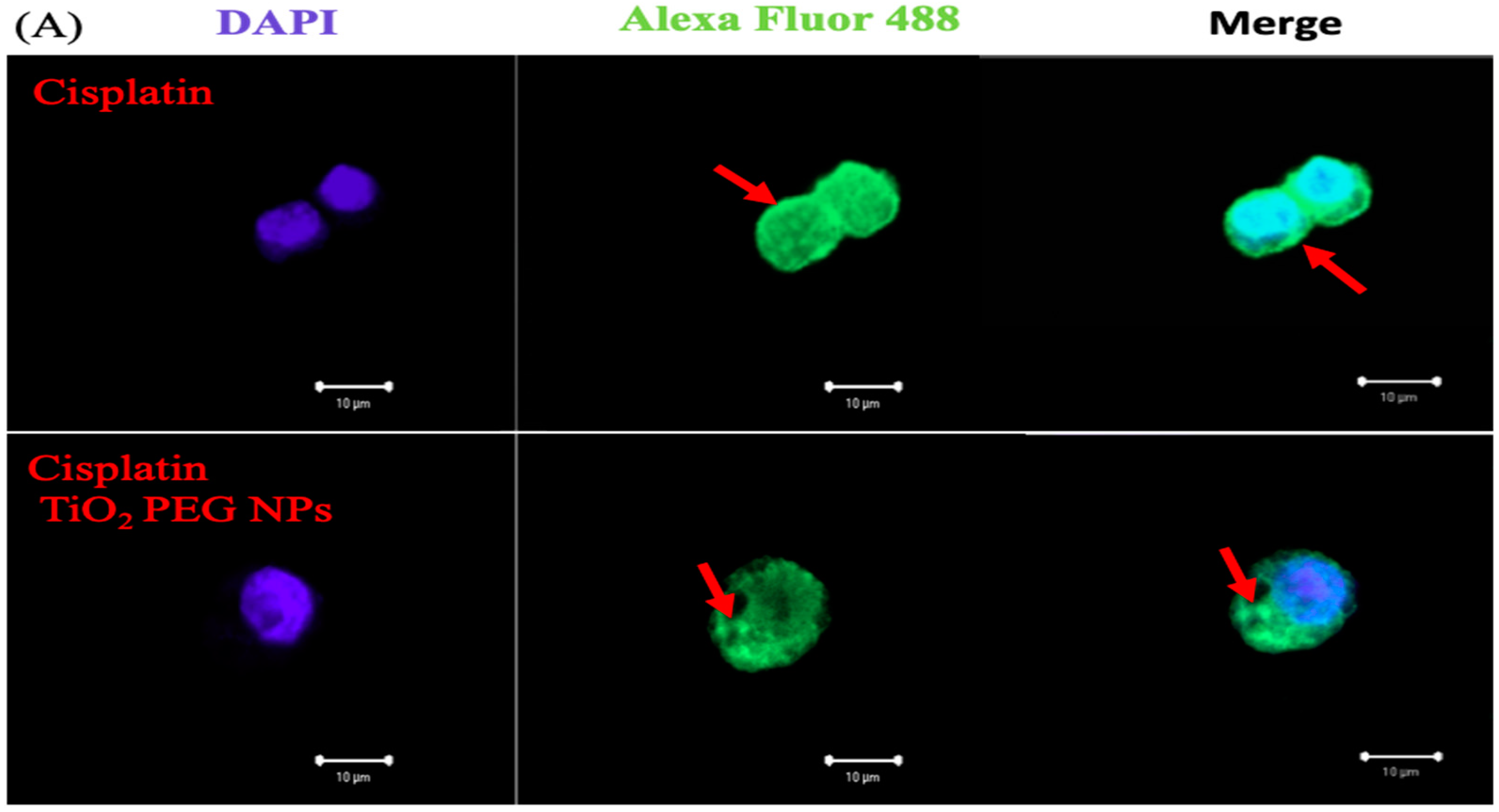
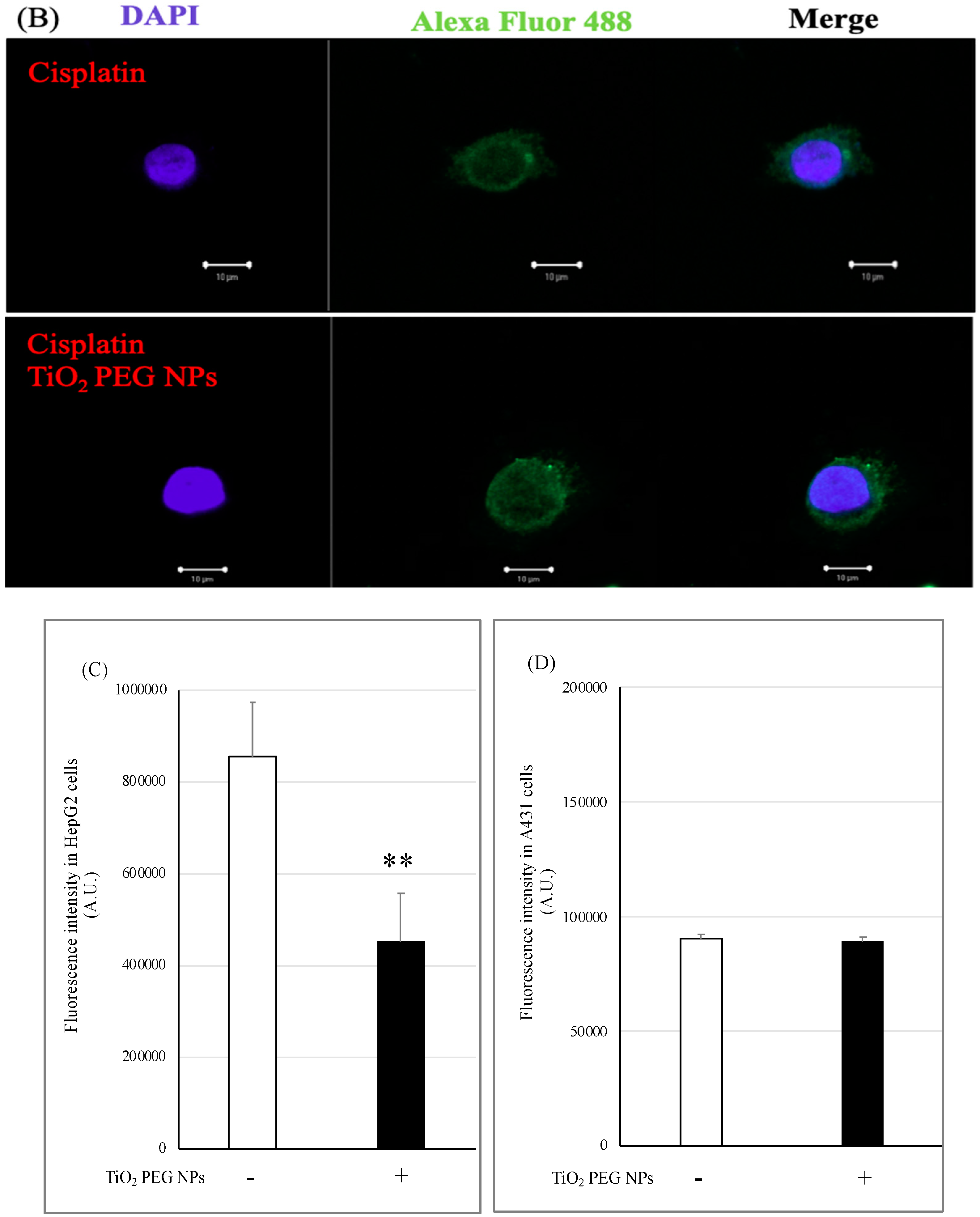
2.5. Localization of P-gp by Immunofluorescence Staining
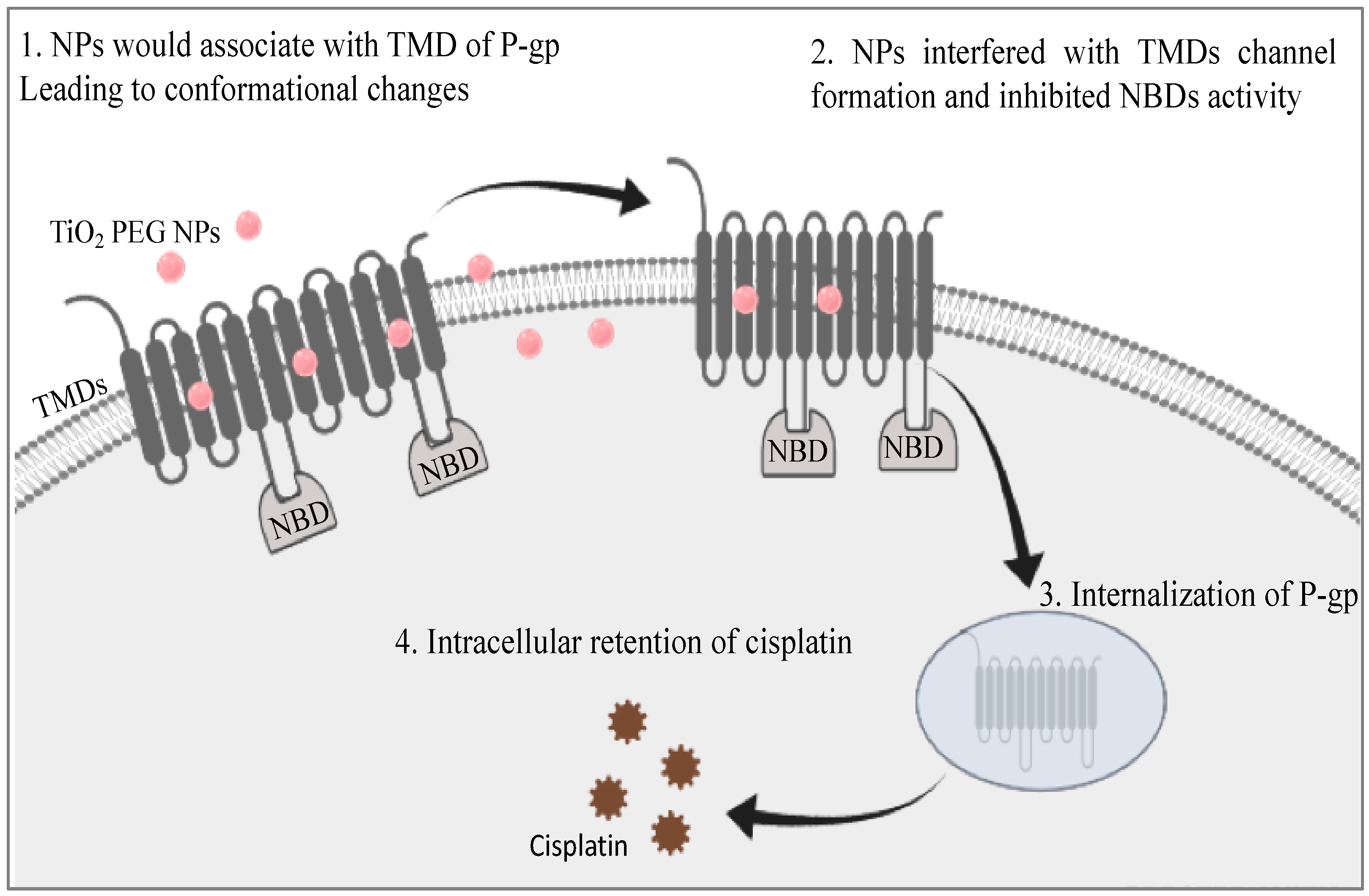
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Preparation of Nanoparticles
4.3. Preparation of Cisplatin
4.4. Cell Viability/Cytotoxicity Assay
4.5. Evaluation of Cellular Cisplatin Uptake by ICP-MS Analysis
4.6. Evaluation of Cellular Uptake of TiO2 PEG NPs by Flow Cytometry
4.7. Immunofluorescence Staining of P-gp
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDR | Multi-drug resistance |
| TiO2 PEG NPs | polyethylene glycol-modified titanium dioxide nanoparticles |
| P-gp | P-glycoprotein |
| TMDs | Trans-membrane domains |
| NBDs | Nucleotide-binding domains |
| HGFRs | Hepatocyte growth factor receptors |
| EGFRs | Epidermal growth factor receptors |
| TNFR1 | Tumor necrosis factor receptor 1 |
References
- Hall, M.D.; Okabe, M.; Shen, D.W.; Liang, X.J.; Gottesman, M.M. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 495–535. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van, S.S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [Google Scholar] [PubMed]
- Ward, A.B.; Szewczyk, P.; Grimard, V.; Lee, C.W.; Martinez, L.; Doshi, R.; Caya, A.; Villaluz, M.; Pardon, E.; Cregger, C.; et al. Structures of P- glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc. Natl. Acad. Sci. USA 2013, 110, 13386–13391. [Google Scholar] [CrossRef]
- Ward, A.; Reyes, C.L.; Yu, J.; Roth, C.B.; Chang, G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc. Natl. Acad. Sci. USA 2007, 104, 19005–19010. [Google Scholar] [CrossRef]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Drug binding in human P-glycoprotein causes conformational changes in both nucleotide- binding domains. J. Biol. Chem. 2003, 278, 1575–1578. [Google Scholar] [CrossRef]
- Fu, D.; Bebawy, M.; Kable, E.P.; Roufogalis, B.D. Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein: Implications in multidrug resistance in cancer. Int. J. Cancer 2004, 109, 174–181. [Google Scholar] [CrossRef]
- Fu, D.; Roufogalis, B.D. Actin disruption inhibits endosomal traffic of P-glycoprotein-EGFP and resistance to daunorubicin accumulation. Am. J. Physiol. Cell Physiol. 2007, 292, C1543–C1552. [Google Scholar] [CrossRef]
- Budnik, A.; Stephens, D.J. ER exit sites—Localization and control of COPII vesicle formation. FEBS Lett. 2009, 583, 3796–3803. [Google Scholar] [CrossRef]
- Katayama, K.; Khyati, K.; Shinobu, O.; Atish, P.; William, S.S.; Suresh, V. Revealing the fate of cell surface human P-glycoprotein (ABCB1): The lysosomal degradation pathway. Biochim. Biophys. Acta 2015, 1853, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, S.; Meijerman, I.; Febus, C.L.; Maas-Bakker, R.F.; Beijnen, J.H.; Schellens, J.H. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother. Pharmacol. 2010, 66, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chinta, G.; Safiulla, B.S.; Mohane, S.C.; Latha, P. Piperine: A Comprehensive Review of Pre-Clinical and Clinical Investigations. Curr. Bioact. Compd. 2015, 11, 156–169. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Amin, M.L. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef]
- Safiulla, B.S.; Hemant, A.; I-Hsuan, F.; Teng-Kuang, Y.; Latha, P.; Hsing-Pang, H.; Mohane, S.C. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Nat. Sci. Rep. 2017, 7, 7972. [Google Scholar]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; Decrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with In Vivo toxicological outcomes. Bioorg. Org. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Devanand, G.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Folate targeted PEGylated titanium dioxide nanoparticles as a nanocarrier for targeted paclitaxel drug delivery. Adv. Powder Technol. 2013, 24, 947–954. [Google Scholar] [CrossRef]
- Tijana, R.; Nada, D.; Marc, B.; Tamara, K.; Vani, K. Titanium Dioxide in the Service of the Biomedical Revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar]
- Kanehira, K.; Yano, Y.; Hasumi, H.; Fukuhara, H.; Inoue, K.; Hanazaki, K.; Yao, M. Fluorescence Enhancement Effect of TiO2 Nanoparticles and Application for Photodynamic Diagnosis. Int. J. Mol. Sci. 2019, 20, 3698. [Google Scholar] [CrossRef] [PubMed]
- Qingqing, S.; Kanehira, K.; Taniguchi, A. Low doses of TiO2-polyethylene glycol nanoparticles stimulate proliferation of hepatocyte cells. Sci. Technol. Adv. Mater. 2016, 17, 669–676. [Google Scholar]
- Le, T.P.; Taniguchi, A. Epidermal Growth Factor Enhances Cellular Uptake of Polystyrene Nanoparticles by Clathrin-Mediated Endocytosis. Int. J. Mol. Sci. 2017, 18, 1301. [Google Scholar]
- Fehaid, A.; Taniguchi, A. Size-dependent effect of silver nanoparticles on the tumor necrosis factor α-induced DNA damage response. Int. J. Mol. Sci. 2019, 20, 1038. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Laurent, G.; Ling, V. P-glycoprotein stability is affected by serum deprivation and high cell density in multidrug-resistant cells. J. Cell Physiol. 1995, 163, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Qingqing, S.; Kanehira, K.; Taniguchi, A. Uniform TiO2 nanoparticles induce apoptosis in epithelial cell lines in a size-dependent manner. Biomater. Sci. 2017, 5, 1014–1021. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, B.; El-Sherbini, E.-S.; El-Sayed, G.; El-Adl, M.; Kanehira, K.; Taniguchi, A. The Effects of TiO2 Nanoparticles on Cisplatin Cytotoxicity in Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 605. https://doi.org/10.3390/ijms21020605
Salama B, El-Sherbini E-S, El-Sayed G, El-Adl M, Kanehira K, Taniguchi A. The Effects of TiO2 Nanoparticles on Cisplatin Cytotoxicity in Cancer Cell Lines. International Journal of Molecular Sciences. 2020; 21(2):605. https://doi.org/10.3390/ijms21020605
Chicago/Turabian StyleSalama, Basma, El-Said El-Sherbini, Gehad El-Sayed, Mohamed El-Adl, Koki Kanehira, and Akiyoshi Taniguchi. 2020. "The Effects of TiO2 Nanoparticles on Cisplatin Cytotoxicity in Cancer Cell Lines" International Journal of Molecular Sciences 21, no. 2: 605. https://doi.org/10.3390/ijms21020605
APA StyleSalama, B., El-Sherbini, E.-S., El-Sayed, G., El-Adl, M., Kanehira, K., & Taniguchi, A. (2020). The Effects of TiO2 Nanoparticles on Cisplatin Cytotoxicity in Cancer Cell Lines. International Journal of Molecular Sciences, 21(2), 605. https://doi.org/10.3390/ijms21020605





