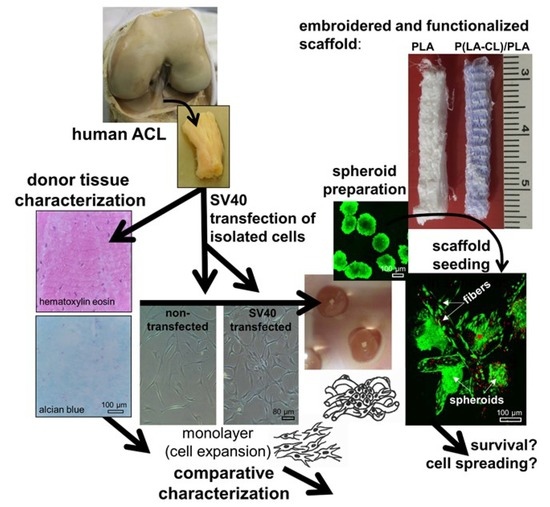
SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes—Suitable as a Human in Vitro Model for Ligament Reconstruction?
Abstract
1. Introduction
2. Results
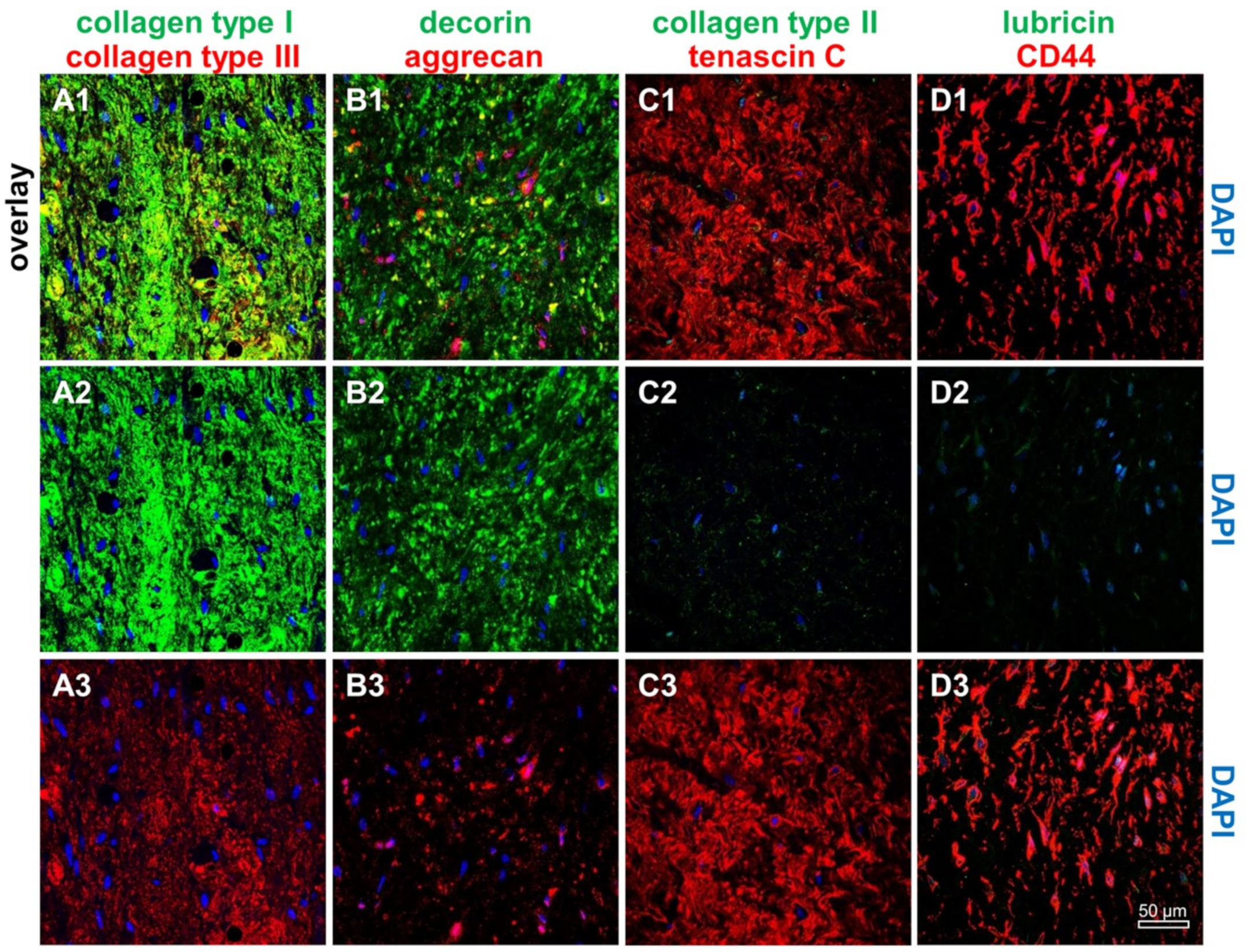
2.1. ACL Donor Tissue Characterization
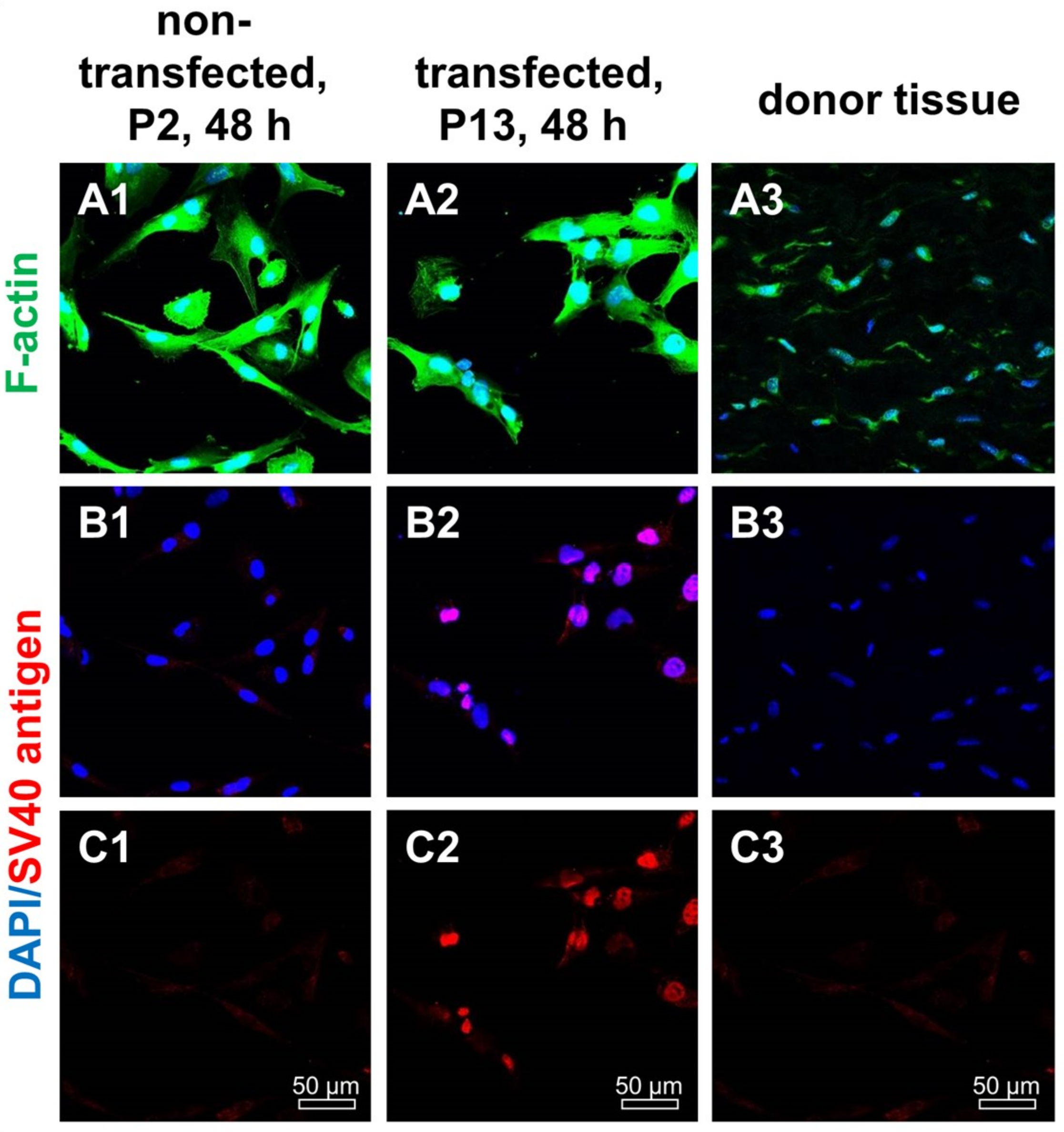
2.2. SV40 T Antigen Expression
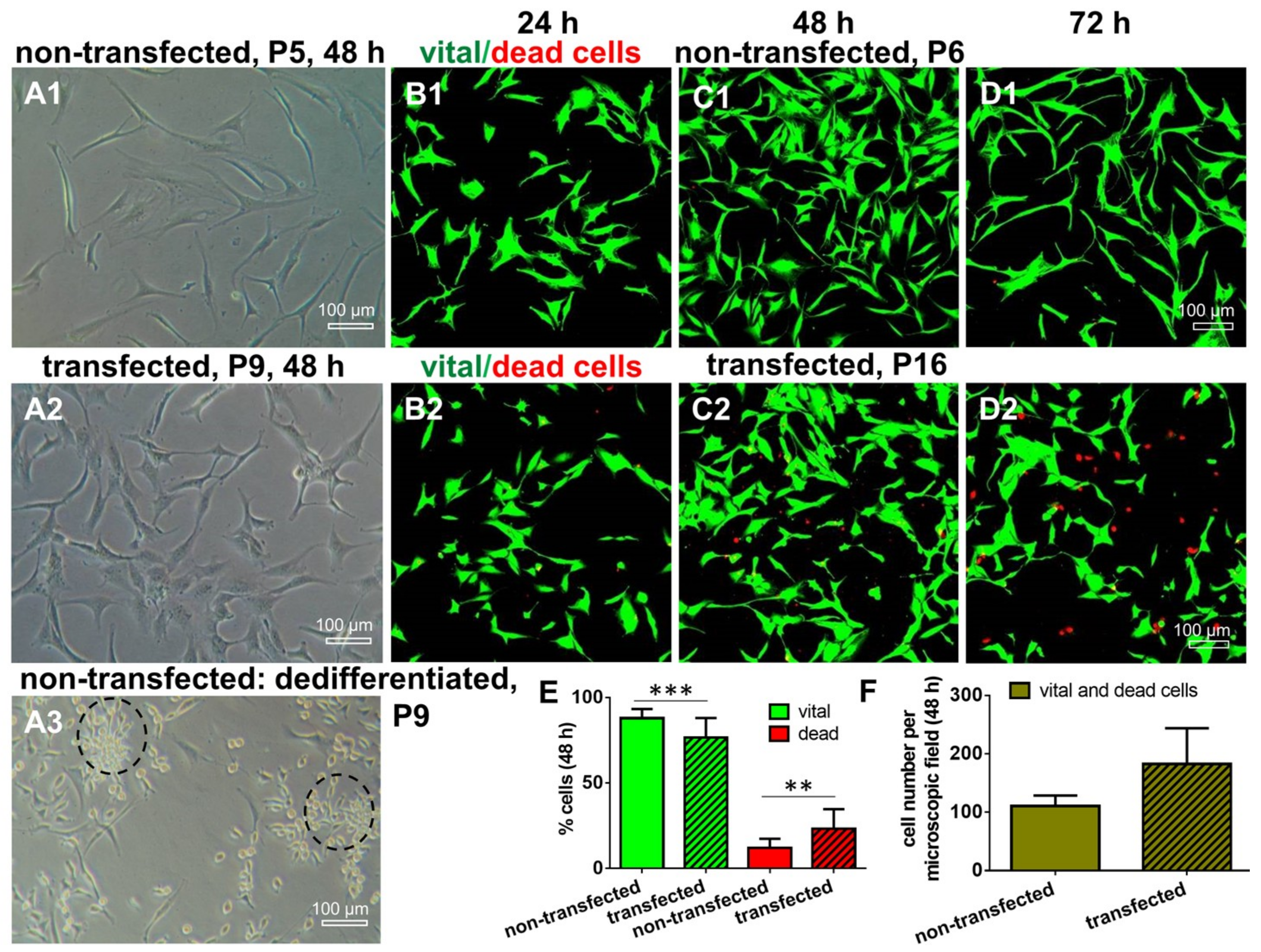
2.3. Morphology and Cell Survival of Non-Transfected and SV40 Transfected ACL Ligamentocytes in Monolayer Culture
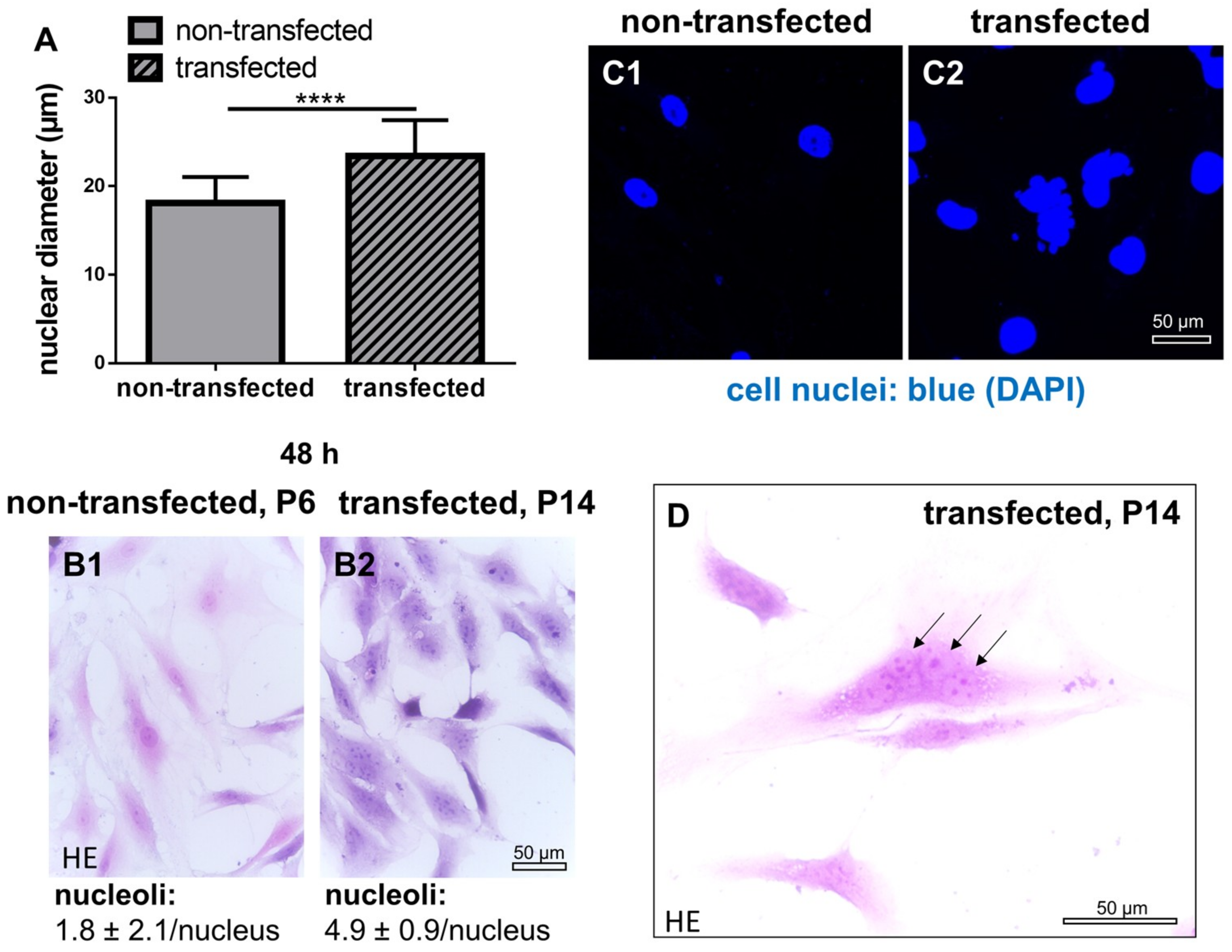
2.4. Cell Nuclei and Numbers of Nucleoli in Non-Transfected and SV40 Transfected ACL Ligamentocytes in Monolayer Culture
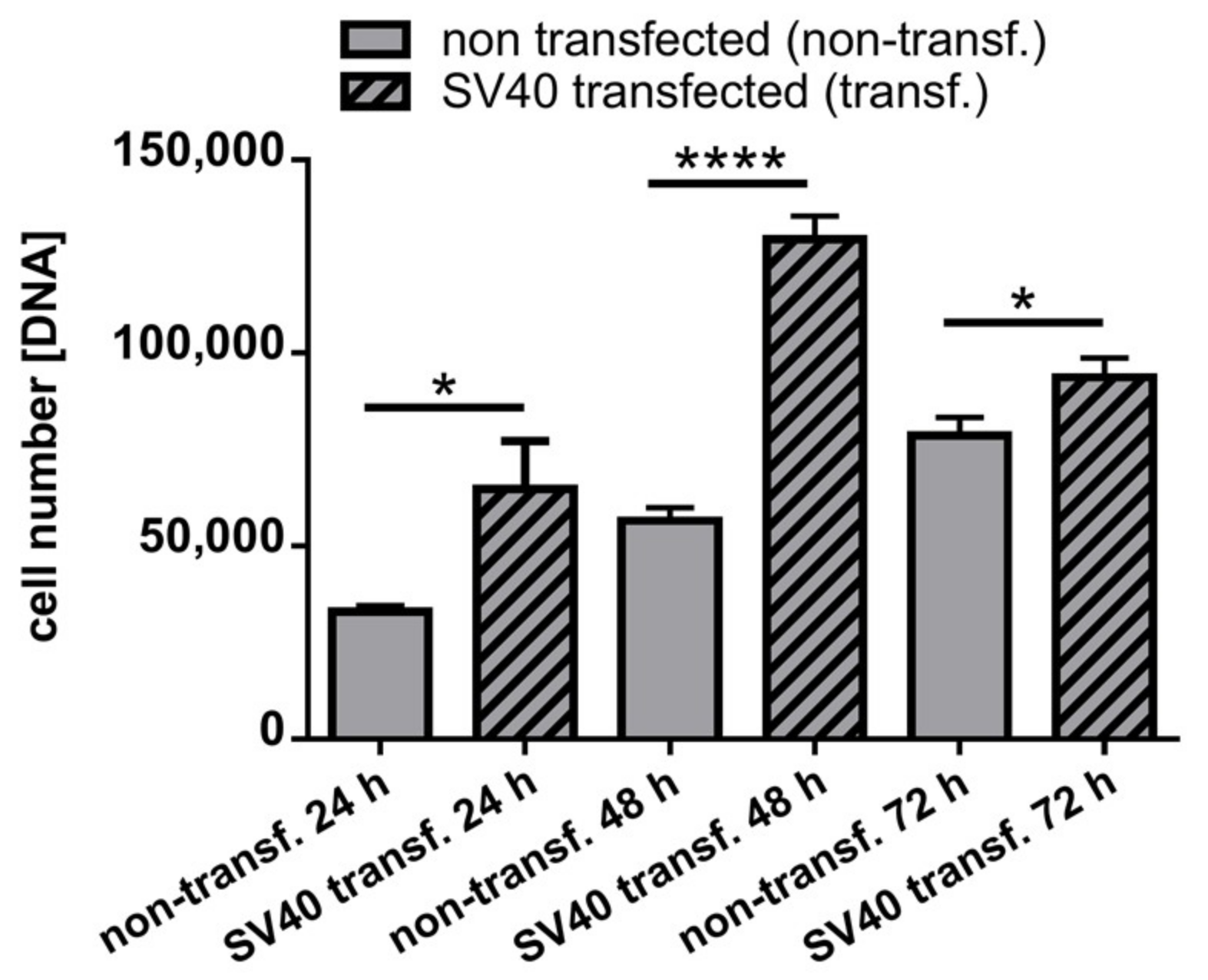
2.5. DNA Content in Monolayer Culture in Non-Transfected and SV40 Transfected ACL Ligamentocytes in Monolayer culture
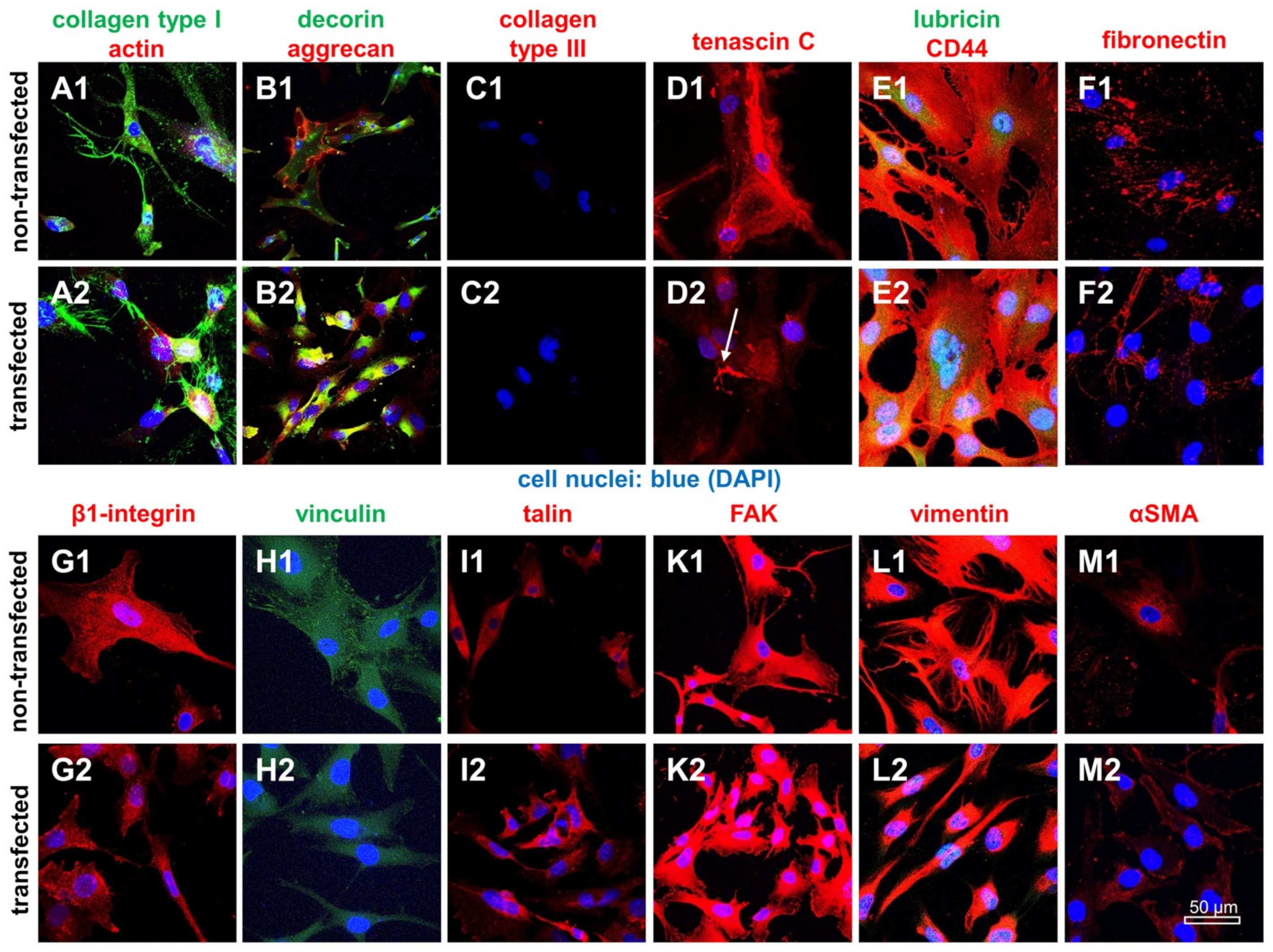
2.6. Protein Expression Analysis in Non-Transfected and SV40 Transfected Ligamentocytes in Monolayer Culture
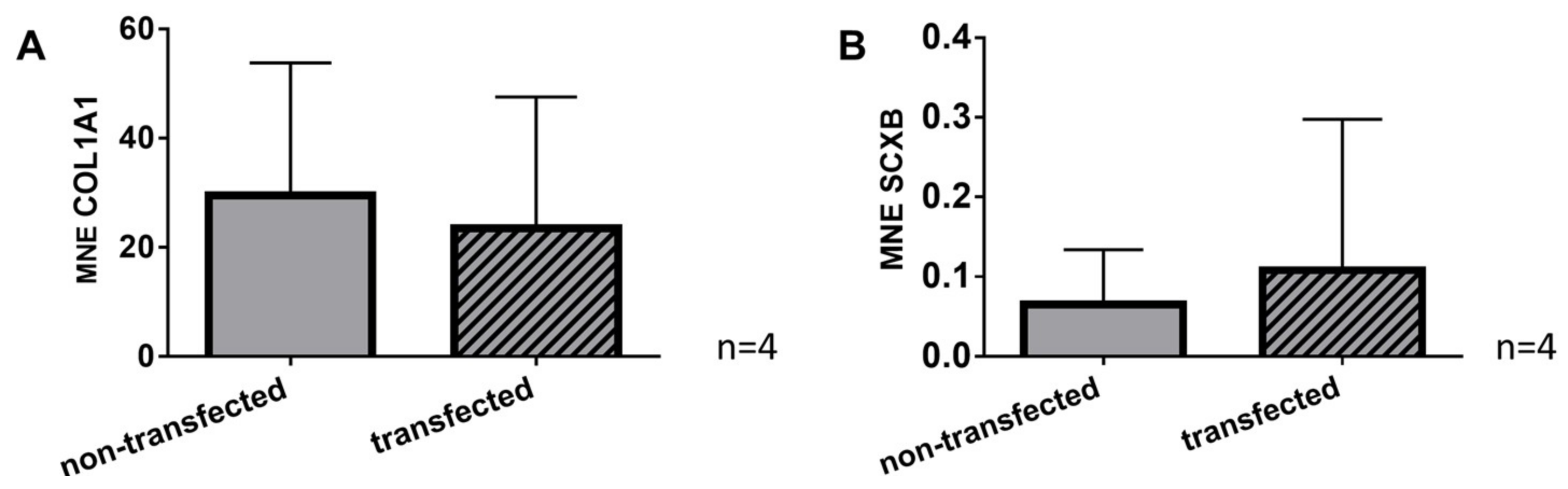
2.7. Gene Expression of Typical Tendon Components in Non-Transfected and SV40 Transfected Ligamentocytes
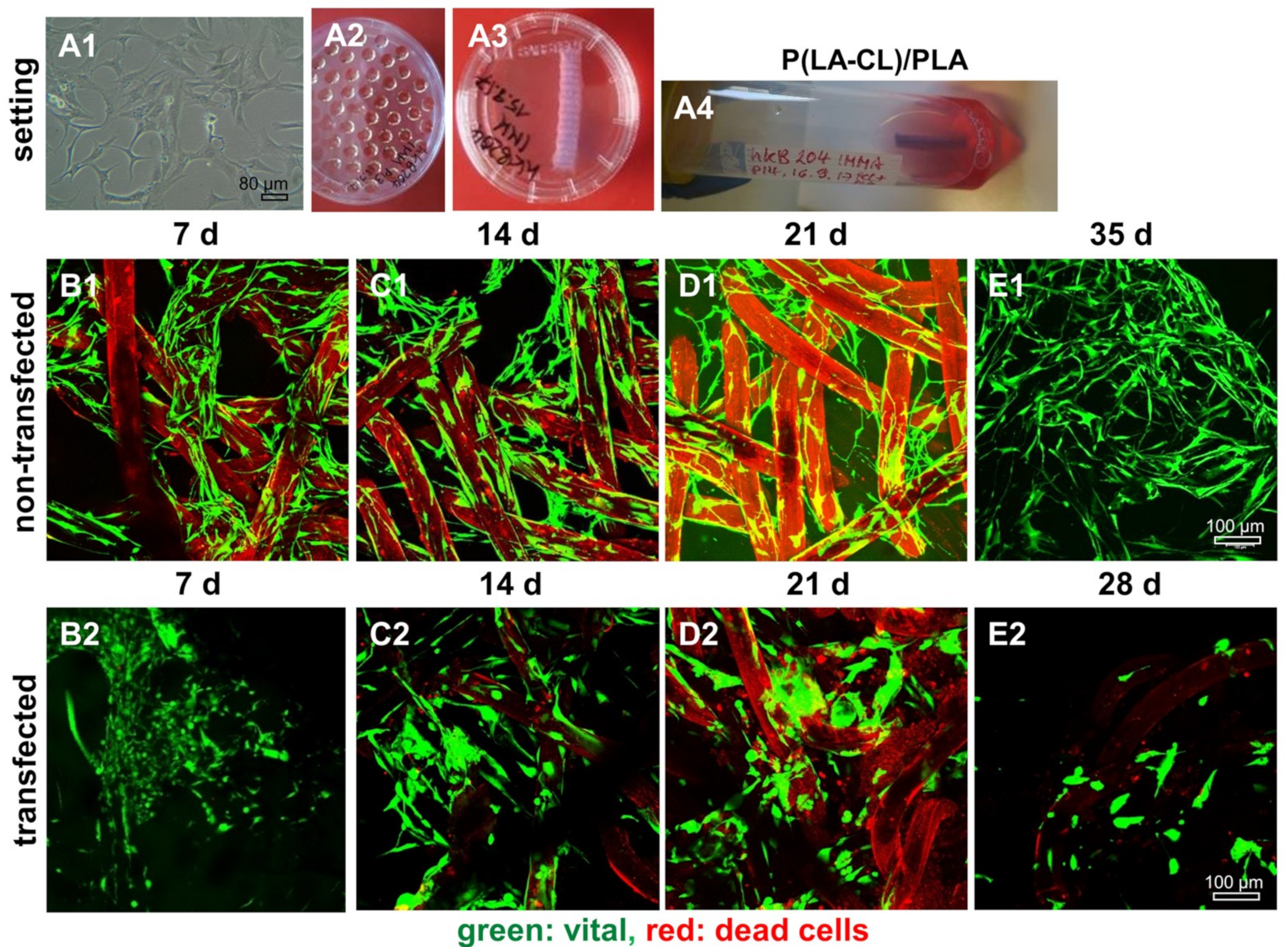
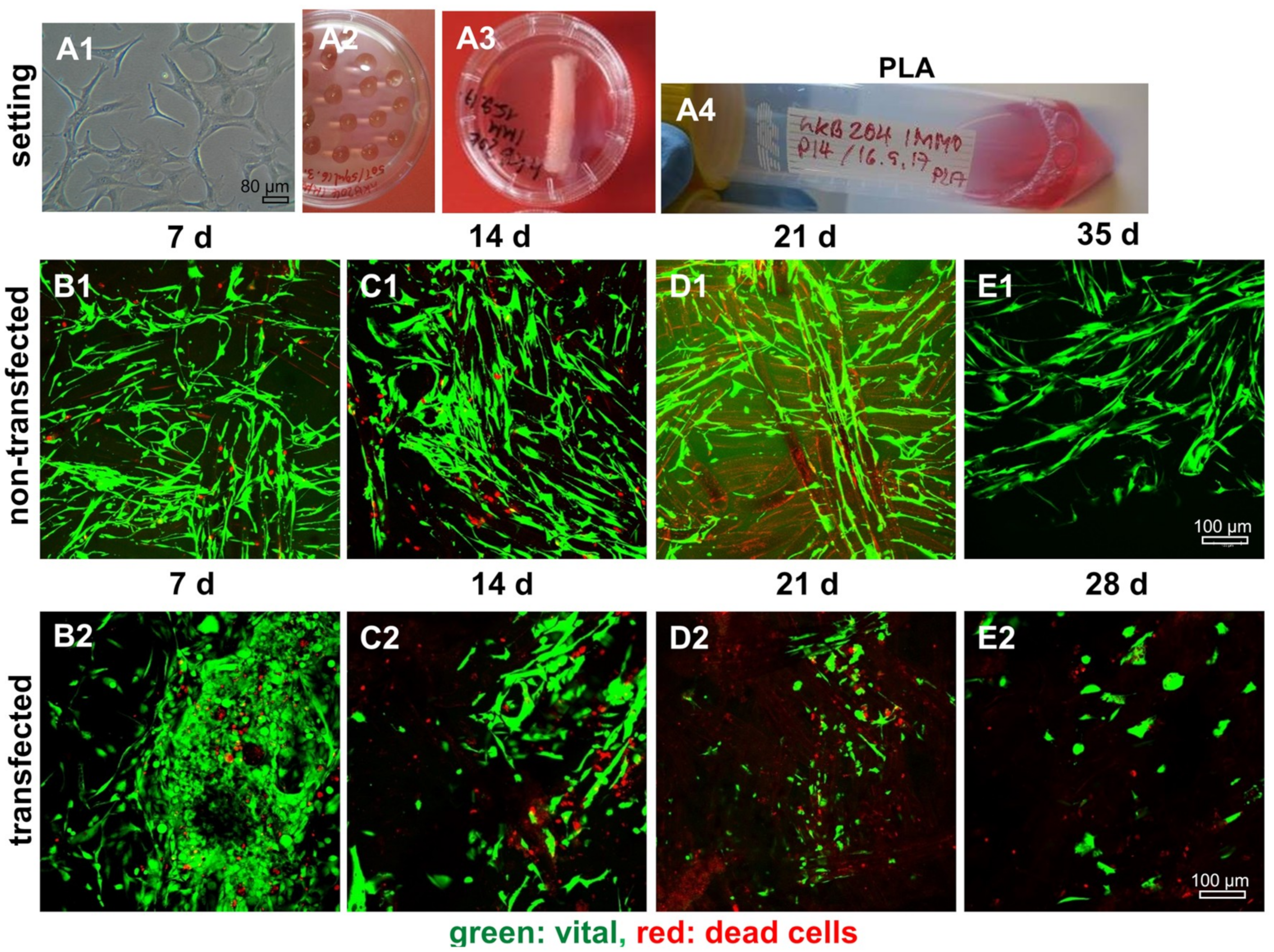
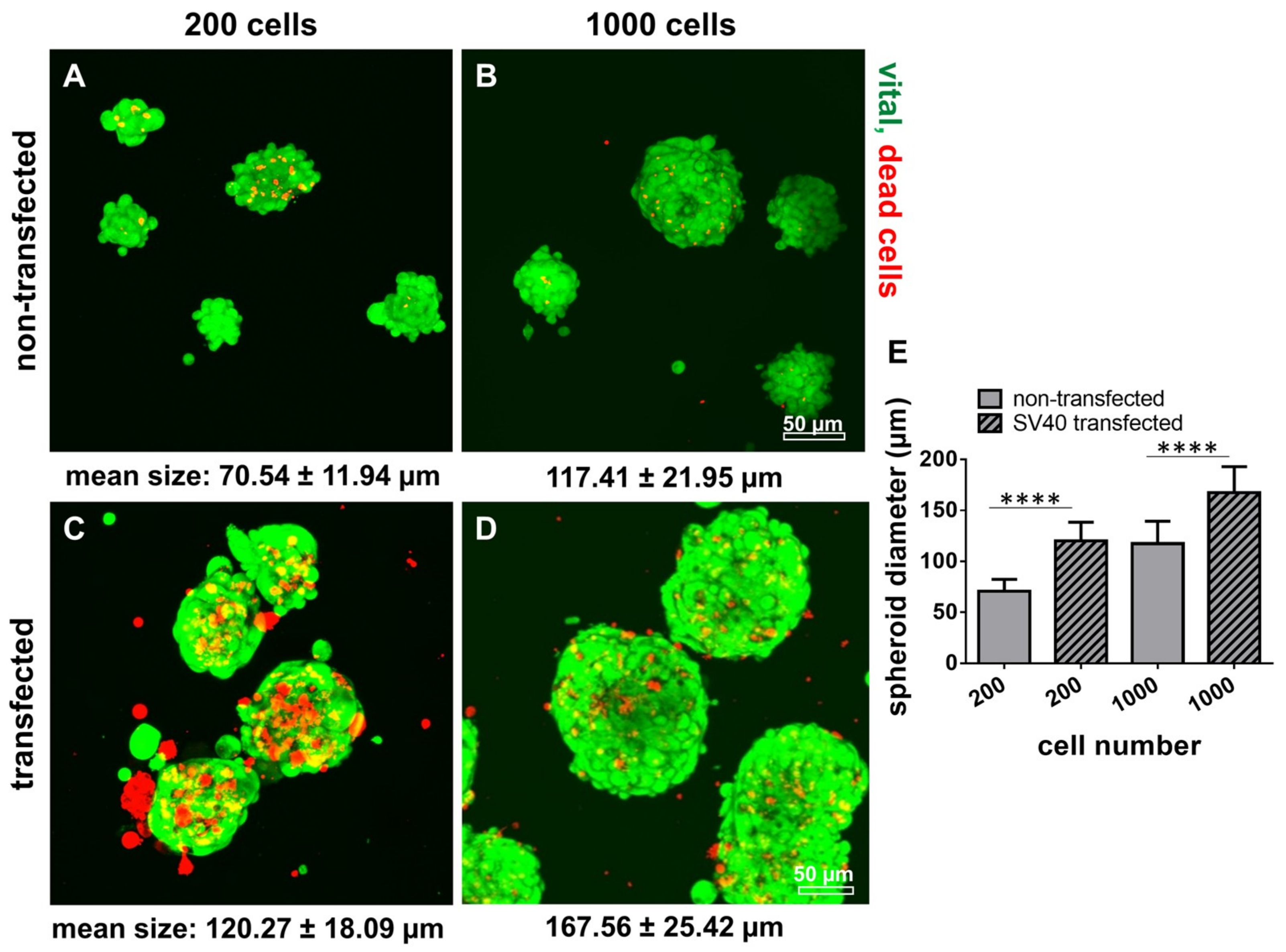
2.8. Survival of Non-Transfected and SV40 Transfected Ligamentocytes in 3D Culture
3. Discussion
4. Materials and Methods
4.1. Cell isolation by Explant Culture
4.2. Transfection with an SV40 Plasmid
4.3. Scaffold Preparation
4.4. Spheroid Culture and Scaffold Seeding
4.5. Culture of ACL Ligamentocytes on Glass Cover Slips
4.6. Histological Staining
4.7. Live/Dead Assay
4.8. Immunofluorescence Analysis of Marker Expression
4.9. Measurement of Cell Nuclei Diameters and Spheroid Diameters
4.10. Quantification of Cell’s DNA Content by CyQuant Assay
4.11. Gene Expression Analysis Using RTD-PCR
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| AB | alcian blue |
| ACL | anterior cruciate ligament |
| DAPI | 4’,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle’s |
| ECM | extracellular matrix |
| FCS | fetal calf serum |
| FDA | fluorescein diacetate |
| HE | hematoxylin eosin staining |
| HMDI | hexamethylene diisocyanate |
| HPRT | hypoxanthine-guanine phosphoribosyltransferase |
| hTERT | human telomerase reverse transcriptase |
| PBS | phosphate buffered saline |
| PFA | paraformaldehyde |
| PI | propidium iodide |
| RT | room temperature |
| SD | standard deviation |
| SV40 | Simian virus 40 |
| sGAGs | sulphated glycosaminoglycans |
| TBS | TRIS buffered saline |
| TE | tissue engineering |
References
- Kudo, Y.; Hiraoka, M.; Kitagawa, S.; Miyauchi, M.; Kakuo, S.; Zhao, M.; Ide, T.; Takata, T. Establishment of human cementifying fibroma cell lines by transfection with temperature-sensitive simian virus-40 T-antigen gene and hTERT gene. Bone 2002, 30, 712–717. [Google Scholar] [CrossRef]
- Fujii, S.; Maeda, H.; Wada, N.; Kano, Y.; Akamine, A. Establishing and characterizing human periodontal ligament fibroblasts immortalized by SV40T-antigen and hTERT gene transfer. Cell Tissue Res. 2006, 324, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Maeda, H.; Wada, N.; Tomokiyo, A.; Saito, M.; Akamine, A. Investigating a clonal human periodontal ligament progenitor/stem cell line in vitro and in vivo. J. Cell. Physiol. 2008, 215, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Parkar, M.H.; Kuru, L.; O’Hare, M.; Newman, H.N.; Hughes, F.; Olsen, I. Retroviral transduction of human periodontal cells with a temperature-sensitive SV40 large T antigen. Arch. Oral Biol. 1999, 44, 823–834. [Google Scholar] [CrossRef]
- McNutt, N.S.; Culp, L.A.; Black, P.H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J. Cell Biol. 1973, 56, 412–428. [Google Scholar] [CrossRef]
- Salingcarnboriboon, R.; Yoshitake, H.; Tsuji, K.; Obinata, M.; Amagasa, T.; Nifuji, A.; Noda, M. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp. Cell Res. 2003, 287, 289–300. [Google Scholar] [CrossRef]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar]
- Liu, H.; Zhu, S.; Zhang, C.; Lu, P.; Hu, J.; Yin, Z.; Ma, Y.; Chen, X.; OuYang, H. Crucial transcription factors in tendon development and differentiation: Their potential for tendon regeneration. Cell Tissue Res. 2014, 356, 287–298. [Google Scholar] [CrossRef]
- Yoon, J.H.; Halper, J. Tendon proteoglycans: Biochemistry and function. J. Musculoskelet. Neuronal Interact. 2005, 5, 22–34. [Google Scholar]
- Kraus, A.; Luetzenberg, R.; Abuagela, N.; Hollenberg, S.; Infanger, M. Spheroid formation and modulation of tenocyte-specific gene expression under simulated microgravity. Muscles Ligaments Tendons J. 2017, 7, 411–417. [Google Scholar] [CrossRef]
- Schwarz, S.; Gogele, C.; Ondruschka, B.; Hammer, N.; Kohl, B.; Schulze-Tanzil, G. Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction. Int. J. Mol. Sci. 2019, 20, 1972. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Otsuki, S.; Pauli, C.; Miyaki, S.; Patil, S.; Steklov, N.; Kinoshita, M.; Koziol, J.; D’Lima, D.D.; Lotz, M.K. Anterior cruciate ligament changes in the human knee joint in aging and osteoarthritis. Arthritis Rheum. 2012, 64, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.M.; Chen, D.; Oates, T.W.; Jiang, C.; Harris, S.E.; Cochran, D.L. Development and characterization of a transformed human periodontal ligament cell line. J. Periodontol. 1997, 68, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Kahn, P.; Topp, W.C.; Shin, S. Tumorigenicity of SV40-transformed human and monkey cells in immunodeficient mice. Virology 1983, 126, 348–360. [Google Scholar] [CrossRef]
- Gish, W.R.; Botchan, M.R. Simian virus 40-transformed human cells that express large T antigens defective for viral DNA replication. J. Virol. 1987, 61, 2864–2876. [Google Scholar] [CrossRef]
- Levine, A.J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology 1990, 177, 419–426. [Google Scholar] [CrossRef]
- Itahana, K.; Dimri, G.P.; Hara, E.; Itahana, Y.; Zou, Y.; Desprez, P.Y.; Campisi, J. A role for p53 in maintaining and establishing the quiescence growth arrest in human cells. J. Biol. Chem. 2002, 277, 18206–18214. [Google Scholar] [CrossRef]
- Pope, J.H.; Rowe, W.P. Detection of Specific Antigen in Sv40-Transformed Cells by Immunofluorescence. J. Exp. Med. 1964, 120, 121–128. [Google Scholar] [CrossRef]
- Hoffschir, F.; Ricoul, M.; Dutrillaux, B. SV40-transformed human fibroblasts exhibit a characteristic chromosomal pattern. Cytogenet. Cell Genet. 1988, 49, 264–268. [Google Scholar] [CrossRef]
- Terriac, E.; Schutz, S.; Lautenschlager, F. Vimentin Intermediate Filament Rings Deform the Nucleus During the First Steps of Adhesion. Front. Cell Dev. Biol. 2019, 7, 106. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Salmenpera, P.; Kankuri, E.; Bizik, J.; Siren, V.; Virtanen, I.; Takahashi, S.; Leiss, M.; Fassler, R.; Vaheri, A. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp. Cell Res. 2008, 314, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; McCoy, G.A.; Folkvord, J.M.; McPherson, J.M. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: A fibronectin matrix-dependent event. J. Cell. Physiol. 1997, 170, 69–80. [Google Scholar] [CrossRef]
- Hahner, J.; Hoyer, M.; Hillig, S.; Schulze-Tanzil, G.; Meyer, M.; Schropfer, M.; Lohan, A.; Garbe, L.A.; Heinrich, G.; Breier, A. Diffusion chamber system for testing of collagen-based cell migration barriers for separation of ligament enthesis zones in tissue-engineered ACL constructs. J. Biomater. Sci. Polym. Ed. 2015, 26, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, M.; Drechsel, N.; Meyer, M.; Meier, C.; Hinuber, C.; Breier, A.; Hahner, J.; Heinrich, G.; Rentsch, C.; Garbe, L.A.; et al. Embroidered polymer-collagen hybrid scaffold variants for ligament tissue engineering. Mater. Sci. Eng. CMater. Biol. Appl. 2014, 43, 290–299. [Google Scholar] [CrossRef]
- Hahn, J.; Schulze-Tanzil, G.; Schropfer, M.; Meyer, M.; Gogele, C.; Hoyer, M.; Spickenheuer, A.; Heinrich, G.; Breier, A. Viscoelastic Behavior of Embroidered Scaffolds for ACL Tissue Engineering Made of PLA and P(LA-CL) After In Vitro Degradation. Int. J. Mol. Sci. 2019, 20, 4655. [Google Scholar] [CrossRef]
- Hoyer, M.; Meier, C.; Breier, A.; Hahner, J.; Heinrich, G.; Drechsel, N.; Meyer, M.; Rentsch, C.; Garbe, L.A.; Ertel, W.; et al. In vitro characterization of self-assembled anterior cruciate ligament cell spheroids for ligament tissue engineering. Histochem. Cell Biol. 2015, 143, 289–300. [Google Scholar] [CrossRef]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Maffulli, N.; Aspden, R.M. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006, 12, 1843–1849. [Google Scholar] [CrossRef]
- Clegg, P.D.; Strassburg, S.; Smith, R.K. Cell phenotypic variation in normal and damaged tendons. Int. J. Exp. Pathol. 2007, 88, 227–235. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Intraarticular Ligament Degeneration Is Interrelated with Cartilage and Bone Destruction in Osteoarthritis. Cells 2019, 8, 990. [Google Scholar] [CrossRef]
- Kamata, N.; Fujimoto, R.; Tomonari, M.; Taki, M.; Nagayama, M.; Yasumoto, S. Immortalization of human dental papilla, dental pulp, periodontal ligament cells and gingival fibroblasts by telomerase reverse transcriptase. J. Oral Pathol. Med. 2004, 33, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.C. Biomedical Textiles for Orthopaedic and Surgical Applications, Fundamentals, Applications and Tissue Engineering: Embroidery technology for hard-tissue scaffolds. Woodhead Publ. Ser. Biomater. 2015, 93, 23–40. [Google Scholar]
- Hahner, J.H.C.; Breier, A.; Siebert, T.; Brünig, H.; Heinrich, G. Adjusting the mechanical behavior of embroidered scaffolds to lapin anterior cruciate ligaments by varying the thread materials. Text. Res. J. 2015, 85, 1431–1444. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sah, R.L.; Doong, J.Y.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Target | Primary Antibody | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| αSMA | mouse-anti-human, Sigma-Aldrich | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| β1-integrin | mouse-anti-human, Merck-Millipore, Darmstadt, Germany | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| aggrecan | mouse anti human R&D systems, Minneapolis, USA | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| CD44 | mouse-anti-human, Cell signalling Technology, Danvers, USA | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| collagen type I | goat anti human, Abcam, Cambridge, UK | 1:50 | donkey anti goat, Alexa Fluor 488, Invitrogen, Carlsbad, USA | 1:200 |
| collagen type II | rabbit anti human, Acris Laboratories, Hiddenhausen, Germany | 1:50 | donkey anti rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| collagen type III | mouse anti human Acris Laboratories | 1:30 | donkey-anti-mouse cyanine-3 (cy3), Invitrogen | 1:200 |
| decorin | rabbit anti human, Acris Laboratories | 1:30 | donkey anti rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| elastin | mouse anti human Acris Laboratories | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| fibronectin | mouse-anti-human, Dianova, Hamburg, Germany | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| focal adhesion kinase | mouse-anti-human, BD Transduction Laboratories, Ca, San Jose, USA | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| lubricin | rabbit-anti-human, Abcam, Cambridge, UK | 1:30 | donkey anti rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| mohawk | rabbit-anti-human, Biozol, Eching, Germany | 1:30 | donkey anti rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| SV40 T antigen | mouse-anti-human, Merck-Millipore | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| talin | mouse-anti-human, Sigma-Aldrich | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| tenascin C | mouse-anti-human, GeneTex Inc. Biozol | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| VEGF | mouse-anti-human, R&D Systems | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| vimentin | mouse-anti-human, Dako Cytomation, Hamburg, Germany | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| vinculin | mouse-anti-human 1:50, Sigma-Aldrich | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Gene Symbol | Species | Gene Name | Amplicon Length (bp *) | Assay ID |
|---|---|---|---|---|
| COL1A1 | Homo sapiens | collagen type I, alpha1 chain | 66 | Hs00164004_m1 |
| SCXB | Homo sapiens | scleraxis homolog B | 63 | Hs03054634_g1 |
| HPRT | Homo sapiens | hypoxanthine-guanine phosphoribosyltransferase | 100 | Hs99999909_m1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze-Tanzil, G.; Arnold, P.; Gögele, C.; Hahn, J.; Breier, A.; Meyer, M.; Kohl, B.; Schröpfer, M.; Schwarz, S. SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes—Suitable as a Human in Vitro Model for Ligament Reconstruction? Int. J. Mol. Sci. 2020, 21, 593. https://doi.org/10.3390/ijms21020593
Schulze-Tanzil G, Arnold P, Gögele C, Hahn J, Breier A, Meyer M, Kohl B, Schröpfer M, Schwarz S. SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes—Suitable as a Human in Vitro Model for Ligament Reconstruction? International Journal of Molecular Sciences. 2020; 21(2):593. https://doi.org/10.3390/ijms21020593
Chicago/Turabian StyleSchulze-Tanzil, Gundula, Philipp Arnold, Clemens Gögele, Judith Hahn, Annette Breier, Michael Meyer, Benjamin Kohl, Michaela Schröpfer, and Silke Schwarz. 2020. "SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes—Suitable as a Human in Vitro Model for Ligament Reconstruction?" International Journal of Molecular Sciences 21, no. 2: 593. https://doi.org/10.3390/ijms21020593
APA StyleSchulze-Tanzil, G., Arnold, P., Gögele, C., Hahn, J., Breier, A., Meyer, M., Kohl, B., Schröpfer, M., & Schwarz, S. (2020). SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes—Suitable as a Human in Vitro Model for Ligament Reconstruction? International Journal of Molecular Sciences, 21(2), 593. https://doi.org/10.3390/ijms21020593