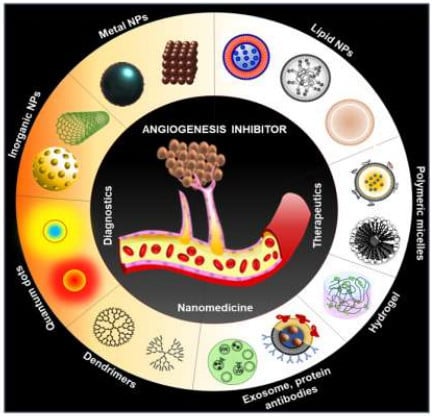
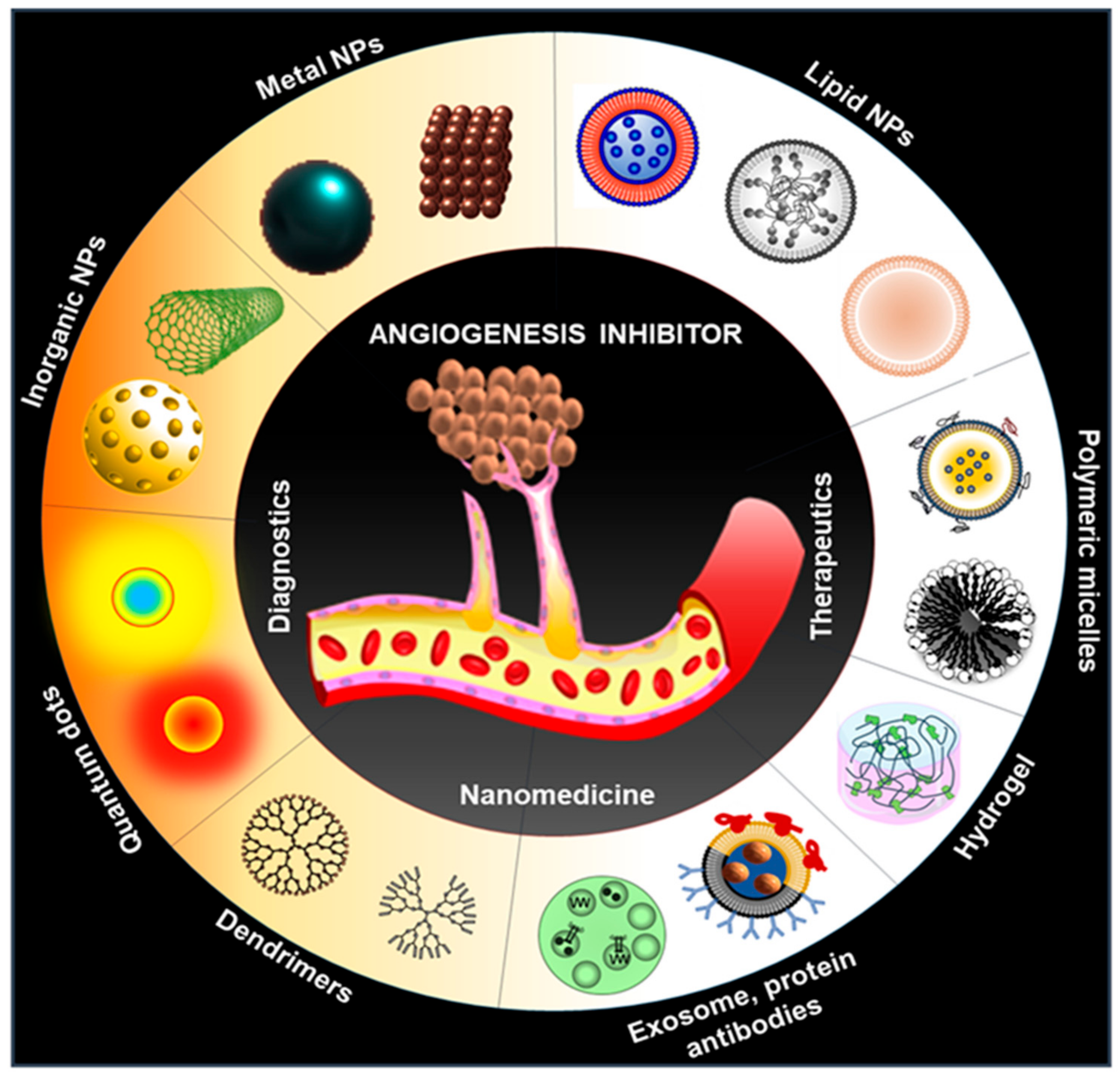
Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer
Abstract
1. Introduction
2. Cancer, Statistics, Conventional Therapy, Challenges
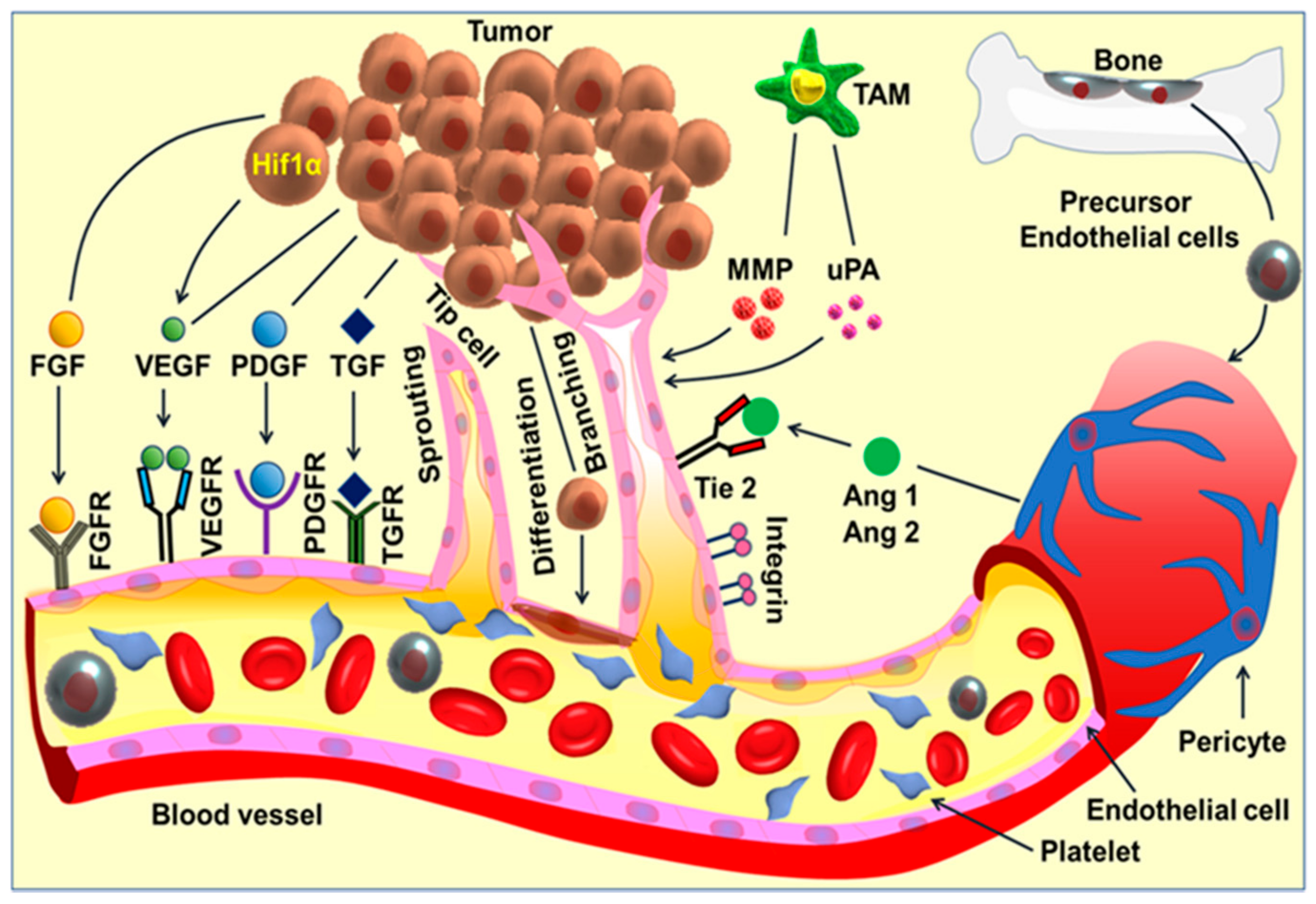
3. Angiogenesis and Cancer
4. Current Antiangiogenic Therapies in Cancer and Their Limitations
5. Alternative Therapy: Nanomedicine
6. Lipid-Based Nanoparticles for Antiangiogenic Therapy
7. Polymeric Nanomedicine
8. Inorganic Nanoparticles
9. Protein Based Nanoparticles
10. Viral and Other Bio-Inspired Nanoparticles
11. Challenges of Nanomedicine, Conclusion, and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Dvorak, H.F. Angiogenesis: Update 2005. J. Thromb. Haemost. 2005, 3, 1835–1842. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Petrillo, M.; Patella, F.; Pesapane, F.; Suter, M.B.; Ierardi, A.M.; Angileri, S.A.; Floridi, C.; de Filippo, M.; Carrafiello, G. Hypoxia and tumor angiogenesis in the era of hepatocellular carcinoma transarterial loco-regional treatments. Future Oncol. 2018, 14, 2957–2967. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular Endothelial Growth Factor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef]
- Ferrara, N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010, 21, 21–26. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, J.; Zhang, C.; Hu, T.; Li, S.; He, S.; Yan, H.; Tan, Y.; Lei, M.; Wen, M.; et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017, 4, 19–24. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, G.Y.; Li, P.P.; Sun, X.; Li, W.M.; Li, Y.; Lu, S.Y.; Liu, P.J. Suppression of hypoxia-induced excessive angiogenesis by metformin via elevating tumor blood perfusion. Oncotarget 2017, 8, 73892–73904. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale 2016, 8, 12444–12470. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Heath, V.L.; Bicknell, R. Anticancer strategies involving the vasculature. Nat. Rev. Clin. Oncol. 2009, 6, 395–404. [Google Scholar] [CrossRef]
- Chung, A.S.; Lee, J.; Ferrara, N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat. Rev. Cancer 2010, 10, 505–514. [Google Scholar] [CrossRef]
- Prager, G.W.; Poettler, M.; Unseld, M.; Zielinski, C.C. Angiogenesis in cancer: Anti-VEGF escape mechanisms. Transl. Lung Cancer Res. 2012, 1, 14–25. [Google Scholar]
- Clarke, S.J.; Sharma, R. Experimental & Clinical Pharmacology: Angiogenesis inhibitors in cancer-mechanisms of action. Aust. Prescr. 2006, 29, 9–12. [Google Scholar]
- Ricart, A.D.; Ashton, E.A.; Cooney, M.M.; Sarantopoulos, J.; Brell, J.M.; Feldman, M.A.; Ruby, K.E.; Matsuda, K.; Munsey, M.S.; Medina, G.; et al. A phase I study of MN-029 (denibulin), a novel vascular-disrupting agent, in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2011, 68, 959–970. [Google Scholar] [CrossRef]
- Ebos, J.M.L.; Lee, C.R.; Kerbel, R.S. Tumor and Host-Mediated Pathways of Resistance and Disease Progression in Response to Antiangiogenic Therapy. Clin. Cancer Res. 2009, 15, 5020–5025. [Google Scholar] [CrossRef]
- Abdalla, A.M.E.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef]
- Mukherjee, S. Recent progress toward antiangiogenesis application of nanomedicine in cancer therapy. Future Sci. OA 2018, 4. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef]
- Huang, S.; Shao, K.; Liu, Y.; Kuang, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; Jiang, C. Tumor-Targeting and Microenvironment-Responsive Smart Nanoparticles for Combination Therapy of Antiangiogenesis and Apoptosis. ACS Nano 2013, 7, 2860–2871. [Google Scholar] [CrossRef]
- Mukherjee, A.; Paul, M.; Mukherjee, S. Recent Progress in the Theranostics Application of Nanomedicine in Lung Cancer. Cancers 2019, 11, 597. [Google Scholar] [CrossRef]
- Nethi, S.K.; Barui, A.K.; Mukherjee, S.; Patra, C.R. Engineered Nanoparticles for Effective Redox Signaling During Angiogenic and Antiangiogenic Therapy. Antioxid. Redox Signal. 2019, 30, 786–809. [Google Scholar] [CrossRef]
- Aslan, C.; Maralbashi, S.; Salari, F.; Kahroba, H.; Sigaroodi, F.; Kazemi, T.; Kharaziha, P. Tumor-derived exosomes: Implication in angiogenesis and antiangiogenesis cancer therapy. J. Cell. Physiol. 2019, 234, 16885–16903. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Wild, C.P. The global cancer burden: Necessity is the mother of prevention. Nat. Rev. Cancer 2019, 19, 123–124. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Harper, J.; Moses, M.A. Molecular regulation of tumor angiogenesis: Mechanisms and therapeutic implications. EXS 2006, 96, 223–268. [Google Scholar]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef]
- Qian, C.N.; Tan, M.H.; Yang, J.P.; Cao, Y. Revisiting tumor angiogenesis: Vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation. Chin. J. Cancer 2016, 35, 10. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Potente, M.; Carmeliet, P. The Link Between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef]
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Sharma, V. Tumour Angiogenesis and Angiogenic Inhibitors: A Review. J. Clin. Diagn. Res. 2015, 9, XE01–XE05. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Minion, L.E.; Tewari, K.S. The safety and efficacy of bevacizumab in the treatment of patients with recurrent or metastatic cervical cancer. Expert Rev. Anticancer Ther. 2017, 17, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Brenner, A.; de Groot, J.F.; Butowski, N.A.; Zach, L.; Campian, J.L.; Ellingson, B.M.; Freedman, L.S.; Cohen, Y.C.; Lowenton-Spier, N.; et al. A randomized controlled phase III study of VB-111 combined with bevacizumab vs. bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2019. [Google Scholar] [CrossRef]
- Falcon, B.L.; Chintharlapalli, S.; Uhlik, M.T.; Pytowski, B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol. Ther. 2016, 164, 204–225. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A. New Insights in Anti-Angiogenesis in Multiple Myeloma. Int. J. Mol. Sci 2018, 19, 2031. [Google Scholar] [CrossRef]
- Montoro, J.; Yerlikaya, A.; Ali, A.; Raza, A. Improving Treatment for Myelodysplastic Syndromes Patients. Curr. Treat. Options Oncol. 2018, 19, 66. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chao, W.T.; Liao, C.C.; Shih, J.H.; Lai, Y.S.; Hsu, Y.H.; Liu, Y.H. The Roles Of Angiogenesis And Cancer Stem Cells In Sorafenib Drug Resistance In Hepatocellular Carcinoma. Onco Targets Ther. 2019, 12, 8217–8227. [Google Scholar] [CrossRef]
- Hao, Z.; Sadek, I. Sunitinib: The antiangiogenic effects and beyond. Onco Targets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, H.; Li, Z.; Xia, Q.; Nie, Y. Temsirolimus as a dual inhibitor of retinoblastoma and angiogenesis via targeting mTOR signalling. Biochem. Biophys. Res. Commun. 2019, 516, 726–732. [Google Scholar] [CrossRef]
- Kamli, H.; Li, L.; Gobe, G.C. Limitations to the Therapeutic Potential of Tyrosine Kinase Inhibitors and Alternative Therapies for Kidney Cancer. Ochsner J. 2019, 19, 138–151. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Chellian, J.; Ng, Z.Y.; Sim, Y.J.; Theng, C.W.; Ling, J.; Wong, M.; Foo, J.H.; Yang, G.J.; Hang, L.Y.; et al. The role of pazopanib on tumour angiogenesis and in the management of cancers: A review. Biomed. Pharmacother. 2017, 96, 768–781. [Google Scholar] [CrossRef]
- Matsuki, M.; Hoshi, T.; Yamamoto, Y.; Ikemori-Kawada, M.; Minoshima, Y.; Funahashi, Y.; Matsui, J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018, 7, 2641–2653. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Vaishampayan, U. Cabozantinib for the treatment of kidney cancer. Expert Rev. Anticancer Ther. 2017, 17, 577–584. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Everolimus in the treatment of neuroendocrine tumors: Efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin. Pharmacother. 2018, 19, 909–928. [Google Scholar] [CrossRef]
- Morabito, A.; Piccirillo, M.C.; Falasconi, F.; De Feo, G.; Del Giudice, A.; Bryce, J.; Di Maio, M.; De Maio, E.; Normanno, N.; Perrone, F. Vandetanib (ZD6474), a dual inhibitor of vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) tyrosine kinases: Current status and future directions. Oncologist 2009, 14, 378–390. [Google Scholar] [CrossRef]
- Javle, M.; Smyth, E.C.; Chau, I. Ramucirumab: Successfully targeting angiogenesis in gastric cancer. Clin. Cancer Res. 2014, 20, 5875–5881. [Google Scholar] [CrossRef]
- Rey, J.B.; Launay-Vacher, V.; Tournigand, C. Regorafenib as a single-agent in the treatment of patients with gastrointestinal tumors: An overview for pharmacists. Target. Oncol. 2015, 10, 199–213. [Google Scholar] [CrossRef]
- Ivanova, J.I.; Saverno, K.R.; Sung, J.; Duh, M.S.; Zhao, C.; Cai, S.; Vekeman, F.; Peevyhouse, A.; Dhawan, R.; Fuchs, C.S. Real-world treatment patterns and effectiveness among patients with metastatic colorectal cancer treated with ziv-aflibercept in community oncology practices in the USA. Med. Oncol. 2017, 34, 193. [Google Scholar] [CrossRef]
- Ribatti, D. Tumor refractoriness to anti-VEGF therapy. Oncotarget 2016, 7, 46668–46677. [Google Scholar] [CrossRef]
- Gacche, R.N.; Assaraf, Y.G. Redundant angiogenic signaling and tumor drug resistance. Drug Resist. Updates 2018, 36, 47–76. [Google Scholar] [CrossRef] [PubMed]
- van Beijnum, J.R.; Nowak-Sliwinska, P.; Huijbers, E.J.; Thijssen, V.L.; Griffioen, A.W. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol. Rev. 2015, 67, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Sakurai, Y.; Hida, Y.; Harashima, H. Heterogeneity of tumor endothelial cells and drug delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt B, 140–147. [Google Scholar] [CrossRef]
- Meka, R.R.; Mukherjee, S.; Patra, C.R.; Chaudhuri, A. Shikimoyl-ligand decorated gold nanoparticles for use in ex vivo engineered dendritic cell based DNA vaccination. Nanoscale 2019, 11, 7931–7943. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, B.; Mukherjee, S.; Patra, C.R.; Iyer, P.K. Amplified Fluorescence from Polyfluorene Nanoparticles with Dual State Emission and Aggregation Caused Red Shifted Emission for Live Cell Imaging and Cancer Theranostics. ACS Appl. Mater. Interfaces 2016, 8, 32220–32229. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Mukherjee, S.; Punugupati, N.; Vasudevan, D.; Patra, C.R.; Narayan, R.; Vsn Kothapalli, R. Facile synthesis of carbon dot and residual carbon nanobeads: Implications for ion sensing, medicinal and biological applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 643–652. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic Properties of Gold Nanoparticles. Clin. Cancer Res. 2005, 11, 3530. [Google Scholar] [CrossRef]
- Barui, A.K.; Veeriah, V.; Mukherjee, S.; Manna, J.; Patel, A.K.; Patra, S.; Pal, K.; Murali, S.; Rana, R.K.; Chatterjee, S.; et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869. [Google Scholar] [CrossRef]
- Afsharzadeh, M.; Abnous, K.; Yazdian-Robati, R.; Ataranzadeh, A.; Ramezani, M.; Hashemi, M. Formulation and evaluation of anticancer and antiangiogenesis efficiency of PLA-PEG nanoparticles loaded with galbanic acid in C26 colon carcinoma, in vitro and in vivo. J. Cell. Physiol. 2019, 234, 6099–6107. [Google Scholar] [CrossRef]
- Min, H.; Wang, J.; Qi, Y.; Zhang, Y.; Han, X.; Xu, Y.; Xu, J.; Li, Y.; Chen, L.; Cheng, K.; et al. Biomimetic Metal-Organic Framework Nanoparticles for Cooperative Combination of Antiangiogenesis and Photodynamic Therapy for Enhanced Efficacy. Adv. Mater. 2019, 31, e1808200. [Google Scholar] [CrossRef]
- Yu, M.; Su, D.; Yang, Y.; Qin, L.; Hu, C.; Liu, R.; Zhou, Y.; Yang, C.; Yang, X.; Wang, G.; et al. D-T7 Peptide-Modified PEGylated Bilirubin Nanoparticles Loaded with Cediranib and Paclitaxel for Antiangiogenesis and Chemotherapy of Glioma. ACS Appl. Mater. Interfaces 2019, 11, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sriram, P.; Barui, A.K.; Nethi, S.K.; Veeriah, V.; Chatterjee, S.; Suresh, K.I.; Patra, C.R. Graphene Oxides Show Angiogenic Properties. Adv. Healthc. Mater. 2015, 4, 1722–1732. [Google Scholar] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
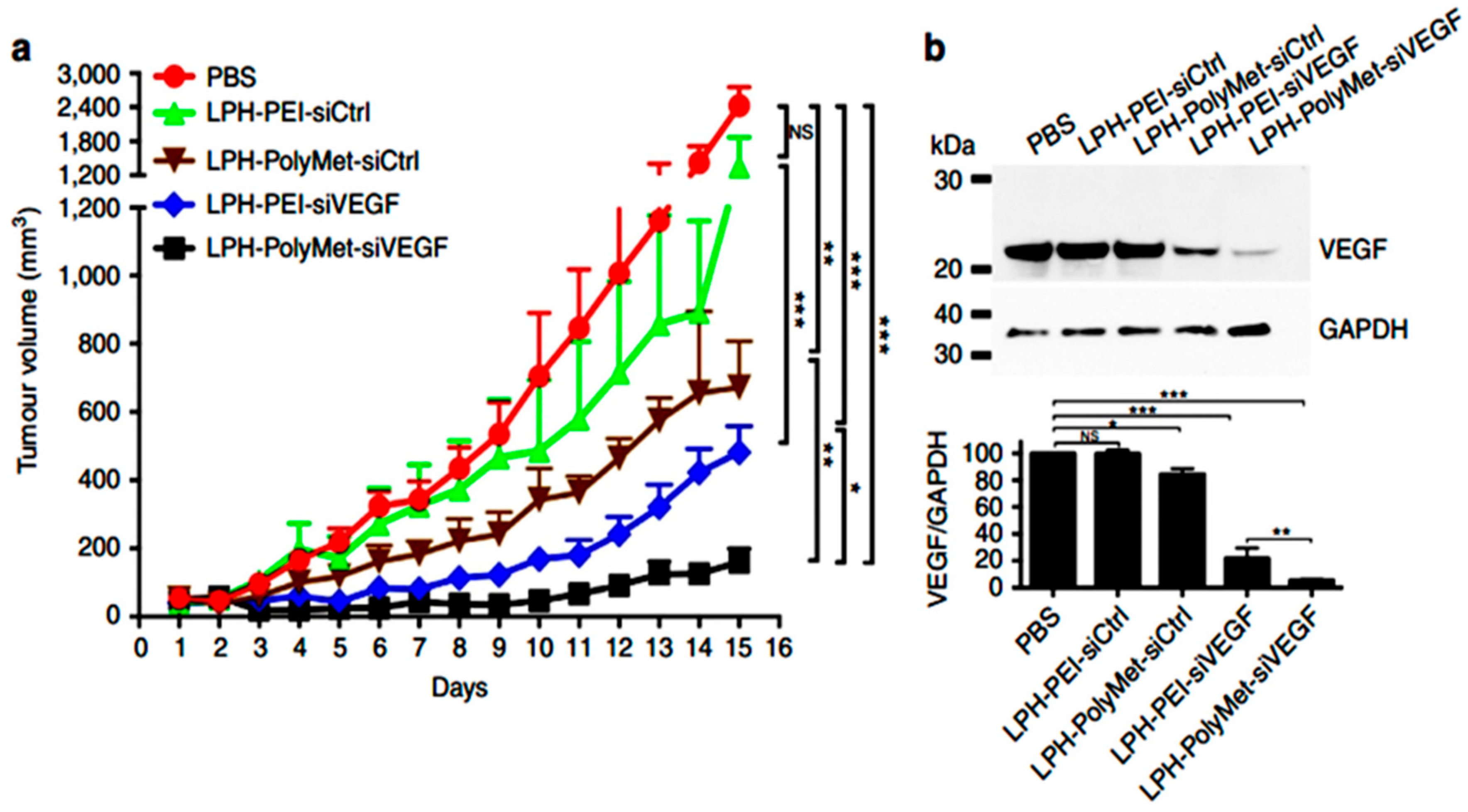
- Zhao, Y.; Wang, W.; Guo, S.; Wang, Y.; Miao, L.; Xiong, Y.; Huang, L. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 2016, 7, 11822. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhao, Y.; Miao, L.; Satterlee, A.; Haynes, M.; Luo, C.; Musetti, S.; Huang, L. Dual Functional LipoMET Mediates Envelope-type Nanoparticles to Combinational Oncogene Silencing and Tumor Growth Inhibition. Mol. Ther. 2017, 25, 1567–1579. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Li, J.-Q.; Wang, Z.-Z.; Dong, D.-W.; Qi, X.-R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014, 35, 5226–5239. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Song, W.-J.; Sun, T.-M.; Zhang, P.-Z.; Wang, J. Targeted Delivery of Antisense Inhibitor of miRNA for Antiangiogenesis Therapy Using cRGD-Functionalized Nanoparticles. Mol. Pharm. 2010, 8, 250–259. [Google Scholar] [CrossRef]
- Yang, Z.; Xiang, B.; Dong, D.; Wang, Z.; Li, J.; Qi, X. Dual Receptor-Specific Peptides Modified Liposomes as VEGF siRNA Vector for Tumor-Targeting Therapy. Curr. Gene Ther. 2014, 14, 289–299. [Google Scholar] [CrossRef]
- Guo, P.; Yang, J.; Jia, D.; Moses, M.A.; Auguste, D.T. ICAM-1-Targeted, Lcn2 siRNA-Encapsulating Liposomes are Potent Anti-angiogenic Agents for Triple Negative Breast Cancer. Theranostics 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Shao, R.; Xu, Y.; Gao, J.-Q.; Liang, W.-Q. VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075–6088. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, J.; Chen, X. Effect of surface properties on liposomal siRNA delivery. Biomaterials 2016, 79, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2017, 46, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Chen, W.-L.; Wang, D.-D.; Zhou, Y.-J.; You, B.-G.; Liu, Y.; Qu, C.-X.; Yang, S.-D.; Chen, M.-T.; et al. Co-delivery of VEGF siRNA and Etoposide for Enhanced Anti-angiogenesis and Anti-proliferation Effect via Multi-functional Nanoparticles for Orthotopic Non-Small Cell Lung Cancer Treatment. Theranostics 2019, 9, 5886–5898. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Hasegawa, M.; Ayano, E.; Maitani, Y.; Kanazawa, H. Effect of Polymer Phase Transition Behavior on Temperature-Responsive Polymer-Modified Liposomes for siRNA Transfection. Int. J. Mol. Sci. 2019, 20, 430. [Google Scholar] [CrossRef]
- Golkar, N.; Samani, S.M.; Tamaddon, A.M. Modulated cellular delivery of anti-VEGF siRNA (bevasiranib) by incorporating supramolecular assemblies of hydrophobically modified polyamidoamine dendrimer in stealth liposomes. Int. J. Pharm. 2016, 510, 30–41. [Google Scholar] [CrossRef]
- Lee, S.J.; Yook, S.; Yhee, J.Y.; Yoon, H.Y.; Kim, M.-G.; Ku, S.H.; Kim, S.H.; Park, J.H.; Jeong, J.H.; Kwon, I.C.; et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J. Control. Release 2015, 220, 631–641. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Ghiyami-Hour, F.; Jahangiri, S.; Negahdari, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. Nanoparticles as new tools for inhibition of cancer angiogenesis. J. Cell. Physiol. 2018, 233, 2902–2910. [Google Scholar] [CrossRef]
- Clavreul, A.; Roger, E.; Pourbaghi-Masouleh, M.; Lemaire, L.; Tétaud, C.; Menei, P. Development and characterization of sorafenib-loaded lipid nanocapsules for the treatment of glioblastoma. Drug Deliv. 2018, 25, 1756–1765. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, T.; Liu, Y.; Zhang, N. Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1374–1383. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Cai, M.; Lin, L.; Chen, X.; Cao, Z.; Zhu, K.; Shuai, X. Codelivery of sorafenib and GPC3 siRNA with PEI-modified liposomes for hepatoma therapy. Biomater. Sci. 2017, 5, 2468–2479. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.-C.; Sung, Y.-C.; Ramjiawan, R.R.; Lin, T.-T.; Chang, C.-C.; Jeng, K.-S.; Chang, C.-F.; Liu, C.-H.; Gao, D.-Y.; et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci. Rep. 2017, 7, 44123. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Madamsetty, V.S.; Dutta, S.K.; Mukhopadhyay, D. Co-delivery of everolimus and vinorelbine via a tumor-targeted liposomal formulation inhibits tumor growth and metastasis in RCC. Int. J. Nanomed. 2019, 14, 5109–5123. [Google Scholar] [CrossRef] [PubMed]
- Abud, M.B.; Louzada, R.N.; Isaac, D.L.C.; Souza, L.G.; dos Reis, R.G.; Lima, E.M.; de Ávila, M.P. In vivo and in vitro toxicity evaluation of liposome-encapsulated sirolimus. Int. J. Retin. Vitr. 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Chang, I.H.; Goo, Y.T.; Kim, C.H.; Kang, T.H.; Kim, S.-Y.; Lee, S.J.; Song, S.H.; Whang, Y.M.; Choi, Y.W. Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. Int. J. Nanomed. 2019, 14, 6249–6268. [Google Scholar] [CrossRef]
- Iwase, Y.; Maitani, Y. Preparation and in Vivo Evaluation of Liposomal Everolimus for Lung Carcinoma and Thyroid Carcinoma. Biol. Pharm. Bull. 2012, 35, 975–979. [Google Scholar] [CrossRef][Green Version]
- Banerjee, I.; De, K.; Mukherjee, D.; Dey, G.; Chattopadhyay, S.; Mukherjee, M.; Mandal, M.; Bandyopadhyay, A.K.; Gupta, A.; Ganguly, S.; et al. Paclitaxel-loaded solid lipid nanoparticles modified with Tyr-3-octreotide for enhanced anti-angiogenic and anti-glioma therapy. Acta Biomater. 2016, 38, 69–81. [Google Scholar] [CrossRef]
- Abu-Lila, A.; Suzuki, T.; Doi, Y.; Ishida, T.; Kiwada, H. Oxaliplatin targeting to angiogenic vessels by PEGylated cationic liposomes suppresses the angiogenesis in a dorsal air sac mouse model. J. Control. Release 2009, 134, 18–25. [Google Scholar] [CrossRef]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef]
- Bhattarai, P.; Hameed, S.; Dai, Z. Recent advances in anti-angiogenic nanomedicines for cancer therapy. Nanoscale 2018, 10, 5393–5423. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor Angiogenesis and Metastasis—Correlation in Invasive Breast Carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
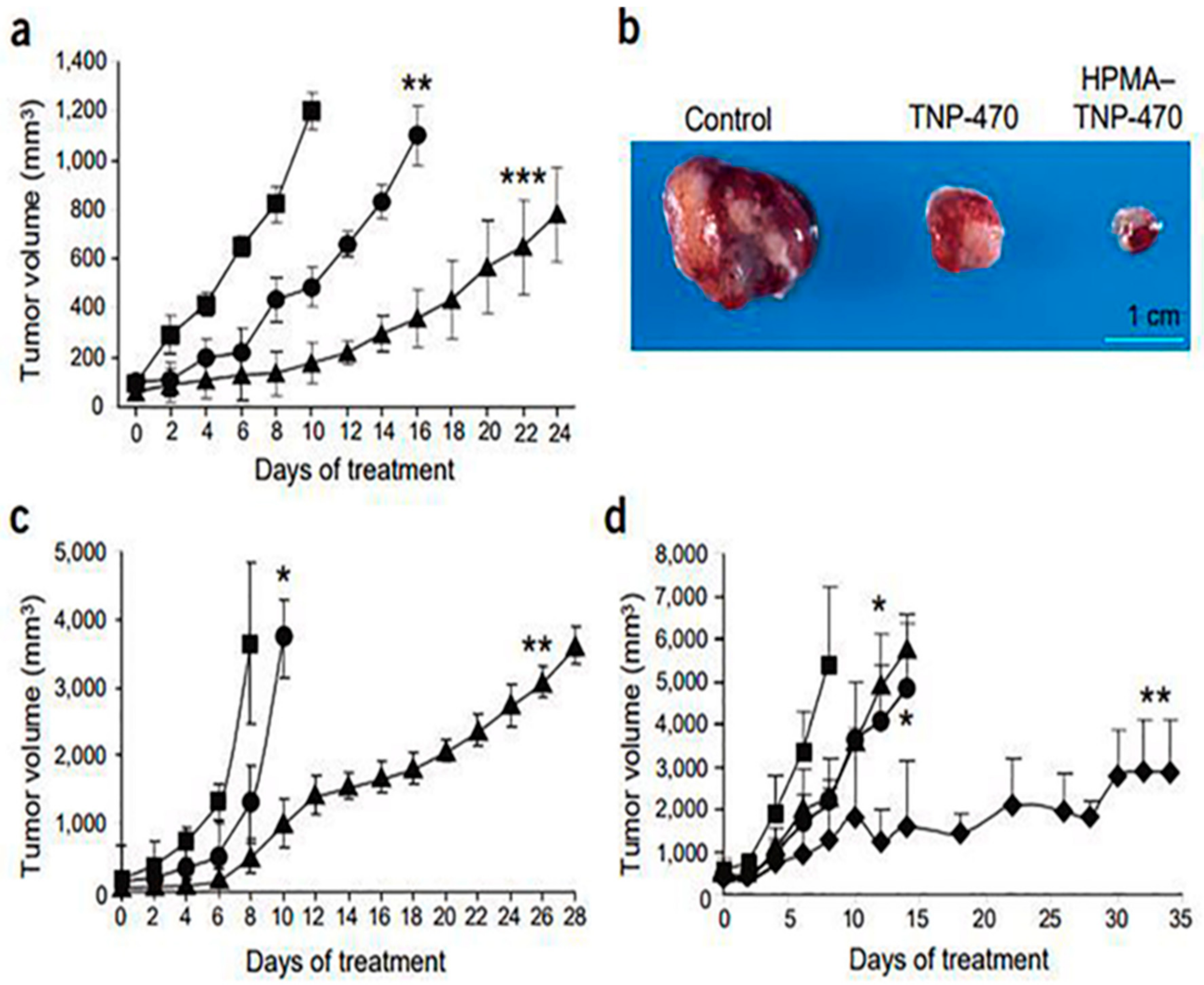
- Satchi-Fainaro, R.; Puder, M.; Davies, J.W.; Tran, H.T.; Sampson, D.A.; Greene, A.K.; Corfas, G.; Folkman, J. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat. Med. 2004, 10, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Benny, O.; Fainaru, O.; Adini, A.; Cassiola, F.; Bazinet, L.; Adini, I.; Pravda, E.; Nahmias, Y.; Koirala, S.; Corfas, G.; et al. An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nat. Biotechnol. 2008, 26, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, R.; Basu, S.; Soni, S.; Hentschel, D.M.; Mashelkar, R.A.; Sengupta, S. Nanoparticle-mediated targeting of phosphatidylinositol-3-kinase signaling inhibits angiogenesis. Angiogenesis 2009, 12, 325–338. [Google Scholar] [CrossRef]
- Dahmani, F.Z.; Xiao, Y.; Zhang, J.; Yu, Y.; Zhou, J.; Yao, J. Multifunctional Polymeric Nanosystems for Dual-Targeted Combinatorial Chemo/Antiangiogenesis Therapy of Tumors. Adv. Healthc. Mater. 2016, 5, 1447–1461. [Google Scholar]
- Tian, F.; Dahmani, F.Z.; Qiao, J.; Ni, J.; Xiong, H.; Liu, T.; Zhou, J.; Yao, J. A targeted nanoplatform co-delivering chemotherapeutic and antiangiogenic drugs as a tool to reverse multidrug resistance in breast cancer. Acta Biomater. 2018, 75, 398–412. [Google Scholar] [CrossRef]
- Prasad, P.; Shuhendler, A.; Cai, P.; Rauth, A.M.; Wu, X.Y. Doxorubicin and mitomycin C co-loaded polymer-lipid hybrid nanoparticles inhibit growth of sensitive and multidrug resistant human mammary tumor xenografts. Cancer Lett. 2013, 334, 263–273. [Google Scholar] [CrossRef]
- Zhang, T.; Prasad, P.; Cai, P.; He, C.; Shan, D.; Rauth, A.M.; Wu, X.Y. Dual-targeted hybrid nanoparticles of synergistic drugs for treating lung metastases of triple negative breast cancer in mice. Acta Pharmacol. Sin. 2017, 38, 835–847. [Google Scholar] [CrossRef]
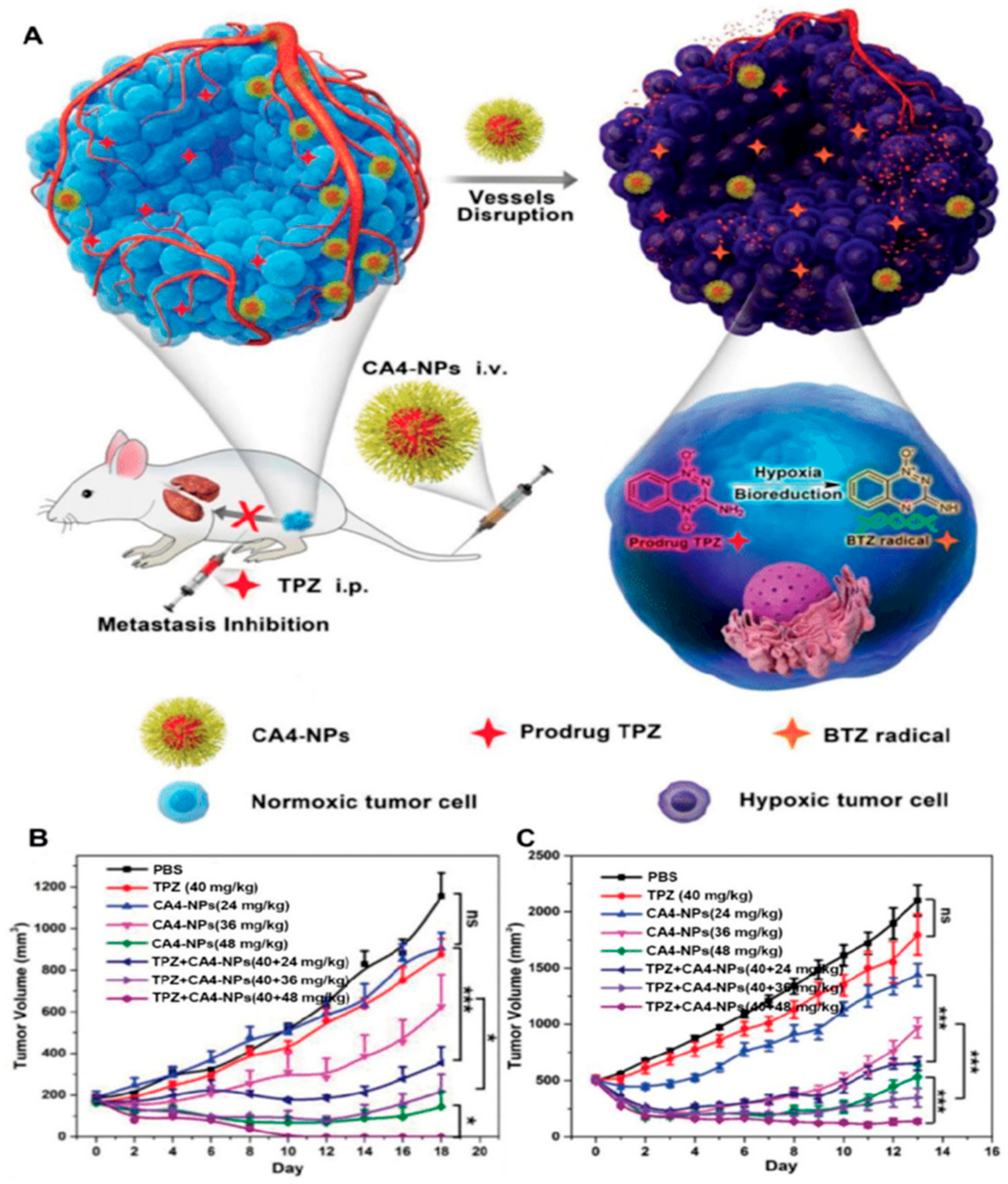
- Yang, S.; Tang, Z.; Hu, C.; Zhang, D.; Shen, N.; Yu, H.; Chen, X. Selectively Potentiating Hypoxia Levels by Combretastatin A4 Nanomedicine: Toward Highly Enhanced Hypoxia-Activated Prodrug Tirapazamine Therapy for Metastatic Tumors. Adv. Mater. 2019, 31, 1805955. [Google Scholar] [CrossRef]
- Tandle, A.; Blazer, D.G.; Libutti, S.K. Antiangiogenic gene therapy of cancer: Recent developments. J. Transl. Med. 2004, 2, 22. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, E.J.; Lee, Y.K.; Kim, K.; Kwon, I.C.; Lee, K.Y. Optical Imaging and Gene Therapy with Neuroblastoma-Targeting Polymeric Nanoparticles for Potential Theranostic Applications. Small 2016, 12, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Osada, K.; Ge, Z.; Uchida, S.; Tockary, T.A.; Dirisala, A.; Matsui, A.; Toh, K.; Takeda, K.M.; Liu, X.; et al. Polyplex micelle installing intracellular self-processing functionalities without free catiomers for safe and efficient systemic gene therapy through tumor vasculature targeting. Biomaterials 2017, 113, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, Y.; Cao, C.; Le, F.; Qin, X.; Sun, D.; Liu, J. The use of pH-sensitive functional selenium nanoparticles shows enhanced in vivo VEGF-siRNA silencing and fluorescence imaging. Nanoscale 2014, 6, 9279–9292. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, Z.; Zeng, X.; Chen, X.; Gu, Z. Advances in nanomedicine for cancer starvation therapy. Theranostics 2019, 9, 8026–8047. [Google Scholar] [CrossRef]
- Sun, H.; Dong, Y.; Feijen, J.; Zhong, Z. Peptide-decorated polymeric nanomedicines for precision cancer therapy. J. Control. Release 2018, 290, 11–27. [Google Scholar] [CrossRef]
- Pan, F.; Yang, W.; Li, W.; Yang, X.-Y.; Liu, S.; Li, X.; Zhao, X.; Ding, H.; Qin, L.; Pan, Y. Conjugation of gold nanoparticles and recombinant human endostatin modulates vascular normalization via interruption of anterior gradient 2–mediated angiogenesis. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, W.; Choi, C.; Kim, S.Y.; Son, A.; Kim, H.; Lee, N.; Park, H.C. Fucoidan-Manganese Dioxide Nanoparticles Potentiate Radiation Therapy by Co-Targeting Tumor Hypoxia and Angiogenesis. Mar. Drugs 2018, 16, 510. [Google Scholar] [CrossRef]
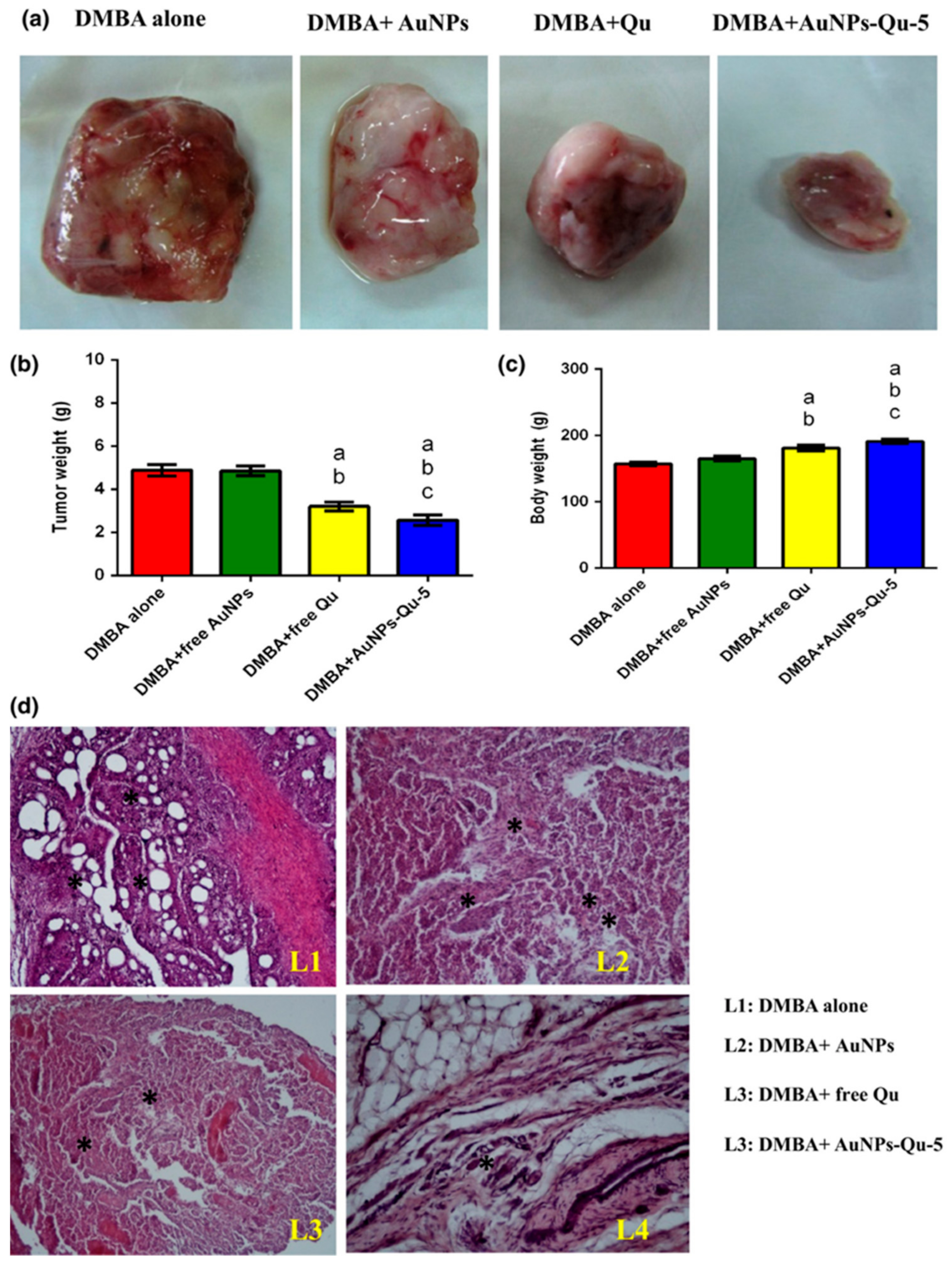
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasivenessviaEGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Pan, Y.; Ding, H.; Qin, L.; Zhao, X.; Cai, J.; Du, B. Gold nanoparticles induce nanostructural reorganization of VEGFR2 to repress angiogenesis. J. Biomed. Nanotechnol. 2013, 9, 1746–1756. [Google Scholar] [CrossRef]
- Gurunathan, S.; Lee, K.-J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Song, H.; Wang, W.; Zhao, P.; Qi, Z.; Zhao, S. Cuprous oxide nanoparticles inhibit angiogenesis via down regulation of VEGFR2 expression. Nanoscale 2014, 6, 3206–3216. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Karakoti, A.; Graham, R.P.; Maguire, J.L.; Reilly, C.M.; Seal, S.; Rattan, R.; Shridhar, V. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS ONE 2013, 8, e54578. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Leong, D.T. Mesoporous Silica Nanoparticles as an Antitumoral-Angiogenesis Strategy. ACS Appl. Mater. Interfaces 2017, 9, 6690–6703. [Google Scholar] [CrossRef] [PubMed]
- Neek, M.; Kim, T.I.; Wang, S.-W. Protein-based nanoparticles in cancer vaccine development. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 164–174. [Google Scholar] [CrossRef]
- Diaz, D.; Care, A.; Sunna, A. Bioengineering Strategies for Protein-Based Nanoparticles. Genes 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, N.K.; Kim, I.-S. Bioengineered protein-based nanocage for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Sandra, F.; Khaliq, N.U.; Sunna, A.; Care, A. Developing Protein-Based Nanoparticles as Versatile Delivery Systems for Cancer Therapy and Imaging. Nanomaterials 2019, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood–Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Yang, K.; Kong, C.; Liu, C.; Chen, H.; Huang, J.; Qian, F. Tumor Progression of Non-Small Cell Lung Cancer Controlled by Albumin and Micellar Nanoparticles of Itraconazole, a Multitarget Angiogenesis Inhibitor. Mol. Pharm. 2017, 14, 4705–4713. [Google Scholar] [CrossRef]
- Kommareddy, S.; Amiji, M. Antiangiogenic gene therapy with systemically administered sFlt-1 plasmid DNA in engineered gelatin-based nanovectors. Cancer Gene Ther. 2007, 14, 488–498. [Google Scholar] [CrossRef]
- Lizotte, P.H.; Wen, A.M.; Sheen, M.R.; Fields, J.; Rojanasopondist, P.; Steinmetz, N.F.; Fiering, S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2015, 11, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.A.; Wang, C.; Fiering, S.; Steinmetz, N.F. In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Mol. Pharm. 2018, 15, 3700–3716. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Beiss, V.; Steinmetz, N.F.; Simon, A.E. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019, 93, e00129-19. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.H.; Lewis, J.D. Cowpea mosaic virus nanoparticles for cancer imaging and therapy. Adv. Drug Deliv. Rev. 2019, 145, 130–144. [Google Scholar] [CrossRef]
- Czapar, A.E.; Steinmetz, N.F. Plant viruses and bacteriophages for drug delivery in medicine and biotechnology. Curr. Opin. Chem. Biol. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Gamper, C.; Spenlé, C.; Boscá, S.; van der Heyden, M.; Erhardt, M.; Orend, G.; Bagnard, D.; Heinlein, M. Functionalized Tobacco Mosaic Virus Coat Protein Monomers and Oligomers as Nanocarriers for Anti-Cancer Peptides. Cancers 2019, 11, 1609. [Google Scholar] [CrossRef]
- Brunel, F.M.; Lewis, J.D.; Destito, G.; Steinmetz, N.F.; Manchester, M.; Stuhlmann, H.; Dawson, P.E. Hydrazone Ligation Strategy to Assemble Multifunctional Viral Nanoparticles for Cell Imaging and Tumor Targeting. Nano Lett. 2010, 10, 1093–1097. [Google Scholar] [CrossRef]
- Lewis, J.D.; Destito, G.; Zijlstra, A.; Gonzalez, M.J.; Quigley, J.P.; Manchester, M.; Stuhlmann, H. Viral nanoparticles as tools for intravital vascular imaging. Nat. Med. 2006, 12, 354–360. [Google Scholar] [CrossRef]
- Steinmetz, N.F. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 634–641. [Google Scholar] [CrossRef]
- Niehl, A.; Appaix, F.; Boscá, S.; van der Sanden, B.; Nicoud, J.-F.; Bolze, F.; Heinlein, M. Fluorescent Tobacco mosaic virus-Derived Bio-Nanoparticles for Intravital Two-Photon Imaging. Front. PlantSci. 2016, 6, 1244. [Google Scholar]
- Cho, C.-F.; Yu, L.; Nsiama, T.K.; Kadam, A.N.; Raturi, A.; Shukla, S.; Amadei, G.A.; Steinmetz, N.F.; Luyt, L.G.; Lewis, J.D. Viral nanoparticles decorated with novel EGFL7 ligands enable intravital imaging of tumor neovasculature. Nanoscale 2017, 9, 12096–12109. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.A.; Lim, V.; Yusof, N.A.; Khor, K.Z.; Rahman, H.S.; Abdul Samad, N. Antiangiogenic properties of nanoparticles: A systematic review. Int. J. Nanomed. 2019, 14, 5135–5146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, Y.; An, Y.; Kim, B.Y.S. Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano 2015, 9, 8689–8696. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Xiong, H.; Xu, C.; Lu, Y.; Yao, J. Attempts to strengthen and simplify the tumor vascular normalization strategy using tumor vessel normalization promoting nanomedicines. Biomater. Sci. 2019, 7, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, H.; Hao, D.; Zhang, X.; Wang, F. The functions and applications of A7R in anti-angiogenic therapy, imaging and drug delivery systems. Asian J. Pharm. Sci. 2019, 14, 595–608. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Pal, K.; Keshavan, S.; Caulfield, T.R.; Dutta, S.K.; Wang, E.; Fadeel, B.; Mukhopadhyay, D. Development of multi-drug loaded PEGylated nanodiamonds to inhibit tumor growth and metastasis in genetically engineered mouse models of pancreatic cancer. Nanoscale 2019, 11, 22006–22018. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, L.-J.; Liu, L.; Chen, X.; Chen, P.; Yang, G.-L.; Hou, W.-L.; Tang, M.-H.; Zhang, F.; Wang, X.-H.; et al. Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity. Exp. Mol. Med. 2008, 40, 617. [Google Scholar] [CrossRef]
- Jiang, Q.-Q.; Fan, L.-Y.; Yang, G.-L.; Guo, W.-H.; Hou, W.-L.; Chen, L.-J.; Wei, Y.-Q. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer 2008, 8, 242. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; He, X.; Fan, L.; Yang, G.; Chen, X.; Lin, X.; Du, L.; Li, Z.; Ye, H.; et al. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int. J. Gynecol. Cancer 2008, 18, 652. [Google Scholar] [CrossRef]
- Strijkers, G.J.; Kluza, E.; Van Tilborg, G.A.F.; van der Schaft, D.W.J.; Griffioen, A.W.; Mulder, W.J.M.; Nicolay, K. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis 2010, 13, 161–173. [Google Scholar] [CrossRef]
- Brandwijk, R.J.M.G.E.; Mulder, W.J.M.; Nicolay, K.; Mayo, K.H.; Thijssen, V.L.J.L.; Griffioen, A.W. Anginex-Conjugated Liposomes for Targeting of Angiogenic Endothelial Cells. Bioconjugate Chem. 2007, 18, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Madamsetty, V.S.; Dutta, S.K.; Wang, E.; Angom, R.S.; Mukhopadhyay, D. Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer. NPJ Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M.; Peira, E.; Chirio, D.; Solazzi, I.; Giordano, S.M.A.; Gigliotti, C.L.; Riganti, C.; Dianzani, C. Bevacizumab loaded solid lipid nanoparticles prepared by the coacervation technique: Preliminary in vitro studies. Nanotechnology 2015, 26, 255102. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, D.; Pastorino, F.; Zuccari, G.; Caffa, I.; Loi, M.; Marimpietri, D.; Brignole, C.; Perri, P.; Cilli, M.; Nico, B.; et al. Enhanced anti-tumor and anti-angiogenic efficacy of a novel liposomal fenretinide on human neuroblastoma. J. Control. Release 2013, 170, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, D.; Ambrogio, C.; Pastorino, F.; Brignole, C.; Martinengo, C.; Carosio, R.; Loi, M.; Pagnan, G.; Emionite, L.; Cilli, M.; et al. Selective Therapeutic Targeting of the Anaplastic Lymphoma Kinase With Liposomal siRNA Induces Apoptosis and Inhibits Angiogenesis in Neuroblastoma. Mol. Ther. 2011, 19, 2201–2212. [Google Scholar] [CrossRef]
- Piaggio, F.; Kondylis, V.; Pastorino, F.; Di Paolo, D.; Perri, P.; Cossu, I.; Schorn, F.; Marinaccio, C.; Murgia, D.; Daga, A.; et al. A novel liposomal Clodronate depletes tumor-associated macrophages in primary and metastatic melanoma: Anti-angiogenic and anti-tumor effects. J. Control. Release 2016, 223, 165–177. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles-conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt-mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef]
- Bhat, F.A.; Sharmila, G.; Balakrishnan, S.; Arunkumar, R.; Elumalai, P.; Suganya, S.; Raja Singh, P.; Srinivasan, N.; Arunakaran, J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J. Nutr. Biochem. 2014, 25, 1132–1139. [Google Scholar] [CrossRef]
- Gautam, S.; Pratheeshkumar, P.; Budhraja, A.; Son, Y.-O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; et al. Quercetin Inhibits Angiogenesis Mediated Human Prostate Tumor Growth by Targeting VEGFR- 2 Regulated AKT/mTOR/P70S6K Signaling Pathways. PLoS ONE 2012, 7, e47516. [Google Scholar]
- Darweesh, R.S.; Ayoub, N.M.; Nazzal, S. Gold nanoparticles and angiogenesis: Molecular mechanisms and biomedical applications. Int. J. Nanomed. 2019, 14, 7643–7663. [Google Scholar] [CrossRef]
- Seo, S.J.; Lee, S.H.; Kim, K.H.; Kim, J.K. Anti-Flt1 peptide and cyanine-conjugated gold nanoparticles for the concurrent antiangiogenic and endothelial cell proton treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Heuer-Jungemann, A.; Fernandes, A.R.; Kanaras, A.G.; Baptista, P.V. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomed. 2016, 11, 2633–2639. [Google Scholar]
- Bartczak, D.; Muskens, O.L.; Sanchez-Elsner, T.; Kanaras, A.G.; Millar, T.M. Manipulation of in vitro angiogenesis using peptide-coated gold nanoparticles. ACS Nano 2013, 7, 5628–5636. [Google Scholar] [CrossRef]
- Banerjee, D.; Harfouche, R.; Sengupta, S. Nanotechnology-mediated targeting of tumor angiogenesis. Vasc. Cell 2011, 3, 3. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2010, 28, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Guan, Y.-Y.; Luan, X.; Lu, Q.; Wang, C.; Liu, H.-J.; Gao, Y.-G.; Yang, S.-C.; Dong, X.; Chen, H.-Z.; et al. Delta-like ligand 4-targeted nanomedicine for antiangiogenic cancer therapy. Biomaterials 2015, 42, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, M.; Das, S.; Mert, I.; Gupta, A.; Al-Wahab, Z.; Tebbe, C.; Dar, S.; Chhina, J.; Giri, S.; Munkarah, A.; et al. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer 2016, 16, 220. [Google Scholar] [CrossRef]
- Vassie, J.A.; Whitelock, J.M.; Lord, M.S. Targeted Delivery and Redox Activity of Folic Acid-Functionalized Nanoceria in Tumor Cells. Mol. Pharm. 2018, 15, 994–1004. [Google Scholar] [CrossRef]
- Mousa, S.A.; Yalcin, M.; Bharali, D.J.; Meng, R.; Tang, H.-Y.; Lin, H.-Y.; Davis, F.B.; Davis, P.J. Tetraiodothyroacetic acid and its nanoformulation inhibit thyroid hormone stimulation of non-small cell lung cancer cells in vitro and its growth in xenografts. Lung Cancer 2012, 76, 39–45. [Google Scholar] [CrossRef]
- Li, W.; Yalcin, M.; Bharali, D.J.; Lin, Q.; Godugu, K.; Fujioka, K.; Keating, K.A.; Mousa, S.A. Pharmacokinetics, Biodistribution, and Anti-Angiogenesis Efficacy of Diamino Propane Tetraiodothyroacetic Acid-conjugated Biodegradable Polymeric Nanoparticle. Sci. Rep. 2019, 9, 9006. [Google Scholar] [CrossRef]
- Chwalibog, A.; Hotowy, A.; Grodzik, M.; Wierzbicki, M.; Mitura, K.; Orlowski, P.; Sawosz, E.; Szmidt, M.; Niemiec, T. Nanoparticles of carbon allotropes inhibit glioblastoma multiforme angiogenesis in ovo. Int. J. Nanomed. 2011, 6, 3041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, T.; Yao, Q.; Cao, F.; Liu, Q.; Liu, B.; Wang, X. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: Insight into the cytotoxicity and antiangiogenesis. Int. J. Nanomed. 2016, 11, 6679–6692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y. Alphastatin-Loaded Chitosan Nanoparticle Preparation and Its Antiangiogenic Effect on Lung Carcinoma. Int. J. Polym. Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 1–29. [Google Scholar] [CrossRef]
- Wierzbicki, M.; Sawosz, E.; Strojny, B.; Jaworski, S.; Grodzik, M.; Chwalibog, A. NF-κB-related decrease of glioma angiogenic potential by graphite nanoparticles and graphene oxide nanoplatelets. Sci. Rep. 2018, 8, 14733. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ponrasu, T.; Chandel, S.; Dixit, M.; Muthuvijayan, V. Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications. R. Soc. Open Sci. 2018, 5, 172017. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Shi, W.; Lang, M. pH-responsive carboxymethyl chitosan-derived micelles as apatinib carriers for effective anti-angiogenesis activity: Preparation and in vitro evaluation. Carbohydr. Polym. 2017, 176, 107–116. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Owaid, I.H.; Singh, M. Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer. Drug Deliv. 2015, 23, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Hrynyk, M.; Ellis, J.P.; Haxho, F.; Allison, S.; Steele, J.A.; Abdulkhalek, S.; Neufeld, R.J.; Szewczuk, M.R. Therapeutic designed poly (lactic-co-glycolic acid) cylindrical oseltamivir phosphate-loaded implants impede tumor neovascularization, growth and metastasis in mouse model of human pancreatic carcinoma. Drug Des. Dev. Ther. 2015, 9, 4573–4586. [Google Scholar]
- Wang, J.; Guo, F.; Yu, M.; Liu, L.; Tan, F.; Yan, R.; Li, N. Rapamycin/DiR loaded lipid-polyaniline nanoparticles for dual-modal imaging guided enhanced photothermal and antiangiogenic combination therapy. J. Control. Release 2016, 237, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, M.; Pan, L.; Shi, J. Tumor vascular-targeted co-delivery of anti-angiogenesis and chemotherapeutic agents by mesoporous silica nanoparticle-based drug delivery system for synergetic therapy of tumor. Int. J. Nanomed. 2015, 11, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Shi, Z.; Yang, Y.; Xie, X.; Lee, S.M.; Wang, Y.; Leong, K.W.; Chen, M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017, 58, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Huang, L.; Miao, L.; Zhou, J.; Satterlee, A.B.; Yao, J. Sigma receptor-mediated targeted delivery of anti-angiogenic multifunctional nanodrugs for combination tumor therapy. J. Control. Release 2016, 228, 107–119. [Google Scholar] [CrossRef] [PubMed]

| Drug (Trade Name) | Structure | Chemical Name, Target, and FDA Approved to Treat Patients with | Ref. |
|---|---|---|---|
| Bevacizumab (Avastin®) | Anti-VEGF monoclonal antibody | Anti-VEGF monoclonal antibody Cervical cancer: Nonresponsive to other treatment/metastastatic/recurrent. Colorectal cancer: metastastatic. Glioblastoma: Nonresponsive to other treatment/recurrent. Nonsquamous non-small cell lung cancer: locally advanced, nonresectable/metastastatic/recurrent. Ovarian epithelial, fallopiantube, orprimary peritoneal cancer: stage III/stage IV/recurrent. Renal cell carcinoma: metastastatic. | [13,43,44,45] |
| Thalidomide (Synovir, Thalomid®) |  | (±)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione Immune modulator and inhibits VEGF and bFGF production Multiple myeloma: newly diagnosed. | [46] |
| Lenalidomide (Revlimid®) | 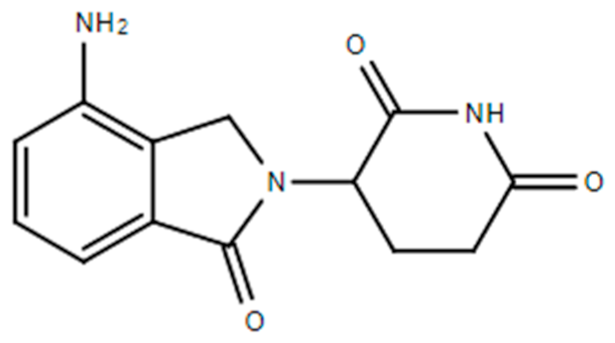 | 1-oxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline VEGF-induced PI3K-Akt pathway signaling and HIF-1α expression Anemia associated with certain types of myelodysplastic syndromes. Follicular lymphoma: Nonresponsive to other treatment. Mantle cell lymphoma: Nonresponsive to other treatment/recurrent. Marginal zone lymphoma: Nonresponsive to other treatment. Multiple myeloma and as maintenance therapy | [13,42,47] |
| Sorafenib (Nexavar®) | 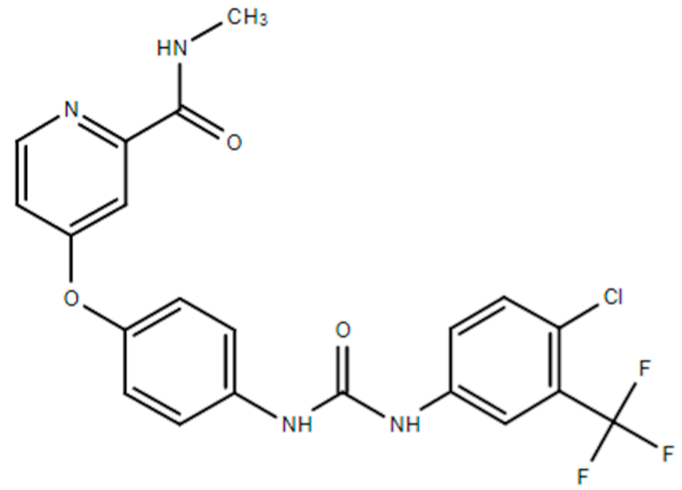 | 4-[4-(([4-chloro-3-(trifluoromethyl)phenyl]carbamoyl)amino)phenoxy]-N-methylpyridine-2-carboxamide Small molecule inhibitors of the VEGFR-2 tyrosine kinase activity. Hepatocellular carcinoma: Nonresectable. Renal cell carcinoma: Advanced. Thyroid cancer: Progressive/metastastatic/recurrent. | [41,48] |
| Sunitinib (Sutent®) | 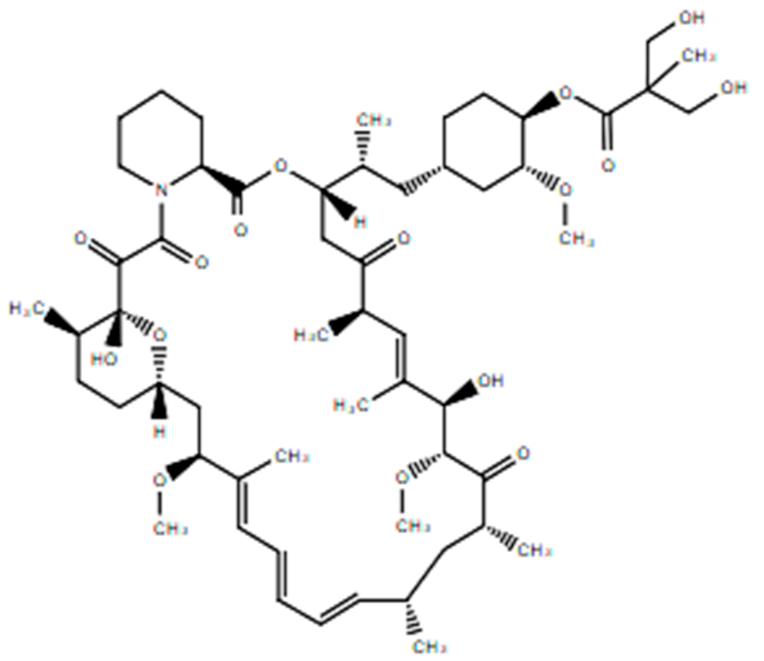 | (Z)-N-(2-(diethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide Small molecule inhibitors of the VEGFR-2 tyrosine kinase Gastrointestinal stromal tumor: nonresponsive to imatinibmesylate. Pancreatic cancer: progressive neuroendocrine tumors that are nonresectable/metastastatic. Renal cell carcinoma: advanced disease. | [13,41,42,49] |
| Temsirolimus | 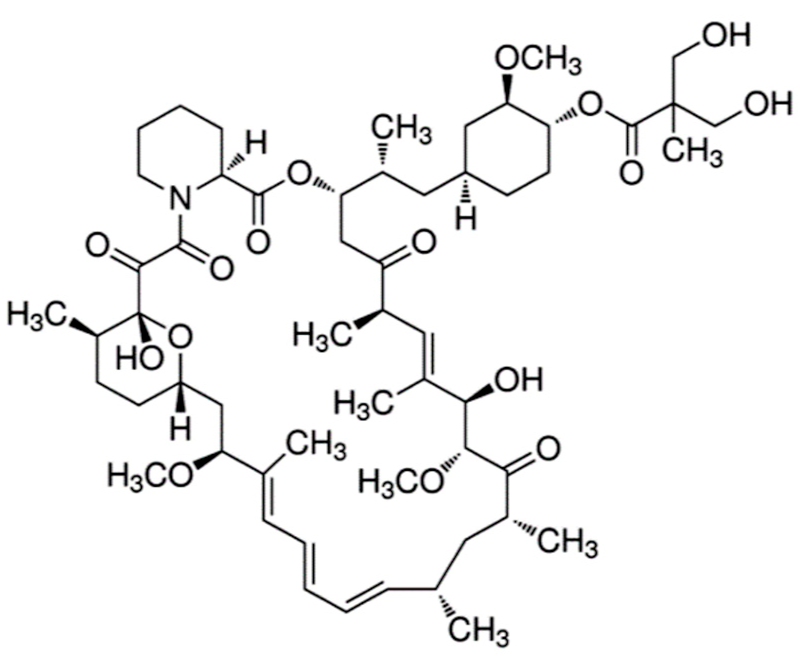 | 42-[3-Hydroxy-2-(hydroxymethyl)-2-methylpropanoate]-rapamycin Reduces synthesis of VEGF and targets the mammalian target of rapamycin (mTOR) Retinoblastoma. Renal cell carcinoma: advanced disease. | [50] |
| Axitinib (Inlyta®) | 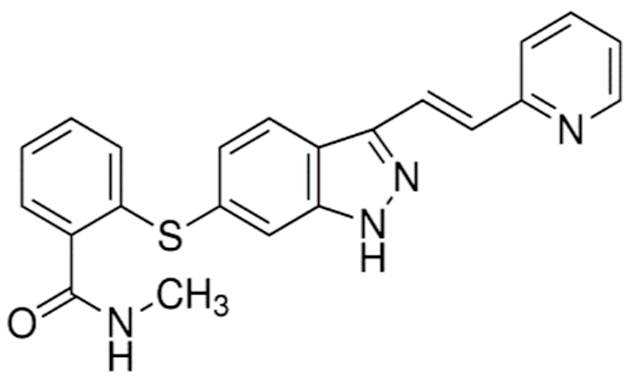 | N-Methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide Inhibitor of VEGF-1, -2, and -3 Renal cell carcinoma: Advanced/nonresponsive to other treatment. | [13,42,51] |
| Pazopanib (Votrient®) | 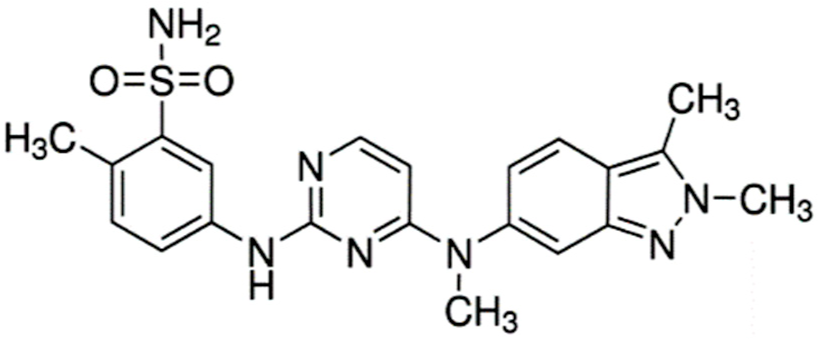 | 5-((4-((2,3-dimethyl-2H-indazol-6-yl)(methyl)amino)pyrimidin-2-yl)amino)-2-methylbenzenesulfonamide Small molecule multi-targeted receptor tyrosine kinase inhibitor Renal cell carcinoma: Advanced. Soft tissue sarcoma: Advanced. Nonresponsive to other treatment. | [13,52] |
| Lenvatinibmesylate (Lenvima®) | 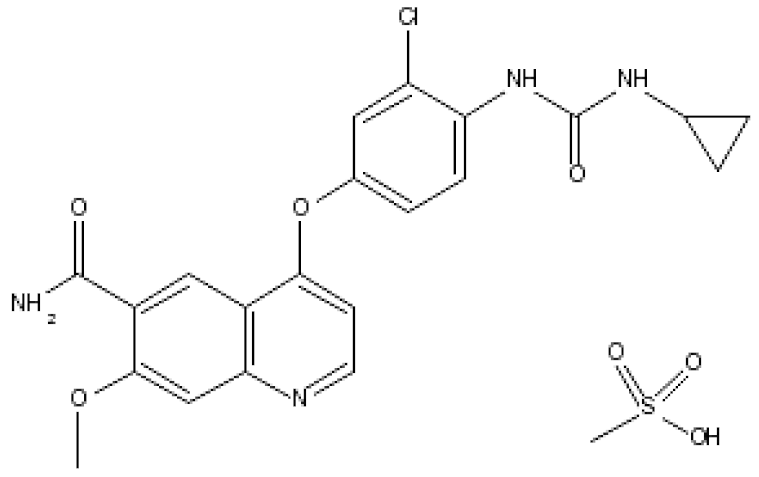 | 4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxamide methane sulfonate Lenvatinib inhibits tyrosine kinase activity of VEGF1, 2 and 3, fibroblast growth factor receptors (FGFRs) 1–4 Endometrial carcinoma: Advanced/nonresponsive to other treatment. Hepatocellular carcinoma: first-line treatment in nonresectable tumor. Renal cell carcinoma: Advanced. Thyroid cancer: Progressive/recurrent/metastatic/nonresponsive to radioactive iodine treatment. | [53] |
| Cabozantinib (Cometriq®) | 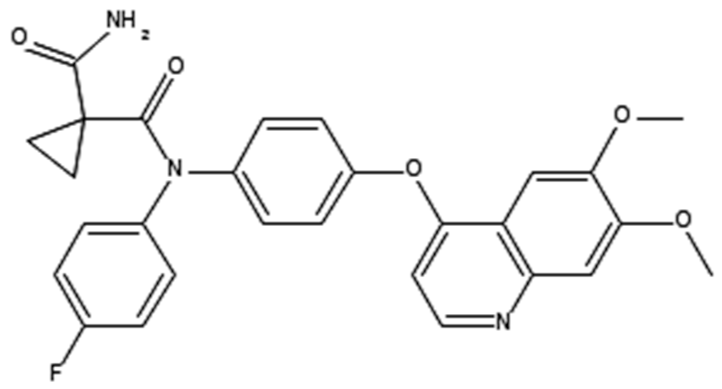 | 1,1-cyclopropanedicarboxamide, n′-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-n-(4-fluorophenyl)- c-MET and VEGFR2 Inhibitor Hepatocellular carcinoma: already been treated with sorafenib. Medullary thyroid cancer: Progressive/metastatic. Renal cell carcinoma: Advanced. | [41,54] |
| Everolimus (Afinitor®) | 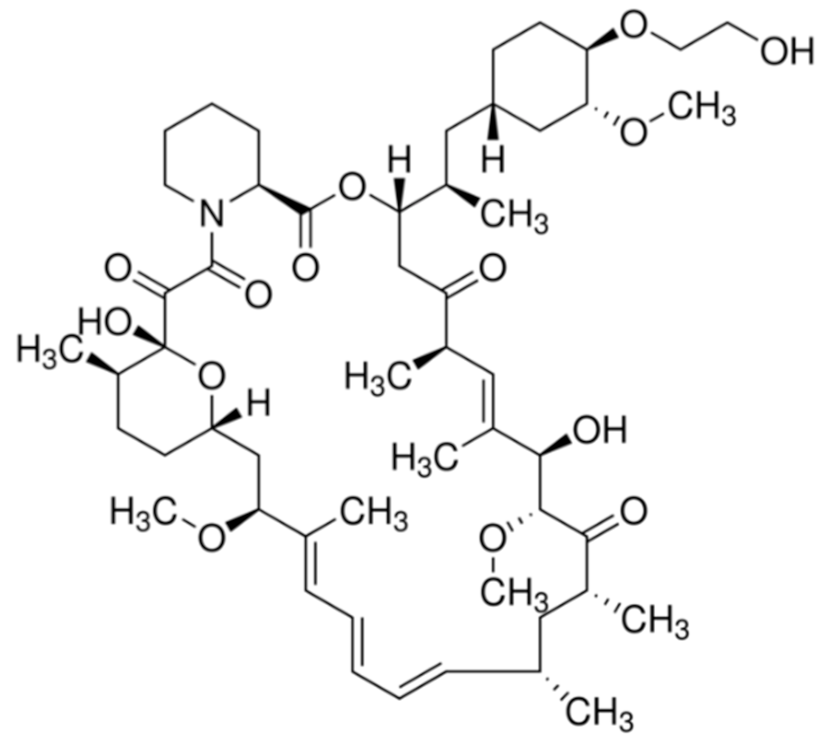 | (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,35R)-1,18-dihydroxy-12-((2R)-1-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl)-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[3 0.3.1.0(4,9)]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone40-O-(2-hydroxyethyl)-rapamycin Immunosuppression and targets the mTOR pathway Breast cancer: Advanced hormone receptor–positive (HR+) breast cancer that is also HER2 negative. Pancreatic cancer, gastrointestinal cancer, and lung cancer: Neuroendocrine tumors/nonresectable/metastatic. Renal cell carcinoma: Advanced. Subependymal giant cell astrocytoma: Nonresectable. | [13,41,55] |
| Vandetanib (Caprelsa®) |  | (4-Bromo-2-fluoro-phenyl)-[6-methoxy-7-(1-methyl-piperidin-4-ylmethoxy)-quinazolin-4-yl]-amine Dual Inhibitor of VEGFR and Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinases and also inhibits the mTOR–HIF-1 alpha–VEGF signaling axis Medullary thyroid cancer: Nonresectable/metastatic. | [13,41,42,56] |
| Ramucirumab (Cyramza®) | Anti-VEGFR2 monoclonal antibody | Anti-VEGFR2 monoclonal antibody Colorectal cancer: Metastatic/nonresponsive to other treatment like bevacizumab, oxaliplatin, and fluoropyrimidine. Hepatocellular carcinoma: Nonresponsive to sorafenib. Non-small cell lung cancer: Metastatic/aggravated after platinum chemotherapy/with a mutation in the EGFR gene or ALK gene. Stomach adenocarcinoma or gastroesophageal junction adenocarcinoma: Advanced/metastatic | [41,57] |
| Regorafenib (Stivarga®) |  | 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide. Dual targeted VEGFR2-TIE2 tyrosine kinase inhibition. Colorectal cancer: Metastatic/nonresponsive to other treatment. Gastrointestinal stromal tumor: Advanced/nonresectable/metastatic/nonresponsive to imatinibmesylate and sunitinib malate. Hepatocellular carcinoma: Nonresponsive to sorafenib. | [41,58] |
| Ziv-aflibercept (Zaltrap®) | A recombinant fusion protein comprising the extracellular domains of human VEGF receptors 1 and 2 | Inhibitor of VEGF Colorectal cancer: Metastatic/nonresponsive to other treatment. | [41,59] |
| S.No | Nanoparticle | Therapeutics | Application | Ref |
|---|---|---|---|---|
| 1 | Liposomes | Honokiol (potent anti-angiogenesis agent) | Liposomal honokiol improved efficacy of radiotherapy and chemotherapy in lung andovarian tumors. | [147,148,149] |
| 2 | Liposomes | Gd-DTPA Rhodamine PE | Gd-RGD-liposomes for target-specific MRI imaging and therapy of tumor angiogenesis. | [150] |
| 3 | Liposomes | Anginex-peptide | Anginex-liposomes used imaging for the angiogenesis-dependent disease. | [151] |
| 4 | Liposomes | EverolimusmTOR) EG00229 (VEGF/NRP1) | Showed effective tumor growth inhibition in a highly aggressive syngeneic immune-competent mouse model. | [152] |
| 5 | Solid-lipid nanoparticle | Bevacizumab | BSLNPs showed highly more effective than the parent in glioblastoma. | [153] |
| 6 | Liposomes | Fenretinide | Fenretinide–liposomes showed enhanced antiangiogenic and antitumor activity on human neuroblastoma. | [154] |
| 7 | Liposomes | ALK-siRNA | ALKsiRNA loaded liposomes induce apoptosis and inhibit angiogenesis. | [155] |
| 8 | Liposomes | Clodronate | Clo-liposomes efficiently deplete tumor-associated macrophages and showed antiangiogenic and antitumor effects in primary and metastatic melanoma. | [156] |
| 9 | Gold nanoparticles | Recombinant human endostatin (antiangiogenic agent) | Endostatin-gold nanoparticles normalized vessels in metastatic colorectal cancer. | [116] |
| 10 | Gold nanoparticles | GNPs | Gold nanoparticles inhibit subsequent angiogenesis-related signaling events. | [67] |
| 11 | Gold nanoparticles | Quercetin | Quercetin-GNPs inhibits EMT, angiogenesis and invasiveness in cancer. | [118,157,158,159,160] |
| 12 | Gold NPs | Peptides | Inhibit angiogenesis. | [161,162,163] |
| 13 | Nanoparticles | Small molecules | Inhibits tumor angiogenesis and tumor growth. | [99,142,143,164,165] |
| 14 | Lipid conjugates | PTX/LGC | IRGD-nanoconjugates improve tumor vessel normalization to achieve optimal chemo drug delivery into solid tumors. | [144] |
| 15 | PLA -NPs | Delta-like ligand 4 (Dll4-GD16-PTX | GD16-PTX-NPdemonstrated significant antiangiogenic and anticancer activity. | [166] |
| 16 | Cerium oxide-NPs | Nanoceria (NCe) | NCe-FA demonstrated excellent antiangiogenic effect in ovarian cancer. | [167,168] |
| 17 | Tetrac-NP | Tetraiodothyroacetic acid | Tetrac-NP significantly suppressed tumor growth and angiogenesis in murine xenograft models. | [169] |
| 18 | Polymeric Nanoparticle | Diamino Propane Tetraiodothyroacetic Acid | NPs showed excellent pharmacokinetics, biodistribution, and antiangiogenesis properties. | [170] |
| 19 | Carbon-NPs | Angiogenesis inhibitors | Inhibits tumor angiogenesis and tumor growth. | [146,171] |
| 20 | Silver nanoparticles (Ag-NPs) | Ag-NPs | Ag-NPs inhibit vascular endothelial growth factor (VEGF) and the formation of new blood microvessels. | [120,172] |
| 21 | Chitosan nanoparticles (CNP) | Alphastatin/CNPs | AsCs-NPs inhibited the SphK1-S1P signaling pathway and enhanced the antiangiogenic effect of Alphastatin both in vitro and in vivo. | [173,174] |
| 22 | Graphene-NPs | Graphite(G), rGOandnGO | Graphite nanoparticles and graphene oxide nanoplatelets showed potential antiangiogenic effects. | [175,176] |
| 23 | Chitosan-derived micelles | Apatinib | Apatinib-micelles showed effective anti-angiogenesis cancer therapy. | [177] |
| 24 | Cationic PEGylated liposomes | Gambogic acid | GAL significantly inhibited angiogenesis against TNBC. | [178] |
| 25 | PLGA copolymer | Osseltamivirphosphate (OP) | PLGA-OP actively impedes tumor neovascularization, growth, and metastasis in a mouse model of human pancreatic carcinoma. | [179] |
| 26 | Lipid-PA nanoparticles | Rapamycin and DiR | RDLPNPs showed an excellent antitumor effect with the enhanced photothermal and antiangiogenic effect. | [180] |
| 27 | Selenium nanoparticles | VEGF siRNA | Showed enhanced in vivo VEGF-siRNA silencing and fluorescence imaging efficacy. | [113] |
| 28 | Mesoporous silica nanoparticle | Combretastatin A4 doxorubicin | Tumor vascular-targeted co-delivery iRGD-NPs presented excellent anti-angiogenesis and antitumor activity. | [181] |
| 29 | pH-sensitive polymeric nanoparticles | Doxorubicin curcumin | Displaced enhanced proapoptotic and antiangiogenic activities. | [182] |
| 30 | Multifunctional nanodrugs | LMWH and ursolic acid | Demonstrated excellent anti-angiogenesis and antitumor activity. | [183] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, A.; Madamsetty, V.S.; Paul, M.K.; Mukherjee, S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. Int. J. Mol. Sci. 2020, 21, 455. https://doi.org/10.3390/ijms21020455
Mukherjee A, Madamsetty VS, Paul MK, Mukherjee S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. International Journal of Molecular Sciences. 2020; 21(2):455. https://doi.org/10.3390/ijms21020455
Chicago/Turabian StyleMukherjee, Anubhab, Vijay Sagar Madamsetty, Manash K. Paul, and Sudip Mukherjee. 2020. "Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer" International Journal of Molecular Sciences 21, no. 2: 455. https://doi.org/10.3390/ijms21020455
APA StyleMukherjee, A., Madamsetty, V. S., Paul, M. K., & Mukherjee, S. (2020). Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. International Journal of Molecular Sciences, 21(2), 455. https://doi.org/10.3390/ijms21020455