The Potential Role of Irisin in Vascular Function and Atherosclerosis: A Review
Abstract
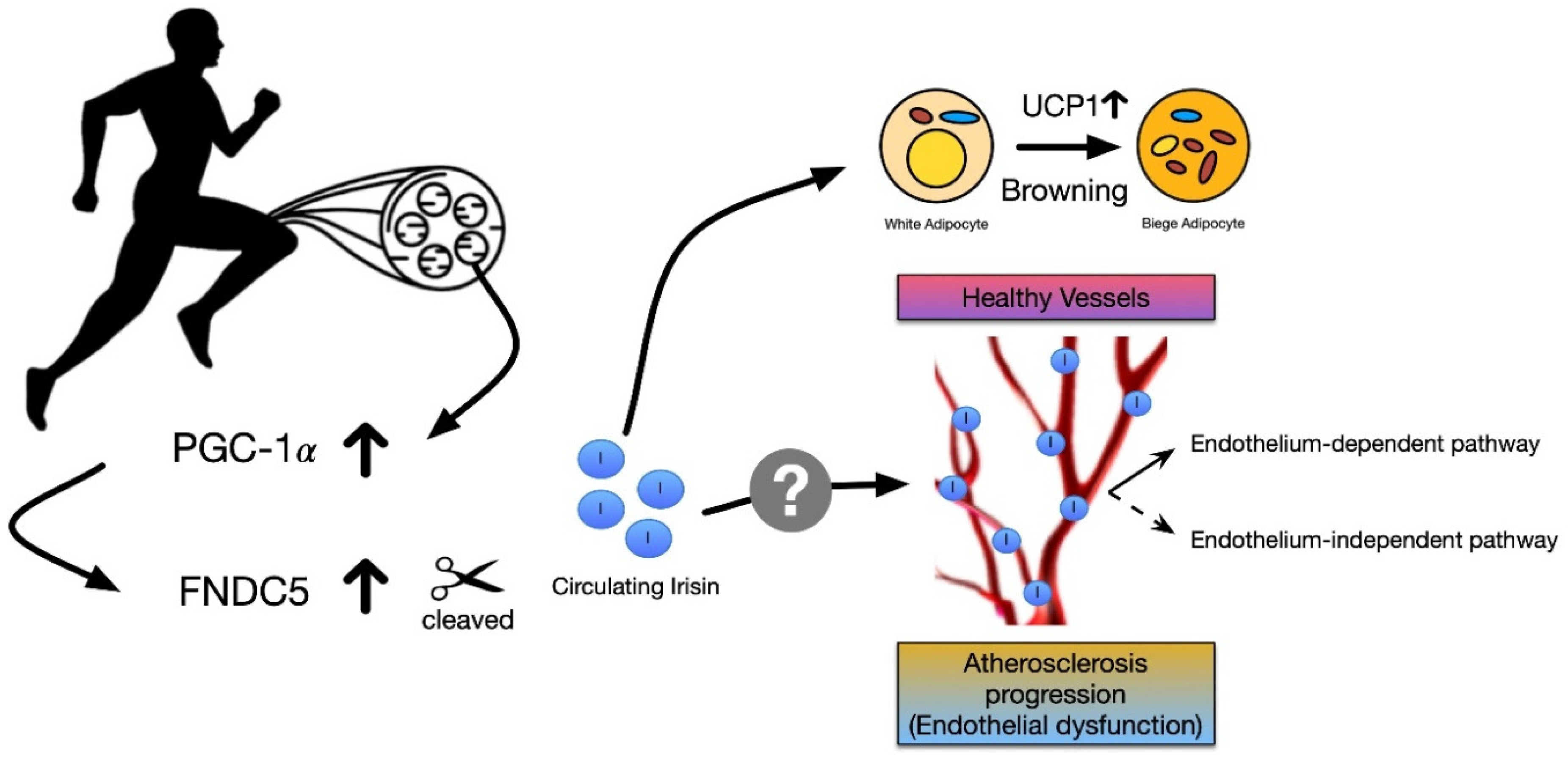
1. Introduction
2. Circulating Irisin Expression in Health and Disease
3. The Potential Role of Irisin in Vascular Reactivity and Atherosclerosis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| BMI | body mass index |
| CAD | coronary artery disease |
| CVD | cardiovascular diseases |
| ELISA | enzyme-linked immunosorbent assay |
| FNDC5 | fibronectin type III domain containing protein 5 |
| MS | metabolic syndrome |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator-1α |
| PVAT | perivascular adipose tissue |
| SHR | spontaneously-hypertensive rat |
| T2D | type 2 diabetes mellitus |
| WAT | white adipose tissue |
References
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Venojarvi, M.; Wasenius, N.; Manderoos, S.; Deruisseau, K.C.; Gidlund, E.K.; Heinonen, O.J.; Lindholm, H.; Aunola, S.; Eriksson, J.G.; et al. Plasma irisin is increased following 12 weeks of Nordic walking and associates with glucose homoeostasis in overweight/obese men with impaired glucose regulation. Eur. J. Sport Sci. 2019, 19, 258–266. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Tam, C.S.; Ban, L.A.; Ehrenfeld-Slater, P.; McLennan, S.V.; Twigg, S.M. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism 2020, 102, 154008. [Google Scholar] [CrossRef]
- Nygaard, H.; Slettalokken, G.; Vegge, G.; Hollan, I.; Whist, J.E.; Strand, T.; Ronnestad, B.R.; Ellefsen, S. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS ONE 2015, 10, e0121367. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Yang, J.; Rao, J.; Wang, H.; Zhang, J.; Wang, S.; Chen, X.; Dong, X. Time-Dependent Changes in Increased Levels of Plasma Irisin and Muscle PGC-1alpha and FNDC5 after Exercise in Mice. Tohoku J. Exp. Med. 2018, 244, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.Y. Aquarobic exercises improve the serum blood irisin and brain-derived neurotrophic factor levels in elderly women. Exp. Gerontol. 2018, 104, 60–65. [Google Scholar] [CrossRef]
- Shimba, Y.; Togawa, H.; Senoo, N.; Ikeda, M.; Miyoshi, N.; Morita, A.; Miura, S. Skeletal Muscle-specific PGC-1alpha Overexpression Suppresses Atherosclerosis in Apolipoprotein E-Knockout Mice. Sci. Rep. 2019, 9, 4077. [Google Scholar] [CrossRef]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Zhang, J.; Zhang, X.; Xin, C.; Zhang, F.; Lee, Y.; Zhang, L.; Lian, K.; Yan, W.; et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J. Mol. Cell. Cardiol. 2015, 87, 138–147. [Google Scholar] [CrossRef]
- Han, F.; Zhang, S.; Hou, N.; Wang, D.; Sun, X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1501–H1508. [Google Scholar] [CrossRef]
- Shoukry, A.; Shalaby, S.M.; El-Arabi Bdeer, S.; Mahmoud, A.A.; Mousa, M.M.; Khalifa, A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life 2016, 68, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Han, F.; Sun, X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin. Endocrinol. (Oxf.) 2015, 83, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Ahn, S.V.; Choi, J.H.; Koh, S.B.; Chung, C.H. High Serum Irisin Level as an Independent Predictor of Diabetes Mellitus: A Longitudinal Population-Based Study. Medicine 2016, 95, e3742. [Google Scholar] [CrossRef]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Yin, H.; Zugel, M.; Sun, Z.; Steinacker, J.M.; Schumann, U. Association between circulating irisin and insulin resistance in non-diabetic adults: A meta-analysis. Metabolism 2016, 65, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Huang, Y.Y.; Gusdon, A.M.; Qu, S. Irisin: A new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015, 14, 2. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef]
- Sesti, G.; Andreozzi, F.; Fiorentino, T.V.; Mannino, G.C.; Sciacqua, A.; Marini, M.A.; Perticone, F. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014, 51, 705–713. [Google Scholar] [CrossRef]
- Wu, H.; Guo, P.; Jin, Z.; Li, X.; Yang, X.; Tang, C.; Wang, Y.; Ke, J. Serum levels of irisin predict short-term outcomes in ischemic stroke. Cytokine 2019, 122, 154303. [Google Scholar] [CrossRef]
- Zugel, M.; Qiu, S.; Laszlo, R.; Bosnyak, E.; Weigt, C.; Muller, D.; Diel, P.; Steinacker, J.M.; Schumann, U. The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine 2016, 54, 101–110. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, R.; Jiang, F.; Wang, J.; Chen, M.; Peng, D.; Yan, J.; Wang, S.; Bao, Y.; Hu, C.; et al. Circulating irisin levels are associated with lipid and uric acid metabolism in a Chinese population. Clin. Exp. Pharmacol. Physiol. 2015, 42, 896–901. [Google Scholar] [CrossRef]
- Liu, J.J.; Wong, M.D.; Toy, W.C.; Tan, C.S.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhang, X.W.; Chen, W.K.; Huang, Q.X.; Chen, Q.Q. Relationship between serum irisin levels and urinary albumin excretion in patients with type 2 diabetes. J. Diabetes Complicat. 2015, 29, 384–389. [Google Scholar] [CrossRef]
- Alis, R.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Hernandez-Mijares, A.; Romagnoli, M.; Victor, V.M.; Rocha, M. Association between irisin and homocysteine in euglycemic and diabetic subjects. Clin. Biochem. 2014, 47, 333–335. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, M.K.; Bae, K.H.; Seo, H.A.; Jeong, J.Y.; Lee, W.K.; Kim, J.G.; Lee, I.K.; Park, K.G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Zhang, H.; Xu, Y.; Wang, G. Exenatide treatment increases serum irisin levels in patients with obesity and newly diagnosed type 2 diabetes. J. Diabetes Complicat. 2016, 30, 1555–1559. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Zhu, Y.J.; Li, C.G.; Tang, Y.Z.; Jiang, Z.H.; Yang, M.; Ni, C.L.; Chen, L.M.; Niu, W.Y. The relationship between circulating irisin levels and tissues AGE accumulation in type 2 diabetes patients. Biosci. Rep. 2017, 37, BSR20170213. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, J.; Song, M.; Zhou, F.; Fu, D.; Ruan, G.; Zhu, X.; Bai, Y.; Huang, L.; Pang, R.; et al. Irisin Increased the Number and Improved the Function of Endothelial Progenitor Cells in Diabetes Mellitus Mice. J Cardiovasc Pharm. 2016, 68, 67–73. [Google Scholar] [CrossRef]
- Deng, W. Association of Serum Irisin Concentrations with Presence and Severity of Coronary Artery Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 4193–4197. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Miura, K.; Arima, H.; Fujiyoshi, A.; Kadota, A.; Kadowaki, S.; Zaid, M.; Miyagawa, N.; Satoh, A.; Kunimura, A.; et al. Relationship of serum irisin levels to prevalence and progression of coronary artery calcification: A prospective, population-based study. Int. J. Cardiol. 2018, 267, 177–182. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Pardo, M.; Arturo, R.R.; Navas-Carretero, S.; Zulet, M.A.; Martinez, J.A.; Casanueva, F.F. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am. J. Hum. Biol. 2014, 26, 198–207. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Anastasilakis, A.D.; Geladari, E.V.; Mantzoros, C.S. Irisin in patients with nonalcoholic fatty liver disease. Metabolism 2014, 63, 207–217. [Google Scholar] [CrossRef]
- Waluga, M.; Kukla, M.; Kotulski, R.; Zorniak, M.; Boryczka, G.; Kajor, M.; Ciupinska-Kajor, M.; Lekstan, A.; Olczyk, P.; Waluga, E. Omentin, vaspin and irisin in chronic liver diseases. J. Physiol. Pharm. 2019, 70, 277–285. [Google Scholar]
- Calan, M.; Demirpence, M. Increased circulating levels of irisin are associated with cardiovascular risk factors in subjects with acromegaly. Hormones (Athens) 2019, 18, 435–442. [Google Scholar] [CrossRef]
- Anaszewicz, M.; Wawrzenczyk, A.; Czerniak, B.; Banas, W.; Socha, E.; Lis, K.; Zbikowska-Gotz, M.; Bartuzi, Z.; Budzynski, J. Leptin, adiponectin, tumor necrosis factor alpha, and irisin concentrations as factors linking obesity with the risk of atrial fibrillation among inpatients with cardiovascular diseases. Kardiol. Pol. 2019, 77, 1055–1061. [Google Scholar]
- Yasar, H.Y.; Demirpence, M.; Colak, A.; Yurdakul, L.; Zeytinli, M.; Turkon, H.; Ekinci, F.; Gunaslan, A.; Yasar, E. Serum irisin and apelin levels and markers of atherosclerosis in patients with subclinical hypothyroidism. Arch. Endocrinol. Metab. 2019, 63, 16–21. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Zhang, Y.; Liu, C.; Wang, Y.; Zhang, L.; Shi, Z.; Wu, Z.; et al. Exercise hormone irisin mitigates endothelial barrier dysfunction and microvascular leakage-related diseases. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Brunner, H.; Cockcroft, J.R.; Deanfield, J.; Donald, A.; Ferrannini, E.; Halcox, J.; Kiowski, W.; Luscher, T.F.; Mancia, G.; Natali, A.; et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 233–246. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Dellsperger, K.C.; Zhang, C. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H306–H314. [Google Scholar] [CrossRef]
- Halvorson, B.D.; Whitehead, S.N.; McGuire, J.J.; Wiseman, R.W.; Frisbee, J.C. Endothelium-dependent impairments to cerebral vascular reactivity with type 2 diabetes mellitus in the Goto-Kakizaki rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R149–R159. [Google Scholar] [CrossRef]
- Nguyen-Tu, M.S.; Nivoit, P.; Orea, V.; Lemoine, S.; Acquaviva, C.; Pagnon-Minot, A.; Fromy, B.; Sethi, J.K.; Sigaudo-Roussel, D. Inflammation-linked adaptations in dermal microvascular reactivity accompany the development of obesity and type 2 diabetes. Int. J. Obes. (Lond.) 2019, 43, 556–566. [Google Scholar] [CrossRef]
- Xiang, L.; Xiang, G.; Yue, L.; Zhang, J.; Zhao, L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis 2014, 235, 328–333. [Google Scholar] [CrossRef]
- Jiang, M.; Wan, F.; Wang, F.; Wu, Q. Irisin relaxes mouse mesenteric arteries through endothelium-dependent and endothelium-independent mechanisms. Biochem. Biophys. Res. Commun. 2015, 468, 832–836. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, L.; Zhang, C.; Zhang, R.; Li, Z.; Chai, B.; Li, J.; Chen, E.; Mulholland, M. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc. Drugs Ther. 2015, 29, 121–127. [Google Scholar] [CrossRef]
- Ye, L.; Xu, M.; Hu, M.; Zhang, H.; Tan, X.; Li, Q.; Shen, B.; Huang, J. TRPV4 is involved in irisin-induced endothelium-dependent vasodilation. Biochem. Biophys. Res. Commun. 2018, 495, 41–45. [Google Scholar] [CrossRef]
- Hou, N.; Du, G.; Han, F.; Zhang, J.; Jiao, X.; Sun, X. Irisin Regulates Heme Oxygenase-1/Adiponectin Axis in Perivascular Adipose Tissue and Improves Endothelial Dysfunction in Diet-Induced Obese Mice. Cell. Physiol. Biochem. 2017, 42, 603–614. [Google Scholar] [CrossRef]
- Hou, N.; Liu, Y.; Han, F.; Wang, D.; Hou, X.; Hou, S.; Sun, X. Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase-1/adiponectin axis in diet-induced obese mice. J. Mol. Cell. Cardiol. 2016, 99, 188–196. [Google Scholar] [CrossRef]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Q.; Zhou, Z.; Song, H.; Zhang, Y.; Wu, F.; Jiang, M.; Wang, F.; Zhang, W.; Li, L.; et al. Protective Effect of Irisin on Atherosclerosis via Suppressing Oxidized Low Density Lipoprotein Induced Vascular Inflammation and Endothelial Dysfunction. PLoS ONE 2016, 11, e0158038. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, H.; Zhang, Y.; Wu, F.; Mu, Q.; Jiang, M.; Wang, F.; Zhang, W.; Li, L.; Shao, L.; et al. Irisin Inhibits Atherosclerosis by Promoting Endothelial Proliferation Through microRNA126-5p. J. Am. Heart Assoc. 2016, 5, e004031. [Google Scholar] [CrossRef]
- Fu, J.; Han, Y.; Wang, J.; Liu, Y.; Zheng, S.; Zhou, L.; Jose, P.A.; Zeng, C. Irisin Lowers Blood Pressure by Improvement of Endothelial Dysfunction via AMPK-Akt-eNOS-NO Pathway in the Spontaneously Hypertensive Rat. J. Am. Heart Assoc. 2016, 5, e003433. [Google Scholar] [CrossRef]
- Aydogdu, N.; Yalcinkaya Yavuz, O.; Tastekin, E.; Tayfur, P.; Kaya, O.; Kandemir, N. The Effects of Irisin on Nomega-Nitro-L-arginine Methyl Ester Hydrochloride-Induced Hypertension in Rats. Balk. Med. J. 2019, 36, 337–346. [Google Scholar]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
| Subject or Animal Model | Disease Condition | Method to Diagnose Disease | Conclusion | Method to Detect Irisin | Refs |
|---|---|---|---|---|---|
| Human | Males and females with T2D | WHO criteria - fasting glucose ≥ 126 mg/dL - or HbA1c ≥ 6.5% - or taking antidiabetic medication | - ↑serum irisin (2.378 vs. control, 1.456 μg/mL) | ELISA (Phoenix Pharmaceuticals, EK-067-52) | [13] |
| Human | Males and females with MS | National Heart, Lung, and Blood Institute/AHA criteria - The presence of at least three of MS risk factors (central obesity, elevated triglyceride, low HDL cholesterol, high fasting glucose or high blood pressure) | - ↑serum irisin (214.4. vs. control, 162.2 ng/mL) | ELISA (Phoenix Pharmaceuticals, EK-067-52) | [17] |
| Human | Males and females with T2D | - fasting glucose level ≥ 126 mg/dL (7.0 mmol/L) - or 2 h postprandial blood glucose level ≥ 200 mg/dL (11.1 mmol/L) - or HbA1c ≥ 6.5% | - ↓serum irisin (237.06 ± 21.22 vs. control, 377.81 ± 27.16 ng/mL) | ELISA (BioVision) | [11] |
| Human | Males and females with obesity | BMI ≥ 30 kg/m2 | - ↑serum irisin (399.84 ± 16.12 vs. control, 340.87 ± 8.40 ng/mL) | ELISA (BioVision) | [11] |
| Human | Males and females with obesity | BMI ≥ 25 kg/m2 | - ↓serum irisin (180.5 ± 22.4 vs. control 194.8 ± 19.9 ng/mL) | ELISA (Phoenix Pharmaceuticals) | [12] |
| Human | CAD | Angiographic evidence of stenosis ≥ 50% in at least one major coronary artery | - ↓ irisin (119.55 vs. control, 146.22 ng/mL) | ELISA (Phoenix Pharmaceuticals) | [30] |
| Human | Males and females with IGR or T2D | - T2D ∙ fasting plasma glucose ≥ 7.0 mmol/L ∙ 2 h post-challenge plasma glucose ≥ 11.1 mmol/L - IGR ∙ 6.1 mmol/L ≤ fasting plasma glucose ≤ 7.0 mmol/L ∙ 7.8 mmol/L ≤ 2 h post-challenge plasma glucose < 11.1 mmol/L | - No difference in serum irisin (6.75 vs. 7.36 vs. 7.08 ng/mL) | ELISA (Phonenix Pharmaceuticals, EK-067-29) | [22] |
| Human | Males and females with T2D | means of HbA1c, 8.3 ± 1.9% | - ↓circulating irisin (204 ± 72 vs. control, 257 ± 24 ng/mL) | ELISA (USCN Life Science) | [23] |
| Human | Males and females with T2D | ADA criteria | - ↓serum irisin (14.12 ± 3.93 vs. control, 28.98 ± 2.56 ng/mL) | ELISA (A Viscera Bioscience) | [24] |
| Human | Males and females with T2D | ADA criteria | - ↓serum irisin (279 ± 58 vs. control, 263 ± 38 ng/mL) | ELISA (Phoenix Pharmaceuticals) | [25] |
| Human | Males and females with T2D | WHO criteria - fasting plasma glucose ≥ 126 mg/dL - or 2 h post-bad plasma glucose ≥ 200 mg/dL - or HbA1c ≥ 6.5% | - ↓serum irisin (24.53 ± 3.53 vs. control, 38.86 ± 2.48 pg/mL) | ELISA (USCN Life Science) | [26] |
| Human | Males and females with T2D | ADA criteria - HbA1c ≥ 7% | - ↓serum irisin (38.06 vs. control, 58.01 ng/mL) | ELISA (Aviscera Bioscience) | [27] |
| Human | Males and females with T2D | WHO criteria - fasting plasma glucose ≥ 7.0 mmol/l - 2 h post-load plasma glucose ≥ 11.1 mmol/l | - ↓serum irisin (16.24 ± 5.16 vs. controls 24.35 ± 2.76 ng/mL) | ELISA (Aviscera Bioscience) | [28] |
| Human | Morbidly obese men and women | - 5 women (BW, 128.7 ± 37.1 kg) - 5 men (BW, 158.8 ± 26.9 kg) | - ↑serum irisin (30% higher than normal weight) | ELISA (Phoenix, EK-067-52) | [21] |
| Human | Male and female subject with acromegaly | GH and IGF-1 concentration | - ↑serum irisin in active acromegaly | ELISA (Sunred Biological Technology, 201-12-5328) | [35] |
| Human | Male and female subjects with chronic liver disease | Abdominal ultrasound and laboratory tests | - ↓serum irisin in primary biliary cholangitis (5.82 ± 2.41), nonalcoholic fatty liver disease (4.98 ± 2.017) and alcoholic cirrhosis (3.13 ± 1.96) compared to control (29.67 ± 19.9 μg/mL) | ELISA (BioVendor) | [34] |
| Human | Male and female subjects with obesity or nonalcoholic fatty liver disease | Liver biopsy and NSFLD Activity Score | - ↓serum irisin in obese controls (34.2 ± 2.0), NAFL (31.4 ± 2.8) and NASH (37.9 ± 3.0) compared to lean control (47.3 ± 2.6 ng/mL) | ELISA (Phoenix Pharmaceuticals) | [33] |
| Human | Male and female patients with atrial fibrillation | Patients hospitalized due to paroxysmal or persistent AF | - No difference in serum irisin | ELISA (BioVendor, RAG018R) | [36] |
| Human | Male and female patients with subclinical hypothyroidism | Autoimmune thyroiditis and anti-Microsome antibody | - No difference in serum irisin | ELISA (Sunred Biological Technology, 201-12-5328) | [37] |
| Human | Male with coronary artery calcification | Electron-beam computed tomography | Higher serum irisin were associated with less burden of coronary atherosclerosis. | ELISA (Adipogen, AG-45A-0046EK-k101) | [31] |
| Human | Patients with ARDS | Chest x-ray or computed tomography and mechanical ventilation | - ↓serum irisin compared to control | ELISA (USCN Life Science) | [38] |
| Mouse (Male C57 BL/6) | T2D (high-fat diet, 60% fat for 8 weeks) | IPGTT | - ↓serum irisin | ELISA (NeoBioscience Technology) | [9] |
| Mouse | Obesity (high-fat diet, 50.1% fat for 8 weeks) | BW↑ 26.4 ± 1.8 vs. 35.3 ± 2.6 | - ↓serum irisin (29.12 ± 3.04 vs. control, 35.87 ± 3.95 ng/mL) | ELISA (Not specified) | [10] |
| Mouse (Male C57BL/6J) | Cerebral ischemia stroke (Middle cerebral artery occlusion model) | 70% ↓ in blood flow perfusion | - ↓serum irisin (~37 vs. sham ~70 ng/mL) | ELISA (Phoenix) | [18] |
| Mouse (Male C57/BL6) | T1D (STZ, 35 mg/kg BW + HFD for 8 weeks) | Plasma glucose level by glucose oxidase method | - ↓serum irisin (30.7 ± 3.5 vs. control, 37.4 ± 4.2 ng/mL) | ELISA (Phoenix Pharmaceuticals) | [29] |
| Subject or Animal Model | Disease Condition | Irisin Treatment or Involvement | Conclusion | Vessels Used | Potential Mechanisms Involved | Refs |
|---|---|---|---|---|---|---|
| Human | Males and females with obesity | Correlation of circulating irisin and EDV | Positive correlation (r = 0.388) | Brachial artery | Endothelium-dependent pathway | [12] |
| Human | Males and females with T2D | Correlation of circulating irisin and FMD | Positive correlation (r = 0.51) | Brachial artery | Endothelium-dependent pathway | [24] |
| Human | Males and females with CAD | Correlation of circulating irisin and CAI score | Negative correlation (r = −0.340) | Coronary artery | - | [30] |
| Mice (male C57 BL/6J) | High fat diet (60% fat) for 8 weeks | IP injection 0.5 mg/kg BW Once a day for 2 weeks | ↑ACh-mediated relaxation | Aorta | PKC-β/NADPH oxidase and NF-κB/iNOS | [9] |
| Mice (male C57 BL/6J) | High fat diet (50.1% fat) for 8 weeks to induce T2D | IP injection 0.5 μg/g−1∙day−1 for 8 weeks | ↑ACh-mediated relaxation | Aorta | AMPK-eNOS pathway | [10] |
| Mice (male C57 BL/6) | High fat diet for 12 weeks to induce obesity | IP injection 0.5 μg/g−1∙day−1 for 12 weeks | ↑ACh-mediated relaxation | Aortas with and without PVAT | HO-1/adiponectin axis | [48] |
| Mice (male C57BL/6) | High fat diet for 8 weeks to induce obesity | IP injection 0.5 μg/g−1∙day−1 for 8 weeks Once daily | ↓PE-induced vasoconstriction | Aorta | HO-1/adiponectin axis | [49] |
| Mice (male C57 BL/6J) | 10–12 weeks old | Irisin-induced relaxation (0.1~100 μM) | relaxes in dose-dependent manner in endothelium-intact and denuded mesenteric arteries | Mesenteric arteries (2nd order) | NO-cGMP-dependent pathway Voltage-dependent Ca2+ channel Intracellular Ca2+ release | [45] |
| Mice (Apo E + STZ) | STZ injected to induce T1D | Tail-vein injection 0.2 μg/g BW for 12 weeks | - ↑ACh-mediated relaxation - ↓Aortic Plaque Area | Aorta | AMPK-PI3K-Akt-eNOS signaling pathway | [50] |
| Mice (ApoE KO) | Atherosclerosis | IP injection 0.5 μg/g BW for 8 weeks | ↓Aortic lesion area | Aorta | ROS-p38 MAPK-NF-κB signaling pathway | [51] |
| Mice (Male ApoE KO) | High cholesterol diet + partial ligation of the left common carotid artery | IP injection 0.5 μg/kg BW for 4 weeks | ↓Carotid lesion area | Carotid artery | ERK signaling pathway(miroRNA126-5p) | [52] |
| Male SD rats (200–250 g) | - | Irisin-induced relaxation (10 nM~100 μM) | ↑Relaxation in dose-dependent manner | Mesenteric arteries (2nd order) | ATP-sensitive K+ channel | [46] |
| Male SD rat | - | - Irisin-induced relaxation (100 nM) | ↑Relaxation | Mesenteric arteries | Endothelium-dependent pathway (TRPV4) | [47] |
| Male Wistar-Kyoto (control) and SHR (hypertension) rats (16–18 weeks old) | Hypertension | 3000 ng/mL pre-incubation (1 h) | - ↑ACh-mediated relaxation - ↓PHE-mediated vasoconstriction - No-direct dilation | Mesentery arteries (3rd order) | AMPK–Akt–eNOS–NO signaling pathway | [53] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, K.; Lee, S. The Potential Role of Irisin in Vascular Function and Atherosclerosis: A Review. Int. J. Mol. Sci. 2020, 21, 7184. https://doi.org/10.3390/ijms21197184
Byun K, Lee S. The Potential Role of Irisin in Vascular Function and Atherosclerosis: A Review. International Journal of Molecular Sciences. 2020; 21(19):7184. https://doi.org/10.3390/ijms21197184
Chicago/Turabian StyleByun, Kyeongho, and Sewon Lee. 2020. "The Potential Role of Irisin in Vascular Function and Atherosclerosis: A Review" International Journal of Molecular Sciences 21, no. 19: 7184. https://doi.org/10.3390/ijms21197184
APA StyleByun, K., & Lee, S. (2020). The Potential Role of Irisin in Vascular Function and Atherosclerosis: A Review. International Journal of Molecular Sciences, 21(19), 7184. https://doi.org/10.3390/ijms21197184






