MCPIP1 RNase and Its Multifaceted Role
Abstract
1. Introduction
1.1. Inflammation
1.2. MCPIP Family
1.3. MCPIP1
2. Broad Roles of MCPIP1 in the Immune System
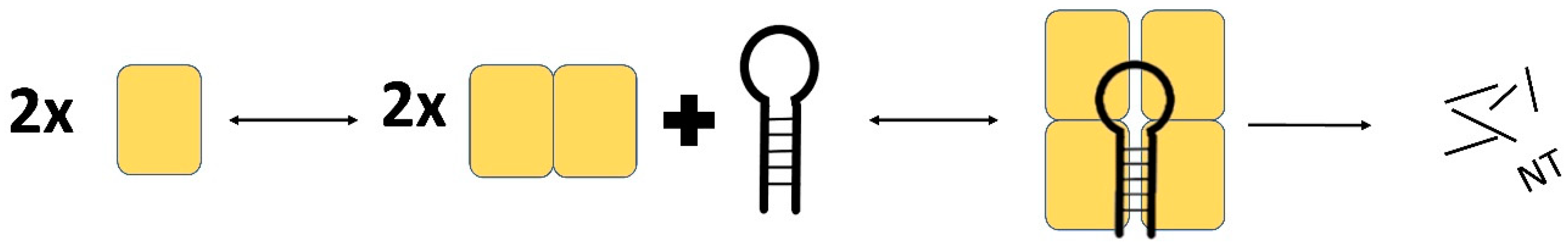
2.1. MCPIP1 Regulation
2.2. Roles of MCPIP1 in the Regulation of Cellular and Bodily Processes
2.3. Adipogenesis
2.4. Angiogenesis
3. Tumor Immune Response
4. Role of MCPIP1 in the Skin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuprash, D.V.; Nedospasov, S.A. Molecular and cellular mechanisms of inflammation. Biochemistry 2016, 81, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1–11. [Google Scholar]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Thornton, C.A.; Morgan, G. Innate and adaptive immune pathways to tolerance. Nestle Nutrition Workshop Series: Pediatric Program; Public Library of Science: San Francisco, CA, USA, 2009; Volume 64, pp. 45–61. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 2010, 327, 291–295. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Bansal, P.; Jialal, I. Chronic Inflammation. In StatPearls (Internet); StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Sasindran, S.J.; Torrelles, J.B. Mycobacterium Tuberculosis Infection and Inflammation: What is Beneficial for the Host and for the Bacterium? Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef]
- Chan, J.Y.W.; Tsui, J.C.C.; Law, P.T.W.; So, W.K.W.; Leung, D.Y.P.; Sham, M.M.K.; Tsui, S.K.W.; Chan, C.W.H. Regulation of TLR4 in silica-induced inflammation: An underlying mechanism of silicosis. Int. J. Med. Sci. 2018, 15, 986–991. [Google Scholar] [CrossRef]
- Ozdogan, H.; Ugurlu, S. Familial Mediterranean Fever. La Presse Med. 2019, 48, e61–e76. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A. Endogenous anti-inflammatory proteins. Biochem. Pharmacol. 1977, 26, 693–698. [Google Scholar] [CrossRef]
- Ferencík, M.; Stvrtinová, V. Endogenous control and modulation of inflammation. Folia Biol. 1996, 42, 47–55. [Google Scholar]
- Delgado, M.; Abad, C.; Martinez, C.; Leceta, J.; Gomariz, R.P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 2001, 7, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Said, S.I.; Mutt, V. Polypeptide with Broad Biological Activity: Isolation from Small Intestine. Science 1970, 169, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Gomariz, R.P.; Martinez, C.; Abad, C.; Leceta, J. Anti-inflammatory Properties of the Type 1 and Type 2 Vasoactive Intestinal Peptide Receptors: Role in Lethal Endotoxic Shock. Eur. J. Immunol. 2000, 30. [Google Scholar] [CrossRef]
- Zhou, L.; Azfer, A.; Niu, J.; Graham, S.; Choudhury, M.; Adamski, F.M.; Younce, C.; Binkley, P.F.; Kolattukudy, P.E. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 2006, 98, 1177–1185. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Azfer, A.; Song, W.; Tromp, G.; Kolattukudy, P.E.; Fu, M. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J. Biol. Chem. 2008, 283, 6337–6346. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Arase, M.; Matsuyama, H.; Choi, Y.L.; Ueno, T.; Mano, H.; Sugimoto, K.; Miyazono, K. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol. Cell 2011, 44, 424–436. [Google Scholar] [CrossRef]
- Lin, R.J.; Chien, H.L.; Lin, S.Y.; Chang, B.L.; Yu, H.P.; Tang, W.C.; Lin, Y.L. MCPIP1 ribonuclease exhibits broad-spectrum antiviral effects through viral RNA binding and degradation. Nucleic Acids Res. 2013, 41, 3314–3326. [Google Scholar] [CrossRef]
- Jura, J.; Skalniak, L.; Koj, A. Monocyte chemotactic protein-1-induced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Mizgalska, D.; Wgrzyn, P.; Murzyn, K.; Kasza, A.; Koj, A.; Jura, J.; Jarzb, B.; Jura, J. Interleukin-1-inducible MCPIP protein has structural and functional properties of RNase and participates in degradation of IL-1β mRNA. FEBS J. 2009, 276, 7386–7399. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Takeuchi, O.; Standley, D.M.; Kumagai, Y.; Kawagoe, T.; Miyake, T.; Satoh, T.; Kato, H.; Tsujimura, T.; Nakamura, H.; et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 2009, 458, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, E.; Wilamowski, M.; Lech, M.; Bugara, B.; Jura, J.; Potempa, J.; Koziel, J. MCPIP-1, Alias Regnase-1, Controls Epithelial Inflammation by Posttranscriptional Regulation of IL-8 Production. J. Innate Immun. 2016, 8, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.V.; Amatya, N.; Chen, K.; Cruz, J.A.; Grover, P.; Whibley, N.; Conti, H.R.; Hernandez Mir, G.; Sirakova, T.; Childs, E.C.; et al. MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation. Immunity 2015, 43, 475–487. [Google Scholar] [CrossRef]
- Omiya, S.; Omori, Y.; Taneike, M.; Murakawa, T.; Ito, J.; Tanada, Y.; Nishida, K.; Yamaguchi, O.; Satoh, T.; Shah, A.M.; et al. Cytokine mRNA Degradation in Cardiomyocytes Restrains Sterile Inflammation in Pressure-Overloaded Hearts. Circulation 2020, 667–677. [Google Scholar] [CrossRef]
- Li, M.; Cao, W.; Liu, H.; Zhang, W.; Liu, X.; Cai, Z.; Guo, J.; Wang, X.; Hui, Z.; Zhang, H.; et al. MCPIP1 Down-Regulates IL-2 Expression through an ARE-Independent Pathway. PLoS ONE 2012, 7, e49841. [Google Scholar] [CrossRef]
- Mino, T.; Murakawa, Y.; Fukao, A.; Vandenbon, A.; Wessels, H.H.; Ori, D.; Uehata, T.; Tartey, S.; Akira, S.; Suzuki, Y.; et al. Regnase-1 and roquin regulate a common element in inflammatory mRNAs by spatiotemporally distinct mechanisms. Cell 2015, 161, 1058–1073. [Google Scholar] [CrossRef]
- Wilamowski, M.; Gorecki, A.; Dziedzicka-Wasylewska, M.; Jura, J. Substrate specificity of human MCPIP1 endoribonuclease. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Senissar, M.; Manav, M.C.; Brodersen, D.E. Structural conservation of the PIN domain active site across all domains of life. Protein Sci. 2017, 26, 1474–1492. [Google Scholar] [CrossRef]
- Xu, J.; Peng, W.; Sun, Y.; Wang, X.; Xu, Y.; Li, X.; Gao, G.; Rao, Z. Structural study of MCPIP1 N-terminal conserved domain reveals a PIN-like RNase. Nucleic Acids Res. 2012, 40, 6957–6965. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Shi, Y.; Xue, J.; Miao, R.; Huang, S.; Wang, T.; Wu, J.; Fu, M.; Wu, Z.H. USP10 inhibits genotoxic NF-κB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO J. 2013, 32, 3206–3219. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Saad, Y.; Lei, T.; Wang, J.; Qi, D.; Yang, Q.; Kolattukudy, P.E.; Fu, M. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-κB signaling. J. Exp. Med. 2010, 207, 2959–2973. [Google Scholar] [CrossRef]
- Muzio, M.; Natoli, G.; Saccani, S.; Levrero, M.; Mantovani, A. The Human Toll Signaling Pathway: Divergence of Nuclear Factor κB and JNK/SAPK Activation Upstream of Tumor Necrosis Factor Receptor–associated Factor 6 (TRAF6). J. Exp. Med. 1998, 187, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.M.T. Multiple modes of RNA recognition by zinc finger proteins. Curr. Opin. Struct. Biol. 2005, 15, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Vogel, K.U.; Edelmann, S.L.; Jeltsch, K.M.; Bertossi, A.; Heger, K.; Heinz, G.A.; Zöller, J.; Warth, S.C.; Hoefig, K.P.; Lohs, C.; et al. Roquin Paralogs 1 and 2 Redundantly Repress the Icos and Ox40 Costimulator mRNAs and Control Follicular Helper T Cell Differentiation. Immunity 2013, 38, 655–668. [Google Scholar] [CrossRef]
- Yokogawa, M.; Tsushima, T.; Noda, N.N.; Kumeta, H.; Enokizono, Y.; Yamashita, K.; Standley, D.M.; Takeuchi, O.; Akira, S.; Inagaki, F. Structural basis for the regulation of enzymatic activity of Regnase-1 by domain-domain interactions. Sci. Rep. 2016, 6, 22324. [Google Scholar] [CrossRef]
- Bergdoll, M.; Remy, M.-H.; Cagnon, C.; Masson, J.-M.; Dumas, P. Proline-dependent oligomerization with arm exchange. Structure 1997, 5, 391–401. [Google Scholar] [CrossRef]
- Happel, C.; Ramalingam, D.; Ziegelbauer, J.M. Virus-Mediated Alterations in miRNA Factors and Degradation of Viral miRNAs by MCPIP1. PLoS Biol. 2016, 14. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, C.; Miao, R.; Zhou, J.; Lee, A.; Liu, B.; Lester, S.N.; Fu, W.; Zhu, L.; Zhang, L.; et al. MCPIP1 restricts HIV infection and is rapidly degraded in activated CD4+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, 19083–19088. [Google Scholar] [CrossRef]
- Saleh, M.; Elson, C.O. Experimental Inflammatory Bowel Disease: Insights into the Host-Microbiota Dialog. Immunity 2011, 34, 293–302. [Google Scholar] [CrossRef]
- Lin, R.-J.; Chu, J.-S.; Chien, H.-L.; Tseng, C.-H.; Ko, P.-C.; Mei, Y.-Y.; Tang, W.-C.; Kao, Y.-T.; Cheng, H.-Y.; Liang, Y.-C.; et al. MCPIP1 Suppresses Hepatitis C Virus Replication and Negatively Regulates Virus-Induced Proinflammatory Cytokine Responses. J. Immunol. 2014, 193, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, J.; Saad, Y.; Warble, L.; Becerra, E.; Kolattukudy, P.E. Participation of MCP-induced protein 1 in lipopolysaccharide preconditioning-induced ischemic stroke tolerance by regulating the expression of proinflammatory cytokines. J. Neuroinflamm. 2011, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-J.; Choi, J.-A.; Lee, J.-H.; Choi, C.H.; Kim, H.-J.; Song, C.-H. Mycobacterium tuberculosis 38-kDa antigen induces endoplasmic reticulum stress-mediated apoptosis via toll-like receptor 2/4. Apoptosis 2015, 20, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Uehata, T.; Iwasaki, H.; Vandenbon, A.; Matsushita, K.; Hernandez-Cuellar, E.; Kuniyoshi, K.; Satoh, T.; Mino, T.; Suzuki, Y.; Standley, D.M.; et al. Malt1-Induced Cleavage of Regnase-1 in CD4+ Helper T Cells Regulates Immune Activation. Cell 2013, 153, 1036–1049. [Google Scholar] [CrossRef]
- Jeltsch, K.M.; Hu, D.; Brenner, S.; Zöller, J.; Heinz, G.A.; Nagel, D.; Vogel, K.U.; Rehage, N.; Warth, S.C.; Edelmann, S.L.; et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote TH17 differentiation. Nat. Immunol. 2014, 15, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Tanaka, H.; Yasuda, K.; Adachi, T.; Fukuoka, A.; Akasaki, S.; Koida, A.; Kuroda, E.; Akira, S.; Yoshimoto, T. Regnase-1 degradation is crucial for IL-33–and IL-25–mediated ILC2 activation. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Qian, Y.; Li, X.; Miao, R.; Liu, S.; Xin, H.-B.; Huang, X.; Wang, T.T.; Fu, M. Selective degradation of plasmid-derived mRNAs by MCPIP1 RNase. Biochem. J. 2019, 476, 2927–2938. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Huang, S.; He, H.; Lei, T.; Saaoud, F.; Yu, X.Q.; Melnick, A.; Kumar, A.; Papasian, C.J.; et al. Central role of myeloid MCPIP1 in protecting against LPS-induced inflammation and lung injury. Signal Transduct. Target. Ther. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.T. MCPIP1/regnase-I inhibits simian immunodeficiency virus and is not counteracted by Vpx. J. Gen. Virol. 2016, 97, 1693–1698. [Google Scholar] [CrossRef]
- Iwasaki, H.; Takeuchi, O.; Teraguchi, S.; Matsushita, K.; Uehata, T.; Kuniyoshi, K.; Satoh, T.; Saitoh, T.; Matsushita, M.; Standley, D.M.; et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR–IL-1R by controlling degradation of regnase-1. Nat. Immunol. 2011, 12, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Okamoto, H.; Toyama, Y.; Momohara, S. Molecular aspects of rheumatoid arthritis: Chemokines in the joints of patients. FEBS J. 2008, 275, 4448–4455. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sato, S. The roles of chemokines in leukocyte recruitment and fibrosis in systemic sclerosis. Front. Biosci. 2008, 13, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Murugan, V.; Peck, M.J. Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Exp. Lung Res. 2009, 35, 439–485. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H. MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2008, 294, F697–F701. [Google Scholar] [CrossRef]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose Tissue: The New Endocrine Organ? A Review Article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Wang, K.; Niu, J.; Kim, H.; Kolattukudy, P.E. Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J. Mol. Cell Biol. 2011, 3, 360–368. [Google Scholar] [CrossRef]
- Da, J.; Zhuo, M.; Qian, M. MCPIP is induced by cholesterol and participated in cholesterol-caused DNA damage in HUVEC. Int. J. Clin. Exp. Pathol. 2015, 8, 10625–10634. [Google Scholar]
- Qi, D.; Huang, S.; Miao, R.; She, Z.-G.; Quinn, T.; Chang, Y.; Liu, J.; Fan, D.; Chen, Y.E.; Fu, M. Monocyte Chemotactic Protein-induced Protein 1 (MCPIP1) Suppresses Stress Granule Formation and Determines Apoptosis under Stress. J. Biol. Chem. 2011, 286, 41692–41700. [Google Scholar] [CrossRef]
- Vrotsos, E.G.; Kolattukudy, P.E.; Sugaya, K. MCP-1 involvement in glial differentiation of neuroprogenitor cells through APP signaling. Brain Res. Bull. 2009, 79, 97–103. [Google Scholar] [CrossRef]
- Niu, J.; Azfer, A.; Zhelyabovska, O.; Fatma, S.; Kolattukudy, P.E. Monocyte Chemotactic Protein (MCP)-1 Promotes Angiogenesis via a Novel Transcription Factor, MCP-1-induced Protein (MCPIP). J. Biol. Chem. 2008, 283, 14542–14551. [Google Scholar] [CrossRef] [PubMed]
- Younce, C.W.; Azfer, A.; Kolattukudy, P.E. MCP-1 (Monocyte Chemotactic Protein-1)-induced Protein, a Recently Identified Zinc Finger Protein, Induces Adipogenesis in 3T3-L1 Pre-adipocytes without Peroxisome Proliferator-activated Receptor γ. J. Biol. Chem. 2009, 284, 27620–27628. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Lipert, B.; Wegrzyn, P.; Sell, H.; Eckel, J.; Winiarski, M.; Budzynski, A.; Matlok, M.; Kotlinowski, J.; Ramage, L.; Malecki, M.; et al. Monocyte chemoattractant protein-induced protein 1 impairs adipogenesis in 3T3-L1 cells. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 780–788. [Google Scholar] [CrossRef]
- Losko, M.; Lichawska-Cieslar, A.; Kulecka, M.; Paziewska, A.; Rumienczyk, I.; Mikula, M.; Jura, J. Ectopic overexpression of MCPIP1 impairs adipogenesis by modulating microRNAs. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 186–195. [Google Scholar] [CrossRef]
- Hong, K.H.; Ryu, J.; Han, K.H. Monocyte chemoattractant protein-1–induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005, 105, 1405–1407. [Google Scholar] [CrossRef]
- Labedz-Maslowska, A.; Lipert, B.; Berdecka, D.; Kedracka-Krok, S.; Jankowska, U.; Kamycka, E.; Sekula, M.; Madeja, Z.; Dawn, B.; Jura, J.; et al. Monocyte Chemoattractant Protein-Induced Protein 1 (MCPIP1) Enhances Angiogenic and Cardiomyogenic Potential of Murine Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0133746. [Google Scholar] [CrossRef]
- Ligeza, J.; Marona, P.; Gach, N.; Lipert, B.; Miekus, K.; Wilk, W.; Jaszczynski, J.; Stelmach, A.; Loboda, A.; Dulak, J.; et al. MCPIP1 contributes to clear cell renal cell carcinomas development. Angiogenesis 2017, 20, 325–340. [Google Scholar] [CrossRef]
- Marona, P.; Górka, J.; Mazurek, Z.; Wilk, W.; Rys, J.; Majka, M.; Jura, J.; Miekus, K. MCPIP1 Downregulation in Clear Cell Renal Cell Carcinoma Promotes Vascularization and Metastatic Progression. Cancer Res. 2017, 77, 4905–4920. [Google Scholar] [CrossRef]
- Lichawska-Cieslar, A.; Pietrzycka, R.; Ligeza, J.; Kulecka, M.; Paziewska, A.; Kalita, A.; Dolicka, D.D.; Wilamowski, M.; Miekus, K.; Ostrowski, J.; et al. RNA sequencing reveals widespread transcriptome changes in a renal carcinoma cell line. Oncotarget 2018, 9, 8597–8613. [Google Scholar] [CrossRef] [PubMed]
- Skalniak, A.; Boratyn, E.; Tyrkalska, S.D.; Horwacik, I.; Durbas, M.; Lastowska, M.; Jura, J.; Rokita, H. Expression of the monocyte chemotactic protein-1-induced protein 1 decreases human neuroblastoma cell survival. Oncol. Rep. 2014, 31, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Boratyn, E.; Nowak, I.; Horwacik, I.; Durbas, M.; Mistarz, A.; Kukla, M.; Kaczówka, P.; Łastowska, M.; Jura, J.; Rokita, H. Monocyte Chemoattractant Protein-Induced Protein 1 Overexpression Modulates Transcriptome, Including MicroRNA, in Human Neuroblastoma Cells. J. Cell. Biochem. 2016, 117, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, F.; Siret, C.; Dobric, A.; Silvy, F.; Soubeyran, P.; Iovanna, J.; Lombardo, D.; Berthois, Y. Ribonuclease MCPiP1 contributes to the loss of micro-RNA-200 family members in pancreatic cancer cells. Oncotarget 2018, 9, 35941–35961. [Google Scholar] [CrossRef][Green Version]
- Lu, W.; Ning, H.; Gu, L.; Peng, H.; Wang, Q.; Hou, R.; Fu, M.; Hoft, D.F.; Liu, J. MCPIP1 selectively destabilizes transcripts associated with an antiapoptotic gene expression program in breast cancer cells that can elicit complete tumor regression. Cancer Res. 2016, 76, 1429–1440. [Google Scholar] [CrossRef]
- Wei, J.; Long, L.; Zheng, W.; Dhungana, Y.; Lim, S.A.; Guy, C.; Wang, Y.; Wang, Y.D.; Qian, C.; Xu, B.; et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 2019, 576, 471–476. [Google Scholar] [CrossRef]
- Gallo, R.L.; Hooper, L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef]
- Kucharzik, T. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut 2005, 54, 1565–1572. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, C. Elevated Serum Interleukin-8 Level as a Preferable Biomarker for Identifying Uncontrolled Asthma and Glucocorticosteroid Responsiveness. Tanafos 2017, 16, 260–269. [Google Scholar]
- Poghosyan, A.; Patel, J.K.; Clifford, R.L.; Knox, A.J. Epigenetic dysregulation of interleukin 8 (CXCL8) hypersecretion in cystic fibrosis airway epithelial cells. Biochem. Biophys. Res. Commun. 2016, 476, 431–437. [Google Scholar] [CrossRef]
- Gabay, C.; Towne, J.E. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J. Leukoc. Biol. 2015, 97, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Satoh, T.; Akira, S.; Sano, S. Regnase-1, an Immunomodulator, Limits the IL-36/IL-36R Autostimulatory Loop in Keratinocytes to Suppress Skin Inflammation. J. Investig. Dermatol. 2018, 138, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Ruiz-Romeu, E.; Ferran, M.; Giménez-Arnau, A.; Bugara, B.; Lipert, B.; Jura, J.; Florencia, E.F.; Prens, E.P.; Celada, A.; Pujol, R.M.; et al. MCPIP1 RNase Is Aberrantly Distributed in Psoriatic Epidermis and Rapidly Induced by IL-17A. J. Investig. Dermatol. 2016, 136, 1599–1607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monin, L.; Gudjonsson, J.E.; Childs, E.E.; Amatya, N.; Xing, X.; Verma, A.H.; Coleman, B.M.; Garg, A.V.; Killeen, M.; Mathers, A.; et al. MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-Dependent Skin Inflammation. J. Immunol. 2016, 198, 767–775. [Google Scholar] [CrossRef]
- Konieczny, P.; Lichawska-Cieslar, A.; Kwiecinska, P.; Cichy, J.; Pietrzycka, R.; Szukala, W.; Declercq, W.; Devos, M.; Paziewska, A.; Rumienczyk, I.; et al. Keratinocyte-specific ablation of Mcpip1 impairs skin integrity and promotes local and systemic inflammation. J. Mol. Med. 2019, 97, 1669–1684. [Google Scholar] [CrossRef]
- Elsholz, F.; Harteneck, C.; Muller, W.; Friedland, K. Calcium—A central regulator of keratinocyte differentiation in health and disease. Eur. J. Dermatol. 2014, 24, 650–661. [Google Scholar] [CrossRef]
- Gibbs, S.; Fijneman, R.; Wiegant, J.; van Kessel, A.G.; van de Putte, P.; Backendorf, C. Molecular Characterization and Evolution of the SPRR Family of Keratinocyte Differentiation Markers Encoding Small Proline-Rich Proteins. Genomics 1993, 16, 630–637. [Google Scholar] [CrossRef]
- Lessard, J.C.; Pina-Paz, S.; Rotty, J.D.; Hickerson, R.P.; Kaspar, R.L.; Balmain, A.; Coulombe, P.A. Keratin 16 regulates innate immunity in response to epidermal barrier breach. Proc. Natl. Acad. Sci. USA 2013, 110, 19537–19542. [Google Scholar] [CrossRef]
- Madlener, M.; Parks, W.C.; Werner, S. Matrix Metalloproteinases (MMPs) and Their Physiological Inhibitors (TIMPs) Are Differentially Expressed during Excisional Skin Wound Repair. Exp. Cell Res. 1998, 242, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Buisson-Legendre, N.; Emonard, H.; Bernard, P.; Hornebeck, W. Relationship Between Cell-Associated Matrix Metalloproteinase 9 and Psoriatic Keratinocyte Growth. J. Investig. Dermatol. 2000, 115, 213–218. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musson, R.; Szukała, W.; Jura, J. MCPIP1 RNase and Its Multifaceted Role. Int. J. Mol. Sci. 2020, 21, 7183. https://doi.org/10.3390/ijms21197183
Musson R, Szukała W, Jura J. MCPIP1 RNase and Its Multifaceted Role. International Journal of Molecular Sciences. 2020; 21(19):7183. https://doi.org/10.3390/ijms21197183
Chicago/Turabian StyleMusson, Richard, Weronika Szukała, and Jolanta Jura. 2020. "MCPIP1 RNase and Its Multifaceted Role" International Journal of Molecular Sciences 21, no. 19: 7183. https://doi.org/10.3390/ijms21197183
APA StyleMusson, R., Szukała, W., & Jura, J. (2020). MCPIP1 RNase and Its Multifaceted Role. International Journal of Molecular Sciences, 21(19), 7183. https://doi.org/10.3390/ijms21197183





