B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role
Abstract
1. Introduction
2. Results
2.1. Analysis of B7-H3 in MB Exosomes
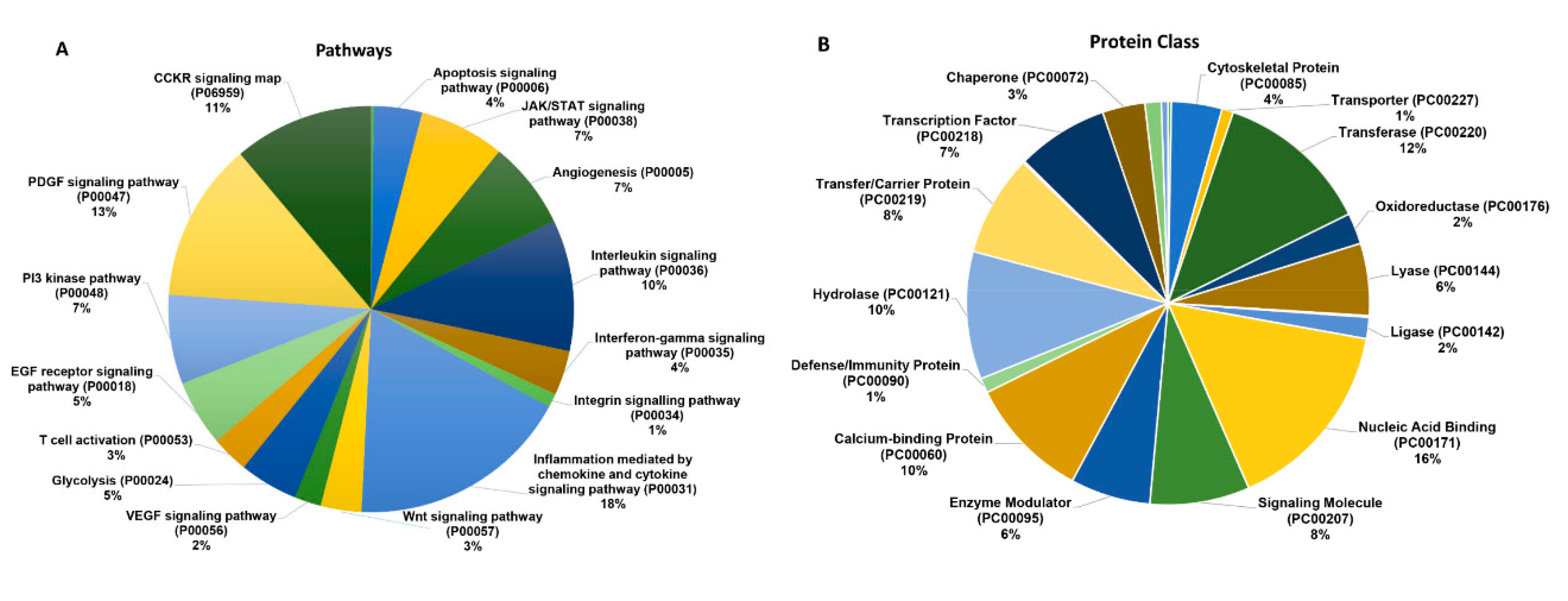
2.2. B7-H3 Overexpressing Exosomes Contain Novel Pro-Tumorigenic Molecules
2.3. B7-H3 Overexpressing Exosomes Translocate into Stromal and Cancer Cells
3. Discussion
4. Methods
4.1. Antibodies and Reagents
4.2. Cell Lines and Transfections
4.3. Exosome Isolation and Fluorescent Nanoparticle Tracking (NTA)
4.4. Mass Spectrometry and Immunoblotting
4.5. F-Actin Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EV | Extracellular vesicle |
| MB | Medulloblastoma |
| B7-H3 | Cluster of Differentiation 276 |
| MYC | V-myc avian myelocytomatosis viral oncogene homolog |
| DMEM | Dulbecco’s Modified Eagle Media |
| FBS | Fetal bovine serum |
| HMEC | Human microvascular endothelial cell |
| OE | Overexpression |
| NTA | Nanoparticle Tracking Analysis |
| mL | Milliliter |
| μL | Microliter |
| min | Minutes |
| h | Hours |
References
- Zhou, X.; Li, T.; Chen, Y.; Zhang, N.; Wang, P.; Liang, Y.; Long, M.; Liu, H.; Mao, J.; Liu, Q.; et al. Mesenchymal stem cellderived extracellular vesicles promote the in vitro proliferation and migration of breast cancer cells through the activation of the ERK pathway. Int. J. Oncol. 2019, 54, 1843–1852. [Google Scholar] [PubMed]
- Xie, C.; Ji, N.; Tang, Z.; Li, J.; Chen, Q. The role of extracellular vesicles from different origin in the microenvironment of head and neck cancers. Mol. Cancer 2019, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- D’Asti, E.; Garnier, D.; Lee, T.H.; Montermini, L.; Meehan, B.; Rak, J. Oncogenic extracellular vesicles in brain tumor progression. Front. Physiol. 2012, 3, 294. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of exosomal proteins in cancer diagnosis. Mol. Cancer 2017, 16, 145. [Google Scholar] [CrossRef]
- Bebawy, M.; Combes, V.; Lee, E.; Jaiswal, R.; Gong, J.; Bonhoure, A.; Grau, G.E. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 2009, 23, 1643–1649. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.W.; Chan, L.C.; Wei, Y.; Hsu, J.M.; Xia, W.; Cha, J.H.; Hou, J.; Hsu, J.L.; Sun, L.; et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018, 28, 862–864. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Yang, L.; Liu, J.; Zhang, X.; Zhang, Y.; Tu, Q.; Yin, D.; Lin, D.; Wong, P.P.; et al. Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Ridder, K.; Sevko, A.; Heide, J.; Dams, M.; Rupp, A.K.; Macas, J.; Starmann, J.; Tjwa, M.; Plate, K.H.; Sultmann, H.; et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology 2015, 4, e1008371. [Google Scholar] [CrossRef] [PubMed]
- Epple, L.M.; Griffiths, S.G.; Dechkovskaia, A.M.; Dusto, N.L.; White, J.; Ouellette, R.J.; Anchordoquy, T.J.; Bemis, L.T.; Graner, M.W. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS ONE 2012, 7, e42064. [Google Scholar] [CrossRef] [PubMed]
- Bisaro, B.; Mandili, G.; Poli, A.; Piolatto, A.; Papa, V.; Novelli, F.; Cenacchi, G.; Forni, M.; Zanini, C. Proteomic analysis of extracellular vesicles from medullospheres reveals a role for iron in the cancer progression of medulloblastoma. Mol. Cell Ther. 2015, 3, 8. [Google Scholar] [CrossRef]
- Ciregia, F.; Urbani, A.; Palmisano, G. Extracellular Vesicles in Brain Tumors and Neurodegenerative Diseases. Front. Mol. Neurosci. 2017, 10, 276. [Google Scholar]
- Zhao, Z.; Fan, J.; Hsu, Y.S.; Lyon, C.J.; Ning, B.; Hu, T.Y. Extracellular vesicles as cancer liquid biopsies: From discovery, validation, to clinical application. Lab. Chip. 2019, 19, 1114–1140. [Google Scholar]
- Purvis, I.J.; Avilala, J.; Guda, M.R.; Venkataraman, S.; Vibhakar, R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. Role of MYC-miR-29-B7-H3 in Medulloblastoma Growth and Angiogenesis. J. Clin. Med. 2019, 8, 1158. [Google Scholar] [CrossRef]
- Castellanos, J.R.; Purvis, I.J.; Labak, C.M.; Guda, M.R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol. 2017, 6, 66–75. [Google Scholar]
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M.; et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef]
- Nagai, S.; Azuma, M. The CD28-B7 Family of Co-signaling Molecules. Adv. Exp. Med. Biol. 2019, 1189, 25–51. [Google Scholar] [PubMed]
- Flem-Karlsen, K.; Fodstad, O.; Tan, M.; Nunes-Xavier, C.E. B7-H3 in Cancer—Beyond Immune Regulation. Trends. Cancer 2018, 4, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, D.; Chen, Q.; Yang, C.; Wang, B.; Wu, H. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-kappaB pathway. Sci. Rep. 2016, 6, 27528. [Google Scholar] [CrossRef] [PubMed]
- Flem-Karlsen, K.; Fodstad, Y.; Nunes-Xavier, C.E. B7-H3 immune checkpoint protein in human cancer. Curr. Med. Chem. 2020, 27, 4062–4086. [Google Scholar] [CrossRef]
- Wang, L.; Kang, F.B.; Zhang, G.C.; Wang, J.; Xie, M.F.; Zhang, Y.Z. Clinical significance of serum soluble B7-H3 in patients with osteosarcoma. Cancer Cell Int. 2018, 18, 115. [Google Scholar] [CrossRef]
- Marimpietri, D.; Petretto, A.; Raffaghello, L.; Pezzolo, A.; Gagliani, C.; Tacchetti, C.; Mauri, P.; Melioli, G.; Pistoia, V. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS ONE 2013, 8, e75054. [Google Scholar] [CrossRef]
- Brosseau, C.; Colas, L.; Magnan, A.; Brouard, S. CD9 Tetraspanin: A New Pathway for the Regulation of Inflammation? Front. Immunol. 2018, 9, 2316. [Google Scholar] [CrossRef]
- Khushman, M.; Bhardwaj, A.; Patel, G.K.; Laurini, J.A.; Roveda, K.; Tan, M.C.; Patton, M.C.; Singh, S.; Taylor, W.; Singh, A.P. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Sandfeld-Paulsen, B.; Jakobsen, K.R.; Baek, R.; Folkersen, B.H.; Rasmussen, T.R.; Meldgaard, P.; Varming, K.; Jorgensen, M.M.; Sorensen, B.S. Exosomal Proteins as Diagnostic Biomarkers in Lung Cancer. J. Thorac. Oncol. 2016, 11, 1701–1710. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Reddy, V.S.; Madala, S.K.; Trinath, J.; Reddy, G.B. Extracellular small heat shock proteins: Exosomal biogenesis and function. Cell Stress Chaperones 2018, 23, 441–454. [Google Scholar] [PubMed]
- Lauwers, E.; Wang, Y.C.; Gallardo, R.; Van der Kant, R.; Michiels, E.; Swerts, J.; Baatsen, P.; Zaiter, S.S.; McAlpine, S.R.; Gounko, N.V.; et al. Hsp90 Mediates Membrane Deformation and Exosome Release. Mol. Cell 2018, 71, 689–702.e9. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, M.; Chanteloup, G.; Isambert, N.; Seigneuric, R.; Fumoleau, P.; Garrido, C.; Gobbo, J. Exosomes in cancer theranostic: Diamonds in the rough. Cell Adh. Migr. 2017, 11, 151–163. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, H.; Zhang, Z.; Dai, X.; Mao, T.; Fan, Y.; Xie, X.; Wen, H.; Yu, P.; Hu, Y.; et al. The interaction of MMP-2/B7-H3 in human osteoporosis. Clin. Immunol. 2016, 162, 118–124. [Google Scholar] [CrossRef]
- Xu, L.; Ding, X.; Tan, H.; Qian, J. Correlation between B7-H3 expression and matrix metalloproteinases 2 expression in pancreatic cancer. Cancer Cell Int. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, G.; Song, J.; Cai, Z.; Yang, J.; Chen, Z.; Wang, Y.; Huang, Y.; Gao, Q. B7-H3 Promotes the Migration and Invasion of Human Bladder Cancer Cells via the PI3K/Akt/STAT3 Signaling Pathway. J. Cancer 2017, 8, 816–824. [Google Scholar] [CrossRef]
- Lee, S.H.; Dominguez, R. Regulation of actin cytoskeleton dynamics in cells. Mol. Cells 2010, 29, 311–325. [Google Scholar] [PubMed]
- Maruthamuthu, V.; Aratyn-Schaus, Y.; Gardel, M.L. Conserved F-actin dynamics and force transmission at cell adhesions. Curr. Opin. Cell Biol. 2010, 22, 583–588. [Google Scholar] [CrossRef]
- Figard, L.; Wang, M.; Zheng, L.; Golding, I.; Sokac, A.M. Membrane Supply and Demand Regulates F-Actin in a Cell Surface Reservoir. Dev. Cell. 2016, 37, 267–278. [Google Scholar] [CrossRef]
- Fremont, S.; Echard, A. Membrane Traffic in the Late Steps of Cytokinesis. Curr. Biol. 2018, 28, R458–R470. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Chen, S.; Cai, J.; Ban, Y.; Peng, Q.; Zhou, Y.; Zeng, Z.; Li, X.; Xiong, W.; et al. FOXA1 reprograms the TGF-beta-stimulated transcriptional program from a metastasis promoter to a tumor suppressor in nasopharyngeal carcinoma. Cancer Lett. 2019, 442, 1–14. [Google Scholar] [PubMed]
- Sun, T.; Man, Z.; Peng, C.; Wang, G.; Sun, S. A specific affinity cyclic peptide enhances the adhesion, expansion and proliferation of rat bone mesenchymal stem cells on betatricalcium phosphate scaffolds. Mol. Med. Rep. 2019, 20, 1157–1166. [Google Scholar] [PubMed]
- Martin, A.M.; Nirschl, C.J.; Polanczyk, M.J.; Bell, W.R.; Nirschl, T.R.; Harris-Bookman, S.; Phallen, J.; Hicks, J.; Martinez, D.; Ogurtsova, A.; et al. PD-L1 expression in medulloblastoma: An evaluation by subgroup. Oncotarget 2018, 9, 19177–19191. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Dudas, J.; Riechelmann, H.; Skvortsova, I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Ruivo, C.F.; Adem, B.; Silva, M.; Melo, S.A. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017, 77, 6480–6488. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Wang, Y.; Dong, F.; Zhu, M.; Wan, W.; Li, H.; Wu, F.; Yan, X.; Ke, X. B7-H3 silencing by RNAi inhibits tumor progression and enhances chemosensitivity in U937 cells. Onco. Targets. Ther. 2015, 8, 1721–1733. [Google Scholar]
- Singh, S.K.; Mishra, M.K.; Eltoum, I.A.; Bae, S.; Lillard, J.W., Jr.; Singh, R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci. Rep. 2018, 8, 1323. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, Y. B7-H3 Induces Ovarian Cancer Drugs Resistance Through An PI3K/AKT/BCL-2 Signaling Pathway. Cancer Manag. Res. 2019, 11, 10205–10214. [Google Scholar] [CrossRef]
- Wei, X.; Li, K.; Zhang, G.; Huang, Y.; Lv, J.; Li, M.; Zhao, L.; Fan, C.; Pu, J.; Hou, J.; et al. B7-H3 promoted proliferation of mouse spermatogonial stem cells via the PI3K signaling pathway. Oncotarget 2017, 9, 1542–1552. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Que, L.; Tang, X. The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J. Cancer 2019, 10, 5770–5784. [Google Scholar] [CrossRef]
- Campa, C.C.; Franco, I.; Hirsch, E. PI3K-C2alpha: One enzyme for two products coupling vesicle trafficking and signal transduction. FEBS Lett. 2015, 589, 1552–1558. [Google Scholar] [PubMed]
- Mountford, S.J.; Zheng, Z.; Sundaram, K.; Jennings, I.G.; Hamilton, J.R.; Thompson, P.E. Class II but Not Second Class-Prospects for the Development of Class II PI3K Inhibitors. ACS Med. Chem. Lett. 2014, 6, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Cui, H.; Wakayama, T.; Takuwa, N.; Okamoto, Y.; Du, W.; Qi, X.; Asanuma, K.; Sugihara, K.; Aki, S.; et al. Endothelial PI3K-C2alpha, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 2012, 18, 1560–1569. [Google Scholar]
- Aki, S.; Yoshioka, K.; Okamoto, Y.; Takuwa, N.; Takuwa, Y. Phosphatidylinositol 3-kinase class II alpha-isoform PI3K-C2alpha is required for transforming growth factor beta-induced Smad signaling in endothelial cells. J. Biol. Chem. 2015, 290, 6086–6105. [Google Scholar] [PubMed]
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Margaria, J.P.; Ratto, E.; Gozzelino, L.; Li, H.; Hirsch, E. Class II PI3Ks at the Intersection between Signal Transduction and Membrane Trafficking. Biomolecules 2019, 9, 104. [Google Scholar] [CrossRef]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The role of JAK-STAT signaling within the CNS. JAKSTAT 2013, 2, e22925. [Google Scholar] [CrossRef]
- Wei, J.; Ma, L.; Li, C.; Pierson, C.R.; Finlay, J.L.; Lin, J. Targeting Upstream Kinases of STAT3 in Human Medulloblastoma Cells. Curr. Cancer Drug Targets 2019, 19, 571–582. [Google Scholar] [CrossRef]
- Lin, L.; Cao, L.; Liu, Y.; Wang, K.; Zhang, X.; Qin, X.; Zhao, D.; Hao, J.; Chang, Y.; Huang, X.; et al. B7-H3 promotes multiple myeloma cell survival and proliferation by ROS-dependent activation of Src/STAT3 and c-Cbl-mediated degradation of SOCS3. Leukemia 2019, 33, 1475–1486. [Google Scholar] [CrossRef]
- Wong, A.L.A.; Bellot, G.L.; Hirpara, J.L.; Pervaiz, S. Understanding the cancer stem cell phenotype: A step forward in the therapeutic management of cancer. Biochem. Pharmacol. 2019, 162, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, M.R.; Wang, L.; Hu, C.J. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene 2014, 33, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wang, B.; Long, M.; Gao, Z.; Zhang, Z.; Wang, H.; Wang, X.; Li, R.; Dong, K.; Zhang, H. The type 1 transmembrane glycoprotein B7-H3 interacts with the glycolytic enzyme ENO1 to promote malignancy and glycolysis in HeLa cells. FEBS Lett. 2018, 592, 2476–2488. [Google Scholar] [CrossRef]
- Shi, T.; Ma, Y.; Cao, L.; Zhan, S.; Xu, Y.; Fu, F.; Liu, C.; Zhang, G.; Wang, Z.; Wang, R.; et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019, 10, 308. [Google Scholar] [CrossRef]
- Diaz-Ramos, A.; Roig-Borrellas, A.; Garcia-Melero, A.; Lopez-Alemany, R. Alpha-Enolase, a multifunctional protein: Its role on pathophysiological situations. J. Biomed. Biotechnol. 2012, 2012, 156795. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006, 25, 4777–4786. [Google Scholar] [CrossRef]
- Niehage, C.; Steenblock, C.; Pursche, T.; Bornhauser, M.; Corbeil, D.; Hoflack, B. The cell surface proteome of human mesenchymal stromal cells. PLoS ONE 2011, 6, e20399. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, M.A.; Gallois, A.; Riedl, T.; Jurdic, P.; Hoflack, B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS ONE 2008, 3, e3537. [Google Scholar] [CrossRef]
- Singhatanadgit, W.; Salih, V.; Olsen, I. Up-regulation of bone morphogenetic protein receptor IB by growth factors enhances BMP-2-induced human bone cell functions. J. Cell Physiol. 2006, 209, 912–922. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Mao, Y.; Zhu, J.; Meng, F.; Chen, Q.; Tao, L.; Li, R.; Fu, F.; Liu, C.; Hu, Y.; et al. TGF-beta1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget 2016, 7, 67196–67211. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, Z. B7-H3 and its role in bone cancers. Pathol. Res. Pract. 2019, 215, 152420. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, A.; Corrias, M.V.; Castriconi, R.; Dondero, A.; Mosconi, M.; Gambini, C.; Moretta, A.; Moretta, L.; Bottino, C. Small round blue cell tumours: Diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 2008, 53, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Gercel-Taylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 2005, 92, 305–311. [Google Scholar] [CrossRef]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef]
- Nanou, A.; Miller, M.C.; Zeune, L.L.; de Wit, S.; Punt, C.J.A.; Groen, H.J.M.; Hayes, D.F.; de Bono, J.S.; Terstappen, L.W.M.M. Tumour-derived extracellular vesicles in blood of metastatic cancer patients associate with overall survival. Br. J. Cancer 2020, 122, 801–811. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, X.; Fang, M.; Li, H.; Su, P.; Tu, Y.; Zhang, L.; Zhou, F. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv. Sci. (Weinh) 2019, 6, 1901779. [Google Scholar] [CrossRef]
- Wu, S.Q.; Song, H.P.; Li, B.; Liu, R.Z.; Yang, H.; He, L.; Li, P. A fast and accurate method for the identification of peroxidase inhibitors from Radix Salvia Miltiorrhizae by on-flow biochemical assay coupled with LC/Q-TOF-MS: Comparison with ultrafiltration-based affinity selection. Anal. Bioanal. Chem. 2018, 410, 4311–4322. [Google Scholar] [CrossRef]
- Asuthkar, S.; Velpula, K.K.; Nalla, A.K.; Gogineni, V.R.; Gondi, C.S.; Rao, J.S. Irradiation-induced angiogenesis is associated with an MMP-9-miR-494-syndecan-1 regulatory loop in medulloblastoma cells. Oncogene 2014, 33, 1922–1933. [Google Scholar] [CrossRef]


| Control EV | B7-H3 OE EV | ||||
|---|---|---|---|---|---|
| Gene | Associated Pathway(s) | Matched Peptide Sequence | Gene | Associated Pathway(s) | Matched Peptide Sequence |
| STAT1 | JAK/STAT, IFN-γ/IL-12, Chemokine signaling | DPIQMSMIIYSCLKE | STAT3 | JAK/STAT, Chemokine signaling, Stem cell/LIF, IL-6/HIF-1 | NQGVPVLIVANK |
| STAT2 | JAK/STAT, Chemokine signaling | LSLDLEPLLKAGLDLGPELE | c-MYC | MAPK, PI3K/AKT, WNT, TGF-β | KQIVAGVNYFLDVE |
| MYCN | Group 4 MB | DAPPQKKIK | AKT2 | MAPK, HIF-1, PI3K/AKT, Chemokine signaling, VEGF, Ras | VSLAKPKHRVTMNE |
| IKKB | NF-κB, MAPK, mTOR, PI3K/AKT | AAMMNLLRNNSCLSKMK | MMP2 | Endothelial migration/angiogenesis, MAPK/ERK, Myc | NVAADIAVQLCE, VWELGGCANKE |
| TANK | NF-κB, NOD-like receptor | GPQQPIWKPFPNQDSDSVVLSGTDSE | MMP9 | TNF, IL-17/MAPK, NF- κB, angiogenesis | NKPTRPVIVSPANETME |
| TGFB1 | TGF-β | LLAPSDSPEWLSFDVTGVVR, RGDLATIHGMNRPFLLLMATPLER | TIMP2 | MMP2/MMP9 | FTTSVVRR |
| TGFR1 | TGF-β | VLDDSINMK | NFKB2 | NF-κB, MAPK, PI3K/AKT, Ras | LAPASPMASPGGSIDERPLSSSPLVRVK, LLTDVQLMK, VVNKLIQFLISLVQSNR |
| CCL2 | JAK/STAT, Src, MAPK, PI3K/AKT, NF-κB | ICADPKQKWVQDSMDHLDK | IL2 | PI3K/AKT, JAK/STAT, MHC/Antigen signaling, ZAP70 | HPRNIQESPF |
| H2A1D | Histone 2 complex | VGAGAPVYLAAVLE | CCL5 | JAK/STAT, PI3K/AKT, TNF, TLR4, LPS/ERK | SSTLIGR |
| CCR9 | CCL25, PI3K/AKT, JAK/STAT, RhoA/ROCK, MAPK | LEVLQDCTFE | |||
| TSG101 | ESCRT complex, vesicle formation | AMLASRSASLLK | |||
| H2A1B | Histone 2 complex | VGAGAPVYLAAVLE | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purvis, I.J.; Velpula, K.K.; Guda, M.R.; Nguyen, D.; Tsung, A.J.; Asuthkar, S. B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role. Int. J. Mol. Sci. 2020, 21, 7050. https://doi.org/10.3390/ijms21197050
Purvis IJ, Velpula KK, Guda MR, Nguyen D, Tsung AJ, Asuthkar S. B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role. International Journal of Molecular Sciences. 2020; 21(19):7050. https://doi.org/10.3390/ijms21197050
Chicago/Turabian StylePurvis, Ian J., Kiran K. Velpula, Maheedhara R. Guda, Daniel Nguyen, Andrew J. Tsung, and Swapna Asuthkar. 2020. "B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role" International Journal of Molecular Sciences 21, no. 19: 7050. https://doi.org/10.3390/ijms21197050
APA StylePurvis, I. J., Velpula, K. K., Guda, M. R., Nguyen, D., Tsung, A. J., & Asuthkar, S. (2020). B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role. International Journal of Molecular Sciences, 21(19), 7050. https://doi.org/10.3390/ijms21197050






