Natural Agents Targeting Mitochondria in Cancer
Abstract
:1. Introduction
2. Mitocans: The Alternative Cancer Therapy
2.1. The Inception
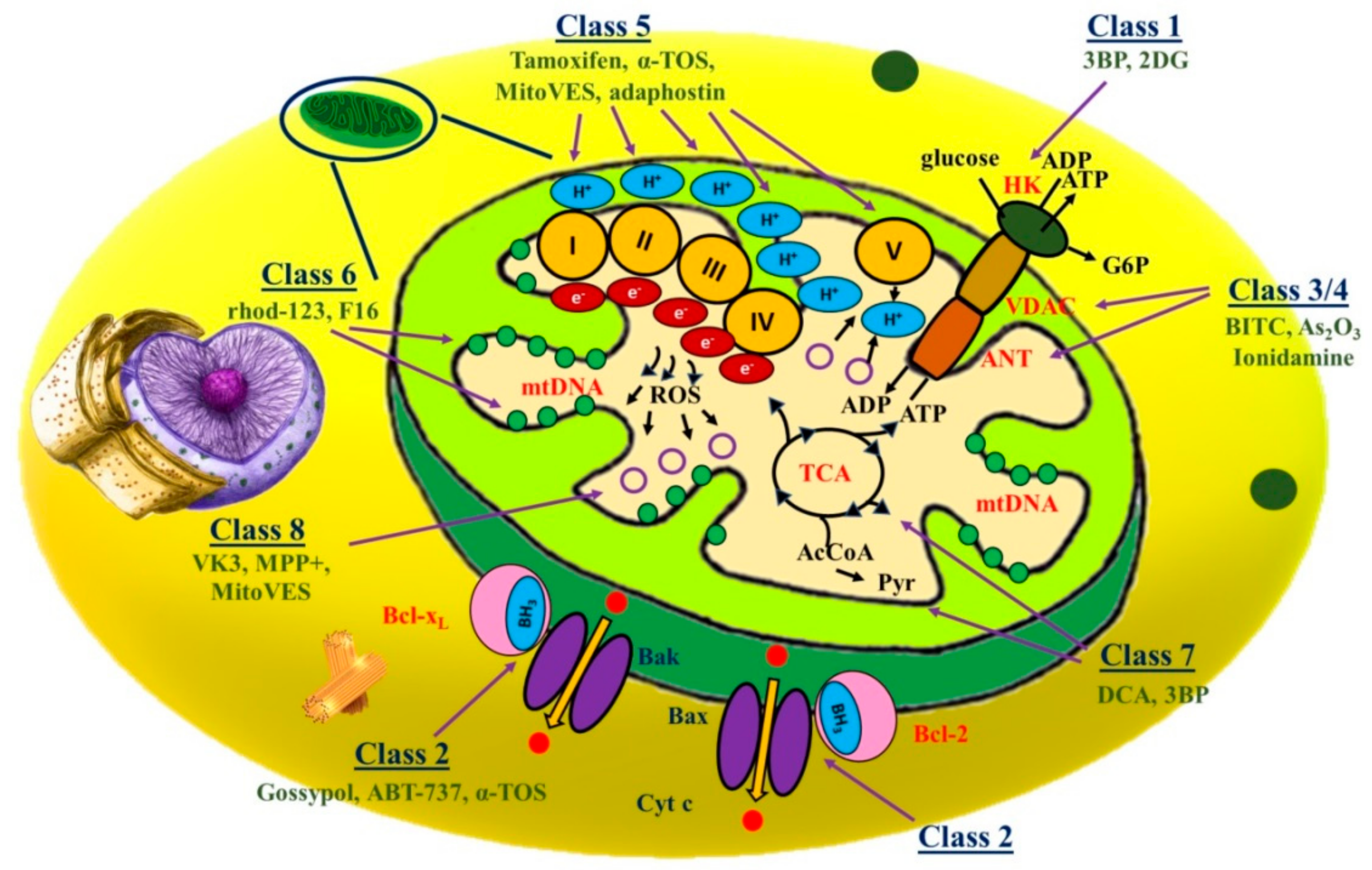
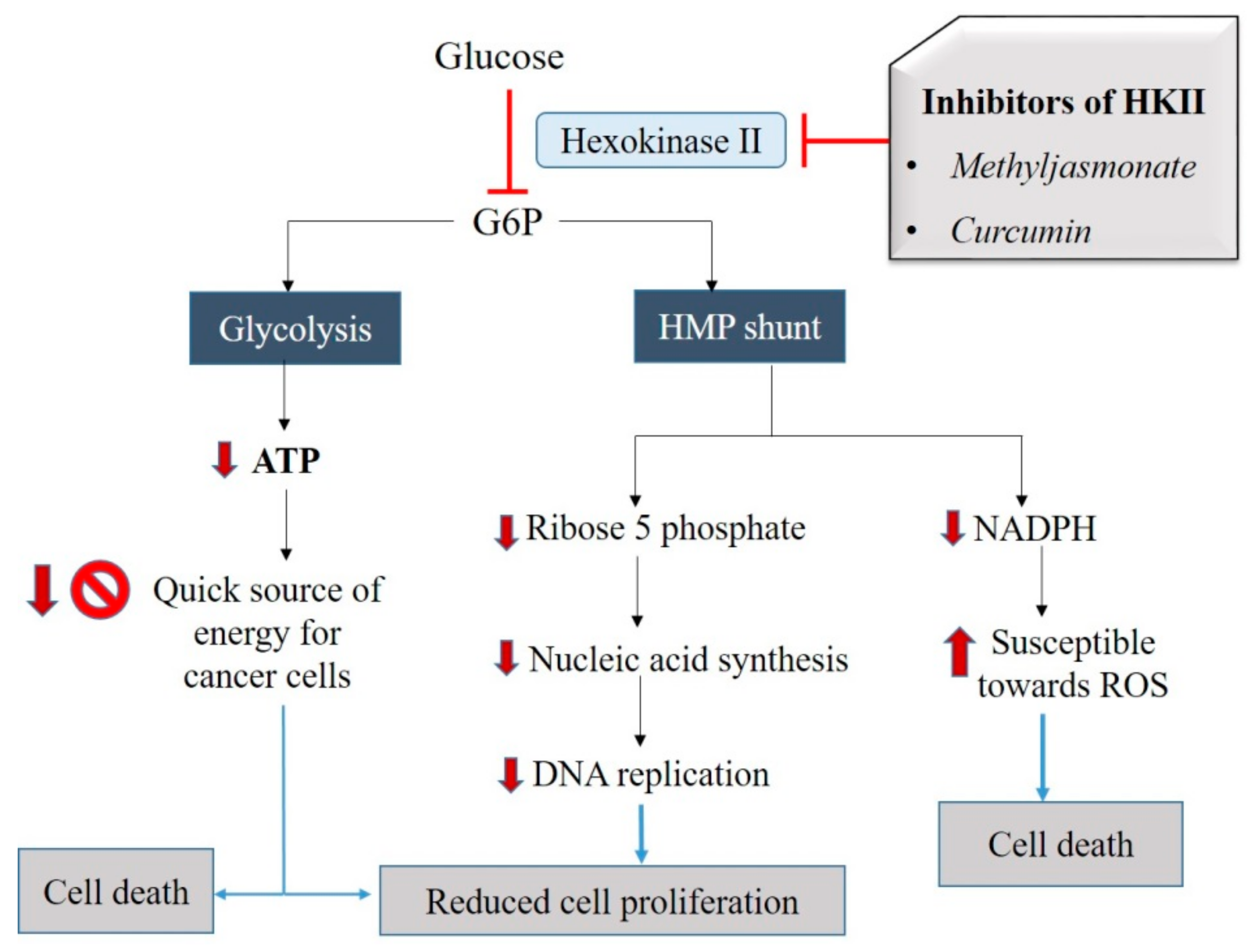
2.1.1. Class 1 Mitocans: Hexokinase Inhibitors
2.1.2. Class 2 Mitocans: Compounds Targeting Bcl-2 Family Proteins
2.1.3. Class 3 Mitocans: Thiol Redox Inhibitors
2.1.4. Class 4 Mitocans: VDAC/ANT Targeting Drugs
2.1.5. Class 5 Mitocans: Electron Transport Chain Targeting Drugs
2.1.6. Class 6 mitocans: Lipophilic Cations Targeting the Inner Membrane
2.1.7. Class 7 Mitocans: Drugs Targeting the Tricarboxylic Acid Cycle
2.1.8. Class 8 Mitocans: Drugs Targeting mtDNA
2.2. The Evolution and Current Status of Mitocans
3. Natural Agents as Mitocans: The Alternative Approach to Overcome the Limitations of Synthetic Mitocans
3.1. Classification of Natural Agents and Plant Extracts as Mitocans
3.2. Natural Agents which Inhibit HKII
3.3. Natural Agents Targeting Bcl-2 Family Proteins
3.4. Natural Agents as ROS Elevators
3.5. Natural Agents Reducing the Mitochondrial Membrane Potential
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer: World Health Organization. Available online: https://www.who.int/health-topics/cancer (accessed on 17 August 2020).
- Shrestha-Bhattarai, T.; Rangnekar, V.M. Cancer-selective apoptotic effects of extracellular and intracellular Par-4. Oncogene 2010, 29, 3873–3880. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.M.; Hossain, M.; Varela-Ramirez, A.; Das, U.; Ayala-Marin, Y.M.; Dimmock, J.R.; Aguilera, R.J. A novel class of piperidones exhibit potent, selective and pro-apoptotic anti-leukemia properties. Oncol. Lett. 2016, 11, 3842–3848. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Alhadlaq, H.A.; Kumar, S.; Alrokayan, S.A.; Ahamed, M. Selective cancer-killing ability of metal-based nanoparticles: Implications for cancer therapy. Arch. Toxicol. 2015, 89, 1895–1907. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vander-Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, S.E.; Chandel, N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015, 11, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sánchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014, 514, 628–632. [Google Scholar] [CrossRef] [Green Version]
- Viale, A.; Corti, D.; Draetta, G.F. Tumors and mitochondrial respiration: A neglected connection. Cancer Res. 2015, 75, 3685–3686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.T.; Supuran, C.; Khalid, A.O. The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med Chem. 2017, 17, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Seyfried, T.N. Cancer as a mitochondrial metabolic disease. Front. Cell Dev. Biol. 2015, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badrinath, N.; Yoo, S.Y. Mitochondria in cancer: In the aspects of tumorigenesis and targeted therapy. Carcinogenesis 2018, 39, 1419–1430. [Google Scholar] [CrossRef]
- Fogal, V.; Richardson, A.D.; Karmali, P.P.; Scheer, I.E.; Smith, J.W.; Ruoslahti, E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell Biol. 2010, 30, 1303–1318. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef] [Green Version]
- Bhat, T.A.; Kumar, S.; Chaudhary, A.K.; Yadav, N.; Chandra, D. Restoration of mitochondria function as a target for cancer therapy. Drug Discov. Today 2015, 20, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging. 2002, 16, 430–450. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell Sci. 2017, 130, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Amith, S.R.; Fliegel, L. Na+/H+ exchanger-mediated hydrogen ion extrusion as a carcinogenic signal in triple-negative breast cancer etiopathogenesis and prospects for its inhibition in therapeutics. Semin. Cancer Biol. 2017, 43, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Counillon, L.; Bouret, Y.; Marchiq, I.; Pouyssegur, J. Na+/H+ antiporter (NHE1) and lactate/H+ symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Heerdt, B.G.; Houston, M.A.; Augenlicht, L.H. Growth properties of colonic tumor cells are a function of the intrinsic mitochondrial membrane potential. Cancer Res. 2006, 66, 1591–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-centric review of polyphenol bioactivity in cancer models. Antioxid Redox Sign. 2018, 29, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Denisenko, T.V.; Gorbunova, A.S.; Zhivotovsky, B. Mitochondrial involvement in migration, invasion and metastasis. Front. Cell Dev. Biol. 2019, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.; Low, P.; Dong, L.; Lawen, A.; Neuzil, J. Mitocans: Mitochondrial Targeted Anti-Cancer Drugs as Improved Therapies and Related Patent Documents. Recent Pat Anticancer Drug Discov. 2006, 1, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Emmings, E.; Mullany, S.; Chang, Z.; Landen, C.N.; Linder, S.; Bazzaro, M. Targeting Mitochondria for Treatment of Chemoresistant Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 229. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Pandey, S. Exploiting mitochondrial vulnerabilities to trigger apoptosis selectively in cancer cells. Cancers 2019, 11, 916. [Google Scholar] [CrossRef] [Green Version]
- Neuzil, J.; Dong, L.F.; Rohlena, J.; Truksa, J.; Ralph, S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 2013, 13, 199–208. [Google Scholar] [CrossRef]
- Mani, S.; Taneja, N.; Jain, S.; Singh, M. Anticancerous Plant Compounds Affecting the Power House of Cancerous Cells: A Possible Herbal Mitocan. In Anticancer Plants: Mechanisms and Molecular Interactions; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2018; pp. 227–258. [Google Scholar]
- Mintah, S.O.; Asafo-Agyei, T.; Archer, M.A.; Junior, P.A.A.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Boakye, Y.D. Medicinal Plants for Treatment of Prevalent Diseases. In Pharmacognosy-Medicinal Plants; Perveen, S., Al-Taweel., A., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/pharmacognosy-medicinal-plants/medicinal-plants-for-treatment-of-prevalent-diseases (accessed on 20 September 2020).
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Magrì, A.; Reina, S.; de Pinto, V. VDAC1 as pharmacological target in cancer and neurodegeneration: Focus on its role in apoptosis. Front. Chem. 2018, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Fan, Z.; Cheng, H.; Huang, Q.; Yang, C.; Jin, K.; Luo, G.; Yu, X.; Liu, C. Hexokinase 2 dimerization and interaction with voltage-dependent anion channel promoted resistance to cell apoptosis induced by gemcitabine in pancreatic cancer. Cancer Med-US. 2019, 8, 5903–5915. [Google Scholar] [CrossRef]
- Camara, A.K.; Zhou, Y.; Wen, P.C.; Tajkhorshid, E.; Kwok, W.M. Mitochondrial VDAC1: A key gatekeeper as potential therapeutic target. Front. Physiol. 2017, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.; Jurgensmeier, J.; Matsuyama, S. Bcl-2 family proteins and mitochondria. BBA Bioenerg. 1998, 1366, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Um, H.D. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: A review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget 2016, 7, 5193. [Google Scholar] [CrossRef] [Green Version]
- Cardaci, S.; Ciriolo, M.R. TCA cycle defects and cancer: When metabolism tunes redox state. Int. J. Cell Biol. 2012, 2012, 161837. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.; Weiss-Lopez, B.; Araya-Maturana, R. Determinants of anti-cancer effect of mitochondrial electron transport chain inhibitors: Bioenergetic profile and metabolic flexibility of cancer cells. Curr. Pharm. Des 2016, 22, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.J.; Neuzil, J. Mitochondria as Targets for Cancer Therapy. In Mitochondria and Cancer; Costello, L., Singh, K., Eds.; Springer: New York, NY, USA, 2009; pp. 211–249. [Google Scholar]
- Rempel, A.; Mathupala, S.P.; Griffin, C.A.; Hawkins, A.L.; Pedersen, P.L. Glucose Catabolism in Cancer Cells: Amplification of the Gene Encoding Type II Hexokinase1. Cancer Res. 1996, 56, 2468–2471. [Google Scholar] [PubMed]
- Shinohara, Y.; Ishida, T.; Hino, M.; Yamazaki, N.; Baba, Y.; Terada, H. Characterization of porin isoforms expressed in tumor cells. Eur. J. Biochem. 2000, 267, 6067–6073. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006, 25, 4777–4786. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Jeong, D.C.; Pak, K.; Han, M.E.; Kim, J.Y.; Liangwen, L.; Kim, H.J.; Kim, T.W.; Kim, T.H.; Hyun, D.W.; et al. SLC2A2 (GLUT2) as a novel prognostic factor for hepatocellular carcinoma. Oncotarget 2017, 8, 68381. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Seino, Y.; Fukumoto, H.; Koh, G.; Yano, H.; Inagaki, N.; Yamada, Y.; Inoue, K.; Manabe, T.; Imura, H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys. Res. Commun. 1990, 170, 223–230. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chi, L.H.; Chang, W.M.; Su, C.Y.; Lin, Y.F.; Chen, C.L.; Chen, M.H.; Chang, P.M.H.; Wu, A.T.; Hsiao, M. Glucose transporter 4 promotes head and neck squamous cell carcinoma metastasis through the TRIM24-DDX58 axis. Oncol. J. Hematol. Oncol. 2017, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Pottosin, I.; Dobrovinskaya, O. Mitochondria as emerging targets for therapies against T cell acute lymphoblastic leukemia. J. Leukoc. Biol. 2019, 105, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2014, 22, 248–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoshan-Barmatz, V.; Mizrachi, D. VDAC1: From structure to cancer therapy. Front. Oncol. 2012, 2, 164. [Google Scholar] [CrossRef] [Green Version]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.M.; El-Abhar, H.S.; Al-Marzabani, M.M.; Shouman, S.A. Targeting glycolysis by 3-bromopyruvate improves tamoxifen cytotoxicity of breast cancer cell lines. BMC Cancer 2015, 15, 838. [Google Scholar] [CrossRef] [Green Version]
- Voss, M.; Lorenz, N.I.; Luger, A.L.; Steinbach, J.P.; Rieger, J.; Ronellenfitsch, M.W. Rescue of 2-Deoxyglucose Side Effects by Ketogenic Diet. Int. J. Mol. Sci. 2018, 19, 2462. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zheng, M.; Wu, S.; Gao, S.; Yang, M.; Li, Z.; Min, Q.; Sun, W.; Chen, L.; Xiang, G.; et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J. Exp. Clin. Cancer Res. 2017, 36, 58. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The Bcl-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Lessene, G.; Czabotar, P.E.; Colman, P. Bcl-2 family antagonists for cancer therapy. Nat. Rev. Drug Discov. 2008, 7, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeitlin, B.D.; Zeitlin, I.J.; Nör, J.E. Expanding circle of inhibition: Small-molecule inhibitors of Bcl-2 as anticancer cell and antiangiogenic agents. J. Clin. Oncol. 2008, 26, 4180–4188. [Google Scholar] [CrossRef] [Green Version]
- Oliver, C.L.; Miranda, M.B.; Shangary, S.; Land, S.; Wang, S.; Johnson, D.E. (−)-Gossypol acts directly on the mitochondria to overcome Bcl-2-and Bcl-XL-mediated apoptosis resistance. Mol. Cancer Ther. 2005, 4, 23–31. [Google Scholar] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [Green Version]
- Shiau, C.W.; Huang, J.W.; Wang, D.S.; Weng, J.R.; Yang, C.C.; Lin, C.H.; Li, C.; Chen, C.S. α-Tocopheryl succinate induces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. J. Biol. Chem. 2006, 281, 11819–11825. [Google Scholar] [CrossRef] [Green Version]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.L.; Lam, S.K.; Li, Y.Y.; Ho, J.C.M. Tumour growth-suppressive effect of arsenic trioxide in squamous cell lung carcinoma. Oncol Lett. 2017, 14, 3748–3754. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Zhivotovsky, B.; Galluzzi, L.; Kepp, O.; Kroemer, G. Adenine nucleotide translocase: A component of the phylogenetically conserved cell death machinery. Cell Death Differ. 2009, 16, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Belzacq, A.S.; el Hamel, C.; Vieira, H.L.; Cohen, I.; Haouzi, D.; Metivier, D.; Marchetti, P.; Brenner, C.; Kroemer, G. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene 2001, 20, 7579–7587. [Google Scholar] [CrossRef] [Green Version]
- Fruehauf, J.P.; Meyskens, F.L. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, Z.; Bunker, E.; Ramirez, A.; Lee, S.; Peng, Y.; Tan, A.C.; Eckhardt, S.G.; Chapnick, D.A.; Liu, X. Sorafenib targets the mitochondrial electron transport chain complexes and ATP synthase to activate the PINK1-Parkin pathway and modulate cellular drug response. J. Biol. Chem. 2017, 292, 15105–15120. [Google Scholar] [CrossRef] [Green Version]
- Rohlenova, K.; Sachaphibulkij, K.; Stursa, J.; Bezawork-Geleta, A.; Blecha, J.; Endaya, B.; Werner, L.; Cerny, J.; Zobalova, R.; Goodwin, J. Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2high Breast Cancer. Antioxid. Redox Signal. 2017, 26, 84–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejewski, S.; Gravel, S.P.; Pollak, M.; St-Pierre, J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014, 2, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modica-Napolitano, J.S.; Aprille, J.R. Basis for the selective cytotoxicity of rhodamine 123. Cancer Res. 1987, 47, 4361–4365. [Google Scholar] [PubMed]
- Modica-Napolitano, J.S.; Aprille, J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 2001, 49, 63–70. [Google Scholar] [CrossRef]
- Alves, I.D.; Carré, M.; Montero, M.P.; Castano, S.; Lecomte, S.; Marquant, R.; Lecorché, P.; Burlina, F.; Schatz, C.; Sagan, S. A proapoptotic peptide conjugated to penetratin selectively inhibits tumor cell growth. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, H.; Xiang, C.; Fan, X.Y.; Yang, L.Y.; Yuan, L.; Jiang, F.L.; Liu, Y. Uncoupling effect of F16 is responsible for its mitochondrial toxicity and anticancer activity. Toxicol. Sci. 2018, 161, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggieri, V.; Agriesti, F.; Scrima, R.; Laurenzana, I.; Perrone, D.; Tataranni, T.; Mazzoccoli, C.; Muzio, L.L.; Capitanio, N.; Piccoli, C. Dichloroacetate, a selective mitochondria-targeting drug for oral squamous cell carcinoma: A metabolic perspective of treatment. Oncotarget 2015, 6, 1217–1230. [Google Scholar] [CrossRef] [Green Version]
- Pereira da Silva, A.P.; El-Bacha, T.; Kyaw, N.; dos Santos, R.S.; Da-Silva, W.S.; Almeida, F.C.; da Poian, A.T.; Galina, A. Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate1. Biochem. J. 2009, 417, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Sasaki, R.; Suzuki, Y.; Yonezawa, Y.; Ota, Y.; Okamoto, Y.; Demizu, Y.; Huang, P.; Yoshida, H.; Sugimura, K.; Mizushina, Y. DNA polymerase γ inhibition by vitamin K3 induces mitochondria-mediated cytotoxicity in human cancer cells. Cancer Sci. 2008, 99, 1040–1048. [Google Scholar] [CrossRef] [Green Version]
- Umeda, S.; Muta, T.; Ohsato, T.; Takamatsu, C.; Hamasaki, N.; Kang, D. The D-loop structure of human mtDNA is destabilized directly by 1-methyl-4-phenylpyridinium ion (MPP+), a parkinsonism-causing toxin. Eur. J. Biochem. 2000, 267, 200–206. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Koya, K.; Weisberg, E.; Brunelli, B.T.; Li, Y.; Chen, L.B. Selective damage to carcinoma mitochondria by the rhodacyanine MKT-077. Cancer Res. 1996, 56, 544–550. [Google Scholar] [PubMed]
- Chunta, J.L.; Vistisen, K.S.; Yazdi, Z.; Braun, R.D. Uptake rate of cationic mitochondrial inhibitor MKT-077 determines cellular oxygen consumption change in carcinoma cells. PLoS ONE 2012, 7, e37471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, C.; Hu, W.; Feng, Z. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett. 2015, 356, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Vousden, K.H.; Ryan, K.M. p53 and metabolism. Nat. Rev. Cancer. 2009, 9, 691–700. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Liang, Y.; Wu, R.; Zhao, Y.; Hong, X.; Lin, M.; Yu, H.; Liu, L.; Levine, A.J.; et al. Tumour associated mutant p53 drives the Warburg effect. Nat. Commun. 2013, 4, 2935. [Google Scholar] [CrossRef] [Green Version]
- Ho, E.; Courtemanche, C.; Ames, B.N. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J. Nutr. 2003, 133, 2543–2548. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [Green Version]
- Virgili, F.; Canali, R.; Figus, E.; Vignolini, F.; Nobili, F.; Mengheri, E. Intestinal damage induced by zinc deficiency is associated with enhanced CuZn superoxide dismutase activity in rats: Effect of dexamethasone or thyroxine treatment. Free Radic. Biol. Med. 1999, 26, 1194–1201. [Google Scholar] [CrossRef]
- Ho, E.; Ames, B.N. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFκB, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.M.; Liang, D.; Jin, J.; Li, D.J.; Zhang, Y.C.; Gao, Z.Y.; He, Y.T. Research progress on the relationship between zinc deficiency, related micro RNA s, and esophageal carcinoma. Thorac Cancer 2017, 8, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Taccioli, C.; Chen, H.; Jiang, Y.; Liu, X.P.; Huang, K.; Smalley, K.J.; Farber, J.L.; Croce, C.M.; Fong, L.Y. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene 2012, 31, 4550–4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, D.K.; Chadha, V.D. Zinc: A promising agent in dietary chemoprevention of cancer. Indian J. Med. Res. 2010, 132, 676–682. [Google Scholar]
- Doerr, T.D.; Prasad, A.S.; Marks, S.C.; Beck, F.W.; Shamsa, F.H.; Penny, H.S.; Mathog, R.H. Zinc deficiency in head and neck cancer patients. J. Am. Coll. Nutr. 1997, 16, 418–422. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 17 August 2020).
- Floridi, A.; Paggi, M.G.; D’Atri, S.; De Martino, C.; Marcante, M.L.; Silvestrini, B.; Caputo, A. Effect of Lonidamine on the Energy Metabolism of Ehrlich Ascites Tumor Cells. Cancer Res. 1981, 41, 4661–4666. [Google Scholar]
- Rozanov, D.; Cheltsov, A.; Sergienko, E.; Vasile, S.; Golubkov, V.; Aleshin, A.E.; Levin, T.; Traer, E.; Hann, B.; Freimuth, J.; et al. TRAIL-based high throughput screening reveals a link between TRAIL-mediated apoptosis and glutathione reductase, a key component of oxidative stress response. PLoS ONE 2015, 10, e0129566. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J. Biol. Chem. 2016, 291, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Zhang, Q.; Pan, J.; Lee, Y.; Ouari, O.; Hardy, M.; Zielonka, M.; Myers, C.R.; Zielonka, J.; Weh, K.; et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rozanov, D.; Cheltsov, A.; Nilsen, A.; Boniface, C.; Forquer, I.; Korkola, J.; Gray, J.; Tyner, J.; Tognon, C.E.; Mills, G.B. Targeting mitochondria in cancer therapy could provide a basis for the selective anti-cancer activity. PLoS ONE 2019, 14, e0205623. [Google Scholar] [CrossRef] [Green Version]
- Schoenwaelder, S.M.; Jackson, S.P. Bcl-x L-inhibitory BH3 mimetics (ABT-737 or ABT-263) and the modulation of cytosolic calcium flux and platelet function. Blood 2012, 119, 1320–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, J.; Letai, A. Why do BCL-2 inhibitorswork and where should we use them in the clinic? Cell Death Differ. 2018, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Mina, D.A.; Latinwo, L.M.; Soliman, K.F. Selective anticancer activity of neurotoxin 1-methyl-4-phenylpyridinium on non-small cell lung adenocarcinoma A549 cells. Anticancer Res. 2014, 34, 5447–5452. [Google Scholar]
- Akiyoshi, T.; Matzno, S.; Sakai, M.; Okamura, N.; Matsuyama, K. The potential of vitamin K3 as an anticancer agent against breast cancer that acts via the mitochondria-related apoptotic pathway. Cancer Chemother Pharmacol. 2009, 65, 143–150. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Sutendra, G.; Dromparis, P.; Webster, L.; Haromy, A.; Niven, E.; Maguire, C.; Gammer, T.L.; Mackey, J.R.; Fulton, D.; et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci. Transl. Med. 2010, 2, ra34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubackova, S.; Davidova, E.; Rohlenova, K.; Stursa, J.; Werner, L.; Andera, L.; Dong, L.; Terp, M.G.; Hodny, Z.; Ditzel, H.J.; et al. Selective elimination of senescent cells by mitochondrial targeting is regulated by ANT2. Cell Death Differ. 2019, 26, 276–290. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.F.; Freeman, R.; Liu, J.; Zobalova, R.; Marin-Hernandez, A.; Stantic, M.; Rohlena, J.; Valis, K.; Rodriguez-Enriquez, S.; Butcher, B.; et al. Suppression of tumor growth in vivo by the mitocan α-tocopheryl succinate requires respiratory complex II. Clin. Cancer Res. 2009, 15, 1593–1600. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Enríquez, S.; Hernández-Esquivel, L.; Marín-Hernández, A.; Dong, L.F.; Akporiaye, E.T.; Neuzil, J.; Ralph, S.J.; Moreno-Sánchez, R. Molecular mechanism for the selective impairment of cancer mitochondrial function by a mitochondrially targeted vitamin e analogue. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1597–1607. [Google Scholar] [CrossRef]
- Wen, R.; Dhar, S. Turn up the cellular power generator with Vitamin E analogue formulation. Chem. Sci. 2016, 7, 5559–5567. [Google Scholar] [CrossRef] [Green Version]
- Aft, R.L.; Zhang, F.W.; Gius, D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: Mechanism of cell death. Br. J. Cancer 2002, 87, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Raez, L.E.; Papadopoulos, K.; Ricart, A.D.; Chiorean, E.G.; DiPaola, R.S.; Stein, M.N.; Lima, C.M.R.; Schlesselman, J.J.; Tolba, K.; Langmuir, V.K.; et al. A phase i dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013, 71, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Fantin, V.R.; Leder, P. F16, a Mitochondriotoxic Compound, Triggers Apoptosis or Necrosis Depending on the Genetic Background of the Target Carcinoma Cell. Cancer Res. 2004, 64, 329–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Li, D.W.; Yang, L.Y.; Fu, L.; Zhu, X.J.; Wong, W.K.; Jiang, F.L.; Liu, Y. A novel bifunctional mitochondria-targeted anticancer agent with high selectivity for cancer cells. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.C.; Board, P.G.; Blackburn, A.C. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol Cancer 2011, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Yedjou, C.G.; Tchounwou, P.B. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J. Exp. Clin. Cancer Res. 2014, 33, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018, 9, 1–14. [Google Scholar]
- Han, Y.H.; Kim, S.H.; Kim, S.Z.; Park, W.H. Antimycin A as a mitochondrial electron transport inhibitor prevents the growth of human lung cancer A549 cells. Oncol. Rep. 2008, 20, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Leanza, L.; Romio, M.; Becker, K.A.; Azzolini, M.; Trentin, L.; Manago, A.; Venturini, E.; Zaccagnino, A.; Mattarei, A.; Carraretto, L.; et al. Direct Pharmacological Targeting of a Mitochondrial Ion Channel Selectively Kills Tumor Cells In Vivo. Cancer Cell. 2017, 31, 516–531. [Google Scholar] [CrossRef]
- Feng, S.H.I.; Xiong, L.; Ji, Z.; Cheng, W.E.I.; Yang, H. Correlation between increased copy number of mitochondrial DNA and clinicopathological stage in colorectal cancer. Oncol. Lett. 2011, 2, 899–903. [Google Scholar]
- Shanafelt, T.D.; Lee, Y.K.; Bone, N.D.; Strege, A.K.; Narayanan, V.L.; Sausville, E.A.; Geyer, S.M.; Kaufmann, S.H.; Kay, N.E. Adaphostin-induced apoptosis in CLL B cells is associated with induction of oxidative stress and exhibits synergy with fludarabine. Blood 2005, 105, 2099–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sattler, M.; Tonon, G.; Grabher, C.; Lababidi, S.; Zimmerhackl, A.; Raab, M.S.; Vallet, S.; Zhou, Y.; Cartron, M.A.; et al. Targeting angiogenesis via a c-Myc/hypoxia-inducible factor-1α- dependent pathway in multiple myeloma. Cancer Res. 2009, 69, 5082–5090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, R.N.; Cheng, T.; Irwin, M.E.; Donnella, H.; Singh, M.M.; Chandra, J. Combinatorial effects of histone deacetylase inhibitors (HDACi), vorinostat and entinostat, and adaphostin are characterized by distinct redox alterations. Cancer Chemother Pharmacol. 2018, 81, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Dell’Antone, P. Targets of 3-Bromopyruvate, A New, Energy Depleting, Anticancer Agent. Med. Chem. 2009, 5, 491–496. [Google Scholar] [CrossRef]
- el Sayed, S.M. Enhancing anticancer effects, decreasing risks and solving practical problems facing 3-bromopyruvate in clinical oncology: 10 years of research experience. Int. J. Nanomed. 2018, 13, 4699–4709. [Google Scholar] [CrossRef] [Green Version]
- Lampidis, T.J.; Bernal, S.D.; Summerhayes, I.C.; Chen, L.B. Selective toxicity of rhodamine 123 in carcinoma cells in vitro. Cancer Res. 1983, 43, 716–720. [Google Scholar]
- Ko, Y.T.; Falcao, C.; Torchilin, V.P. Cationic liposomes loaded with proapoptotic peptide D-(KLAKLAK)2 and Bcl-2 antisense oligodeoxynucleotide G3139 for enhanced anticancer therapy. Mol Pharm. 2009, 6, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Toyama, K.; Nomura, W.; Kobayakawa, T.; Tamamura, H. Delivery of a Proapoptotic Peptide to EGFR-Positive Cancer Cells by a Cyclic Peptide Mimicking the Dimerization Arm Structure of EGFR. Bioconjug Chem. 2018, 29, 2050–2057. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Cui, G.; Chan, J.Y.W.; Wang, L.; Li, C.; Shan, L.; Xu, C.; Zhang, Q.; Wang, Y.; et al. A novel agent exerts antitumor activity in breast cancer cells by targeting mitochondrial complex II. Oncotarget 2016, 7, 32054–32064. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, A.; Wang, L.; Wang, L.; Di, L.; Hoi, P.M.M.; Shan, L.; Wu, X.; Wang, Y. A Danshensu-Tetramethylpyrazine Conjugate DT-010 Overcomes Multidrug Resistance in Human Breast Cancer. Front Pharmacol. 2019, 10, 722. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, J.; Amorim, R.; Santos, K.; Soares, P.; Datta, S.; Cortopassi, G.A.; Serafim, T.L.; Sardão, V.A.; Garrido, J.; Borges, F.; et al. Disruption of mitochondrial function as mechanism for anti-cancer activity of a novel mitochondriotropic menadione derivative. Toxicology 2018, 393, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.F.; Jameson, V.J.; Tilly, D.; Cerny, J.; Mahdavian, E.; Marín-Hernández, A.; Hernández-Esquivel, L.; Rodríguez-Enríquez, S.; Stursa, J.; Witting, P.K.; et al. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J. Biol. Chem. 2011, 286, 3717–3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Stantic, M.; Zobalova, R.; Bezawork-Geleta, A.; Stapelberg, M.; Stursa, J.; Prokopova, K.; Dong, L.; Neuzil, J. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Cancer 2015, 15, 401. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, B.; Warude, D.; Palpu, P.; Narendra, B. Ayurveda and Traditional Chinese Medicine: A Comparative Overview. Evid. Based Complement Altern Med. 2005, 2, 2465–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, M. Taking traditional knowledge to the market: The commoditization of Indian medicine. Anthropol. Med. 2006, 13, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.Z.; Khan, R.A.; Latif, A. Importance of pharmacovigilance in Unani system of medicine. Indian J. Pharmacol. 2008, 40, 17–20. [Google Scholar]
- Xu, K.; Thornalley, P.J. Involvement of glutathione metabolism in the cytotoxicity of the phenethyl isothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. Biochem. Pharmacol. 2001, 61, 165–177. [Google Scholar] [CrossRef]
- Azmi, A.S.; Mohammad, R.M. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J. Cell Physiol. 2009, 218, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.F.; Chiang, N.N.; Lu, Y.H.; Huang, Y.S.; Yang, J.S.; Tsai, S.C.; Lu, C.C.; Chen, F.A. Benzyl isothiocyanate (BITC) triggers mitochondria-mediated apoptotic machinery in human cisplatin-resistant oral cancer CAR cells. BioMedicine 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Kawakami, M.; Yoshihiro, A.; Miyoshi, N.; Ohigashi, H.; Kawai, K.; Osawa, T.; Uchida, K. Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. J. Biol. Chem. 2002, 277, 8492–8499. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Zhang, Y. Mitochondria are the primary target in isothiocyante-induced apoptosis in human bladder cancer cells. Mol. Cancer Ther. 2005, 4, 1250–1259. [Google Scholar] [CrossRef] [Green Version]
- Cheung, H.Y.; Cheung, S.H.; Li, J.; Cheung, C.S.; Lai, W.P.; Fong, W.F.; Leung, F.M. Andrographolide isolated from Andrographis paniculata induces cell cycle arrest and mitochondrial-mediated apoptosis in human leukemic HL-60 cells. Planta Med. 2005, 71, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, D.; Luo, K.; Wu, S.; Wu, P. Andrographolide enhances 5-fluorouracil-induced apoptosis via caspase-8-dependent mitochondrial pathway involving p53 participation in hepatocellular carcinoma (SMMC-7721) cells. Cancer Lett. 2009, 276, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Banerjee, V.; Czinn, S.; Blanchard, T. Increased reactive oxygen species levels cause ER stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget 2017, 8, 26142–26153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.C.; Bosire, K.O.; Lee, E.S.; Lee, Y.S.; Kim, J.A. Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett. 2005, 218, 81–90. [Google Scholar] [CrossRef]
- Tang, X.L.; Yang, X.Y.; Jung, H.J.; Kim, S.Y.; Jung, S.Y.; Choi, D.Y.; Park, W.C.; Park, H. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol. Pharm. Bull. 2009, 32, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Geng, J.; Guo, W.; Gao, J.; Zhu, X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm. Sin B. 2017, 7, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Yang, J.S.; Chen, J.T.; Fan, S.; Yu, F.S.; Yang, J.L.; Lu, C.C.; Kao, M.C.; Huang, A.C.; Lu, H.F. Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Anticancer Res. 2007, 27, 3371–3378. [Google Scholar]
- Patil, J.B.; Kim, J.; Jayaprakasha, G.K. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway. Eur. J. Pharmacol. 2010, 645, 70–78. [Google Scholar] [CrossRef]
- Yip, N.K.Y.; Ho, W.S. Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells. Oncol. Rep. 2013, 30, 1107–1112. [Google Scholar] [CrossRef]
- Klawitter, J.; Klawitter, J.; Gurshtein, J.; Corby, K.; Fong, S.; Tagliaferri, M.; Quattrochi, L.; Cohen, I.; Shtivelman, E.; Christians, U. Bezielle (BZL101)-induced oxidative stress damage followed by redistribution of metabolic fluxes in breast cancer cells: A combined proteomic and metabolomic study. Int. J. Cancer 2011, 129, 2945–2957. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.; Staub, R.E.; Fong, S.; Tagliaferri, M.; Cohen, I.; Shtivelman, E. Bezielle selectively targets mitochondria of cancer cells to inhibit glycolysis and OXPHOS. PLoS ONE 2012, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M.; et al. Curcumin induces apoptotic cell death via inhibition of PI3-kinase/Akt pathway in B-precursor acute lymphoblastic leukemia. Front Oncol. 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Zulfadli, A.J.; Omar, A.R.; Sulaiman, M.R.; Abdullah, M.P.; Alitheen, N.B. Flavokawain a induces apoptosis in MCF-7 and MDA-MB231 and inhibits the metastatic process in vitro. PLoS ONE 2014, 9, e105244. [Google Scholar] [CrossRef]
- Zi, X.; Simoneau, A.R.; Flavokawain, A. A novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and tumor growth in mice. Cancer Res. 2005, 65, 3479–3486. [Google Scholar] [CrossRef] [Green Version]
- Goldin, N.; Arzoine, L.; Heyfets, A.; Israelson, A.; Zaslavsky, Z.; Bravman, T.; Bronner, V.; Notcovich, A.; Shoshan-Barmatz, V.; Flescher, E. Methyl jasmonate binds to and detaches mitochondria-bound hexokinase. Oncogene 2008, 27, 4636–4643. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, K.; Wang, F.; Dai, W.; Li, S.; Feng, J.; Wu, L.; Liu, T.; Xu, S.; Xia, Y.; et al. Methyl jasmonate leads to necrosis and apoptosis in hepatocellular carcinoma cells via inhibition of glycolysis and represses tumor growth in mice. Oncotarget 2017, 8, 45965–45980. [Google Scholar] [CrossRef]
- Widodo, N.; Priyandoko, D.; Shah, N.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by Ashwagandha leaf extract and its component withanone involves ROS signaling. PLoS ONE 2010, 5, e13536. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Akao, Y.; Yi, H.; Ohguchi, K.; Ito, T.; Tanaka, T.; Kobayashi, E.; Iinuma, M.; Nozawa, Y. Preferential target is mitochondria in α-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorganic Med. Chem. 2004, 12, 5799–5806. [Google Scholar] [CrossRef]
- Aisha, A.F.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M.S.A. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement Altern Med. 2012, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Z.; Li, X.L.; Wang, Q.F.; Mehendale, S.R.; Fishbein, A.B.; Han, A.H.; Sun, S.; Yuan, C.S. The mitochondrial pathway is involved in American ginseng-induced apoptosis of SW-480 colon cancer cells. Oncol. Rep. 2009, 21, 577–584. [Google Scholar] [PubMed] [Green Version]
- Dai, Z.J.; Wang, X.J.; Li, Z.F.; Ji, Z.Z.; Ren, H.T.; Tang, W.; Liu, X.X.; Kang, H.F.; Guan, H.T.; Song, L.Q. Scutellaria barbate extract induces apoptosis of hepatoma H22 cells via the mitochondrial pathway involving caspase-3. World J. Gastroenterol. 2008, 14, 7321–7328. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Tsang, S.F.; Tsai, C.H.; Tsai, H.Y.; Chyuan, J.H.; Hsu, H.Y. Momordica charantia Extract Induces Apoptosis in Human Cancer Cells through Caspase-and Mitochondria-Dependent Pathways. Evid. Based Complement Altern Med. 2012, 4, 261971. [Google Scholar]
- Wu, S.J.; Ng, L.T.; Lin, D.L.; Huang, S.N.; Wang, S.S.; Lin, C.C. Physalis peruviana extract induces apoptosis in human Hep G2 cells through CD95/CD95L system and the mitochondrial signaling transduction pathway. Cancer Lett. 2004, 215, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Koppikar, S.J.; Choudhari, A.S.; Suryavanshi, S.A.; Kumari, S.; Chattopadhyay, S.; Kaul-Ghanekar, R. Aqueous Cinnamon Extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer 2010, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.J.; Yang, J.S.; Lin, C.F.; Shyu, W.C.; Tsuzuki, M.; Lu, C.C.; Chen, Y.F.; Lai, K.C. Houttuynia cordata thunb extract induces apoptosis through mitochondrial-dependent pathway in HT-29 human colon adenocarcinoma cells. Oncol. Rep. 2009, 22, 1051–1056. [Google Scholar]
- Karna, P.; Chagani, S.; Gundala, S.R.; Rida, P.C.; Asif, G.; Sharma, V.; Gupta, M.V.; Aneja, R. Benefits of whole ginger extract in prostate cancer. Br. J. Nutr. 2012, 107, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Babykutty, S.; Padikkala, J.; Sathiadevan, P.; Vijayakurup, V.; Azis, T.; Srinivas, P.; Gopala, S. Apoptosis induction of Centella asiatica on human breast cancer cells. Afr. J. Tradit Complement Altern Med. 2009, 6, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Kong, X.; Yan, S.; Yuan, C.; Yang, Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci. 2010, 101, 2375–2383. [Google Scholar] [CrossRef]
- Naidu, V.G.M.; Bandari, U.M.; Giddam, A.K.; Babu, K.R.D.; Ding, J.; Babu, K.S.; Ramesh, B.; Pragada, R.R.; Gopalakrishnakone, P. Apoptogenic activity of ethyl acetate extract of leaves of Memecylon edule on human gastric carcinoma cells via mitochondrial dependent pathway. Asian Pac J. Trop Med. 2013, 6, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.Y.; Kim, G.Y.; Choi, Y.H. Induction of apoptosis by aqueous extract of Cordyceps militaris through activation of caspases and inactivation of Akt in human breast cancer MDA-MB-231 cells. J. Microbiol. Biotechnol. 2008, 18, 1997–2003. [Google Scholar] [PubMed]
- Yeh, C.C.; Tseng, C.N.; Yang, J.I.; Huang, H.W.; Fang, Y.; Tang, J.Y.; Chang, F.R.; Chang, H.W. Antiproliferation and Induction of Apoptosis in Ca9-22 Oral Cancer Cells by Ethanolic Extract of Gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef] [Green Version]
- Bhujade, A.; Gupta, G.; Talmale, S.; Das, S.K.; Patil, M.B. Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food Funct. 2013, 4, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Heyfets, A.; Flescher, E. Cooperative cytotoxicity of methyl jasmonate with anti-cancer drugs and 2-deoxy-d-glucose. Cancer Lett. 2007, 250, 300–310. [Google Scholar] [CrossRef]
- Fingrut, O.; Flescher, E. Plant stress hormones suppress the proliferation and induce apoptosis in human cancer cells. Leukemia 2002, 6, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Reischer, D.; Heyfets, A.; Shimony, S.; Nordenberg, J.; Kashman, Y.; Flescher, E. Effects of natural and novel synthetic jasmonates in experimental metastatic melanoma. Br. J. Pharmacol. 2007, 150, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Lopes, J.E.F.; Barbosa, M.R.; Stella, C.N.; Santos, W.A.; Pereira, E.M.; Nogueira-Neto, J.; Augusto, E.M.; Silva, L.V.; Smaili, S.S.; Gomes, L.F. In vivo anti-angiogenic effects further support the promise of the antineoplasic activity of methyl jasmonate. Braz. J. Biol. 2010, 70, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, B.; Iannitti, T.; Capone, S.; Flescher, E. A preliminary study of the local treatment of preneoplastic and malignant skin lesions using methyl jasmonate. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 333–336. [Google Scholar] [PubMed]
- Klippel, S.; Jakubikova, J.; Delmore, J.; Ooi, M.; McMillin, D.; Kastritis, E.; Laubach, J.; Richardson, P.G.; Anderson, K.C.; Mitsiades, C.S. Methyl jasmonate displays in vitro and in vivo activity against multiple myeloma cells. Br. J. Haematol. 2012, 159, 340–351. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Yan, L.J. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J. Biochem. Pharmacol. Res. 2013, 1, 15–26. [Google Scholar]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Electron transport and oxidative phosphorylation. In Molecular Cell Biology, 4th ed.; Freeman, W.H., Ed.; Academic Press: New York, NY, USA, 2000; Available online: https://www.ncbi.nlm.nih.gov/books/NBK21528/ (accessed on 20 September 2020).
- Driver, J.A.; Beiser, A.; Au, R.; Kreger, B.E.; Splansky, G.L.; Kurth, T.; Kiel, D.P.; Lu, K.P.; Seshadri, S.; Wolf, P.A. Inverse association between cancer and Alzheimer’s disease: Results from the Framingham Heart Study. BMJ 2012, 344, e1442. [Google Scholar] [CrossRef] [Green Version]
- Roe, C.M.; Fitzpatrick, A.L.; Xiong, C.; Sieh, W.; Kuller, L.; Miller, J.P.; Williams, M.M.; Kopan, R.; Behrens, M.I.; Morris, J.C. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010, 74, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Tabarés-Seisdedos, R.; Dumont, N.; Baudot, A.; Valderas, J.M.; Climent, J.; Valencia, A.; Crespo-Facorro, B.; Vieta, E.; Gómez-Beneyto, M.; Martínez, S.; et al. No paradox, no progress: Inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011, 12, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Driver, J.A.; Logroscino, G.; Buring, J.E.; Gaziano, J.M.; Kurth, T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson’s disease. Cancer Epidemiol Biomark. Prev. 2007, 16, 1260–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.B.; Tang, B.; Liu, Y.W.; Wang, X.F.; Chen, G.J. Alzheimer disease and cancer risk: A meta-analysis. J. Cancer Res. Clin. Oncol. 2015, 141, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Stanga, S.; Lanni, C.; Sinforiani, E.; Mazzini, G.; Racchi, M. Searching for predictive blood biomarkers: Misfolded p53 in mild cognitive impairment. Curr. Alzheimer Res. 2012, 9, 1191–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| (a) | |||||
|---|---|---|---|---|---|
| S. No. | Synthetic Compound | Mode of Action | Mitocan Class * | Current Status | References |
| 1. | ABT-263 (Navitoclax) | Inhibits anti-apoptotic Bcl-2 family proteins (Bcl-XL, Bcl-2, and Bcl-w). Induces translocation of Bax, release of cytochrome c. | 2 | Phase II of the clinical trial | [71,117,118] |
| 2. | 1-methyl-4-phenyl-pyridinium | Affects the overall function of mitochondria by inhibiting ATP generation | 8 | Failed to cross the blood-brain barrier | [119] |
| 3. | Vitamin K3 or Menadione | Inhibits DNA polymerase γ and increased ROS generation | 8 | Failed in phase II of the clinical trial (https://clinicaltrials.gov) | [93,120] |
| 4. | Dichloroacetate, (DCA) | Unbalance the redox homeostasis and overproduction of ROS | 7 | Terminated from phase II clinical trial (https://clinicaltrials.gov | [90,121] |
| 5. | Mito-Tam (a mitochondrial-targeted derivative of tamoxifen.) | Inhibits complex I- driven respiration | 5 | Phase I of the clinical trial | [83,122] |
| 6. | α-TOS | Targets Complex II and accumulation of ROS | 5 & 2 | Completed Phase III of clinical trial | [123,124,125] |
| 7. | 2-Deoxy-D-glucose (2DG) | Increased levels of glucose transporter expression and glucose uptake. Inhibits hexokinase and hexose phosphate isomerase | 1 | Terminated after phase I clinical trial Study for combination with other drugs. | [64,126,127] |
| 8. | F16 | Accumulation in cancer cell mitochondria leading to apoptosis | 6 | Terminated from phase II clinical trial (https://clinicaltrials.gov) | [88,128,129] |
| 9. | Arsenic trioxide | Induces oxidative stress, DNA damage, change in mitochondrial membrane potential, translocation, and upregulation of apoptotic proteins | 3 | Phase II of a clinical trial (https://clinicaltrials.gov) | [76,130,131] |
| 10. | Benserazide | Reduce the uptake of glucose, production of lactate, level of ATP and causes apoptosis | 1 | Phase IV of the clinical trial | [65] |
| 11. | Metformin | Target mitochondrial ETC; Exerts oxidative stress | 5 | Phase I of clinical trials | [83,84,132] |
| 12. | Sorafenib | Inhibition of ATP synthase | 5 | Phase III of the clinical trial | [82] |
| (b) | |||||
| S. No. | Synthetic Compound | Mode of action | Mitocan Class * | Current status | References |
| 1. | Antimycin A | Inhibits succinate, nicotinamide adenine dinucleotide (NADH) oxidase, and electron transport between cytochrome b & c | 2 & 5 | Preclinical studies | [133,134] |
| 2. | MKT-007 | Targets mtDNA, a metabolic inhibitor | 8 | Preclinical studies and in vitro studies | [96,135] |
| 3. | Adaphostin | Inhibits complex II and III of ETC and accumulation of ROS | 5 | Preclinical stage and combinatorial study with other drugs. | [136,137,138] |
| 4. | 3 Bromopyruvate (3BP) | Inhibition of HK II, glyceraldehyde-3-phosphate dehydrogenase and LDH, Induces the mitochondrial protein leakage and block the electron transport system | 1 & 5 | Under research to improve its specificity, sensitivity & efficacy by combining with other anticancer agents or formulating with targeted liposomes to enhance its delivery. | [63,139,140] |
| 5. | Rhodamine-123 | Accumulation in mitochondria | 6 | No current update as a mitocan | [85,141] |
| 6. | (KLAKKLAK)2 (Pro-apoptotic Peptide) | Disrupt mitochondrial membrane | 6 | Understudy for improving its efficacy | [87,142,143] |
| 7. | Mito-LND | Inhibition of mitochondrial complexes I and II, and stimulation of ROS production | 5 | Pre-clinical studies | [112,114,115] |
| 8. | Danshensu-Tetramethylpyrazine (DT-010) | Inhibition of mitochondrial complex II | 7 | Preclinical studies | [144,145] |
| 9. | NSC13062 | Target mitochondrial ETC | 5 | Preclinical studies and in vitro studies | [113,116] |
| 10. | Mito-K3 (derivative of Menadione) | Accumulates in mitochondria, interferes with redox property, and causes mitochondrial dysfunction | 8 | Preclinical studies | [146] |
| 11. | MitoVE11S (CNC332) | Targets mitochondrial complex II | 5 & 8 | Preclinical and in vitro studies | [50,147,148] |
| S. No. | Natural Agent (Source) | Mode of action | Mitocan Class | Current status | References |
|---|---|---|---|---|---|
| 1. | Phenethyl isothiocyanates (cruciferous vegetables) | Induction of oxidative stress and triggering of Ca2+ flux, which leads to mitochondrial cell death mechanisms | 3 | Phase I clinical trial (https://clinicaltrials.gov) | [154,155,156] |
| 2. | Benzyl isothiocyanate (brassicas) | Intrinsic apoptosis is mediated via ROS production and mitochondrial dysfunction. | 3 | In vitro studies going on | [154,155] |
| 3. | Gossypol (cotton plant) | Inhibits Bcl-2, Bcl-XL, Bcl-W, Mcl-1 | 2 | Phase II of the clinical trial (https://clinicaltrials.gov) | [70,153] |
| 4. | Andrographolide (Andrographis paniculata) | Targets Bcl-2 family protein and cyclophilin D; Increased ROS production | 2 | In vitro study | [157,158,159] |
| 5. | Asiatic acid (Centella asiatica) | Increases mitochondria membrane permeability, ROS generation, alteration of Bax/Bcl-2 ratio, and activation of caspase-3 | 2 | In vitro study | [160,161,162] |
| 6. | Berberine (Coptidis rhizoma) | Increases expression of Bax; Decreases Bcl-2 expression level; Induces ROS and Ca2+ production; Loss of mitochondria membrane permeability | 2 | In vitro study | [163,164,165] |
| 7. | Bezielle (Scutellaria barbata) | Inhibits Glycolysis and OXPHOS by increasing the ROS level | 3 | In vitro study | [166,167] |
| 8. | Curcumin (Curcuma longa) | Downregulation of expression and activity of HK II; Loss of mitochondria membrane potential | 1 and 2 | In vitro study | [168,169] |
| 9. | Flavokawain A (Piper methysticum) | Induces mitochondrial-dependent apoptosis by increasing the expression of Bax | 2 | In vitro and in vivo study | [170,171] |
| 10. | Methyl jasmonate (most of the plants) | Detaches HKII from VDAC and causes loss of mitochondrial function; | 1 | In vitro and in vivo study in mice model | [172,173] |
| 11. | Withanone (Withania somnifera) | Acts as a ROS-producing agent causing DNA and mitochondrial damage | 3 | In vitro study | [174] |
| 12. | Xanthones (Garcinia mangostana) | Causes loss of mitochondria membrane potential | 6 | In vitro and in vivo study | [175,176] |
| S. No. | Anticancer Plant | Extract | Cancer Type/ Cell Lines | Mode of Action | Proposed Class | References |
|---|---|---|---|---|---|---|
| 1. | American Ginseng (Panax quinquefolis) | Steamed and extracted by ethanol | Colon cancer (SW-480) | Decreases the expression of Bcl-2 and induce mitochondrial-mediated apoptosis | 2 | [177] |
| 2. | Ashwagandha (Withania somnifera) | Methanolic | Breast cancer (MCF-7) | Acts as a ROS-producing agent causing DNA and mitochondrial damage | 3 | [174] |
| 3. | Barbed skullcap (Scutellaria barbate) | Aqueous | Primary liver cancer mouse hepatoma cells (H22) | Apoptosis via loss of mitochondrial transmembrane potential, the release of cytochrome c, and activation of caspase-3 | 6 | [178] |
| 4. | Bitter gourd (Momordica charantia) | Methanolic | Human nasopharyngeal carcinoma cells (Hone-1), gastric adenocarcinoma cells (AGS), colon cancer cells (HCT-116), and lung adenocarcinoma cell (CL1-0) | Increased Bax/Bcl-2 ratio and mitochondria-dependent apoptosis | 2 | [179] |
| 5. | Cape gooseberry (Physalis peruviana) | Ethanol | Human hepatocellular carcinoma (Hep G2 cells) + mouse model | Apoptosis mediated through a mitochondrial signaling transduction pathway | 2 | [180] |
| 6. | Cinnamon (Cinnamomum cassia) | Aqueous | Cervical cancer (SiHa) | Induces apoptosis by loss of mitochondrial membrane potential (MMP) | 6 | [181] |
| 7. | Fish mint (Houttuynia cordata) | Ethanol | Human colon adenocarcinoma (HT-29 cells) | Loss of mitochondria membrane potential increased ROS production and alterations ofmitochondrial proteins such as cytochrome c, Apaf-1, AIFand pro-caspase-9 | 6 | [182] |
| 8. | Ginger (Zingiber officinale) | Methanol | Prostate cancer | Altered Bax/Bcl-2ratio and collapse of mitochondrial membrane potential | 2 | [183] |
| 9. | Gotu Kola (Centella Asiatica) | Methanol | Breast cancer (MCF-7) | Loss of mitochondria membrane potential due to increased expression of Bax and decreased expression of Bcl-2 | 2 | [184] |
| 10. | Huaier (Trametes robiniophila murr) | Aqueous | Breast cancer (MCF-7 and MDA-MB-231) | Suppresses the Bcl-2 expression and up-regulate Bax expression and leads to mitochondrial-mediated apoptosis | 2 | [185] |
| 11. | Ironwood (Memecylon edule) | Ethyl acetate | Human gastric carcinoma | Apoptosis by decreasing the expression of anti-apoptotic protein Bcl-2 | 2 | [186] |
| 12. | Pupa grass (Cordyceps militaris) | Aqueous | Breast cancer (MDA-MB-231 cells) | Activation of caspase-3 and mitochondria dysfunctions | 2 | [187] |
| 13. | Slender red seaweed (Gracilaria tenuistipitata) | Ethanol | Oral squamous cell cancer (Ca9-22 cell) | Inhibition and apoptosis by increased ROS level, GSH depletion, caspase activation, and mitochondrial depolarization | 6 | [188] |
| 14. | Veldt grape (Cissus quadrangularis Linn) | Acetone | Skin cancer (A431) | Altered Bax/Bcl-2ratio, release of cytochrome c from mitochondria | 2 | [189] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, S.; Swargiary, G.; Singh, K.K. Natural Agents Targeting Mitochondria in Cancer. Int. J. Mol. Sci. 2020, 21, 6992. https://doi.org/10.3390/ijms21196992
Mani S, Swargiary G, Singh KK. Natural Agents Targeting Mitochondria in Cancer. International Journal of Molecular Sciences. 2020; 21(19):6992. https://doi.org/10.3390/ijms21196992
Chicago/Turabian StyleMani, Shalini, Geeta Swargiary, and Keshav K. Singh. 2020. "Natural Agents Targeting Mitochondria in Cancer" International Journal of Molecular Sciences 21, no. 19: 6992. https://doi.org/10.3390/ijms21196992
APA StyleMani, S., Swargiary, G., & Singh, K. K. (2020). Natural Agents Targeting Mitochondria in Cancer. International Journal of Molecular Sciences, 21(19), 6992. https://doi.org/10.3390/ijms21196992






