Antimicrobial Photoinactivation Approach Based on Natural Agents for Control of Bacteria Biofilms in Spacecraft
Abstract
1. Introduction
2. Biofilms in Spacecraft
2.1. Effects of Microbial Biofilms on Spacecraft Crew Health
2.2. Microbial Biofilm Damage and Corrosion of Indoor Spacecraft Equipment
2.3. Biofilm Formation Investigation during Spaceflight under Assessed Conditions
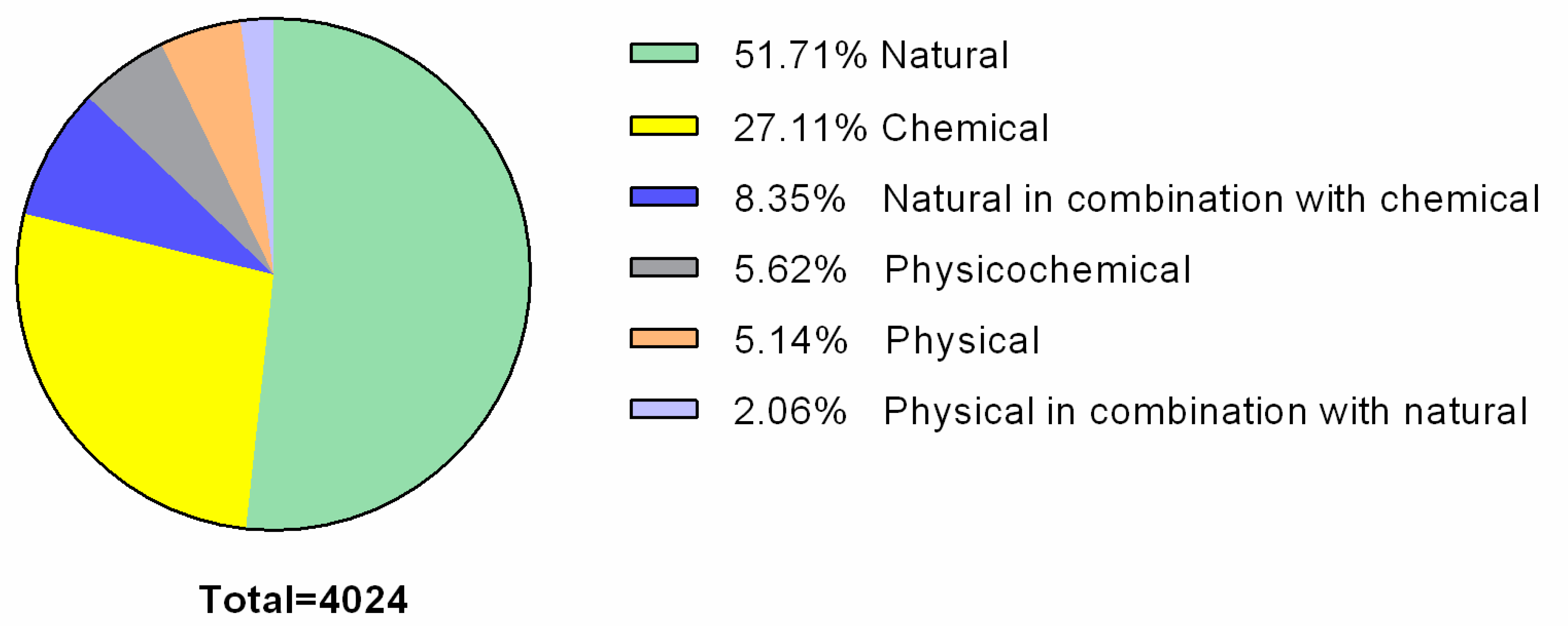
2.4. Biofilm Control Methods
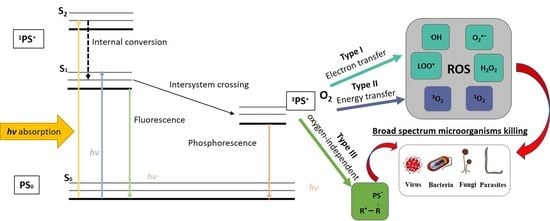
3. Antimicrobial Photoinactivation
- (i)
- The time between administration of the PS and API is too short for the pathogen to develop resistance;
- (ii)
- The PSs exhibit no dark toxicity (or very low toxicity), as a result of which bacteria do nothave to engage adaptive survival mechanisms against the PSs;
- (iii)
- The cells are too damaged after API, disabling them to confer cross-generation additivity;
- (iv)
- API does not target a single site in bacteria, so the ROS generated by this treatment target various pathogen cell structures and different metabolic pathways.
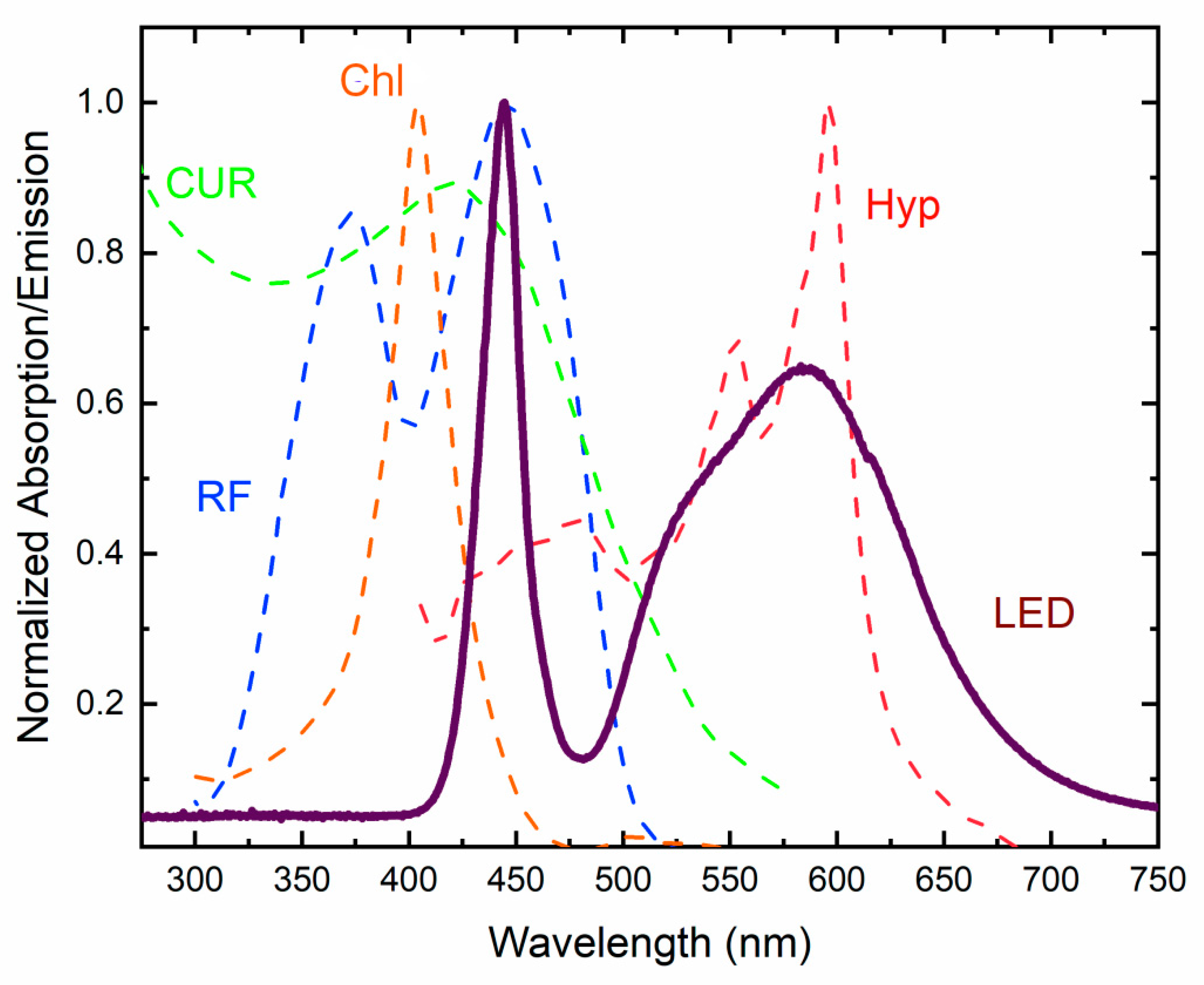
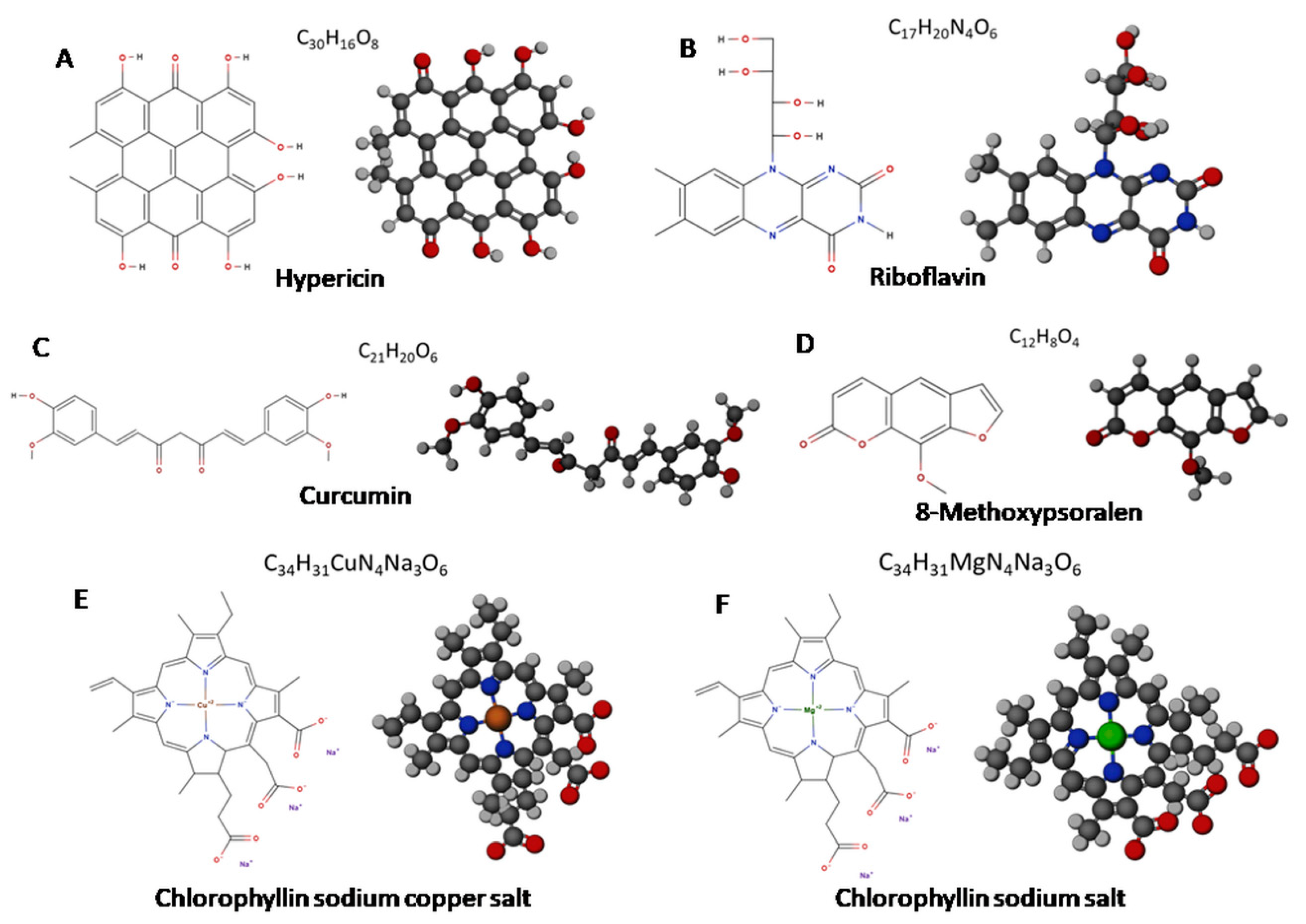
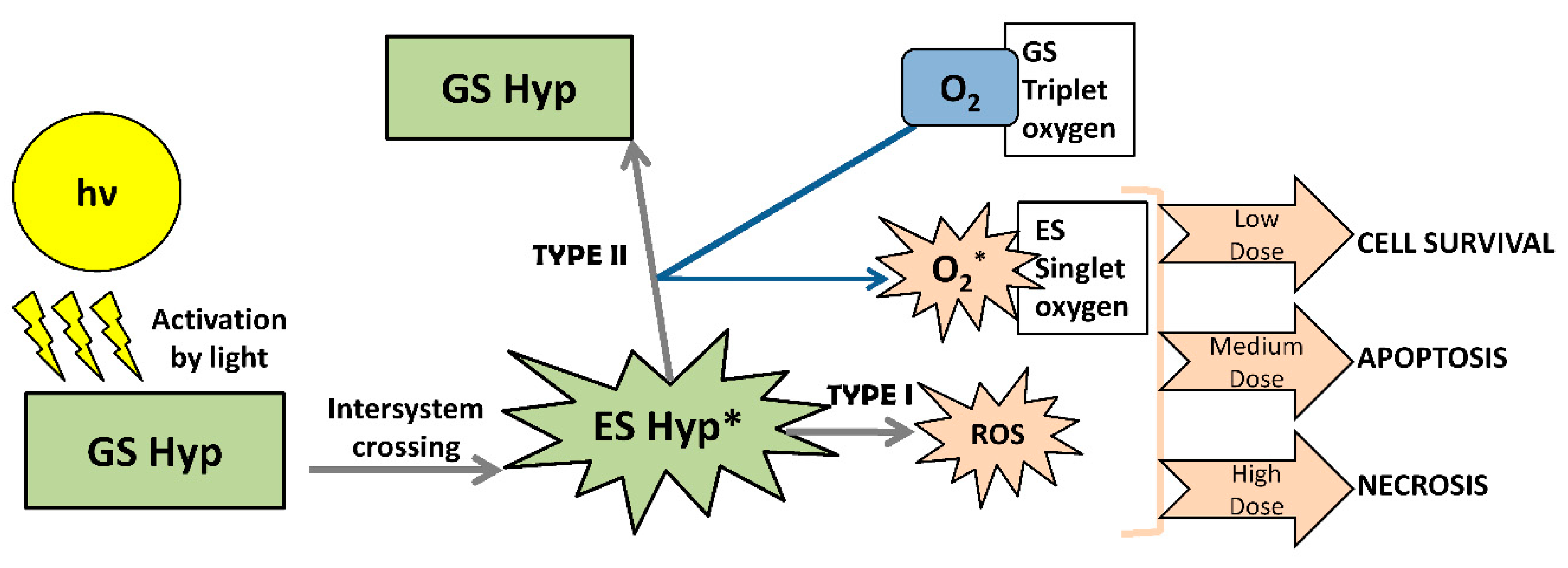
3.1. Perylenequinones
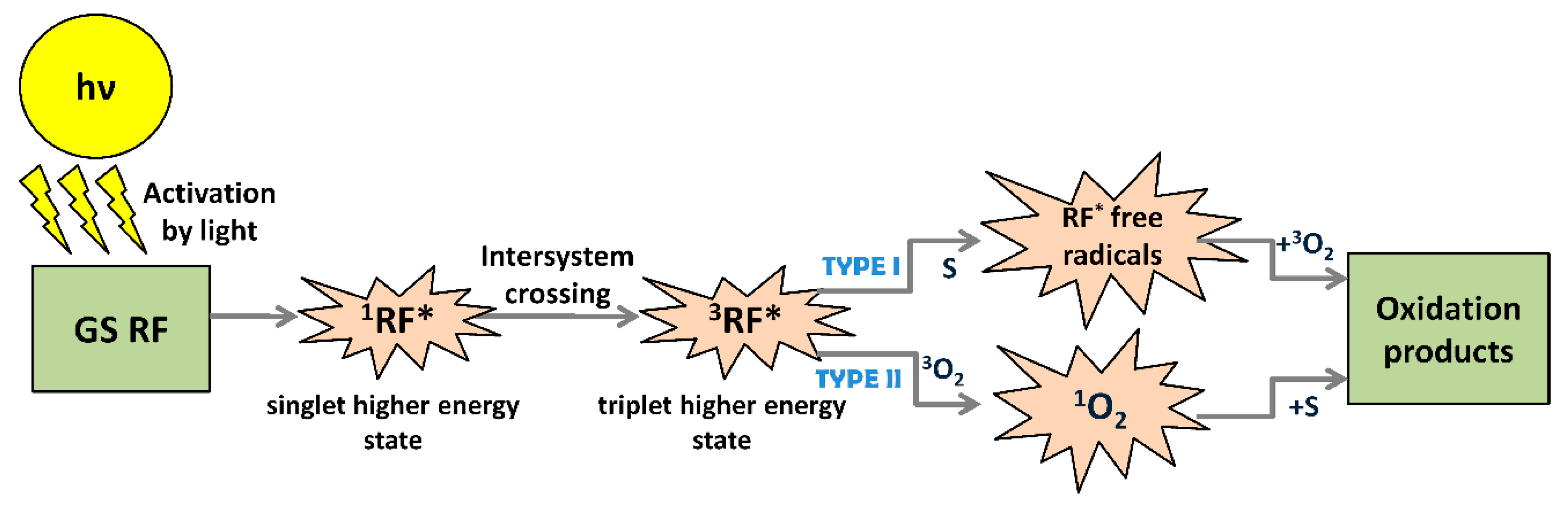
3.2. Riboflavin
3.3. Curcumin
3.4. Psoralens
3.5. Chlorophyllin
4. Illumination Requirements for API
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ISS | International Space Station |
| API | Antimicrobial photoinactivation |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| CUR | Curcumin |
| RF | Riboflavin |
| Hyp | Hypericin |
| Chl | Chlorophyllin |
| EPS | Extracellular polymeric substances |
| LSMMG | Low-shear modeled microgravity |
| MRSA | Methicillin-resistant S. aureus |
| PWD | Portable water dispenser |
| TSB | Tryptic soy broth |
| PDT | Photodynamic therapy |
| DMSO | Dimethyl sulfoxyde |
| GS | Ground state |
| ES | Excited state |
| MSSA | Methicillin-susceptible S. aureus |
| PIA | Polysaccharide intercellular adhesin |
| HA | Hypocrellin A |
| HB | Hypocrellin B |
| FAD | Flavine adenine dinucleotide |
| FMN | Flavine mononucleotide |
| GRAS | Generally regarded as safe |
| FDA | Food and Drug Administration |
| FMF | Formylmethylflavine |
| LC | Lemichrome |
| LF | Lemiflavin |
| CMF | Carboxymethylflavin |
| PBS | Phosphate buffered saline |
References
- Mora, M.; Wink, L.; Kögler, I.; Mahnert, A.; Rettberg, P.; Schwendner, P.; DeMets, R.; Cockell, C.S.; Alekhova, T.; Klingl, A.; et al. Space Station conditions are selective but do not alter microbial characteristics relevant to human health. Nat. Commun. 2019, 10, 3990. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Vaishampayan, P.; Cisneros, J.; Pierson, D.L.; Rogers, S.O.; Perry, J. International Space Station environmental microbiome—Microbial inventories of ISS filter debris. Appl. Microbiol. Biotechnol. 2014, 98, 6453–6466. [Google Scholar] [CrossRef] [PubMed]
- Zea, L.; Nisar, Z.; Rubin, P.; Cortesão, M.; Luo, J.; McBride, S.A.; Moeller, R.; Klaus, D.; Müller, D.; Varanasi, K.K.; et al. Design of a spaceflight biofilm experiment. Acta Astronaut. 2018, 148, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sobisch, L.Y.; Rogowski, K.M.; Fuchs, J.; Schmieder, W.; Vaishampayan, A.; Oles, P.; Novikova, N.; Grohmann, E. Biofilm forming antibiotic resistant gram-positive pathogens isolated from surfaces on the international space station. Front. Microbiol. 2019, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Tengra, F.K.; Shong, J.; Marchand, N.; Chan, H.K.; Young, Z.; Pangule, R.C.; Parra, M.; Dordick, J.S.; Plawsky, J.; et al. Effect of spaceflight on Pseudomonas aeruginosa final cell density is modulated by nutrient and oxygen availability. BMC Microbiol. 2013, 13, 241. [Google Scholar] [CrossRef]
- Kim, W.; Tengra, F.K.; Young, Z.; Shong, J.; Marchand, N.; Chan, H.K.; Pangule, R.C.; Parra, M.; Dordick, J.S.; Plawsky, J.L.; et al. Spaceflight Promotes Biofilm Formation by Pseudomonas aeruginosa. PLoS ONE 2013, 8, e62437. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.T.; Li, Z.D.; Xin, C.X.; Li, X.Q.; Wang, X.; Deng, Y. Microbiomes of China’s space station during assembly, integration, and test operations. Microb. Ecol. 2019, 78, 631–650. [Google Scholar] [CrossRef]
- Novikova, N.D.; De Boever, P.; Poddubko, S.; Deshevaya, E.; Polikarpov, N.; Rakova, N.; Coninx, I.; Mergeay, M. Survey of environmental biocontamination on board the International Space Station. Res. Microbiol. 2006, 157, 5–12. [Google Scholar] [CrossRef]
- Wong, W.C.; Dudinsky, L.A.; Garcia, V.M.; Ott, C.M.; Castro, V.A. Efficacy of various chemical disinfectants on biofilms formed in spacecraft potable water system components. Biofouling 2010, 26, 583–586. [Google Scholar] [CrossRef]
- Thompson, A.F.; English, E.L.; Nock, A.M.; Willsey, G.G.; Eckstrom, K.; Cairns, B.; Bavelock, M.; Tighe, S.; Foote, A.; Shulman, H.; et al. Characterizing species interactions that contribute to biofilm formation in a multispecies model of a potable water bacterial community. Microbiology 2019, 166, 34–43. [Google Scholar] [CrossRef]
- Taylor, P.W. Impact of space flight on bacterial virulence and antibiotic susceptibility. Infect. Drug Resist. 2015, 8, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.J.; Vaishampayan, P. Are we there yet? Understanding interplanetary microbial hitchhikers using molecular methods. Curr. Issues Mol. Biol. 2020, 38, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Klintworth, R.; Reher, H.; Viktorov, A.; Bohle, D. Biological induced corrosion of materials II: New test methods and experiences from MIR station. Acta Astronaut. 1999, 44, 569–578. [Google Scholar] [CrossRef]
- Schiwon, K.; Arends, K.; Rogowski, K.M.; Fürch, S.; Prescha, K.; Sakinc, T.; Van Houdt, R.; Werner, G.; Grohmann, E. Comparison of antibiotic resistance, biofilm formation and conjugative transfer of staphylococcus and enterococcus isolates from international space station and antarctic research station Concordia. Microb. Ecol. 2013, 65, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Nakonechny, F.; Nisnevitch, M. Aspects of photodynamic inactivation of bacteria. In Microorganisms; Blumenberg, M., Shaaban, M., Elgaml, A., Eds.; IntechOpen: London, UK, 2019; Chapter 7; pp. 1–21. [Google Scholar]
- Brovko, L.; Meyer, A.; Tiwana, A.S.; Chen, W.; Liu, H.; Filipe, C.D.; Griffiths, M.W. Photodynamic treatment: A novel method for sanitation of food handling and food processing surfaces. J. Food Prot. 2009, 5, 926–1138. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Woźniak, A.; Grinholc, M. Combined antimicrobial activity of photodynamic inactivation and antimicrobials—State of the art. Front. Microbiol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Milo, R. Towards a quantitative view of the global ubiquity of biofilms. Nat. Rev. Genet. 2019, 17, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K. What drives bacteria to produce biofilms? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Genet. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- La Duc, M.T.; Kern, R.; Venkateswaran, K. Microbial monitoring of spacecraft and associated environments. Microb. Ecol. 2004, 47, 150–158. [Google Scholar] [CrossRef]
- Checinska, A.; Probst, A.J.; Vaishampayan, P.; White, J.R.; Kumar, D.; Stepanov, V.G.; Fox, G.E.; Nilsson, R.H.; Pierson, D.L.; Perry, J.; et al. Microbiomes of the dust particles collected from the international space station and spacecraft assembly facilities. Microbiome 2015, 3, 50. [Google Scholar] [CrossRef]
- Koskinen, K.; Rettberg, P.; Pukall, R.; Auerbach, A.; Wink, L.; Barczyk, S.; Perras, A.; Mahnert, A.; Margheritis, D.; Kminek, G.; et al. Microbial biodiversity assessment of the European Space Agency’s ExoMars 2016 mission. Microbiome 2017, 5, 143. [Google Scholar] [CrossRef]
- Perrin, E.; Bacci, G.; Garrelly, L.; Canganella, F.; Bianconi, G.; Fani, R.; Mengoni, A. The Biowyse Consortium Furnishing spaceship environment: Evaluation of bacterial biofilms on different materials used inside International Space Station. Res. Microbiol. 2018, 169, 289–295. [Google Scholar] [CrossRef]
- Ichijo, T.; Yamaguchi, N.; Tanigaki, F.; Shirakawa, M.; Nasu, M. Four-year bacterial monitoring in the International Space Station—Japanese Experiment Module “Kibo” with culture-independent approach. NPJ Microgravity 2016, 2, 16007. [Google Scholar] [CrossRef]
- Ichijo, T.; Hieda, H.; Ishihara, R.; Yamaguchi, N.; Nasu, M. Bacterial Monitoring with Adhesive Sheet in the International Space Station “Kibo”, the Japanese Experiment Module. Microbes Environ. 2013, 28, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Be, N.A.; Avila-Herrera, A.; Allen, J.E.; Singh, N.; Sielaff, A.C.; Jaing, C.; Venkateswaran, K. Whole metagenome profiles of particulates collected from the International Space Station. Microbiome 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Sielaff, A.C.; Urbaniak, C.; Mohan, G.B.M.; Stepanov, V.G.; Tran, Q.; Wood, J.M.; Minich, J.; McDonald, D.; Mayer, T.; Knight, R.; et al. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 2019, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Perras, A.; Alekhova, T.A.; Wink, L.; Krause, R.; Aleksandrova, A.; Novozhilova, T.; Moissl-Eichinger, C. Resilient microorganisms in dust samples of the International Space Station—Survival of the adaptation specialists. Microbiome 2016, 4, 65. [Google Scholar] [CrossRef]
- Cogoli, A. The effect of space flight on human cellular immunity. Environ. Med. 1993, 37, 107–116. [Google Scholar]
- Crucian, B.E.; Chouker, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune system dysregulation during spaceflight: Potential countermeasures for deep space exploration missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Decelle, J.G.; Taylor, G.R. Autoflora in the upper respiratory tract of Apollo astronauts. Appl. Environ. Microbiol. 1976, 32, 659–665. [Google Scholar] [CrossRef]
- Castro, S.L.; Nelman-Gonzalez, M.; Nickerson, C.A.; Ott, C.M. Induction of attachment-independent biofilm formation and repression ofhfqexpression by low-fluid-shear culture of Staphylococcus aureus. Appl. Environ. Microbiol. 2011, 77, 6368–6378. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Stodieck, L.; Birdsall, H.H.; Becker, J.L.; Koenig, P.; Hammond, J.S.; Gunter, M.A.; Allen, P.L. Effects of microgravity on the virulence of Listeria monocytogenes, Enterococcus faecalis, Candida albicans, and methicillin-resistant Staphylococcus aureus. Astrobiology 2013, 13, 1081–1090. [Google Scholar] [CrossRef]
- O’Rourke, A.; Lee, M.D.; Nierman, W.C.; Everroad, R.C.; Dupont, C.L. Genomic and phenotypic characterization of Burkholderia isolates from the potable water system of the International Space Station. PLoS ONE 2020, 15, e0227152. [Google Scholar] [CrossRef]
- La Duc, M.T.; Sumner, R.; Pierson, D.; Venkat, P.; Venkateswaran, K. Evidence of pathogenic microbes in the international space station drinking water: Reason for concern? Habitation 2004, 10, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.J.; Ott, C.M.; Skuratov, V.M.; Pierson, D.L. Microbial surveillance of potable water sources of the international space station. SAE Trans. 2005, 114, 283–292. [Google Scholar] [CrossRef]
- Mora, M.; Mahnert, A.; Koskinen, K.; Păuşan, M.R.; Oberauner-Wappis, L.; Krause, R.; Perras, A.K.; Gorkiewicz, G.; Berg, G.; Moissl-Eichinger, C. Microorganisms in confined habitats: Microbial monitoring and control of intensive care units, operating rooms, cleanrooms and the international space station. Front. Microbiol. 2016, 7, 1573. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, N.; Huin-Schohn, C.; Bascove, M.; Bueb, J.L.; Tschirhart, E.; Legrand-Frossi, C.; Frippiat, J.P. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J. Leukoc. Biol. 2009, 86, 1027–1038. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Grohmann, E. Multi-resistant biofilm-forming pathogens on the international space station. J. Biosci. 2019, 44, 125. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Sielaff, A.C.; Frey, K.G.; Allen, J.E.; Singh, N.; Jaing, C.; Wheeler, K.; Venkateswaran, K. Detection of antimicrobial resistance genes associated with the International Space Station environmental surfaces. Sci. Rep. 2018, 8, 814. [Google Scholar] [CrossRef]
- Pyle, B.H.; McFeters, G.A.; Broadaway, S.C.; Johnsrud, C.K.; Storga, R.T.; Borkowski, J. Bacterial Growth on Surfaces and in Suspensions. Biorack on Spacehab. Biological Experiments on Shuttle to Mir Missions 03, 05, and 06; European Space Agency: Noordwijk, The Netherlands, 1999; pp. 95–99. [Google Scholar]
- McLean, R.J.C.; Cassanto, J.M.; Barnes, M.B.; Koo, J.H. Bacterial biofilm formation under microgravity conditions. FEMS Microbiol. Lett. 2001, 195, 115–119. [Google Scholar] [CrossRef]
- Lynch, S.V.; Mukundakrishnan, K.; Benoit, M.R.; Ayyaswamy, P.S.; Matin, A.C. Escherichia coli biofilms formed under low-shear modeled microgravity in a ground-based system. Appl. Environ. Microbiol. 2006, 72, 7701–7710. [Google Scholar] [CrossRef]
- Wilson, J.W.; Ott, C.M.; Zu Bentrup, K.H.; Ramamurthy, R.; Quick, L.; Porwollik, S.; Cheng, P.; McClelland, M.; Tsaprailis, G.; Radabaugh, T.; et al. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc. Natl. Acad. Sci. USA 2007, 104, 16299–16304. [Google Scholar] [CrossRef]
- Aunins, T.R.; Erickson, K.E.; Prasad, N.; Levy, S.E.; Jones, A.; Shrestha, S.; Mastracchio, R.; Stodieck, L.; Klaus, D.; Zea, L.; et al. Spaceflight modifies Escherichia coli gene expression in response to antibiotic exposure and reveals role of oxidative stress response. Front. Microbiol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Fernandez-Barat, L.; Motos, A.; Panigada, M.; Álvarez-Lerma, F.; Viña, L.; Lopez-Aladid, R.; Ceccato, A.; Bassi, G.L.; Nicolau, D.P.; Lopez, Y.; et al. Comparative efficacy of linezolid and vancomycin for endotracheal tube MRSA biofilms from ICU patients. Crit. Care 2019, 23, 251. [Google Scholar] [CrossRef] [PubMed]
- Akturk, E.; Oliveira, H.; Santos, S.B.; Costa, S.; Kuyumcu, S.; Melo, L.D.R.; Azeredo, J. Synergistic action of phage and antibiotics: Parameters to enhance the killing efficacy against mono and dual-species biofilms. Antibiotics 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Melian, C.; Segli, F.; Gonzalez, R.; Vignolo, G.; Castellano, P. Lactocin AL705 as quorum sensing inhibitor to control Listeria monocytogenes biofilm formation. J. Appl. Microbiol. 2019, 127, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.N.; Khan, F.; Phan, T.T.V.; Park, S.K.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Biofilm inhibition, modulation of virulence and motility properties by FeOOH nanoparticle in Pseudomonas aeruginosa. Braz. J. Microbiol. 2019, 50, 791–805. [Google Scholar] [CrossRef]
- Fallatah, H.; Elhaneid, M.; Ali-Boucetta, H.; Overton, T.; El Kadri, H.; Gkatzionis, K. Antibacterial effect of graphene oxide (GO) nano-particles against Pseudomonas putida biofilm of variable age. Environ. Sci. Pollut. Res. 2019, 26, 25057–25070. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Liu, Z.; Deng, Q.; Sun, P.; Ren, J.; Qu, X. Depriving bacterial adhesion-related molecule to inhibit biofilm formation using CeO2-decorated metal-organic frameworks. Small 2019, 15, e1902522. [Google Scholar] [CrossRef]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial blue light inactivation of polymicrobial biofilms. Front. Microbiol. 2019, 10, 721. [Google Scholar] [CrossRef]
- Prado, D.B.D.; Szczerepa, M.M.D.A.; Capeloto, O.A.; Astrath, N.G.C.; Dos Santos, N.C.A.; Previdelli, I.T.S.; Nakamura, C.V.; Mikcha, J.M.G.; Filho, B.A.D.A.; Dos Santos, N.C.A. Effect of ultraviolet (UV-C) radiation on spores and biofilms of Alicyclobacillus spp. in industrialized orange juice. Int. J. Food Microbiol. 2019, 305, 108238. [Google Scholar] [CrossRef]
- Li, C.H.; Chen, X.; Landis, R.F.; Geng, Y.; Makabenta, J.M.; Lemnios, W.; Gupta, A.; Rotello, V. Phytochemical-based nanocomposites for the treatment of bacterial biofilms. ACS Infect. Dis. 2019, 5, 1590–1596. [Google Scholar] [CrossRef]
- De Oliveira, A.B.; Ferrisse, T.M.; Marques, R.S.; De Annunzio, S.R.; Brighenti, F.L.; Fontana, C.R. Effect of photodynamic therapy on microorganisms responsible for dental caries: A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 3585. [Google Scholar] [CrossRef]
- Vögeling, H.; Plenagl, N.; Seitz, B.S.; Duse, L.; Pinnapireddy, S.R.; Dayyoub, E.; Jedelska, J.; Brüßler, J.; Bakowsky, U.; Pinapireddy, S.R. Synergistic effects of ultrasound and photodynamic therapy leading to biofilm eradication on polyurethane catheter surfaces modified with hypericin nanoformulations. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109749. [Google Scholar] [CrossRef] [PubMed]
- Picco, D.D.C.R.; Cavalcante, L.L.R.; Trevisan, R.L.B.; Souza-Gabriel, A.E.; Borsatto, M.C.; Corona, S.A.M. Effect of curcumin-mediated photodynamic therapy on Streptococcus mutans and Candida albicans: A systematic review of in vitro studies. Photodiagnosis Photodyn. Ther. 2019, 27, 455–461. [Google Scholar] [CrossRef]
- Lim, E.S.; Koo, O.K.; Kim, M.J.; Kim, J.S. Bio-enzymes for inhibition and elimination of Escherichia coli O157:H7 biofilm and their synergistic effect with sodium hypochlorite. Sci. Rep. 2019, 9, 9920. [Google Scholar] [CrossRef] [PubMed]
- Landau, U.; Lisowsky, T.; Karlheinz, E.; Mehler, K. Bioactive, Ruthenium-Containing Coating and Device. U.S. Patent No. EP 2 077 976 B1, 18 May 2016. [Google Scholar]
- Berg, G.; Liebminger, S.; Oberauner, L.; Klein, T.; Stampf, R. Photodynamic Control of Microbal Growth on Surfaces. U.S. Patent No. 201401 19985A1, 1 May 2014. [Google Scholar]
- Luksienė, Z.; Buchovec, I. Method of Decontamination of Food and Food Related Surfaces. Lithuania Patent No. LT 5567 B, 25 February 2009. [Google Scholar]
- Denis, T.G.S.; Hamblin, M.R. An introduction to photoantimicrobials: Photodynamic therapy as a novel method of microbial pathogen eradication. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances, 3rd ed.; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 675–683. [Google Scholar]
- Luksiene, Z.; Brovko, L. Antibacterial photosensitization-based treatment for food safety. Food Eng. Rev. 2013, 5, 185–199. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Tome, J.P.C.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, 49–55. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Huang, L.; Denis, T.G.S.; Xuan, Y.; Huang, Y.-Y.; Tanaka, M.; Zadlo, A.; Sarna, T.; Hamblin, M.R. Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: Role of ambient oxygen and azide radicals. Free Radic. Biol. Med. 2012, 53, 2062–2071. [Google Scholar] [CrossRef]
- Fotinos, N.; Convert, M.; Piffaretti, J.C.; Gurny, R.; Lange, N. Effects on gram-negative and gram-positive bacteria mediated by 5-aminolevulinic acid and 5-aminolevulinic acid derivatives. Antimicrob. Agents Chemother. 2008, 52, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Oxygen-independent antimicrobial photoinactivation: Type III photochemical mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef]
- Brovko, L. Photodynamic treatment: A new efficient alternative for surface sanitation. In Advances in Food and Nutrition Research; Taylor, S., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 120–147. [Google Scholar]
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Malik, Z.; Ladan, H.; Nitzan, Y. Photodynamic inactivation of Gram-negative bacteria: Problems and possible solutions. J. Photochem. Photobiol. B Biol. 1992, 14, 262–266. [Google Scholar] [CrossRef]
- Nitzan, Y.; Salmon-Divon, M.; Shporen, E.; Malik, Z. ALA induced photodynamic effects on Gram positive and negative bacteria. Photochem. Photobiol. Sci. 2004, 3, 430–435. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Callaghan, S.; Senge, M.O. The good, the bad, and the ugly—Controlling singlet oxygen through design of photosensitizers and delivery systems for photodynamic therapy. Photochem. Photobiol. Sci. 2018, 17, 1490–1514. [Google Scholar] [CrossRef]
- Maisch, T. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J. Photochem. Photobiol. B Biol. 2015, 150, 2–10. [Google Scholar] [CrossRef]
- Yin, R.; Hamblin, M. Antimicrobial photosensitizers: Drug discovery under the spotlight. Curr. Med. Chem. 2015, 22, 2159–2185. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, M.; Huang, Y.-Y.; Landi, G.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: Oxygen-independent photokilling in presence of azide and new mechanistic insights. Free Radic. Biol. Med. 2014, 79, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Pechatnikov, I. Approaches to kill gram-negative bacteria by photosensitized process. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Hamblin, M.R., Ed.; RSC Publishing: Cambridge, UK, 2011; Volume 87, pp. 47–67. [Google Scholar]
- Saw, C.L.L. Science against microbial pathogens: Photodynamic therapy approaches. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Publishing: Badajor, India, 2011; pp. 668–674. [Google Scholar]
- Demidova, T.N.; Hamblin, M.R. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 2005, 49, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Buchovec, I.; Paskeviciute, E. Inactivation of Bacillus cereus by Na-chlorophyllin-based photosensitization on the surface of packaging. J. Appl. Microbiol. 2010, 109, 1540–1548. [Google Scholar] [CrossRef]
- Krüger, M.; Richter, P.R.; Strauch, S.M.; Nasir, A.; Burkovski, A.; Antunes, C.A.; Meißgeier, T.; Schluecker, E.; Schwab, S.; Lebert, M. What an escherichia coli mutant can teach us about the antibacterial effect of chlorophyllin. Microorganisms 2019, 7, 59. [Google Scholar] [CrossRef]
- Mamone, L.; Di Venosa, G.; Gándara, L.; Sáenz, D.; Vallecorsa, P.; Schickinger, S.; Rossetti, M.; Batlle, A.; Buzzola, F.R.; Casas, A. Photodynamic inactivation of Gram-positive bacteria employing natural resources. J. Photochem. Photobiol. B Biol. 2014, 133, 80–89. [Google Scholar] [CrossRef]
- Majiya, H.; Galstyan, A. Dye extract of calyces of Hibiscus sabdariffa has photodynamic antibacterial activity: A prospect for sunlight-driven fresh produce sanitation. Food Sci. Nutr. 2020, 8, 3200–3211. [Google Scholar] [CrossRef]
- Falk, H.; Meyer, J.; Oberreiter, M. A convenient semisynthetic route to hypericin. Monatshefte Chem. 1993, 124, 339–341. [Google Scholar] [CrossRef]
- Kubin, A.; Loew, H.G.; Burner, U.; Jessner, G.; Kolbabek, H.; Wierrani, F. How to make hypericin water-soluble. Die Pharm. 2008, 63, 263–269. [Google Scholar]
- Ehrenberg, B.; Anderson, J.; Foote, C. Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media. Photochem. Photobiol. 1998, 68, 135–140. [Google Scholar] [CrossRef]
- Alam, S.T.; Le, T.A.N.; Park, J.S.; Kwon, H.C.; Kang, K. Antimicrobial biophotonic treatment of ampicillin-resistant Pseudomonas aeruginosa with hypericin and ampicillin cotreatment followed by orange light. Pharmaceutics 2019, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Comas-Barceló, J.; Rodríguez-Amigo, B.; Abbruzzetti, S.; Del Rey-Puech, P.; Agut, M.; Nonell, S.; Viappiani, C. A self-assembled nanostructured material with photosensitising properties. RSC Adv. 2013, 3, 17874–17879. [Google Scholar] [CrossRef]
- Delcanale, P.; Rodríguez-Amigo, B.; Jiménez, J.J.; Luque, F.J.; Abbruzzetti, S.; Agut, M.; Nonell, S.; Viappiani, C. Tuning the local solvent composition at a drug carrier surface: The effect of dimethyl sulfoxide/water mixture on the photofunctional properties of hypericin-β-lactoglobulin complexes. J. Mater. Chem. B 2017, 5, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Delcanale, P.; Hally, C.; Nonell, S.; Bonardi, S.; Viappiani, C.; Abbruzzetti, S. Photodynamic action of Hypericum perforatum hydrophilic extract against Staphylococcus aureus. Photochem. Photobiol. Sci. 2020, 19, 324–331. [Google Scholar] [CrossRef]
- Engelhardt, V.; Krammer, B.; Plaetzer, K. Antibacterial photodynamic therapy using water-soluble formulations of hypericin or mTHPC is effective in inactivation of Staphylococcus aureus. Photochem. Photobiol. Sci. 2010, 9, 365–369. [Google Scholar] [CrossRef]
- García, I.; Ballesta, S.; Gilaberte, Y.; Rezusta, A.; Pascual, A. Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms. Future Microbiol. 2015, 10, 347–356. [Google Scholar] [CrossRef]
- Kashef, N.; Borghei, Y.S.; Djavid, G.E. Photodynamic effect of hypericin on the microorganisms and primary human fibroblasts. Photodiagnosis Photodyn. Ther. 2013, 10, 150–155. [Google Scholar] [CrossRef]
- Kashef, N.; Karami, S.; Djavid, G.E. Phototoxic effect of hypericin alone and in combination with acetylcysteine on Staphylococcus aureus biofilms. Photodiagn. Photodyn. Ther. 2015, 12, 186–192. [Google Scholar] [CrossRef]
- Melo, W.C.M.A.; Lee, A.N.; Perussi, J.R.; Hamblin, M.R. Electroporation enhances antimicrobial photodynamic therapy mediated by the hydrophobic photosensitizer, hypericin. Photodiagn. Photodyn. Ther. 2013, 10, 647–650. [Google Scholar] [CrossRef]
- Rodríguez-Amigo, B.; Delcanale, P.; Rotger, G.; Jiménez, J.J.; Abbruzzetti, S.; Summer, A.; Agut, M.; Luque, F.J.; Nonell, S.; Viappiani, C. The complex of hypericin with β-lactoglobulin has antimicrobial activity with potential applications in dairy industry. J. Dairy Sci. 2015, 98, 89–94. [Google Scholar] [CrossRef]
- Yow, C.M.N.; Tang, H.M.; Chu, E.S.; Huang, Z. Hypericin-mediated Photodynamic Antimicrobial Effect on Clinically Isolated Pathogens†. Photochem. Photobiol. 2012, 88, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Lüthi, M.; Gyenge, E.B.; Engstrüm, M.; Bredell, M.; Gratz, K.; Walt, H.; Gmür, R.; Maake, C. Hypericin- and mTHPC-mediated photodynamic therapy for the treatment of cariogenic bacteria. Med. Laser Appl. 2009, 24, 227–236. [Google Scholar] [CrossRef]
- Kairyte, K.; Lapinskas, S.; Gudelis, V.; Luksiene, Z. Effective inactivation of food pathogens Listeria monocytogenes and Salmonella enterica by combined treatment of hypericin-based photosensitization and high power pulsed light. J. Appl. Microbiol. 2012, 112, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Aponiene, K.; Paskeviciute, E.; Reklaitis, I.; Luksiene, Z. Reduction of microbial contamination of fruits and vegetables by hypericin-based photosensitization: Comparison with other emerging antimicrobial treatments. J. Food Eng. 2015, 144, 29–35. [Google Scholar] [CrossRef]
- Jankowski, A.; Jankowski, S.; Mirończyk, A.; Niedbach, J. The action of photosensitizers and serum in a bactericidal process. II. The effects of dyes: Hypericin, eosin Y and saphranine O. Pol. J. Microbiol. 2005, 54, 323–330. [Google Scholar] [PubMed]
- Zhang, J.N.; Zhang, F.; Tang, Q.J.; Xu, C.S.; Meng, X.H. Effect of photodynamic inactivation of Escherichia coli by hypericin. World J. Microbiol. Biotechnol. 2018, 34, 100. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, V.; Ponnusamy, C.; Sankareswaran, S.; Paulsamy, M.; Madiyalakan, R.; Palanichamy, R.; Kandasamy, R.; Natesan, S.; Madiyalakan, M.; Palanisamy, R. Development of copolymeric nanoparticles of hypocrellin B: Enhanced phototoxic effect and ocular distribution. Eur. J. Pharm. Sci. 2018, 116, 26–36. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Inactivation of Staphylococcus aureus by photodynamic action of hypocrellin B. Photodiagn. Photodyn. Ther. 2013, 10, 600–606. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, W.-N.; Tang, Q.; Zhang, H.; Xu, C. Effect of light-activated hypocrellin b on the growth and membrane permeability of gram-negative Escherichia coli cells. Int. J. Photoenergy 2014, 2014, 521209. [Google Scholar] [CrossRef]
- Otieno, W.; Liu, C.; Deng, H.; Li, J.; Zeng, X.; Ji, Y. Hypocrellin B-mediated photodynamic inactivation of gram-positive antibiotic-resistant bacteria: An in vitro study. Photobiomodul. Photomed. Laser Surg. 2020, 38, 36–42. [Google Scholar] [CrossRef]
- Su, Y.; Sun, J.; Rao, S.; Cai, Y.; Yang, Y. Photodynamic antimicrobial activity of hypocrellin A. J. Photochem. Photobiol. B Biol. 2011, 103, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rezusta, A.; Rezusta-López, A.; Paz-Cristobal, M.P.; Alemany-Ribes, M.; Royo-Díez, D.; Agut, M.; Semino, C.; Nonell, S.; Revillo, M.J.; Aspiroz, C.; et al. In vitro fungicidal photodynamic effect of hypericin on Candida species. Photochem. Photobiol. 2011, 88, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Paz-Cristobal, M.P.; Gilaberte, Y.; Alejandre, C.; Pardo, J.; Revillo, M.J.; Rezusta, A. In vitro fungicidal photodynamic effect of hypericin on Trichophyton spp. Mycopathologia 2014, 178, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.; Harris, L.; Towers, G. The importance of light in the anti-HIV effect of hypericin. Antivir. Res. 1993, 20, 173–178. [Google Scholar] [CrossRef]
- Prince, A.M.; Pascual, D.; Meruelo, D.; Liebes, L.; Mazur, Y.; Dubovi, E.; Mandel, M.; Lavie, G. Strategies for evaluation of enveloped virus inactivation in red cell concentrates using hypericin. Photochem. Photobiol. 2000, 71, 188–195. [Google Scholar] [CrossRef]
- Gulick, R.M.; McAuliffe, V.; Holden-Wiltse, J.; Crumpacker, C.; Liebes, L.; Stein, D.S.; Meehan, P.; Hussey, S.; Forcht, J.; Valentine, F.T. Phase I studies of hypericin, the active compound in St. John’s Wort, as an antiretroviral agent in HIV-infected adults. AIDS Clinical Trials Group Protocols 150 and 258. Ann. Intern. Med. 1999, 130, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.M.; Feinman, L.; Liebes, L.; Ostrow, N.; Koslowski, V.; Tobia, A.; Cabana, B.E.; Lee, D.H.; Spritzler, J.; Prince, A.M. Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John’s wort plant, in patients with chronic hepatitis c virus infection. Antimicrob. Agents Chemother. 2001, 45, 517–524. [Google Scholar] [CrossRef]
- Jendželovská, Z.; Jendželovský, R.; Kuchárová, B.; Fedoročko, P. Hypericin in the light and in the dark: Two sides of the same coin. Front. Plant Sci. 2016, 7, 560. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef]
- Huang, R.; Choe, E.; Min, D. Kinetics for singlet oxygen formation by riboflavin photosensitization and the reaction between riboflavin and singlet oxygen. J. Food Sci. 2006, 69, C726–C732. [Google Scholar] [CrossRef]
- Ahmad, I.; Anwar, Z.; Ahmed, S.; Sheraz, M.A.; Bano, R.; Hafeez, A. Solvent effect on the photolysis of riboflavin. AAPS PharmSciTech 2015, 16, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef]
- Ahgilan, A.; Sabaratnam, V.; Periasamy, V. Antimicrobial properties of vitamin B2. Int. J. Food Prop. 2016, 19, 1173–1181. [Google Scholar] [CrossRef]
- Choe, E.; Huang, R.; Min, D.B. Chemical reactions and stability of riboflavin in foods. J. Food Sci. 2004, 70, 726–732. [Google Scholar] [CrossRef]
- Silva, E.; Edwards, A.M.; Pacheco, D. Visible light-induced photooxidation of glucose sensitized by riboflavin. J. Nutr. Biochem. 1999, 10, 181–185. [Google Scholar] [CrossRef]
- Ionita, P.; Matei, I. Application of Riboflavin Photochemical Properties in Hydrogel Synthesis. In Biophysical Chemistry—Advance Application; Khalid, M.A.A., Ed.; IntechOpen: London, UK, 2019; Chapter 2; pp. 1–15. [Google Scholar]
- Chan, T.C.Y.; Lau, T.W.S.; Lee, J.W.Y.; Wong, I.Y.H.; Jhanji, V.; Wong, R.L. Corneal collagen cross-linking for infectious keratitis: An update of clinical studies. Acta Ophthalmol. 2015, 93, 689–696. [Google Scholar] [CrossRef]
- Ettinger, A.; Miklauz, M.M.; Bihm, D.J.; Maldonado-Codina, G.; Goodrich, R.P. Preparation of cryoprecipitate from riboflavin and UV light-treated plasma. Transfus. Apher. Sci. 2012, 46, 153–158. [Google Scholar] [CrossRef]
- Halili, F.; Arboleda, A.; Durkee, H.; Taneja, M.; Miller, D.; Alawa, K.A.; Aguilar, M.C.; Amescua, G.; Flynn, H.W.; Parel, J.M. Rose Bengal and riboflavin-mediated photodynamic therapy to inhibit methicillin-resistant Staphylococcus aureus keratitis isolates. Am. J. Ophthalmol. 2016, 166, 194–202. [Google Scholar] [CrossRef]
- O’Rourke, J.F.; Dowds, B.C. Dye-mediated photodynamic inactivation of Bacillus subtilis. Biochem. Soc. Trans. 1992, 20, 76S. [Google Scholar] [CrossRef]
- Kashiwabuchi, R.T.; Khan, Y.; Carvalho, F.R.D.S.; Hirai, F.; Campos, M.; McDonnell, P.J. Antimicrobial susceptibility of photodynamic therapy (UVA/riboflavin) against Staphylococcus aureus. Arq. Bras. Ophthalmol. 2012, 75, 423–426. [Google Scholar] [CrossRef]
- Bäckman, A.; Makdoumi, K.; Mortensen, J.; Crafoord, S. The efficiency of cross-linking methods in eradication of bacteria is influenced by the riboflavin concentration and the irradiation time of ultraviolet light. Acta Ophthalmol. 2014, 92, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Makdoumi, K.; Bäckman, A.; Mortensen, J.; Crafoord, S. Evaluation of antibacterial efficacy of photo-activated riboflavin using ultraviolet light (UVA). Graefes Arch. Clin. Exp. Ophthalmol. 2009, 248, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Makdoumi, K.; Bäckman, A. Photodynamic UVA-riboflavin bacterial elimination in antibiotic resistant bacteria. Clin. Exp. Ophthalmol. 2016, 44, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Makdoumi, K.; Goodrich, R.P.; Bäckman, A. Photochemical eradication of methicillin-resistant Staphylococcus aureus by blue light activation of riboflavin. Acta Ophthalmol. 2017, 95, 498–502. [Google Scholar] [CrossRef]
- Makdoumi, K.; Hedin, M.; Bäckman, A. Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes in vitro. Lasers Med. Sci. 2019, 34, 1799–1805. [Google Scholar] [CrossRef]
- Maisch, T.; Eichner, A.; Späth, A.; Gollmer, A.; Koenig, B.; Regensburger, J.; Bäumler, W. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS ONE 2014, 9, e111792. [Google Scholar] [CrossRef]
- Eitenmiller, R.; Ye, L.; Landen, W. Vitamin Analysis for the Health and Food Sciences, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 664. [Google Scholar]
- Thakuri, P.S.; Joshi, R.; Basnet, S.; Pandey, S.; Taujale, S.D.; Mishra, N. Antibacterial photodynamic therapy on Staphylococcus aureus and Pseudomonas aeruginosa in-vitro. Nepal. Med. Coll. J. 2011, 13, 281–284. [Google Scholar]
- Liang, J.Y.; Cheng, C.W.; Yu, C.H.; Chen, L.Y. Investigations of blue light-induced reactive oxygen species from flavin mononucleotide on inactivation of E. coli. J. Photochem. Photobiol. B Biol. 2015, 143, 82–88. [Google Scholar] [CrossRef]
- Wong, T.; Cheng, C.; Hsieh, Z.; Liang, J. Effects of blue or violet light on the inactivation of Staphylococcus aureus by riboflavin-5′-phosphate photolysis. J. Photochem. Photobiol. B Biol. 2013, 173, 672–680. [Google Scholar] [CrossRef]
- Cozzolino, M.; Delcanale, P.; Montali, C.; Tognolini, M.; Giorgio, C.; Corrado, M.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. Enhanced photosensitizing properties of protein bound curcumin. Life Sci. 2019, 223, 116710. [Google Scholar] [CrossRef]
- Das, K.C.; Das, C.K. Curcumin (diferuloylmethane), a singlet oxygen (1O2) quencher. Biochem. Biophys. Res. Commun. 2002, 295, 62–66. [Google Scholar] [CrossRef]
- De Annunzio, S.R.; De Freitas, L.M.; Blanco, A.L.; Da Costa, M.M.; Carmona-Vargas, C.C.; De Oliveira, K.T.; Fontana, C.R. Susceptibility of Enterococcus faecalis and Propionibacterium acnes to antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2018, 178, 545–550. [Google Scholar] [CrossRef]
- Da Frota, M.F.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Bagnato, V.S.; Espir, C.G.; Berbert, F.L.C.V. Photodynamic therapy in root canals contaminated with Enterococcus faecalis using curcumin as photosensitizer. Lasers Med. Sci. 2014, 30, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Haukvik, T.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Photokilling of bacteria by curcumin in different aqueous preparations. Studies on curcumin and curcuminoids XXXVII. Pharmazie 2009, 64, 666–673. [Google Scholar] [PubMed]
- Pileggi, G.; Wataha, J.C.; Girard, M.; Grad, I.; Schrenzel, J.; Lange, N.; Bouillaguet, S.D.M.D. Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagn. Photodyn. Ther. 2013, 10, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Manoil, D.; Filieri, A.; Gameiro, C.; Lange, N.; Schrenzel, J.; Wataha, J.C.; Bouillaguet, S. Flow cytometric assessment of Streptococcus mutans viability after exposure to blue light-activated curcumin. Photodiagn. Photodyn. Ther. 2014, 11, 372–379. [Google Scholar] [CrossRef]
- Paschoal, M.A.B.; Lin, M.; Santos-Pinto, L.; Duarte, S. Photodynamic antimicrobial chemotherapy on Streptococcus mutans using curcumin and toluidine blue activated by a novel LED device. Lasers Med. Sci. 2015, 30, 885–890. [Google Scholar] [CrossRef]
- Paschoal, M.A.B.; Tonon, C.C.; Spolidorio, D.M.P.; Bagnato, V.S.; Giusti, J.S.; Santos-Pinto, L. Photodynamic potential of curcumin and blue LED against Streptococcus mutans in a planktonic culture. Photodiagn. Photodyn. Ther. 2013, 10, 313–319. [Google Scholar] [CrossRef]
- Araújo, N.C.; De Menezes, R.F.; Carneiro, V.S.M.; Dos Santos-Neto, A.P.; Fontana, C.R.; Bagnato, V.S.; Harvey, C.M.; Gerbi, M.E.M. Photodynamic inactivation of cariogenic pathogens using curcumin as photosensitizer. Photomed. Laser Surg. 2017, 35, 259–263. [Google Scholar] [CrossRef]
- Penha, C.B.; Bonin, E.; Da Silva, A.F.; Hioka, N.; Zanqueta, É.B.; Ueda-Nakamura, T.; Filho, B.A.D.A.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT Food. Sci. Technol. 2017, 76, 198–202. [Google Scholar] [CrossRef]
- Aldred, E.M.; Buck, C.H.; Vall, K. Phenols. In Pharmacology, 1st ed.; Aldred, E.M., Buck, C.H., Vall, K., Eds.; Churchill Livingstone: London, UK, 2009; Chapter 21; pp. 149–166. [Google Scholar]
- Llano, J.; Raber, J.; Eriksson, L.A. Theoretical study of phototoxic reactios of psoralens. J. Photoch. Photobiol. A 2003, 154, 235–243. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; Psoralen; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547880/ (accessed on 16 January 2020).
- Harding, A.S.; Schwab, K.J. Using limes and synthetic psoralens to enhance solar disinfection of water (SODIS): A laboratory evaluation with norovirus, Escherichia coli, and MS2. Am. J. Trop. Med. Hyg. 2012, 86, 566–572. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Böhm, V.; Courtney, P.; Schwartz, S.J. Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. J. Food Sci. 2002, 67, 2589–2595. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Singh, K.; Sngh, V. Therapeutic and pharmacological aspects of photodynamic product chlorophyllin. Eur. J. Biol. Res. 2019, 9, 64–76. [Google Scholar]
- Roca, M.; Chen, K.; Pérez-Gálvez, A. Chlorophylls. In Handbook on Natural Pigments in Food and Beverages; Chapter 6, Introduction to Plant Pharmacology; Reinhold, C., Schweiggert, R., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2016; pp. 125–158. [Google Scholar]
- Rastogi, S.; Dwivedi, U.N. Porphyrin. In Biomolecules (Introduction, Structure and Functions); E-Book by Niscair; CSIR Publication: New Delhi, India, 2008; pp. 1–17. [Google Scholar]
- Marquez, U.M.L.; Sinnecker, P. Chlorophylls: Properties, biosynthesis, degradation and functions. In Food Colorants: Chemical and Functional Properties, 1st ed.; Socaciu, C., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 25–45. [Google Scholar]
- Fahey, J.W.; Stephenson, K.K.; Dinkova-Kostova, A.T.; Egner, P.A.; Kensler, T.; Talalay, P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis 2005, 26, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Wohllebe, S.; Richter, R.; Richter, P.; Häder, D.P. Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol. Res. 2019, 104, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Lopezcarballo, G.; Hernandezmunoz, P.; Gavara, R.; Ocio, M. Photoactivated chlorophyllin-based gelatin films and coatings to prevent microbial contamination of food products. Int. J. Food Microbiol. 2008, 126, 65–70. [Google Scholar] [CrossRef]
- Viera-Alcaide, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Buchovec, I.; Lukseviciūtė, V.; Kokstaite, R.; Labeikyte, D.; Kaziukonyte, L.; Luksiene, Z. Inactivation of Gram (−) bacteria Salmonella enterica by chlorophyllin-based photosensitization: Mechanism of action and new strategies to enhance the inactivation efficiency. J. Photochem. Photobiol. B Biol. 2017, 172, 1–10. [Google Scholar] [CrossRef]
- Kreitner, M.; Wagner, K.H.; Alth, G.; Ebermann, R.; Foißy, H.; Elmadfa, I. Hematoporphyrin- and sodium chlorophyllin-induced phototoxicity towards bacteria and yeasts—A new approach for safe foods. Food Control 2001, 12, 529–533. [Google Scholar] [CrossRef]
- Luksiene, Z.; Kokstaite, R.; Katauskis, P.; Skakauskas, V. Novel approach to effective and uniform inactivation of gram-positive Listeria monocytogenes and gram-negative Salmonella enterica by photosensitization. Food Technol. Biotechnol. 2013, 51, 338–344. [Google Scholar]
- Luksiene, Z.; Buchovec, I.; Paskeviciute, E. Inactivation of several strains of Listeria monocytogenes attached to the surface of packaging material by Na-Chlorophyllin-based photosensitization. J. Photochem. Photobiol. B Biol. 2010, 101, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Paskeviciute, E. Microbial control of food-related surfaces: Na-Chlorophyllin-based photosensitization. J. Photochem. Photobiol. B Biol. 2011, 105, 69–74. [Google Scholar] [CrossRef]
- López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R. Photoactivated self-sanitizing chlorophyllin-containing coatings to prevent microbial contamination in packaged food. Coatings 2018, 8, 328. [Google Scholar] [CrossRef]
- Luksiene, Z.; Buchovec, I. Impact of chlorophyllin-chitosan coating and visible light on the microbial contamination, shelf life, nutritional and visual quality of strawberries. Innov. Food Sci. Emerg. Technol. 2019, 52, 463–472. [Google Scholar] [CrossRef]
- Richter, P.R.; Krüger, M.; Prasad, B.; Gastiger, S.; Bodenschatz, M.; Wieder, F.; Burkovski, A.; Geißdörfer, W.; Lebert, M.; Strauch, S.M. Using colistin as a trojan horse: Inactivation of gram-negative bacteria with chlorophyllin. Antibiotics 2019, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Foggiato, A.A.; Silva, D.F.; Castro, R.C.F.R. Effect of photodynamic therapy on surface decontamination in clinical orthodontic instruments. Photodiagn. Photodyn. Ther. 2018, 24, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Foggiato, A.A.; Garcez, A.S.; Fuziy, A.; Silva, D.F.; Foggiato, A.A.; Garcez, A.S.; Fuziy, A.; Silva, D.F. Photodynamic therapy: Alternative in decontamination of surfaces. In Proceedings of the SPIE 11223, Photonic Diagnosis, Monitoring, Prevention, and Treatment of Infections and Inflammatory Diseases 2020, San Francisco, CA, USA, 28 February 2020. 112230K. [Google Scholar]
- Tiwana, A.S. Antimicrobial Photodynamic Treatment for Surface Sanitation. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2006. [Google Scholar]
- Vollmer, A.; Al-Ahmad, A.; Argyropoulou, A.; Thurnheer, T.; Hellwig, E.; Attin, T.; Vach, K.; Wittmer, A.; Ferguson, K.; Skaltsounis, A.L.; et al. Antimicrobial photoinactivation using visible light plus water-filtered infrared-a (vis + wira) and hypericum perforatum modifies in situ oral biofilms. Sci. Rep. 2019, 9, 20325. [Google Scholar] [CrossRef]
- Josewin, S.W.; Ghate, V.; Kim, M.-J.; Yuk, H.-G. Antibacterial effect of 460 nm light-emitting diode in combination with riboflavin against Listeria monocytogenes on smoked salmon. Food Control. 2018, 84, 354–361. [Google Scholar] [CrossRef]
- Khan, S.; Rayis, M.; Rizvi, A.; Alam, M.; Rizvi, M.; Naseem, I. ROS mediated antibacterial activity of photoilluminated riboflavin: A photodynamic mechanism against nosocomial infections. Toxicol. Rep. 2019, 6, 136–142. [Google Scholar] [CrossRef]
- Ruane, P.H.; Edrich, R.; Gampp, D.; Keil, S.D.; Leonard, R.L.; Goodrich, R.P. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004, 44, 877–885. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; Li, H.; Zeng, Q.-H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Enhanced antibacterial and antibiofilm functions of the curcumin-mediated photodynamic inactivation against Listeria monocytogenes. Food Control. 2020, 108, 106886. [Google Scholar] [CrossRef]
- Méndez, D.A.C.; Gutierrez, E.; Lamarque, G.C.C.; Rizzato, V.L.; Buzalaf, M.A.R.; Machado, M.A.A.M.; Cruvinel, T. The effectiveness of curcumin-mediated antimicrobial photodynamic therapy depends on pre-irradiation and biofilm growth times. Photodiagn. Photodyn. Ther. 2019, 27, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Chang, K.C.; Chen, L.Y.; Hu, A. Low-dose blue light irradiation enhances the antimicrobial activities of curcumin against Propionibacterium acnes. J. Photochem. Photobiol. B Biol. 2018, 189, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leung, W.-N.; Hua, H.; Rao, X.; Xu, C. Photodynamic action of LED-activated curcumin against Staphylococcus aureus involving intracellular ROS increase and membrane damage. Int. J. Photoenergy 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Ribeiro, A.P.D.; Pavarina, A.C.; Dovigo, L.N.; Brunetti, I.L.; Bagnato, V.S.; Vergani, C.E.; Costa, C.A.D.S. Phototoxic effect of curcumin on methicillin-resistant Staphylococcus aureus and L929 fibroblasts. Lasers Med. Sci. 2012, 28, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, D.; Martins, C.; David, B.; Lemos, C.; Almeida, A.; Cunha, Â.; Neves, M.; Pinto, D.; Faustino, M.A.F. Photodynamic inactivation of Listeria innocua biofilms with food-grade photosensitizers: A curcumin-rich extract of Curcuma longa vs. commercial curcumin. J. Appl. Microbiol. 2018, 125, 282–294. [Google Scholar] [CrossRef]
- Bulit, F.; Grad, I.; Manoil, D.; Simon, S.; Wataha, J.C.; Filieri, A.; Feki, A.; Schrenzel, J.; Lange, N.; Bouillaguet, S. Antimicrobial activity and cytotoxicity of 3 photosensitizers activated with blue light. J. Endod. 2014, 40, 427–431. [Google Scholar] [CrossRef]
- Walther, J.; Bröcker, M.J.; Wätzlich, D.; Nimtz, M.; Rohde, M.; Jahn, D.; Moser, J. Protochlorophyllide: A new photosensitizer for the photodynamic inactivation of Gram-positive and Gram-negative bacteria. FEMS Microbiol. Lett. 2008, 290, 156–163. [Google Scholar] [CrossRef]
- Caires, A.R.; Leal, C.R.B.; Ramos, C.A.N.; Bogo, D.; Lima, A.R.; Arruda, E.J.; Oliveira, S.L.; Melnikov, P. Photoinactivation effect of eosin methylene blue and chlorophyllin sodium-copper against Staphylococcus aureus and Escherichia coli. Lasers Med. Sci. 2017, 3, 1081–1088. [Google Scholar] [CrossRef]
- Aponiene, K.; Luksiene, Z. Effective combination of LED-based visible light, photosensitizer and photocatalyst to combat Gram (−) bacteria. J. Photochem. Photobiol. B Biol. 2015, 142, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Pamedytyte, R.; Gruskiene, R.; Luksiene, Z. Novel approach to the microbial decontamination of wheat sprouts: Photoactivated chlorophyllin-chitosan complex. In Industrial, Medical and Environmental Applications of Microorganisms; Mendez-Vilas, A., Ed.; Vageningen Academic: Madrid, Spain, 2013; pp. 352–356. [Google Scholar]
- Buchovec, I.; Lukseviciute, V.; Marsalka, A.; Reklaitis, I.; Luksiene, Z. Effective photosensitization-based inactivation of Gram (−) food pathogens and molds using the chlorophyllin–chitosan complex: Towards photoactive edible coatings to preserve strawberries. Photochem. Photobiol. Sci. 2016, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchovec, I.; Gricajeva, A.; Kalėdienė, L.; Vitta, P. Antimicrobial Photoinactivation Approach Based on Natural Agents for Control of Bacteria Biofilms in Spacecraft. Int. J. Mol. Sci. 2020, 21, 6932. https://doi.org/10.3390/ijms21186932
Buchovec I, Gricajeva A, Kalėdienė L, Vitta P. Antimicrobial Photoinactivation Approach Based on Natural Agents for Control of Bacteria Biofilms in Spacecraft. International Journal of Molecular Sciences. 2020; 21(18):6932. https://doi.org/10.3390/ijms21186932
Chicago/Turabian StyleBuchovec, Irina, Alisa Gricajeva, Lilija Kalėdienė, and Pranciškus Vitta. 2020. "Antimicrobial Photoinactivation Approach Based on Natural Agents for Control of Bacteria Biofilms in Spacecraft" International Journal of Molecular Sciences 21, no. 18: 6932. https://doi.org/10.3390/ijms21186932
APA StyleBuchovec, I., Gricajeva, A., Kalėdienė, L., & Vitta, P. (2020). Antimicrobial Photoinactivation Approach Based on Natural Agents for Control of Bacteria Biofilms in Spacecraft. International Journal of Molecular Sciences, 21(18), 6932. https://doi.org/10.3390/ijms21186932