Effects of p-Cresol on Senescence, Survival, Inflammation, and Odontoblast Differentiation in Canine Dental Pulp Stem Cells
Abstract
1. Introduction
2. Results
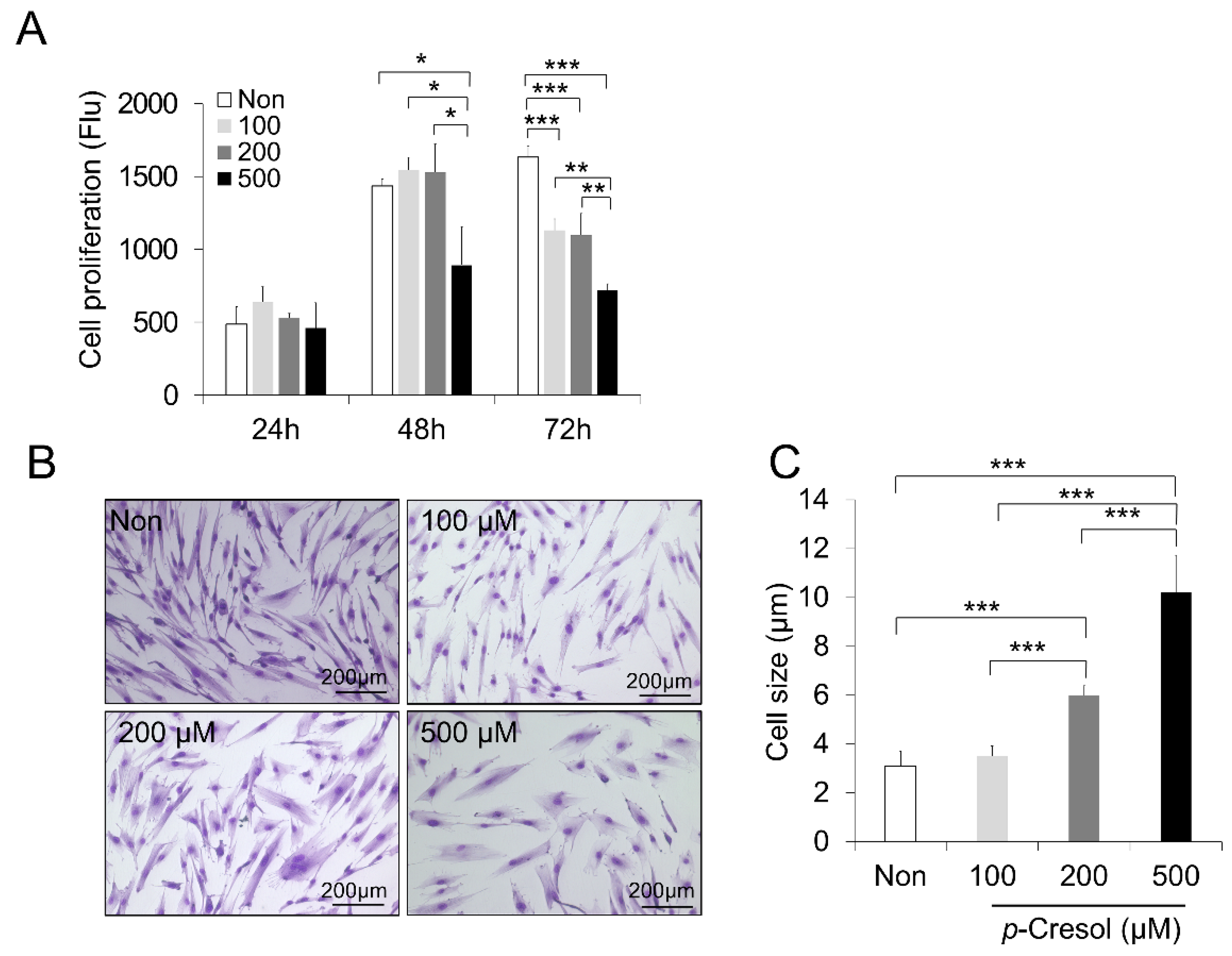
2.1. Reduced Proliferation Rate and Increased Cell Size and Senescence Induced by PC
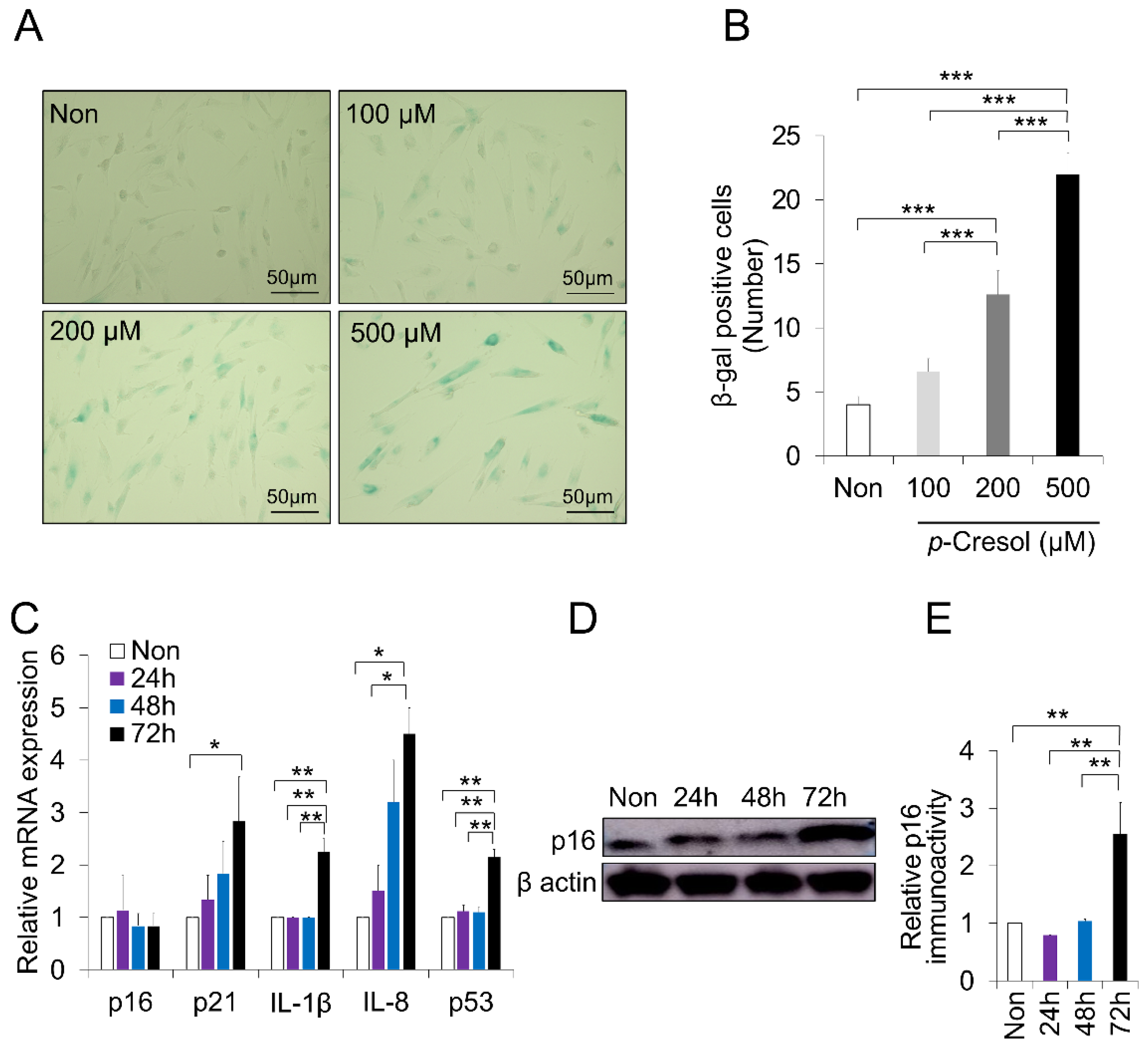
2.2. Increased Senescence Markers Induced by PC
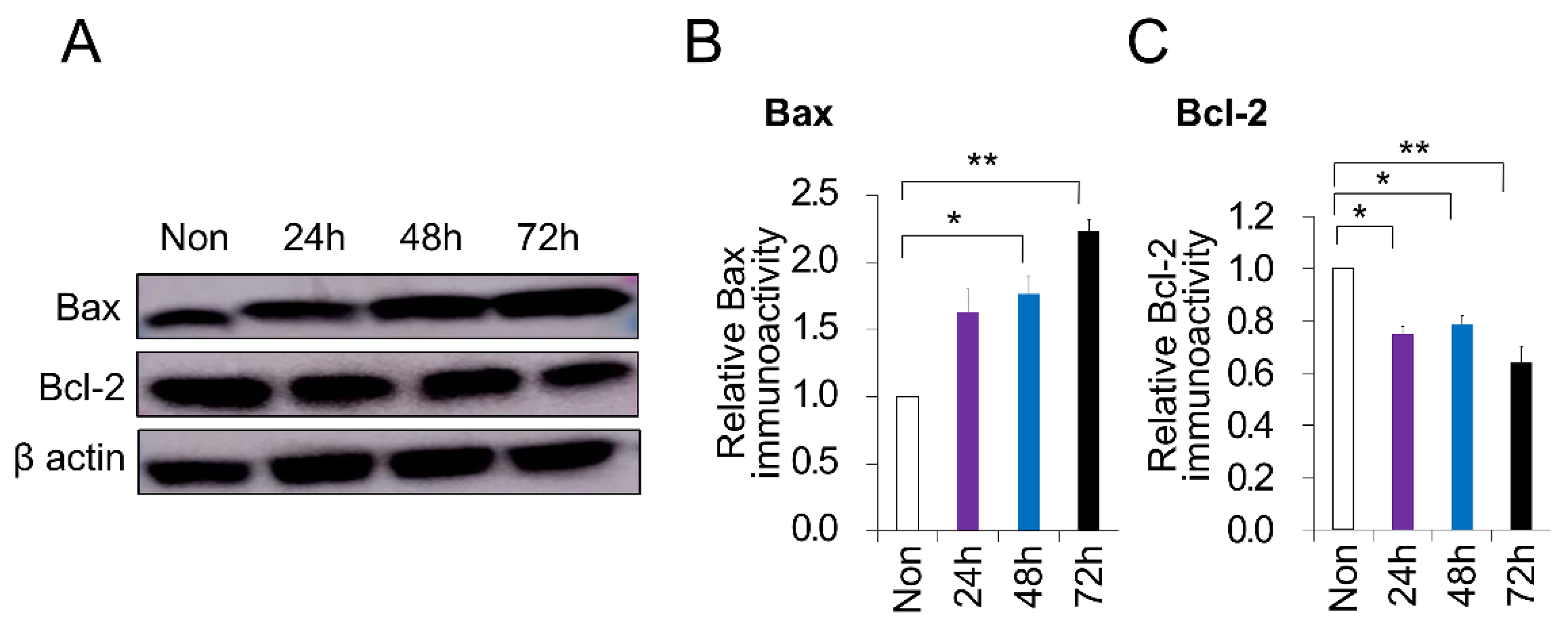
2.3. PC-Induced Apoptosis in DPSCs
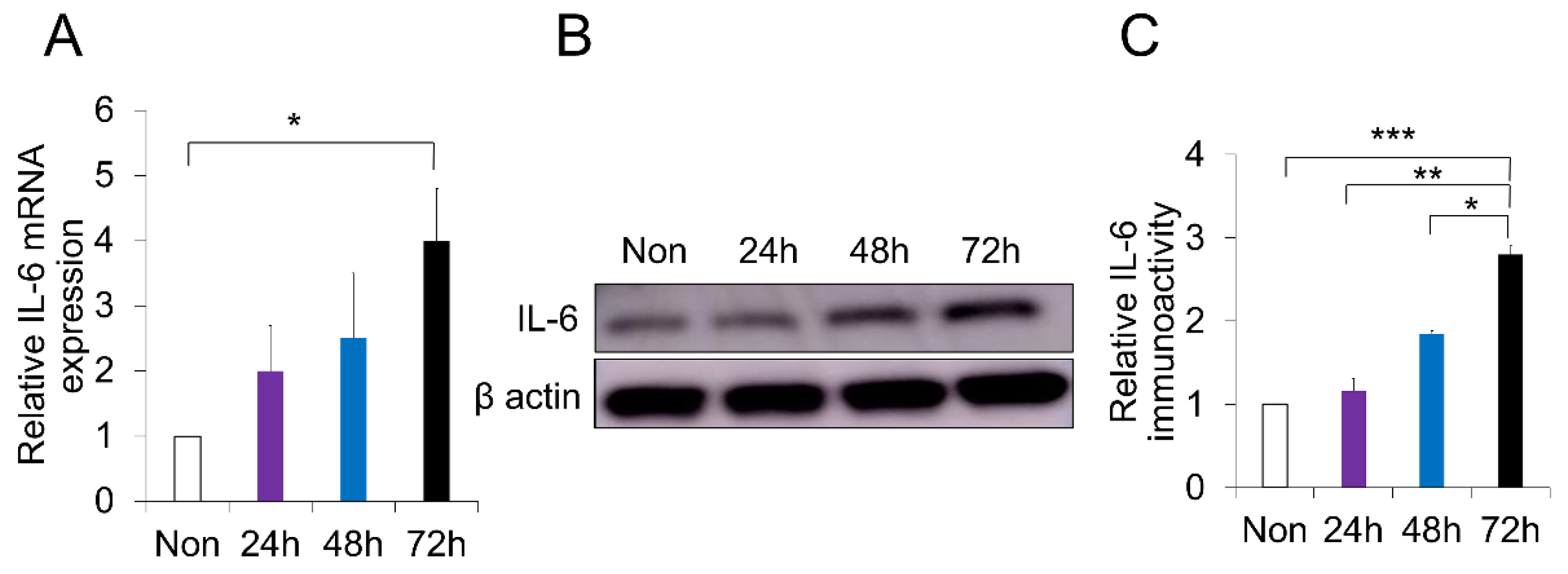
2.4. p-Cresol-Induced Inflammation in DPSCs
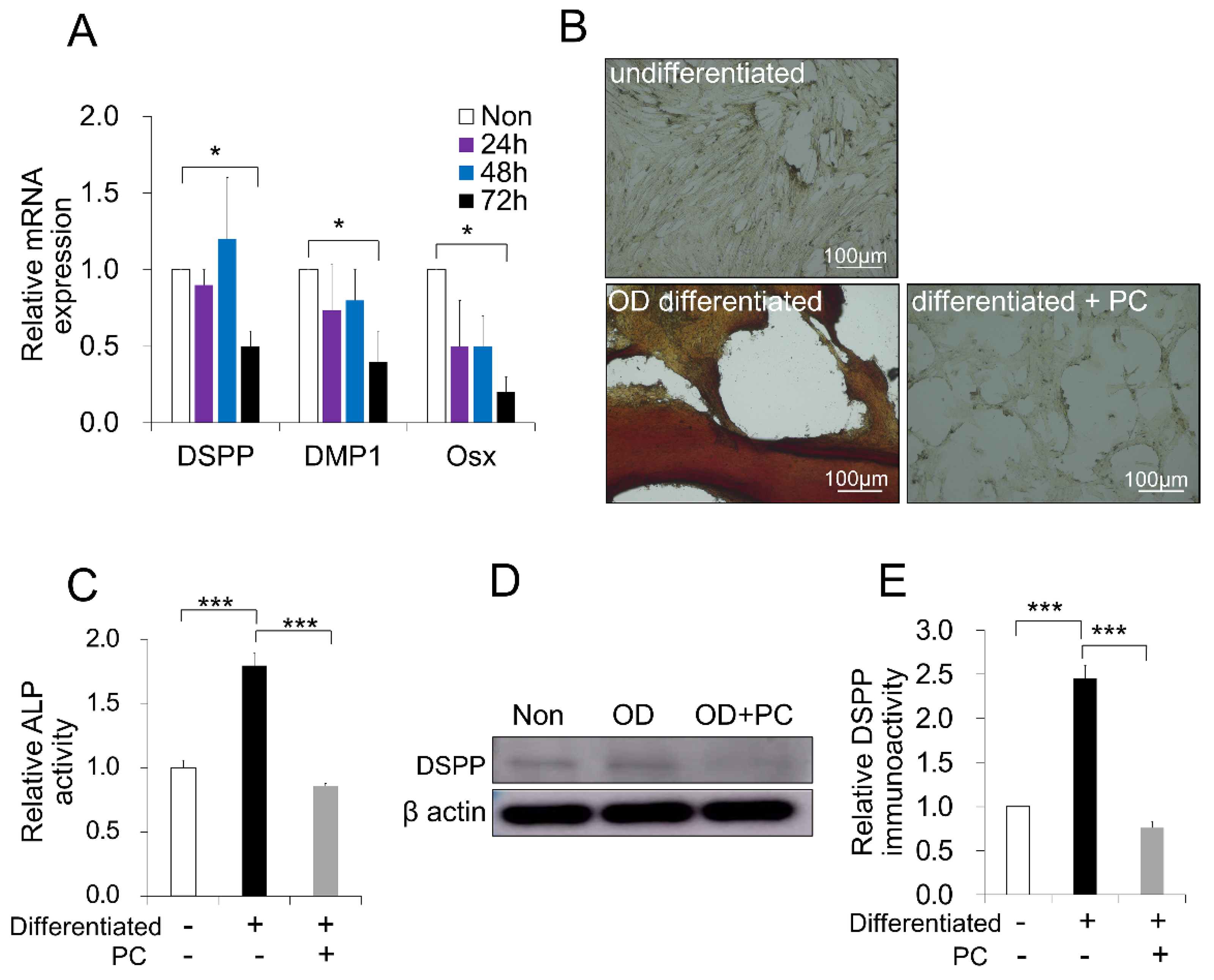
2.5. p-Cresol-Inhibited Odontoblast Differentiation in DPSCs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Determination of Proliferation and Cell Size
4.3. β-Galactosidase Staining
4.4. Quantitative Reverse Transcription Real-Time PCR
4.5. Western Blot Analysis
4.6. Odontoblast Differentiation and Alkaline Phosphatase Assay
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| ALP | Alkaline phosphatase |
| Bax | BCL2 associated X |
| Bcl-2 | B-cell lymphoma 2 |
| DMEM | Dulbecco’s modified eagle’s medium |
| DMP1 | Dentin matrix protein 1 |
| DPSCs | Dental pulp stem cells |
| DSPP | Dentin sialophosphoprotein |
| FBS | Fetal bovine serum |
| MSCs | Mesenchymal stem cells |
| Osx | Osterix |
| PC | p-Cresol |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SA-β-Gal | Senescence-associated beta-galactosidase |
References
- Gil-Montoya, J.A.; de Mello, A.L.F.; Barrios, R.; Gonzalez-Moles, M.A.; Bravo, M. Oral health in the elderly patient and its impact on general well-being: A nonsystematic review. Clin. Interv. Aging 2015, 10, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.W. Dental pulp stem cells: Advances to applications. Stem Cells Cloning 2020, 13, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; Brunetti, G.; Oranger, A.; Carbone, C.; Ballini, A.; Lo Muzio, L.; Colucci, S.; Mori, C.; Grassi, F.R.; Grano, M. Dental pulp stem cells: Osteogenic differentiation and gene expression. Ann. N. Y. Acad. Sci. 2011, 1237, 47–52. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Fanton, Y.; Struys, T.; Gervois, P.; Politis, C.; Martens, W.; Lambrichts, I. Angiogenic properties of human dental pulp stem cells. PLoS ONE 2013, 8, e71104. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.W.; Moon, H.J.; Hong, J.Y.; Hyun, J.K. Characterization of neurogenic potential of dental pulp stem cells cultured in xeno/serum-free condition: In vitro and in vivo assessment. Stem Cells Int. 2016, 2016, 6921097. [Google Scholar] [CrossRef]
- Carnevale, G.; Pisciotta, A.; Riccio, M.; Bertoni, L.; De Biasi, S.; Gibellini, L.; Zordani, A.; Cavallini, G.M.; La Sala, G.B.; Bruzzesi, G.; et al. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e774–e785. [Google Scholar] [CrossRef]
- Yao, L.; Flynn, N. Dental pulp stem cell-derived chondrogenic cells demonstrate differential cell motility in type I and type II collagen hydrogels. Spine J. 2018, 18, 1070–1080. [Google Scholar] [CrossRef]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; De Biasi, S.; Gibellini, L.; La Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell. Res. Ther. 2015, 6, 156. [Google Scholar] [CrossRef]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Robey, P.G.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp stem cell-mediated functional pulp regeneration. J. Dent. Res. 2019, 98, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Iohara, K. Immunomodulation and regeneration properties of dental pulp stem cells: A potential therapy to treat coronavirus disease 2019. Cell Transplant. 2020, 29, 0963689720952089. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Murakami, M. Dental pulp stem cells and regeneration. Endod. Topics 2013, 28, 38–50. [Google Scholar] [CrossRef]
- Ratajczak, J.; Bronckaers, A.; Dillen, Y.; Gervois, P.; Vangansewinkel, T.; Driesen, R.B.; Wolfs, E.; Lambrichts, I.; Hilkens, P. The neurovascular properties of dental stem cells and their importance in dental tissue engineering. Stem Cells Int. 2016, 2016, 9762871. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, I.; Pagella, P.; Mattioli-Belmonte, M.; Mitsiadis, T.A. The effects of ageing on dental pulp stem cells, the tooth longevity elixir. Eur. Cell Mater. 2019, 37, 175–185. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Chung, H.Y.; Lee, E.K.; Choi, Y.J.; Kim, J.M.; Kim, D.H.; Zou, Y.; Kim, C.H.; Lee, J.; Kim, H.S.; Kim, N.D.; et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J. Dent. Res. 2011, 90, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, G.E.; Cho, H.J.; Yu, M.K.; Bhattarai, G.; Lee, N.H.; Yi, H.K. Aging of in vitro pulp illustrates change of inflammation and dentinogenesis. J. Endod. 2013, 39, 340–345. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Takeuchi, N.; Osako, Y.; Ito, M.; Ishizaka, R.; Utunomiya, S.; Nakamura, H.; Matsushita, K.; Nakashima, M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem cells Transl. Med. 2013, 2, 521–533. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014, 52, 39–45. [Google Scholar] [CrossRef]
- Alessio, N.; Del Gaudio, S.; Capasso, S.; Di Bernardo, G.; Cappabianca, S.; Cipollaro, M.; Peluso, G.; Galderisi, U. Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget 2015, 6, 8155–8166. [Google Scholar] [CrossRef]
- Neri, S.; Borzì, R.M. Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules 2020, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wei, R.; Liu, J.; Wang, H.; Cai, W.; Zhao, M.; Hu, Y.; Wang, S.; Yang, T.; Liu, X.; et al. Drug-induced premature senescence model in human dental follicle stem cells. Oncotarget 2017, 8, 7276–7293. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.Y.; Park, S.; Jang, H.O.; Takata, T.; Bae, M.K.; Kim, Y.D.; Ryu, M.H.; Bae, S.K. Visfatin induces senescence of human dental pulp cells. Cells 2020, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G.; De Smet, R.; Lameire, N. New insights in uremic toxins. Kidney Int. Suppl. 2003. [Google Scholar] [CrossRef]
- Chang, M.C.; Chang, H.H.; Chan, C.P.; Yeung, S.Y.; Hsien, H.C.; Lin, B.R.; Yeh, C.Y.; Tseng, W.Y.; Tseng, S.K.; Jeng, J.H. P-cresol affects reactive oxygen species generation, cell cycle arrest, cytotoxicity and inflammation/atherosclerosis-related modulators production in endothelial cells and mononuclear cells. PLoS ONE 2014, 9, e114446. [Google Scholar] [CrossRef]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Idziak, M.; Pędzisz, P.; Burdzińska, A.; Gala, K.; Pączek, L. Uremic toxins impair human bone marrow-derived mesenchymal stem cells functionality in vitro. Exp. Toxicol. Pathol. 2014, 66, 187–194. [Google Scholar] [CrossRef]
- Lee, J.H.; Yun, C.W.; Hur, J.; Lee, S.H. Fucoidan rescues p-cresol-induced cellular senescence in mesenchymal stem cells via FAK-Akt-TWIST Axis. Mar. Drugs 2018, 16, 121. [Google Scholar] [CrossRef]
- Zayed, M.; Iohara, K.; Watanabe, H.; Nakashima, M. CCR3 antagonist protects against induced cellular senescence and promotes rejuvenation in periodontal ligament cells for stimulating pulp regeneration in the aged dog. Sci. Rep. 2020, 10, 8631. [Google Scholar] [CrossRef]
- Tanaka, S.; Yano, S.; Sheikh, A.M.; Nagai, A.; Sugimoto, T. Effects of uremic toxin p-cresol on proliferation, apoptosis, differentiation, and glucose uptake in 3T3-L1 cells. Artif. Organs 2014, 38, 566–571. [Google Scholar] [CrossRef]
- Dodig, S.; Čepelak, I.; Pavić, I. Hallmarks of senescence and aging. Biochem. Med. 2019, 29, 030501. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.; Chun, C.; Strojny, C.; Narayanan, R.; Bartholomew, A.; Sundivakkam, P.; Alapati, S. Chronic inflammation and angiogenic signaling axis impairs differentiation of dental-pulp stem cells. PLoS ONE 2014, 9, e113419. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; Jeanneau, C.; Dejou, J.; About, I. Sources of dentin-pulp regeneration signals and their modulation by the local microenvironment. J. Endod. 2014, 40, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Giebultowicz, J.; Wroczynski, P.; Paczek, L. Age-dependent increase in serum levels of indoxyl sulphate and p-cresol sulphate is not related to their precursors: Tryptophan and tyrosine. Geriatr. Gerontol. Int. 2017, 17, 1022–1026. [Google Scholar] [CrossRef]
- Sankowski, B.; Księżarczyk, K.; Raćkowska, E.; Szlufik, S.; Koziorowski, D.; Giebułtowicz, J. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with parkinson’s disease. Clin. Chim. Acta 2020, 501, 165–173. [Google Scholar] [CrossRef]
- Morgunova, G.V.; Kolesnikov, A.V.; Klebanov, A.A.; Khokhlov, A.N. Senescence-associated β-galactosidase—a biomarker of aging, DNA damage, or cell proliferation restriction? Moscow Univ. Biol. Sci. Bull. 2015, 70, 165–167. [Google Scholar] [CrossRef]
- Kubben, N.; Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat. Rev. Mol. Cell Biol. 2017, 18, 595–609. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Small molecule compounds that induce cellular senescence. Aging Cell 2016, 15, 999–1017. [Google Scholar] [CrossRef]
- Schepers, E.; Meert, N.; Glorieux, G.; Goeman, J.; Van der Eycken, J.; Vanholder, R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transplant. 2007, 22, 592–596. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-z.; Zhang, J.; Yang, K.; Du, R.; Jing, Y.-j.; Lu, L.; Zhang, R.-y. P-cresol, but not p-cresylsulphate, disrupts endothelial progenitor cell function in vitro. Nephrol. Dial. Transplant. 2012, 27, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Aprile, D.; Squillaro, T.; Di Bernardo, G.; Finicelli, M.; Melone, M.A.; Peluso, G.; Galderisi, U. The senescence-associated secretory phenotype (SASP) from mesenchymal stromal cells impairs growth of immortalized prostate cells but has no effect on metastatic prostatic cancer cells. Aging (Albany N. Y.) 2019, 11, 5817–5828. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Deryabin, P.I.; Giukova, A.A.; Nikolsky, N.N. “Social life” of senescent cells: What is SASP and why study it? Acta Naturae 2018, 10, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N. Deciphering the mechanism for induction of senescence-associated secretory phenotype (SASP) and its role in aging and cancer development. J. Biochem. 2019. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in mesenchymal stem cells: Functional alterations, molecular mechanisms, and rejuvenation strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Liu, O.; Yan, F.; Lin, X.; Diao, S.; Wang, L.; Jin, L.; Wang, S.; Lu, Y.; Fan, Z. Analysis of senescence-related differentiation potentials and gene expression profiles in human dental pulp stem cells. Cells Tissues Organs 2017, 203, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rufini, A.; Tucci, P.; Celardo, I.; Melino, G. Senescence and aging: The critical roles of p53. Oncogene 2013, 32, 5129–5143. [Google Scholar] [CrossRef]
- Wang, X.; Kua, H.Y.; Hu, Y.; Guo, K.; Zeng, Q.; Wu, Q.; Ng, H.H.; Karsenty, G.; de Crombrugghe, B.; Yeh, J.; et al. P53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 2006, 172, 115–125. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, G.; Fu, J.; Chen, Z.; Yuan, G. Mdm2 promotes odontoblast-like differentiation by ubiquitinating Dlx3 and p53. J. Dent. Res. 2020, 99, 320–328. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Hansen, G.; Murray, D. Role of p16(INK4A) in replicative senescence and DNA damage-induced premature senescence in p53-deficient human cells. Biochem. Res. Int. 2012, 2012, 951574. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal stem cell senescence and rejuvenation: Current status and challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J. Endod. 2014, 40, S46–S51. [Google Scholar] [CrossRef]
- Yoon, Y.M.; Han, Y.S.; Yun, C.W.; Lee, J.H.; Kim, R.; Lee, S.H. Pioglitazone protects mesenchymal stem cells against p-cresol-induced mitochondrial dysfunction via up-regulation of PINK-1. Int. J. Mol. Sci. 2018, 19, 2898. [Google Scholar] [CrossRef]
- Kim, S.G.; Zheng, Y.; Zhou, J.; Chen, M.; Embree, M.C.; Song, K.; Jiang, N.; Mao, J.J. Dentin and dental pulp regeneration by the patient’s endogenous cells. Endod. Topics 2013, 28, 106–117. [Google Scholar] [CrossRef]
- Iohara, K.; Zayed, M.; Takei, Y.; Watanabe, H.; Nakashima, M. Treatment of pulpectomized teeth with trypsin prior to transplantation of mobilized dental pulp stem cells enhances pulp regeneration in aged dogs. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Hao, J.; Ramachandran, A.; George, A. Temporal and spatial localization of the dentin matrix proteins during dentin biomineralization. J. Histochem. Cytochem. 2009, 57, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Narayanan, K.; Ramachandran, A.; He, G.; Almushayt, A.; Evans, C.; George, A. Odontoblast cells immortalized by telomerase produce mineralized dentin-like tissue both in vitro and in vivo. J. Biol. Chem. 2002, 277, 19976–19981. [Google Scholar] [CrossRef]
- Iezzi, I.; Cerqueni, G.; Licini, C.; Lucarini, G.; Mattioli Belmonte, M. Dental pulp stem cells senescence and regenerative potential relationship. J. Cell. Physiol. 2019, 234, 7186–7197. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Kim, S.M.; Lee, J.H.; Lee, S.H. Co-administration of melatonin effectively enhances the therapeutic effects of pioglitazone on mesenchymal stem cells undergoing indoxyl sulfate-induced senescence through modulation of cellular prion protein expression. Int. J. Mol. Sci. 2018, 19, 1367. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Primer Sequence | Size | |
|---|---|---|---|
| p16 | Forward | CGGAAGGTCACGCAGACAGC | 124 bp |
| Reverse | GCAGGGAAGAGTTGGGTTGGGT | ||
| p21 | Forward | ACCTCTCAGGGCCGAAAAC | 89 bp |
| Reverse | TAGGGCTTCCTCTTGGAGAA | ||
| IL-1β | Forward | CAAGAGTCTGAGGCATTTC | 214 bp |
| Reverse | GGTATTTGTGGCTTATGTCC | ||
| IL-8 | Forward | ACACTCCACACCTTCCAT | 143 bp |
| Reverse | CTTTTGTACCCATTTTTCC | ||
| p53 | Forward | CGCAAAAGAAGAAGCCACTA | 118 bp |
| Reverse | TCCACTCTGGGCATCCTT | ||
| IL-6 | Forward | TCCAGAACAACTATGAGGGTGA | 100 bp |
| Reverse | TCCTGATTCTTTACCTTGCTCTT | ||
| DSPP | Forward | GTCCTAGTGGGAATGGAGCA | 190 bp |
| Reverse | TCTTCAGGGCCATCATCTTC | ||
| DMP1 | Forward | GATAGTGCCCAAGATACCAC | 120 bp |
| Reverse | TCCTACCCAGTGTTCCTTAC | ||
| Osx | Forward | ACCAATGGGCTCCTCTCAC | 162 bp |
| Reverse | CACTGGGCAGGCAGTCAGGA | ||
| β-actin | Forward | AAGTACCCCATTGAGCACGG | 257 bp |
| Reverse | ATCACGATGCCAGTGGTGCG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.; Iohara, K. Effects of p-Cresol on Senescence, Survival, Inflammation, and Odontoblast Differentiation in Canine Dental Pulp Stem Cells. Int. J. Mol. Sci. 2020, 21, 6931. https://doi.org/10.3390/ijms21186931
Zayed M, Iohara K. Effects of p-Cresol on Senescence, Survival, Inflammation, and Odontoblast Differentiation in Canine Dental Pulp Stem Cells. International Journal of Molecular Sciences. 2020; 21(18):6931. https://doi.org/10.3390/ijms21186931
Chicago/Turabian StyleZayed, Mohammed, and Koichiro Iohara. 2020. "Effects of p-Cresol on Senescence, Survival, Inflammation, and Odontoblast Differentiation in Canine Dental Pulp Stem Cells" International Journal of Molecular Sciences 21, no. 18: 6931. https://doi.org/10.3390/ijms21186931
APA StyleZayed, M., & Iohara, K. (2020). Effects of p-Cresol on Senescence, Survival, Inflammation, and Odontoblast Differentiation in Canine Dental Pulp Stem Cells. International Journal of Molecular Sciences, 21(18), 6931. https://doi.org/10.3390/ijms21186931





