The Effect of Low Temperatures on Environmental Radiation Damage in Living Systems: Does Hypothermia Show Promise for Space Travel?
Abstract
:1. Introduction
2. Environmental Radiation Damage in Living Systems
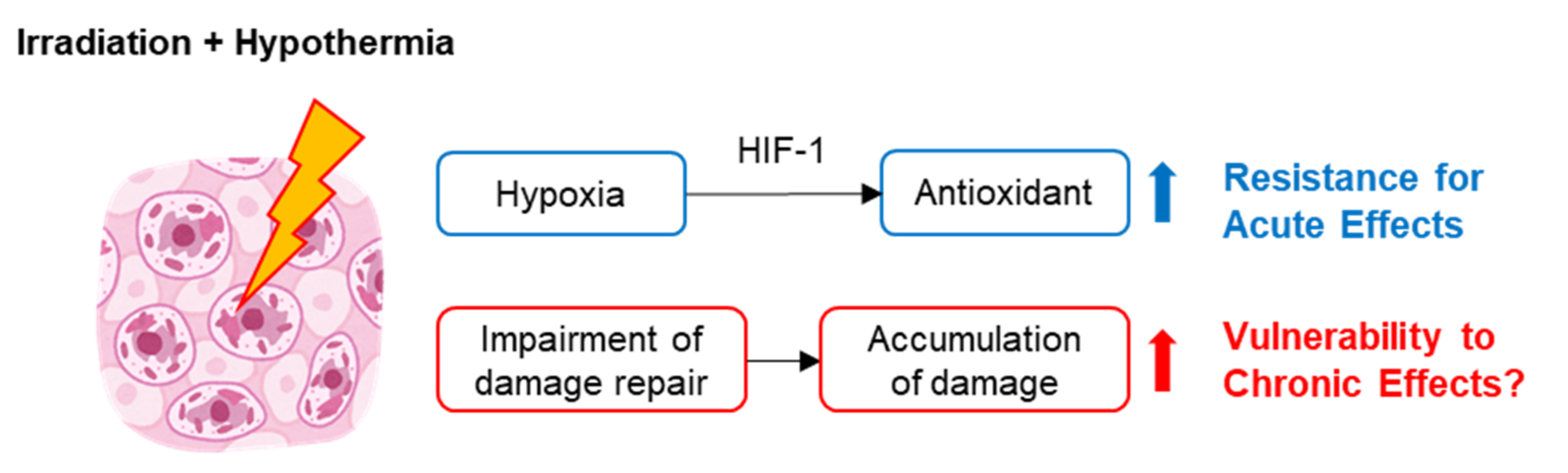
3. Mechanisms of the Effect of Hypothermia-Induced Radioprotection
4. Impairment of Radiation Damage Repair under Hypothermic Conditions
5. Possible Issues Related to Human Space Exploration and the Induction of Hibernation
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| IR | Ionizing radiation |
| IARC | International Agency for Research on Cancer |
| UV | Ultraviolet radiation |
| ICRP | International Commission on Radiological Protection |
| SI | International System of Units |
| Gy | Gray |
| Sv | Sievert |
| LET | Linear energy transfer |
| RIBE | Radiation induced bystander effect |
| SCE | Sister chromatid exchanges |
| TSE | Tissue-sparing effect |
| MRT | Microbeam radiotherapy |
| ROS | Reactive oxygen species |
| HIF | Hypoxia inducible factor |
| CIRP | Cold-inducible RNA binding protein |
| UNSCEAR | United Nations Scientific Committee on the Effects of Atomic Radiation |
| LEO | Low-Earth orbit |
| HZE | High atomic number and energy |
| GCR | Galactic cosmic ray |
| NASA | National Aeronautics and Space Administration |
| MPS | Million Person Study of Low-Dose Health Effects |
References
- Little, J.B. Radiation carcinogenesis. Carcinogenesis 2000, 21, 397–404. [Google Scholar]
- Shah, D.J.; Sachs, R.K.; Wilson, D.J. Radiation-induced cancer: A modern view. Br. J. Radiol. 2012, 85, e1166–e1173. [Google Scholar]
- Muller, H.J. Artificial Transumutation of the Gene. Science 1927, 66, 84–87. [Google Scholar]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar]
- Jacobi, W. The concept of the effective dose—A proposal for the combination of organ doses. Radiat. Environ. Biophys. 1975, 12, 101–109. [Google Scholar]
- International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- Barnett, G.C.; West, C.M.L.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.P.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar]
- Mettler, F.A.; Huda, W.; Yoshizumi, T.T.; Mahesh, M. Effective Doses in Radiology and Diagnostic Nuclear Medicine: A Catalog. Radiology 2008, 248, 254–263. [Google Scholar]
- Sazykina, T.G.; Kryshev, A.I. Manifestation of radiation effects in cold environment: Data review and modeling. Radiat. Environ. Biophys. 2011, 50, 105–114. [Google Scholar]
- Gros, C.M.; Keiling, R.; Bloch, J. Radiation protection effect of cold in fish. Strahlentherapie 1959, 109, 241–245. [Google Scholar]
- Patt, H.M.; Swift, M.N. Influence of temperature on the response of frogs to X irradiation. Am. J. Physiol. 1948, 155, 388–393. [Google Scholar]
- Smith, F.; Grenan, M.M. Effect of hibernation upon survival time following whole-body irradiation in the marmot (Marmota monax). Science 1951, 113, 686–688. [Google Scholar]
- Storer, J.B.; Hempelmann, L.H. Hypothermia and increased survival rate of infant mice irradiated with X-rays. Am. J. Physiol. 1952, 171, 341–348. [Google Scholar]
- Hornsey, S. Protection from whole-body x-irradiation afforded to adult mice by reducing the body temperature. Nature 1956, 178, 87. [Google Scholar]
- Hornsey, S. Discussion -The effect of hypothermia on the radiosensitivity of mice to whole-body X-irradiation. Proc. R. Soc. London Ser. B Biol. Sci. 1957, 147, 547–549. [Google Scholar]
- Hajduković, S.; Hervé, A.; Vidović, V. Diminution de radiosensibilité du rat adulte en hypothermie profonde. Experientia 1954, 10, 343–344. [Google Scholar]
- Musacchia, X.J.; Barr, R.E. Survival of whole-body-irradiated hibernating and active ground squirrels; Citellus tridecemlineatus. Radiat. Res. 1968, 33, 348–356. [Google Scholar]
- Barr, R.E.; Musacchia, X.J. The Effect of Body Temperature and Postirradiation Cold Exposure on the Radiation Response of the Hibernator Citellus tridecemlineatus. Radiat. Res. 1969, 38, 448. [Google Scholar]
- Weiss, L. Alteration of radio-sensitivity of the testis by extreme hypothermia. J. Endocrinol. 1959, 19, 22–28. [Google Scholar]
- Weiss, L. The alteration in radiosensitivity of the intact mouse spleen by extreme hypothermia. Br. J. Radiol. 1960, 33, 32–35. [Google Scholar]
- Hornsey, S. Fertility and lifespan of mice protected by hypothermia against total-body irradiation. Gerontology 1959, 3, 128–136. [Google Scholar]
- Weiss, L. Alterations in radiosensitivity of the haemopoietic system of the mouse produced by extreme hypothermia. Int. J. Radiat. Biol. 1960, 2, 409–423. [Google Scholar]
- Bloch, M.; Bloom, H.J.; Penman, J.; Walsh, L. Irradiation of cerebral astrocytomata under whole-body hypothermia. Lancet 1961, 2, 906–909. [Google Scholar]
- Bloch, M.; Bloom, H.J.; Penman, J.; Walsh, L. Observations on patients with cerebral astrocytoma (Glioblastoma multiforme) treated by irradiation under whole-body hypothermia. Br. J. Cancer 1966, 20, 722–728. [Google Scholar]
- Weiss, L. Sensitivity of Hypothermic Mammals to X Irradiation. Br. Med. Bull. 1961, 17, 70–73. [Google Scholar]
- Hrycushko, B.A.; Bing, C.; Futch, C.; Wodzak, M.; Stojadinovic, S.; Medin, P.M.; Chopra, R. Technical Note: System for evaluating local hypothermia as a radioprotector of the rectum in a small animal model. Med. Phys. 2017, 44, 3932–3938. [Google Scholar]
- Hrycushko, B.A.; Chopra, R.; Sayre, J.W.; Richardson, J.A.; Folkert, M.R.; Timmerman, R.D.; Medin, P.M. Local Hypothermia as a Radioprotector of the Rectal Wall During Prostate Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 75–82. [Google Scholar]
- Averbeck, D.; Salomaa, S.; Bouffler, S.; Ottolenghi, A.; Smyth, V.; Sabatier, L. Progress in low dose health risk research. Mutat. Res. Mutat. Res. 2018, 776, 46–69. [Google Scholar]
- Goodhead, D.T. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int. J. Radiat. Biol. 1994, 65, 7–17. [Google Scholar]
- Booz, J.; Feinendegen, L.E. A microdosimetric understanding of low-dose radiation effects. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1988, 53, 13–21. [Google Scholar]
- International Commission on Radiation Units and Measurements. Microdosimetry. ICRU Report 36; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1983. [Google Scholar]
- Prise, K.M.; Folkard, M.; Michael, B.D. Bystander responses induced by low LET radiation. Oncogene 2003, 22, 7043–7049. [Google Scholar]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar]
- Lorimore, S.A.; Kadhim, M.A.; Pocock, D.A.; Papworth, D.; Stevens, D.L.; Goodhead, D.T.; Wright, E.G. Chromosomal instability in the descendants of unirradiated surviving cells after alpha-particle irradiation. Proc. Natl. Acad. Sci. USA 1998, 95, 5730–5733. [Google Scholar]
- Belyakov, O.V.; Prise, K.M.; Trott, K.R.; Michael, B.D. Delayed lethality, apoptosis and micronucleus formation in human fibroblasts irradiated with X-rays or alpha-particles. Int. J. Radiat. Biol. 1999, 75, 985–993. [Google Scholar]
- Nagasawa, H.; Little, J.B. Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: Evidence for a bystander effect. Radiat. Res. 1999, 152, 552–557. [Google Scholar]
- Belyakov, O.V.; Folkard, M.; Mothersill, C.; Prise, K.M.; Michael, B.D. Bystander-induced apoptosis and premature differentiation in primary urothelial explants after charged particle microbeam irradiation. Radiat. Prot. Dosim. 2002, 99, 249–251. [Google Scholar]
- Iyer, R.; Lehnert, B.E. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat. Res. 2002, 503, 1–9. [Google Scholar]
- Goldberg, Z.; Lehnert, B.E. Radiation-induced effects in unirradiated cells: A review and implications in cancer. Int. J. Oncol. 2002, 21, 337–349. [Google Scholar]
- Schültke, E.; Balosso, J.; Breslin, T.; Cavaletti, G.; Djonov, V.; Esteve, F.; Grotzer, M.; Hildebrandt, G.; Valdman, A.; Laissue, J. Microbeam radiation therapy—grid therapy and beyond: A clinical perspective. Br. J. Radiol. 2017, 90, 20170073. [Google Scholar]
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.A.; Gebbers, J.O.; Laissue, J.A. Subacute neuropathological effects of microplanar beams of x-rays from a synchrotron wiggler. Proc. Natl. Acad. Sci. USA 1995, 92, 8783–8787. [Google Scholar]
- Laissue, J.A.; Geiser, G.; Spanne, P.O.; Dilmanian, F.A.; Gebbers, J.O.; Geiser, M.; Wu, X.Y.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; et al. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays. Int. J. Cancer 1998, 78, 654–660. [Google Scholar]
- Dilmanian, F.A.; Zhong, Z.; Bacarian, T.; Benveniste, H.; Romanelli, P.; Wang, R.; Welwart, J.; Yuasa, T.; Rosen, E.M.; Anschel, D.J. Interlaced x-ray microplanar beams: A radiosurgery approach with clinical potential. Proc. Natl. Acad. Sci. USA 2006, 103, 9709–9714. [Google Scholar]
- van der Sanden, B.; Bräuer-Krisch, E.; Siegbahn, E.A.; Ricard, C.; Vial, J.-C.; Laissue, J. Tolerance of arteries to microplanar X-ray beams. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1545–1552. [Google Scholar]
- Bouchet, A.; Lemasson, B.; Christen, T.; Potez, M.; Rome, C.; Coquery, N.; Le Clec’h, C.; Moisan, A.; Bräuer-Krisch, E.; Leduc, G.; et al. Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain. Radiother. Oncol. 2013, 108, 143–148. [Google Scholar]
- Grotzer, M.A.; Schültke, E.; Bräuer-Krisch, E.; Laissue, J.A. Microbeam radiation therapy: Clinical perspectives. Phys. Med. 2015, 31, 564–567. [Google Scholar]
- Fukunaga, H.; Kaminaga, K.; Sato, T.; Butterworth, K.T.; Watanabe, R.; Usami, N.; Ogawa, T.; Yokoya, A.; Prise, K.M. High-precision microbeam radiotherapy reveals testicular tissue-sparing effects for male fertility preservation. Sci. Rep. 2019, 9, 12618. [Google Scholar]
- Dilmanian, F.A.; Qu, Y.; Feinendegen, L.E.; Peña, L.A.; Bacarian, T.; Henn, F.A.; Kalef-Ezra, J.; Liu, S.; Zhong, Z.; McDonald, J.W. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: Is a bystander effect involved? Exp. Hematol. 2007, 35, 69–77. [Google Scholar]
- Fukunaga, H.; Prise, K.M. Non-uniform radiation-induced biological responses at the tissue level involved in the health risk of environmental radiation: A radiobiological hypothesis. Environ. Health 2018, 17, 93. [Google Scholar]
- Di Gregorio, A.; Bowling, S.; Rodriguez, T.A. Cell Competition and Its Role in the Regulation of Cell Fitness from Development to Cancer. Dev. Cell 2016, 38, 621–634. [Google Scholar]
- Maruyama, T.; Fujita, Y. Cell competition in mammals—Novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol. 2017, 48, 106–112. [Google Scholar]
- Biteau, B.; Hochmuth, C.E.; Jasper, H. Maintaining Tissue Homeostasis: Dynamic Control of Somatic Stem Cell Activity. Cell Stem Cell 2011, 9, 402–411. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar]
- Turk, E.E. Hypothermia. Forensic Sci. Med. Pathol. 2010, 6, 106–115. [Google Scholar]
- Wood, S.C. Interactions Between Hypoxia and Hypothermia. Annu. Rev. Physiol. 1991, 53, 71–85. [Google Scholar]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar]
- Conger, A.D. The effect of oxygen on the radiosensitivity of mammalian cells. Radiology 1956, 66, 63–69. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; ISBN 0781741513. [Google Scholar]
- Morse, M.L.; Dahl, R.H. Cellular glutathione is a key to the oxygen effect in radiation damage. Nature 1978, 271, 660–662. [Google Scholar]
- Weiss, L. Some effects of hypothermia and hypoxia on the sensitivity of hela cells to x-rays. Int. J. Radiat. Biol. 1960, 2, 20–27. [Google Scholar]
- Weiss, L. Decrease in radiosensitivity of the Intact Mouse Spleen produced by Hypoxia. Nature 1959, 184, 1156–1157. [Google Scholar]
- Harada, H. How Can We Overcome Tumor Hypoxia in Radiation Therapy? J. Radiat. Res. 2011, 52, 545–556. [Google Scholar]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar]
- Fukuda, R.; Zhang, H.; Kim, J.W.; Shimoda, L.; Dang, C.V.; Semenza, G.L.L. HIF-1 Regulates Cytochrome Oxidase Subunits to Optimize Efficiency of Respiration in Hypoxic Cells. Cell 2007, 129, 111–122. [Google Scholar]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible Factor-1a by reactive oxygen species: New developments in an old debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar]
- Pereira, T.; Zheng, X.; Poellinger, L. Degradation of the hypoxia-inducible factor 1α: Where does it happen? Cell Cycle 2006, 5, 2720–2722. [Google Scholar]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic radioresistance: Can ROS be the key to overcome it? Cancers 2019, 11, 112. [Google Scholar]
- Butterworth, K.T.; McGarry, C.K.; Trainor, C.; O’Sullivan, J.M.; Hounsell, A.R.; Prise, K.M. Out-of-Field Cell Survival Following Exposure to Intensity-Modulated Radiation Fields. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1516–1522. [Google Scholar]
- Thompson, H.F.; Butterworth, K.T.; McMahon, S.J.; Ghita, M.; Hounsell, A.R.; Prise, K.M. The Impact of Hypoxia on Out-of-Field Cell Survival after Exposure to Modulated Radiation Fields. Radiat. Res. 2017, 188, 636–644. [Google Scholar]
- Kempner, E.S.; Haigler, H.T. The influence of low temperature on the radiation sensitivity of enzymes. J. Biol. Chem. 1982, 257, 13297–13299. [Google Scholar]
- Liao, Y.; Tong, L.; Tang, L.; Wu, S. The role of cold-inducible RNA binding protein in cell stress response. Int. J. Cancer 2017, 141, 2164–2173. [Google Scholar]
- Egami, N.; Etoh, H. Effect of Temperature on the Rate of Recovery from Radiation-Induced Damage in the Fish Oryzias latipes. Radiat. Res. 1966, 27, 637. [Google Scholar]
- Egami, N. Kinetics of recovery from injury after whole-body x-irradiation of the fish Oryzias latipes at different temperatures. Radiat. Res. 1969, 37, 192–201. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Ionizing Radiation: Sources and Biological Effects: 1982 Report to the General Assembly, with Annexes; United Nations: New York, NY, USA, 1982. [Google Scholar]
- Baird, B.J.; Dickey, J.S.; Nakamura, A.J.; Redon, C.E.; Parekh, P.; Griko, Y.V.; Aziz, K.; Georgakilas, A.G.; Bonner, W.M.; Martin, O.A. Hypothermia postpones DNA damage repair in irradiated cells and protects against cell killing. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 711, 142–149. [Google Scholar]
- Dang, L.; Lisowska, H.; Manesh, S.S.; Sollazzo, A.; Deperas-Kaminska, M.; Staaf, E.; Haghdoost, S.; Brehwens, K.; Wojcik, A. Radioprotective effect of hypothermia on cells a multiparametric approach to delineate the mechanisms. Int. J. Radiat. Biol. 2012, 88, 507–514. [Google Scholar]
- Lisowska, H.; Wegierek-Ciuk, A.; Banasik-Nowak, A.; Braziewicz, J.; Wojewodzka, M.; Wojcik, A.; Lankoff, A. The dose-response relationship for dicentric chromosomes and γ-H2AX foci in human peripheral blood lymphocytes: Influence of temperature during exposure and intra- and inter-individual variability of donors. Int. J. Radiat. Biol. 2013, 89, 191–199. [Google Scholar]
- Lisowska, H.; Brehwens, K.; Zölzer, F.; Wegierek-Ciuk, A.; Czub, J.; Lankoff, A.; Haghdoost, S.; Wojcik, A. Effect of hypothermia on radiation-induced micronuclei and delay of cell cycle progression in TK6 cells. Int. J. Radiat. Biol. 2014, 90, 318–324. [Google Scholar]
- Cheng, L.; Lisowska, H.; Sollazzo, A.; Wegierek-Ciuk, A.; Stepień, K.; Kuszewski, T.; Lankoff, A.; Haghdoost, S.; Wojcik, A. Modulation of radiation-induced cytogenetic damage in human peripheral blood lymphocytes by hypothermia. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 793, 96–100. [Google Scholar]
- Lisowska, H.; Cheng, L.; Sollazzo, A.; Lundholm, L.; Wegierek-Ciuk, A.; Sommer, S.; Lankoff, A.; Wojcik, A. Hypothermia modulates the DNA damage response to ionizing radiation in human peripheral blood lymphocytes. Int. J. Radiat. Biol. 2018, 94, 551–557. [Google Scholar]
- Chancellor, J.C.; Blue, R.S.; Cengel, K.A.; Auñón-Chancellor, S.M.; Rubins, K.H.; Katzgraber, H.G.; Kennedy, A.R. Limitations in predicting the space radiation health risk for exploration astronauts. npj Microgravity 2018, 4, 8. [Google Scholar]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar]
- Fukunaga, H.; Yokoya, A.; Taki, Y.; Butterworth, K.T.; Prise, K.M. Precision Radiotherapy and Radiation Risk Assessment: How Do We Overcome Radiogenomic Diversity? Tohoku J. Exp. Med. 2019, 247, 223–235. [Google Scholar]
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space Radiation Biology for “Living in Space”. Biomed Res. Int. 2020, 2020, 4703286. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Effects of Ionizing Radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2006; United Nations: New York, NY, USA, 2008. [Google Scholar]
- Boice, J.D. The Million Person Study relevance to space exploration and mars. Int. J. Radiat. Biol. 2019, 1–9. [Google Scholar]
- Cucinotta, F.A. Review of NASA approach to space radiation risk assessments for mars exploration. Health Phys. 2015, 108, 131–142. [Google Scholar]
- Boice, J.D.; Cohen, S.S.; Mumma, M.T.; Ellis, E.D. The Million Person Study, whence it came and why. Int. J. Radiat. Biol. 2019, 1–14. [Google Scholar]
- Tinganelli, W.; Hitrec, T.; Romani, F.; Simoniello, P.; Squarcio, F.; Stanzani, A.; Piscitiello, E.; Marchesano, V.; Luppi, M.; Sioli, M.; et al. Hibernation and radioprotection: Gene expression in the liver and testicle of rats irradiated under synthetic torpor. Int. J. Mol. Sci. 2019, 20, 352. [Google Scholar]
- Kumar, D.; Salian, S.R.; Kalthur, G.; Uppangala, S.; Kumari, S.; Challapalli, S.; Chandraguthi, S.G.; Krishnamurthy, H.; Jain, N.; Kumar, P.; et al. Semen abnormalities, sperm DNA damage and global hypermethylation in health workers occupationally exposed to ionizing radiation. PLoS ONE 2013, 8, e69927. [Google Scholar]
- Fukunaga, H.; Butterworth, K.T.; Yokoya, A.; Ogawa, T.; Prise, K.M. Low-dose radiation-induced risk in spermatogenesis. Int. J. Radiat. Biol. 2017, 93, 1291–1298. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukunaga, H. The Effect of Low Temperatures on Environmental Radiation Damage in Living Systems: Does Hypothermia Show Promise for Space Travel? Int. J. Mol. Sci. 2020, 21, 6349. https://doi.org/10.3390/ijms21176349
Fukunaga H. The Effect of Low Temperatures on Environmental Radiation Damage in Living Systems: Does Hypothermia Show Promise for Space Travel? International Journal of Molecular Sciences. 2020; 21(17):6349. https://doi.org/10.3390/ijms21176349
Chicago/Turabian StyleFukunaga, Hisanori. 2020. "The Effect of Low Temperatures on Environmental Radiation Damage in Living Systems: Does Hypothermia Show Promise for Space Travel?" International Journal of Molecular Sciences 21, no. 17: 6349. https://doi.org/10.3390/ijms21176349
APA StyleFukunaga, H. (2020). The Effect of Low Temperatures on Environmental Radiation Damage in Living Systems: Does Hypothermia Show Promise for Space Travel? International Journal of Molecular Sciences, 21(17), 6349. https://doi.org/10.3390/ijms21176349




