Aryl Hydrocarbon Receptor Signaling Is Functional in Immune Cells of Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Results
2.1. Fluorescent In Situ Hybridization (FISH) of AhR2
2.2. Cyp1a Gene Expression
2.3. ahR2α Gene Expression
2.4. ahr2β Gene Expression
3. Discussion
4. Material and Methods
4.1. Fish Maintenance and Benzo[a]Pyrene (BaP) Exposure
4.2. Sampling of Organs and Cells
4.3. In Vitro Exposure of Isolated Head Kidney Immune Cells
4.4. Fluorescence In Situ Hybridization (FISH)
4.5. RNA Extraction and Quantitative Real-Time RT-PCR
- CYP1AForward: 5′-TCCGGCACTCTTCCTTCCT-3′Reverse: 5′-GCCATTGAGGGATGTGTCCTT-3′Probe: 5′-CCGTTCATCATCCCACACTGCACG-3′
- AhR2αForward: 5′-TGTCAGATCCTCCCAATTTAAATG-3′Reverse: 5′-CTGAGGGAGACAAGAGATGAGTGA-3′Probe: 5′-CCCCTGACACTGAAGGCTCCCGT-3′
- AhR2βForward: 5′-GAATGTTATTTTGTTGGTGTTGTTGAAC-3′Reverse: 5′-GCATAGACTCCAGCGTTGTTACTC-3′Probe: 5′-TATAGCCGATTTACAGCAGAAGCGTCACCA-3′
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hahn, M.E. Aryl hydrocarbon receptors: Diversity and evolution. Chem. Biol. Interact. 2002, 141, 131–160. [Google Scholar] [CrossRef]
- Tian, J.; Feng, Y.; Fu, H.; Xie, H.Q.; Jiang, J.X.; Zhao, B. The aryl hydrocarbon receptor: A key bridging molecule of external and internal chemical signals. Environ. Sci. Technol. 2015, 49, 9518–9531. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 2017, 67, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.J.; Pereira de Castro, K.; Joshi, A.D.; Elferink, C.J. Canonical and non-canonical aryl hydrocarbon signaling pathways. Curr. Opin. Toxicol. 2017, 2, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Bradford, C.A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2007, 21, 102–116. [Google Scholar] [CrossRef]
- Gutierrez-Vazquez, C.; Quintana, F.J. Regulation of the immune responses by the aryl hydrocarbon receptor. Immunity 2017, 48, 19–33. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Safe, S. Molecular biology of the Ah receptor and its role in carcinogenesis. Toxicol. Lett. 2001, 120, 1–7. [Google Scholar] [CrossRef]
- Okey, A.B. An aryl hydrocarbon receptor odyssey to the shores of toxicology: The Deichmann Lecture, International Congress of Toxicology-XI. Toxicol. Sci. 2007, 98, 5–38. [Google Scholar] [CrossRef]
- Tillitt, D.E.; Cook, P.M.; Giesy, J.P.; Heideman, W.; Peterson, R.E. Reproductive impairment of Great Lakes lake trout by dioxin-like chemicals. In The Toxicology of Fishes; Di Giulio, R.T., Hinton, D.E., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 819–876. [Google Scholar]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunity and toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946. [Google Scholar] [PubMed]
- Bock, K.W. From TCCD-mediated toxicity to searches of physiological AHR functions. Biochem. Pharm. 2018, 155, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.C.; Wittzell, H.; Persson, K.; von Schantz, T. Unprecedented genomic diversity of AhR1 and AhR2 genes in Atlantic salmon (Salmo salar L.). Aquat. Toxicol. 2004, 68, 219–232. [Google Scholar] [CrossRef]
- Hahn, M.E. Unexpected diversity of aryl hydrocarbon receptors in non-mammalian vertebrates: Insights from comparative genomics. J. Exp. Zool. 2006, 305A, 693–706. [Google Scholar] [CrossRef]
- Hahn, M.E.; Woodin, B.R.; Stegeman, J.J.; Tillitt, D.E. Aryl hydrocarbon receptor function in early vertebrates: Inducibility of cytochrome P4501A in agnathan and elasmobranch fish. Comp. Biochem. Physiol. 1998, 120C, 65–75. [Google Scholar]
- Hahn, M.E.; Hestermann, E.V. Receptor mediated mechanisms of toxicity. In The Toxicology of Fishes; Di Giulio, R.T., Hinton, D.E., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 235–272. [Google Scholar]
- Prasch, A.L.; Teraoka, H.; Carney, S.A.; Dong, W.; Hiraga, J.J.; Stegeman, J.J.; Heideman, W.; Peterson, R.E. Aryl hydrocarbon receptor 2 mediates 23,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003, 76, 138–150. [Google Scholar] [CrossRef]
- Hahn, M.E.; Merson, R.R.; Karchner, S.I. Xenobiotic receptors in fish: Structural and functional diversity and evolutionary insights. In Biochemistry and Molecular Biology of Fishes, Volume 6: Environmental Toxicology; Mommsen, T.P., Moon, T.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 191–228. [Google Scholar]
- Hansson, M.C.; Hahn, M.E. Functional properties of the four Atlantic salmon (Salmo salar) aryl hydrocarbon receptor type 2 (AHR2) isoforms. Aquat. Toxicol. 2008, 86, 121–130. [Google Scholar] [CrossRef]
- Vos, J.G.; Moore, J.A.; Zinkl, J.G. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the immune system of laboratory animals. Environ. Health Persp. 1973, 5, 149–162. [Google Scholar] [CrossRef]
- Ross, P.S. The role of immunotoxic environmental contaminants in facilitating the emergence of infectious diseases in marine mammals. Hum. Ecol. Risk Assesm. 2002, 8, 277–292. [Google Scholar] [CrossRef]
- Stockinger, B.; Hirota, K.; Duarte, J.; Veldhoen, M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Sem. Immunol. 2011, 23, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kerkvliet, N.I. TCDD: An environmental immunotoxicant reveals a novel pathway of immunoregulation: A 30-year-odyssee. Toxicol. Pathol. 2012, 40, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.P.; Hall, A.; McConnell, B.; Rosing-Asvid, A.; Barber, J.L.; Brownlow, A.; De Guise, S.; Eulaers, I.; Jepson, P.D.; Letcher, R.J.; et al. Predicting global killer whale population collapse from PCB pollution. Science 2018, 361, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F. The aryl hydrocarbon receptor: A molecular pathway for the environmental control of the immune response. Immunology 2012, 138, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Rothammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Zhou, L. AHR function in lymphocytes: Emerging concepts. Trends Imunol. 2016, 37, 17–31. [Google Scholar] [CrossRef]
- De Abrew, K.N.; Phadnis, A.N.; Crawford, R.B.; Kaminski, N.E.; Thomas, R.S. Regulation of Bach2 by the aryl hydrocarbon receptor as a mechanism for suppression of B cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 2011, 252, 150–158. [Google Scholar] [CrossRef]
- Spitsbergen, J.M.; Schat, K.A.; Kleeman, J.M.; Peterson, R.E. Interactions of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with immune responses of rainbow trout. Vet. Immunol. Immunopathol. 1986, 12, 263–280. [Google Scholar] [CrossRef]
- Rice, C.D.; Schlenk, D. Immune function and cytochrome P4501A activity after acute exposure to 3,3’,4,4^,5-pentachlorobiphenly (PCB 126) in channel catfish. J. Aquat Anim. Health 1995, 7, 195–204. [Google Scholar] [CrossRef]
- Arkoosh, M.R.; Casillas, E.; Clemons, E.; Kagley, A.N.; Olson, R.; Reno, P.; Stein, J.E. Effect of pollution on fish disease: Potential impacts on salmonid population. J. Aquat. Anim. Health 1998, 10, 182–190. [Google Scholar] [CrossRef]
- Arkoosh, M.R.; Casillas, E.; Clemons, E.; McCain, B. Suppression of immunological memory in juvenile chinook salmon (Oncorhynchus tshawytscha) from an urban estuary. Dev. Comp. Immunol. 1991, 15, 578. [Google Scholar] [CrossRef]
- Hutchinson, T.H.; Field, M.D.R.; Manning, M.J. Evaluation of non-specific immune functions in dab, Limanda limanda, following short-term exposure to sediments contaminated with polyaromatic hydrocarbons and/or polychlorinated biphenyls. Mar. Environ. Res. 2003, 55, 193–202. [Google Scholar] [CrossRef]
- Carlson, E.A.; Li, Y.; Zelikoff, J.T. Exposure of Japanese medaka (Oryzias latipes) to benzo[a]pyrene suppresses immune function and host resistance against bacterial challenge. Aquat. Toxicol. 2002, 56, 289–301. [Google Scholar] [CrossRef]
- Reynaud, S.; Deschaux, P. The effects of polycyclic hydrocarbons on the immune system of fish: A review. Aquat. Toxicol. 2006, 77, 229–238. [Google Scholar] [CrossRef]
- Nakayama, K.; Kitamura, S.I.; Murakami, Y.; Song, J.Y.; Oh, M.J.; Iwata, H.; Tanabe, S. Toxicogenomic analysis of immune system-related genes in Japanese flounder (Paralichthys oliveaceus) expoed to heavy oil. Mar. Poll. Bull. 2008, 57, 445–452. [Google Scholar] [CrossRef]
- Iwanowicz, L.R.; Balzer, V.S.; McCormick, S.D.; van Veld, P.A.; Ottinger, C.A. Aroclor 1248 exposure leads to immunomodulation, decreased disease resistance, and endocrine disruption in the brown bullhead, Ameiurus nebulosus. Aquat. Toxicol. 2009, 93, 70–82. [Google Scholar] [CrossRef]
- Song, Y.Y.; Ohta, S.; Nakayama, K.; Muralami, Y.; Kitamura, S.I. A time course study of immune response in Japanese flounder Paralichthys oliveaceus exposed to heavy oil. Environ. Sci. Poll. Res. 2012, 19, 2300–2304. [Google Scholar] [CrossRef]
- Hur, D.; Jeon, J.K.; Hong, S. Analysis of immune gene expression modulated by benzo[a]pyrene in head kidney of olive flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. 2013, 165B, 49–57. [Google Scholar] [CrossRef]
- Rehberger, K.; Werner, I.; Hitzfeld, B.; Segner, H.; Baumann, L. 20 years of fish immunotoxicology–what we know and where we are. Crit. Rev. Toxicol. 2017, 47, 509–535. [Google Scholar] [CrossRef]
- Valdez-Domingos, F.X.; Oliveiro Ribeira, C.A.; Pelletier, E.; Rouleau, C. Tissue distribution and depuration kinetics of 14C-labeled light PAHs in mummichog (Fundulus heteroclitus). Environ. Sci. Technol. 2011, 42, 2684–2690. [Google Scholar] [CrossRef]
- Möller, A.M.; Hermsen, C.; Floehr, T.; Lamoree, M.H.; Segner, H. Tissue-specific metabolism of benzo(a)pyrene in rainbow trout (Oncorhynchus mykiss)–a comparison between liver and immune organs. Drug Metab. Dispos. 2014, 42, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Pollenz, R.S. The mechanisms of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem. Biol. Interact. 2002, 141, 41–61. [Google Scholar] [CrossRef]
- Jönsson, M.E.; Jenny, M.J.; Woodin, B.R.; Hahn, M.E.; Stegeman, J.J. Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebra fish exposed to 3,3’,4,4’,5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2007, 100, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Antkiewicz, D.S.; Peterson, R.E.; Heideman, W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006, 94, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Souder, J.P.; Gorelick, D.A. ahr2, but not ahr1a or ahr1b, is required for craniofacial and fin development and TCDD-dependent cardiotoxicity in zebrafish. Toxicol. Sci. 2019, 170, 25–44. [Google Scholar] [CrossRef]
- King Heiden, T.; Struble, C.A.; Rise, M.L.; Hessner, M.J.; Hutz, R.J.; Carvan III, M.J. Molecular targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin within the zebrafish ovary: Insights into TCDDs ovarian toxicity. Reprod. Toxicol. 2008, 25, 47–57. [Google Scholar] [CrossRef]
- Abnet, C.C.; Tanguay, R.L.; Hahn, M.E.; Heideman, W.; Peterson, R.E. Two forms of aryl hydrocarbon receptor type 2 in rainbow trout (Oncorhynchus mykiss). Evidence for differential expression and enhancer specifity. J. Biol. Chem. 1999, 274, 15159–15166. [Google Scholar] [CrossRef]
- Curtis, L.R.; Garzon, C.B.; Arkoosh, M.; Collier, T.; Myers, M.S.; Buzitis, J.; Hahn, M.E. Reduced cytochrome P4501A activity and recovery from oxidative stress during subchronic benzo[a]pyrene and benzo[e]pyrene treatment of rainbow trout. Toxicol. Appl. Pharmacol. 2011, 254, 1–7. [Google Scholar] [CrossRef]
- Nakayama, A.; Riesen, I.; Köllner, B.; Eppler, E.; Segner, H. Surface marker-defined head kidney granulocytes and B lymphocytes of rainbow trout express benzo[a]pyrene-inducible cytochrome P4501A protein. Toxicol. Sci. 2008, 103, 86–96. [Google Scholar] [CrossRef][Green Version]
- Möller, A.M.; Koellner, B.; Schmidt-Posthaus, H.; Segner, H. The teleostean liver as an immunological organ: Intrahepatic immune cells (IHIC) in healthy and benzo(a)pyrene challenged rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2014, 46, 518–529. [Google Scholar] [CrossRef]
- Faisal, M.; Weeks, B.A.; Vogelbein, W.K.; Huggett, R.J. Evidence of aberration of natural cytotoxic cell activity in Fundulus heteroclitus from the Elizabeth River. Vet. Immunol. Immunpathol. 1991, 29, 339–351. [Google Scholar] [CrossRef]
- Song, J.Y.; Nakayama, K.; Murakami, Y.; Kitamura, S.I. Heavy oil exposure induces high mortalities in virus carrier Japanese flounder (Paralichthys oliveaceus). Mar. Poll. Bull. 2011, 63, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Vorderstrasse, B.A.; Steppan, L.B.; Silverstone, A.E.; Kerkvliet, N.I. Aryl hydrocarbon receptor-deficient mice generate normal immune response to model antigens and are resistant to TCDD-induced immune suppression. Toxicol. Appl. Phamacol. 2001, 171, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.C.; Stockinger, B. The aryl hydrocarbon receptor links Th17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Marionet, D.; Taysse, L.; Chambras, C.; Deschaux, P. 3-methylcholanthrene-induced EROD activity and cytochrome P450 in immune organs of carp (Cyprinus carpio). Comp. Biochem. Physiol. 1997, 118C, 165–170. [Google Scholar] [CrossRef]
- Grinwis, G.C.; Besselink, H.T.; Van den Brandhof, E.J.; Bulder, A.S.; Engelsma, M.Y.; Kuiper, R.V.; Wester, P.W.; Vaal, M.A.; Vethaak, A.D.; Vos, J.G. Toxicity of TCDD in European flounder (Platichthys flesus) with emphasis on histopathology and cytochrome P4501A induction in several organ systems. Aquat. Toxicol. 2000, 75, 80–87. [Google Scholar]
- Sarasquete, C.; Segner, H. Cytochrome P4501A (CYP1A) in teleostean fishes. A review of immunohistochemical studies. STOTEN 2000, 47, 313–332. [Google Scholar] [CrossRef]
- Quabius, E.S.; Nolan, D.T.; Segner, H.; Wendelaar Bonga, S.E. Confinement stress and starvation modulate the induction of EROD activity after dietary exposure to PCB 126 in the Mozambique tilapia (Oreochromis mossambicus). Fish. Physiol. Biochem. 2002, 25, 109–119. [Google Scholar] [CrossRef]
- Carlson, E.A.; Li, Y.; Zelikoff, J. T Benzo[a]pyrene-induced immunotoxicity in Japanese medaka (Oryzias latipes): Relationship between lymphoid CYP1A activity and humoral immune suppression. Toxicol. Appl. Pharm. 2004, 201, 40–52. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kim, E.Y.; Iwata, H.; Tanabe, S. Molecular characterization of the aryl hydrocarbon receptor (AHR1 and AHR2) from red seabream (Pagrus major). Comp. Biochem. Physiol. 2005, 141C, 77–187. [Google Scholar] [CrossRef]
- Lu, M.; Chang, Z.; Bae, M.J.; Oh, S.M.; Chung, K.H.; Park, J.S. Molecular characterization of the aryl hydrocarbon receptor (AhR) pathway in goldfish (Carassius auratus) exposure to TCDD: The mRNA and protein levels. Fish. Shellfish Immunol. 2013, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Holen, E.; Olsvik, P.A. Aryl hydrocarbon receptor protein and CYP1A gene induction by LPS and phenanthrene in Atlantic cod (Gadus morhua) head kidney cells. Fish. Shellfish Immunol. 2014, 40, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Tijet, N.; Boutros, P.C.; Moffat, I.D.; Okey, A.B.; Tuomisto, J.; Pohjanvirta, R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Molec. Pharmacol. 2006, 69, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Bols, N.C.; Schirmer, K.; Joyce, E.M.; Dixon, D.G.; Greenberg, G.M.; Whyte, J.J. Ability of polycyclic aromatic hydrocarbons to induce 7-ethoxyresorufin-o-deethylase activity in a trout liver cell line. Ecotox. Environ. Saf. 1999, 44, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Billiard, S.M.; Hahn, M.E.; Franks, D.G.; Peterson, R.E.; Bols, N.C.; Hodson, P.V. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs). Comp. Biochem. Physiol. 2002, 133B, 55–68. [Google Scholar] [CrossRef]
- Holen, E.; Olsvik, P.A. β-naphthoflavone interferes with cy1c1, cox2 and IL-8 gene transcription and leukotriene B4 secretion in Atlantic cod (Gadus morhua) head kidney cells during inflammation. Fish. Shellfish Immunol. 2016, 54, 128–134. [Google Scholar] [CrossRef]
- Gasiewicz, T.A.; Singh, K.P.; Casado, F.L. The aryl hydrocarbon receptor has an important role in the regulation of hematopoiesis: Implications for benzene-induced hematopoietic toxicity. Chem. Biol. Interact. 2010, 184, 146–251. [Google Scholar] [CrossRef]
- Spitsbergen, J.M.; Kleeman, J.M.; Peterson, R.E. 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in yellow perch (Perca flavescens). J. Toxicol. Env. Health 1988, 23, 359–383. [Google Scholar] [CrossRef]
- Bookout, A.L.; Mangelsdorf, D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Rec. Signal. 2003, 1, e012. [Google Scholar] [CrossRef]


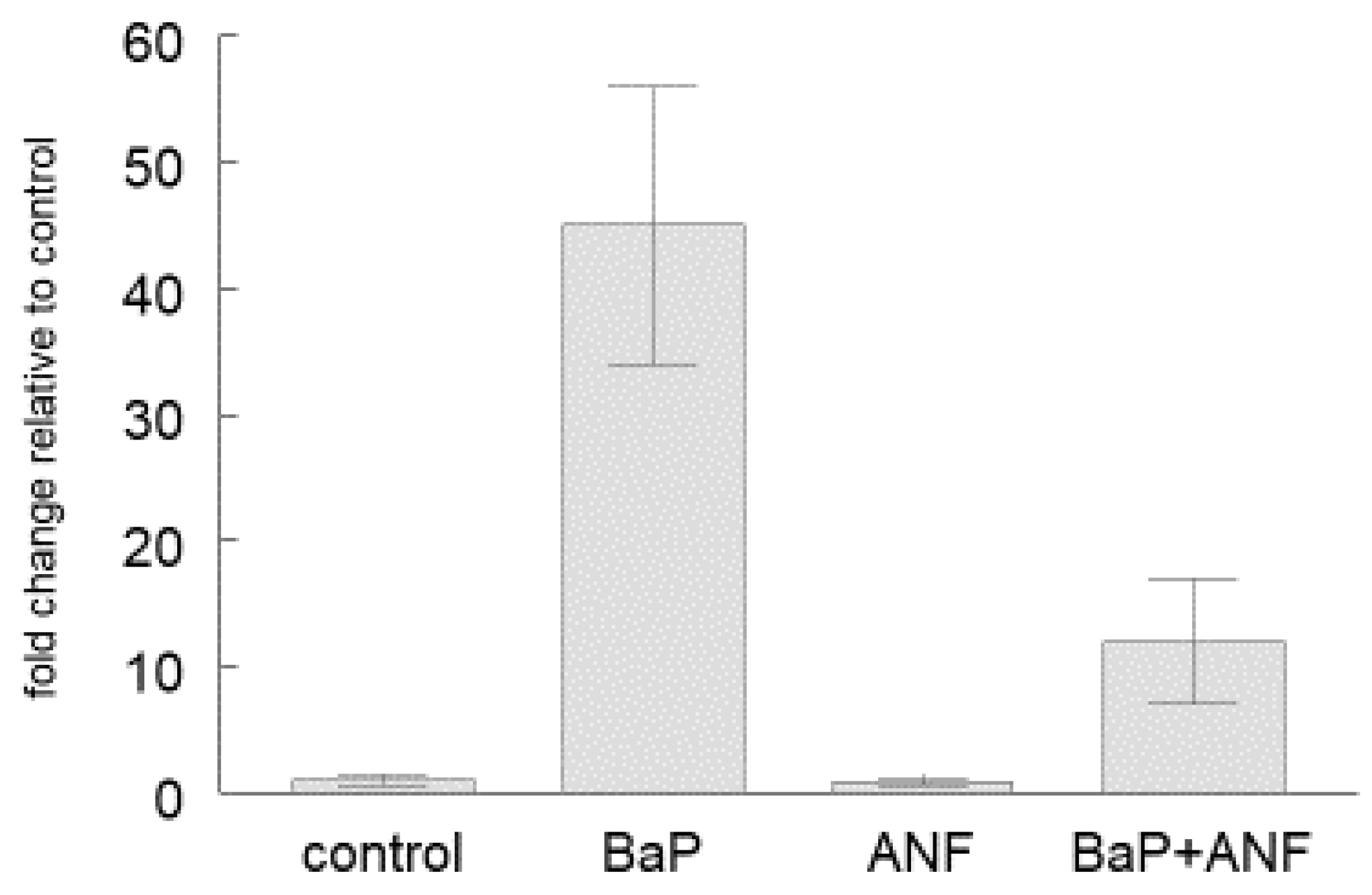
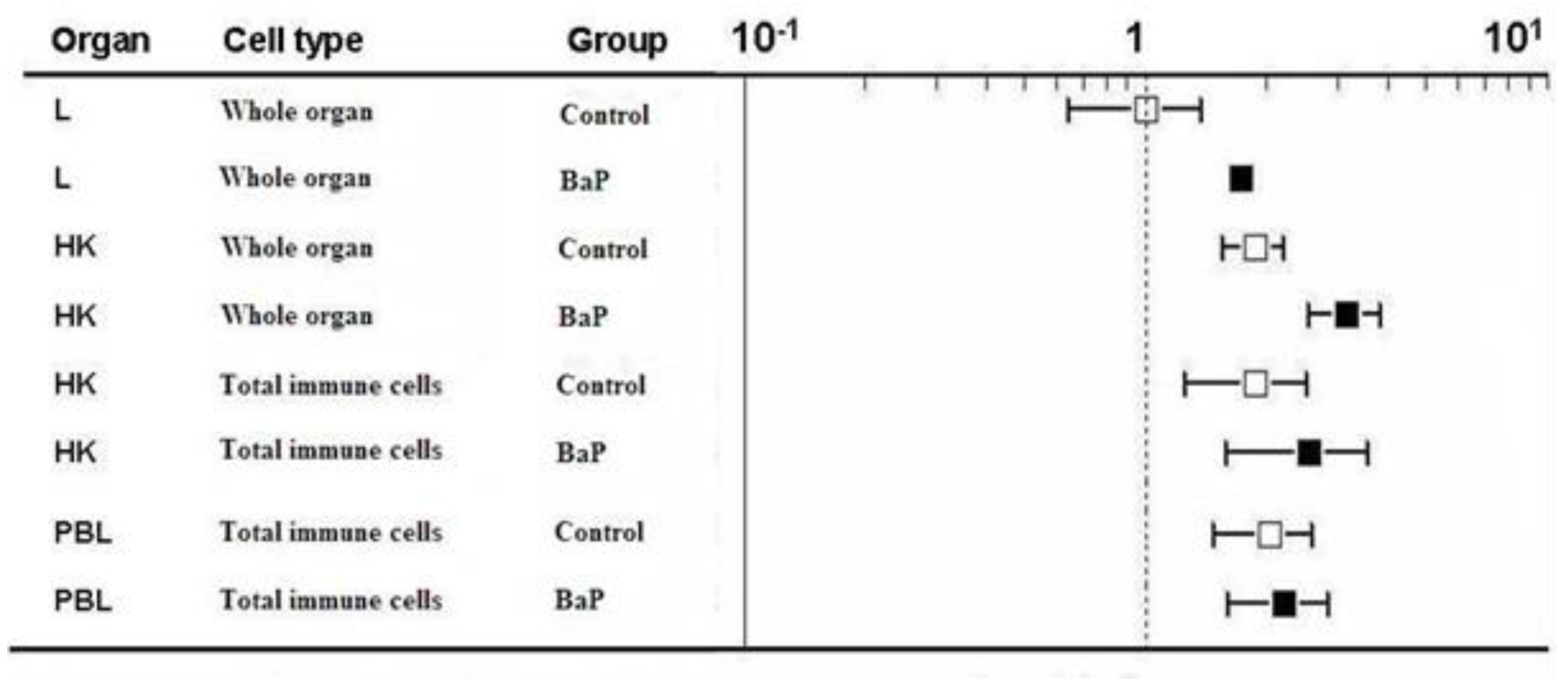

| Organ | Tissue/Cell | Control | BaP |
|---|---|---|---|
| L | Whole organ | 1 ± 0.37 | 59 ±16 * |
| HK | Whole organ | 1 ± 0.47 | 21 ± 1 * |
| HK | Total immune cells | 1 ± 0.27 | 56 ± 16 * |
| PBL | Total immune cells | 1 ± 0.60 | 92 ± 24 * |
| Organ | Tissue/Cell | Control | BaP |
|---|---|---|---|
| L | Whole organ | 1 ± 0.36 | 1.73 ± 0.05 * |
| HK | Whole organ | 1 ± 0.18 | 1.69 ± 0.35 * |
| HK | Total immune cells | 1 ± 0.34 | 1.37 ± 0.53 |
| PBL | Total immune cells | 1 ± 0.28 | 1.09 ± 0.30 |
| Organ | Cell Type | Control | BaP |
|---|---|---|---|
| L | Whole organ | 1 ± 0.31 | 1.48 ± 0.33 |
| HK | Whole organ | 1 ± 0.17 | 1.32 ± 0.46 |
| HK | Total immune cells | 1 ± 0.43 | 1.36 ± 0.56 |
| PBL | Total immune cells | 1 ± 0.31 | 0.90 ± 0.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.-Y.; Casanova-Nakayama, A.; Möller, A.-M.; Kitamura, S.-I.; Nakayama, K.; Segner, H. Aryl Hydrocarbon Receptor Signaling Is Functional in Immune Cells of Rainbow Trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2020, 21, 6323. https://doi.org/10.3390/ijms21176323
Song J-Y, Casanova-Nakayama A, Möller A-M, Kitamura S-I, Nakayama K, Segner H. Aryl Hydrocarbon Receptor Signaling Is Functional in Immune Cells of Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences. 2020; 21(17):6323. https://doi.org/10.3390/ijms21176323
Chicago/Turabian StyleSong, Jun-Young, Ayako Casanova-Nakayama, Anja-Maria Möller, Shin-Ichi Kitamura, Kei Nakayama, and Helmut Segner. 2020. "Aryl Hydrocarbon Receptor Signaling Is Functional in Immune Cells of Rainbow Trout (Oncorhynchus mykiss)" International Journal of Molecular Sciences 21, no. 17: 6323. https://doi.org/10.3390/ijms21176323
APA StyleSong, J.-Y., Casanova-Nakayama, A., Möller, A.-M., Kitamura, S.-I., Nakayama, K., & Segner, H. (2020). Aryl Hydrocarbon Receptor Signaling Is Functional in Immune Cells of Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences, 21(17), 6323. https://doi.org/10.3390/ijms21176323






