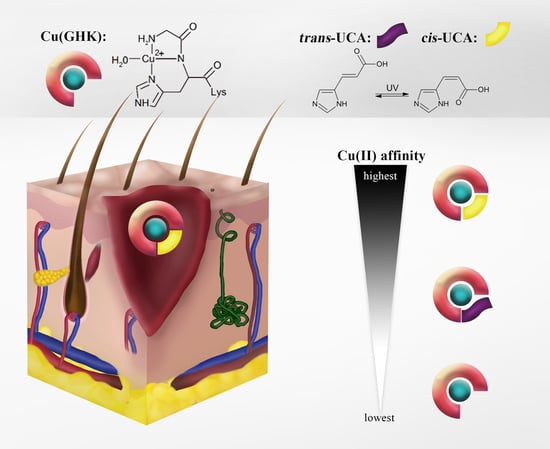
Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate
Abstract
1. Introduction
2. Results and Discussion
2.1. Interaction of Cu(II) with GHK
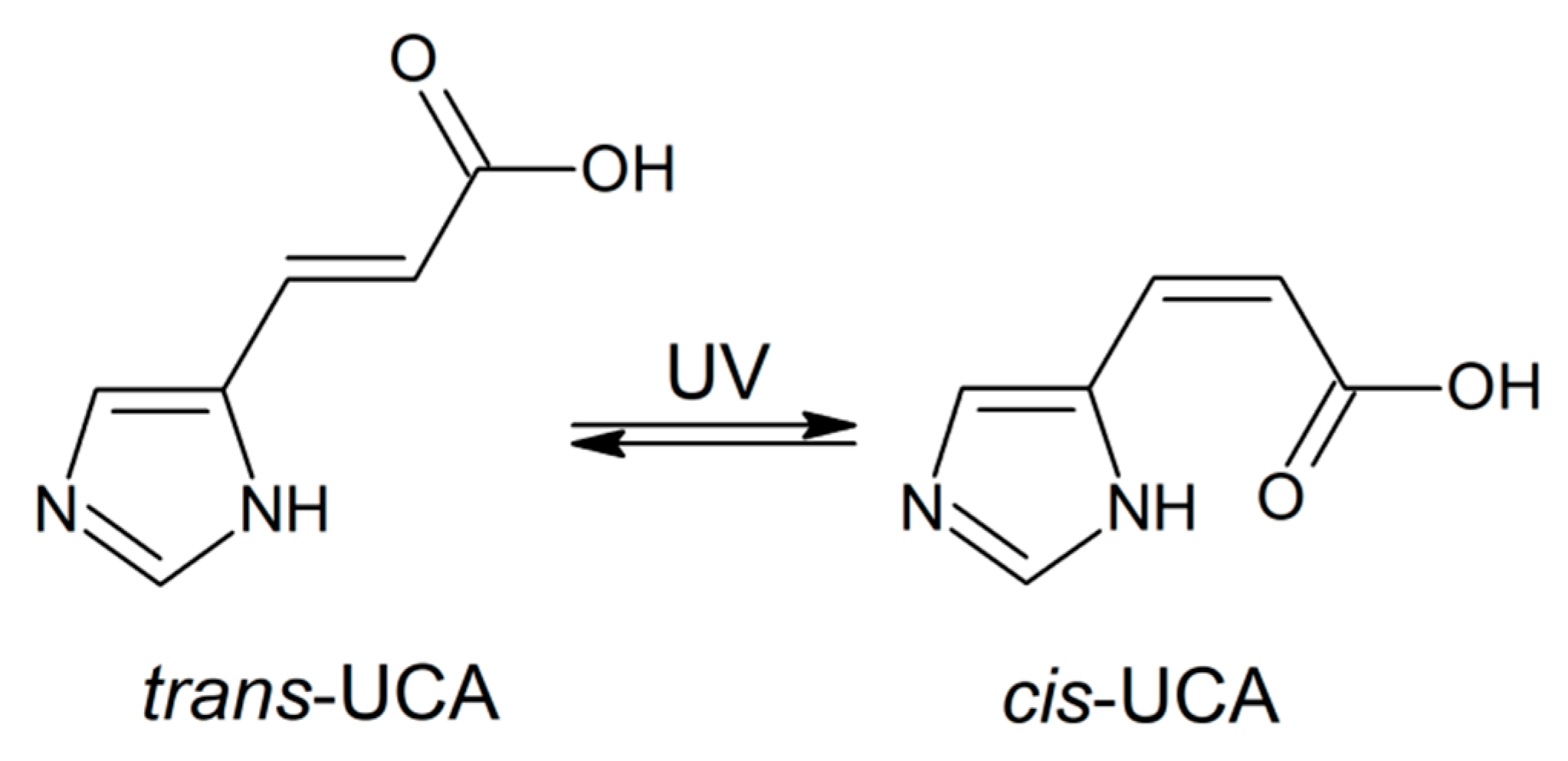
2.2. Interaction of Cu(II) with UCA
2.3. Ternary Complex Formation of Cu/GHK/Imidazole
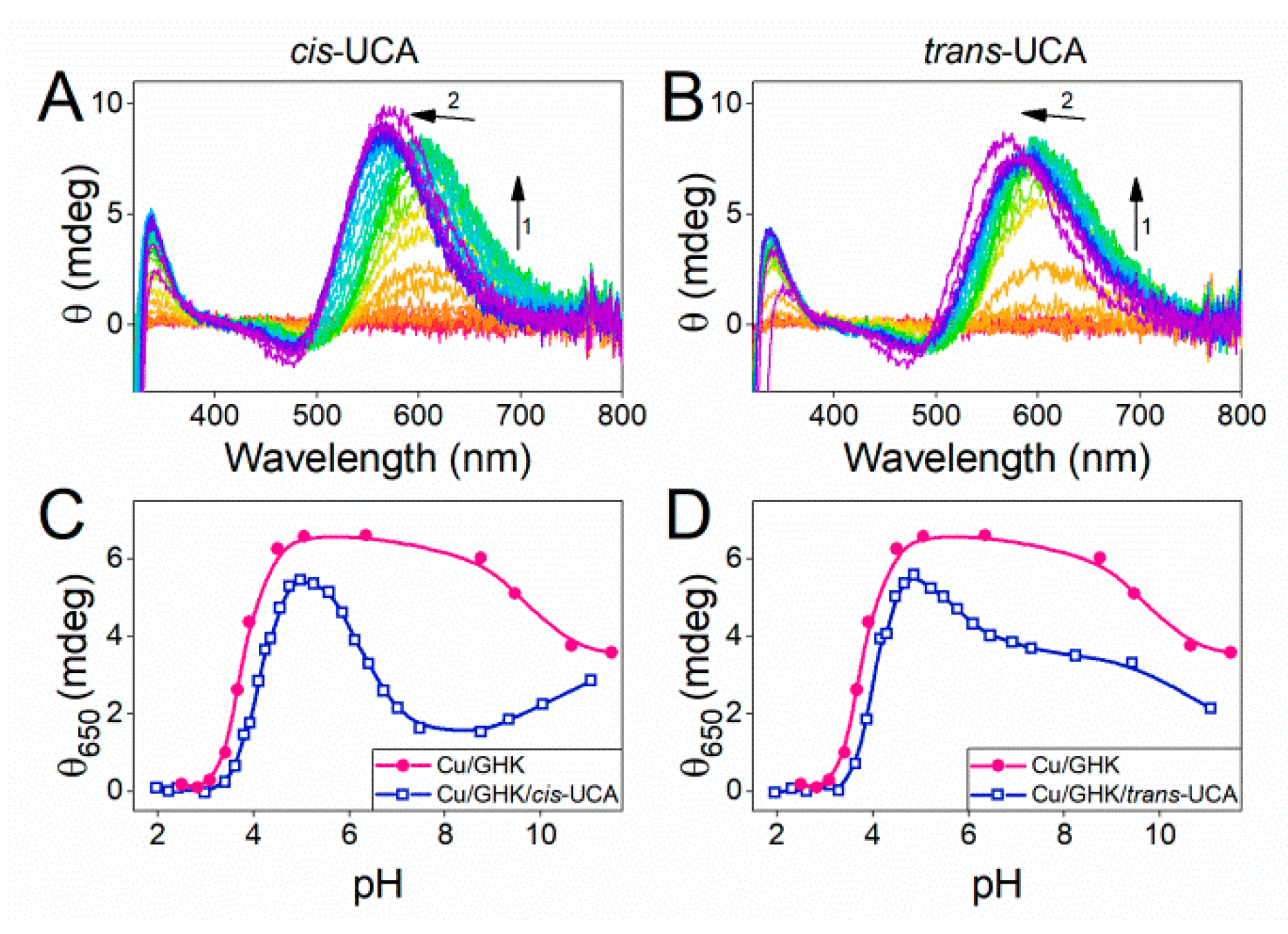
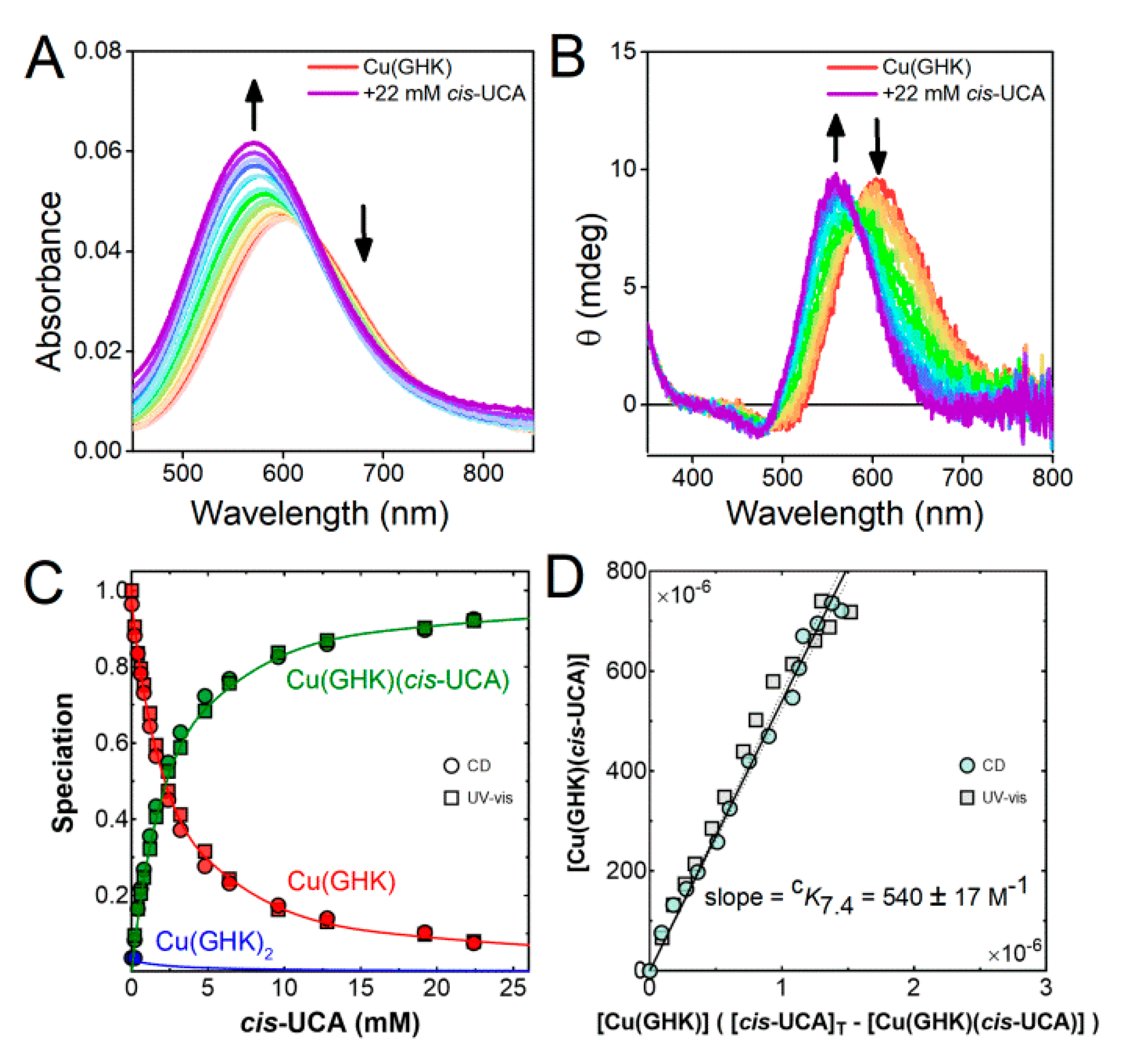
2.4. Ternary Complex Formation of Cu/GHK/UCA
2.5. Biological Relevance
3. Materials and Methods
3.1. Materials
3.2. UV-Vis & Circular Dichroism Spectroscopy
3.2.1. pH-Metric Titrations
3.2.2. Ligand Titrations
3.3. EPR Spectroscopy
3.4. Potentiometry
3.4.1. GHK Preparation
3.4.2. Ligands
3.4.3. Binary Complexes
3.4.4. Ternary Complexes
3.5. Isothermal Titration Calorimetry
3.6. Binding Constant Calculations
Cu(GHK)(Im), Cu(GHK)(trans-UCA), and Cu(GHK)(cis-UCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GHK | Gly-His-Lys peptide |
| Im | imidazole |
| UCA | urocanic acid |
References
- Pickart, L.; Margolina, A. Regenerative and Protective Actions of the GHK-Cu Peptide in the Light of the New Gene Data. Int. J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Xu, Q.; Sun, H.; Shi, M.; Wang, D.; Guo, M.; Yu, J.; Zhao, C.; Feng, B. GHK-Cu-liposomes accelerate scald wound healing in mice by promoting cell proliferation and angiogenesis. Wound Repair Regen. 2017, 25, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Lee, H.; Kim, S.I.; Yang, S.R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget 2016, 7, 58405–58417. [Google Scholar] [CrossRef] [PubMed]
- Bobyntsev, I.I.; Chernysheva, O.I.; Dolgintsev, M.E.; Smakhtin, M.Y.; Belykh, A.E. Anxiolytic Effects of Gly-His-Lys Peptide and Its Analogs. Bull. Exp. Biol. Med. 2015, 158, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK and DNA: Resetting the Human Genome to Health. Biomed. Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Vasquez-Soltero, J.; Margolina, A. The Effect of the Human Peptide GHK on Gene Expression Relevant to Nervous System Function and Cognitive Decline. Brain Sci. 2017, 7, 20. [Google Scholar] [CrossRef]
- Mohammad, T.; Morrison, H.; HogenEsch, H. Urocanic Acid Photochemistry and Photobiology. Photochem. Photobiol. 1999, 69, 115–135. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M.F. Modulation of multiple sclerosis by sunlight exposure: Role of cis-urocanic acid. J. Neuroimmunol. 2013, 261, 134–140. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, N.; Yao, L.; Chen, Q.; Zhang, R.; Qian, J.; Hou, Y.; Guo, W.; Fan, S.; Liu, S.; et al. Moderate UV Exposure Enhances Learning and Memory by Promoting a Novel Glutamate Biosynthetic Pathway in the Brain. Cell 2018, 173, 1716–1727. [Google Scholar] [CrossRef]
- Gibbs, N.K.; Tye, J.; Norval, M. Recent advances in urocanic acid photochemistry, photobiology and photoimmunology. Photochem. Photobiol. Sci. 2008, 7, 655–667. [Google Scholar] [CrossRef]
- Visscher, M.O.; Tolia, G.T.; Wickett, R.R.; Hoath, S.B. Effect of soaking and natural moisturizing factor on stratum corneum water-handling properties. J. Cosmet. Sci. 2003, 54, 289–300. [Google Scholar]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dawson, B.; Favaloro, E.J. High Rate of Deficiency in the Amino Acids Tryptophan and Histidine in People with Wounds. Adv. Skin Wound Care 2009, 22, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, E.; Kim, Y. l-histidine and l-carnosine accelerate wound healing via regulation of corticosterone and PI3K/Akt phosphorylation in d-galactose-induced aging models in vitro and in vivo. J. Funct. Foods 2019, 58, 227–237. [Google Scholar] [CrossRef]
- Bruhs, A.; Eckhart, L.; Tschachler, E.; Schwarz, T.; Schwarz, A. Urocanic Acid: An Endogenous Regulator of Langerhans Cells. J. Investig. Dermatol. 2016, 136, 1735–1737. [Google Scholar] [CrossRef]
- Krien, P.M.; Kermici, M. Evidence for the Existence of a Self-Regulated Enzymatic Process Within the Human Stratum Corneum—An Unexpected Role for Urocanic Acid. J. Investig. Dermatol. 2000, 115, 414–420. [Google Scholar] [CrossRef]
- Wezynfeld, N.E.; Goch, W.; Bal, W.; Frączyk, T. Cis-Urocanic acid as a potential nickel binding molecule in the human skin. Dalton Trans. 2014, 43, 3196–3201. [Google Scholar] [CrossRef]
- Conato, C.; Gavioli, R.; Guerrini, R.; Kozłowski, H.; Młynarz, P.; Pasti, C.; Pulidori, F.; Remelli, M. Copper complexes of glycyl-histidyl-lysine and two of its synthetic analogues: Chemical behaviour and biological activity. Biochim. Biophys. Acta Gen. Subj. 2001, 1526, 199–210. [Google Scholar] [CrossRef]
- Burger, K. Biocoordination Chemistry. In Equilibria in Biologically Active Systems; Burger, K., Ed.; Ellis Horwood: Hemel Hempstead, UK, 1990; p. 349. [Google Scholar]
- Bossak, K.; Mital, M.; Poznański, J.; Bonna, A.; Drew, S.; Bal, W. Interactions of α-Factor-1, a Yeast Pheromone, and Its Analogue with Copper(II) Ions and Low-Molecular-Weight Ligands Yield Very Stable Complexes. Inorg. Chem. 2016, 55, 7829–7831. [Google Scholar] [CrossRef]
- Kotuniak, R.; Frączyk, T.; Skrobecki, P.; Płonka, D.; Bal, W. Gly-His-Thr-Asp-Amide, an Insulin-Activating Peptide from the Human Pancreas Is a Strong Cu(II) but a Weak Zn(II) Chelator. Inorg. Chem. 2018, 57, 15507–15516. [Google Scholar] [CrossRef]
- Farkas, E.; Sóvágó, I.; Kiss, T.; Gergely, A. Studies on transition-metal–peptide complexes. Part 9. Copper(II) complexes of tripeptides containing histidine. J. Chem. Soc. Dalton Trans. 1984, 3, 611–614. [Google Scholar] [CrossRef]
- Daniele, P.G.; Zerbinati, O.; Zelano, V.; Ostacoli, G. Thermodynamic and spectroscopic study of copper(II)-glycyl-L-histidylglycine complexes in aqueous solution. J. Chem. Soc. Dalton Trans. 1991, 2711–2715. [Google Scholar] [CrossRef]
- Krężel, A.; Wójcik, J.; Maciejczyk, M.; Bal, W. May GSH and l-His contribute to intracellular binding of zinc? Thermodynamic and solution structural study of a ternary complex. Chem. Commun. 2003, 3, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Jezowska-Bojczuk, M.; Kaczmarek, P.; Bal, W.; Kasprzak, K.S. Coordination mode and oxidation susceptibility of nickel(II) complexes with 2′-deoxyguanosine 5′-monophosphate and L-histidine. J. Inorg. Biochem. 2004, 98, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Trapaidze, A.; Hureau, C.; Bal, W.; Winterhalter, M.; Faller, P. Thermodynamic study of Cu2+ binding to the DAHK and GHK peptides by isothermal titration calorimetry (ITC) with the weaker competitor glycine. J. Biol. Inorg. Chem. 2012, 17, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bruni, S.; Cariati, F.; Daniele, P.; Prenesti, E. Speciation and structure of copper(II) complexes with histidine-containing peptides in aqueous medium: A combined potentiometric and spectroscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 815–827. [Google Scholar] [CrossRef]
- Lau, S.J.; Sarkar, B. The interaction of copper(II) and glycyl-L-histidyl-L-lysine, a growth-modulating tripeptide from plasma. Biochem. J. 1981, 199, 649–656. [Google Scholar] [CrossRef]
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Kenche, V.B.; Zawisza, I.; Masters, C.L.; Bal, W.; Barnham, K.J.; Drew, S.C. Mixed Ligand Cu2+ Complexes of a Model Therapeutic with Alzheimer’s Amyloid-β Peptide and Monoamine Neurotransmitters. Inorg. Chem. 2013, 52, 4303–4318. [Google Scholar] [CrossRef]
- Mital, M.; Zawisza, I.A.; Wiloch, M.Z.; Wawrzyniak, U.E.; Kenche, V.; Wróblewski, W.; Bal, W.; Drew, S.C. Copper Exchange and Redox Activity of a Prototypical 8-Hydroxyquinoline: Implications for Therapeutic Chelation. Inorg. Chem. 2016, 55, 7317–7319. [Google Scholar] [CrossRef]
- Drew, S.C. The N Terminus of α-Synuclein Forms CuII-Bridged Oligomers. Chem. A Eur. J. 2015, 21, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.C. Probing the quaternary structure of metal-bridged peptide oligomers. J. Inorg. Biochem. 2016, 158, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Bossak-Ahmad, K.; Vileno, B.; Wezynfeld, N.E.; El Khoury, Y.; Hellwig, P.; Hureau, C.; Bal, W.; Faller, P. Triggering Cu-coordination change in Cu(ii)-Ala-His-His by external ligands. Chem. Commun. 2019, 55, 8110–8113. [Google Scholar] [CrossRef] [PubMed]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Lane, T.F.; Iruela-Arispe, M.L.; Johnson, R.S.; Sage, E.H. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J. Cell Biol. 1994, 125, 929–943. [Google Scholar] [CrossRef]
- Maquart, F.X.; Pickart, L.; Laurent, M.; Gillery, P.; Monboisse, J.C.; Borel, J.P. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988, 238, 343–346. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Karrer, S.; Heinlin, J.; Landthaler, M.; Babilas, P. The impact of the pH value on skin integrity and cutaneous wound healing. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 373–378. [Google Scholar] [CrossRef]
- Sharpe, J.R.; Booth, S.; Jubin, K.; Jordan, N.R.; Lawrence-Watt, D.J.; Dheansa, B.S. Progression of wound pH during the course of healing in burns. J. Burn Care Res. 2013, 34, 201–208. [Google Scholar] [CrossRef]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Peltonen, J.; Pylkkänen, L.; Jansén, C.; Volanen, I.; Lehtinen, T.; Laihia, J.; Leino, L. Three Randomised Phase I/IIa Trials of 5% Cis-urocanic Acid Emulsion Cream in Healthy Adult Subjects and in Patients with Atopic Dermatitis. Acta Derm. Venereol. 2014, 94, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anwar, M.F.; Dinda, A.; Singhal, M.; Malik, A. In Vitro and in Vivo Studies of pH-Sensitive GHK-Cu-Incorporated Polyaspartic and Polyacrylic Acid Superabsorbent Polymer. ACS Omega 2019, 4, 20118–20128. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, A.; Hu, Y.; Li, Y.; Shang, L.; Zhang, L. Self-Assembled Fluorescent and Antibacterial GHK-Cu Nanoparticles for Wound Healing Applications. Part. Part. Syst. Charact. 2019, 36, 1–6. [Google Scholar] [CrossRef]
- Jauhonen, H.M.; Laihia, J.; Oksala, O.; Viiri, J.; Sironen, R.; Alajuuma, P.; Kaarniranta, K.; Leino, L. Topical cis-urocanic acid prevents ocular surface irritation in both IgE -independent and -mediated rat model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Mulder, G.; Patt, L.; Sanders, L.; Rosenstock, J.; Altman, M.; Hanley, M.; Duncan, G. Enhanced healing of ulcers in patients with diabetes by topical treatment with glycyl-L-histidyl-L-lysine copper. Wound Repair Regen. 1994, 2, 259–269. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakasuka, N.; Tanaka, M. Complexation of thyrotropin-releasing hormone and its related compounds with copper(II) and nickel(II) ions. Inorg. Chim. Acta 1991, 185, 49–56. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1982; Volume 5, ISBN 9781461567639. [Google Scholar]
- Pickart, L.; Thaler, M.M. Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver. Nat. New Biol. 1973, 243, 85–87. [Google Scholar]
- Miller, D.M.; DeSilva, D.; Pickart, L.; Aust, S.D. Effects of glycyl-histidyl-lysyl chelated Cu(II) on ferritin dependent lipid peroxidation. Adv. Exp. Med. Biol. 1990, 264, 79–84. [Google Scholar] [CrossRef]
- Bossak-Ahmad, K.; Frączyk, T.; Bal, W.; Drew, S.C. The Sub-picomolar Cu2+ Dissociation Constant of Human Serum Albumin. ChemBioChem 2020, 21, 331–334. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. SUPERQUAD: An improved general program for computation of formation constants from potentiometric data. J. Chem. Soc. Dalton Trans. 1985, 1195–1200. [Google Scholar] [CrossRef]
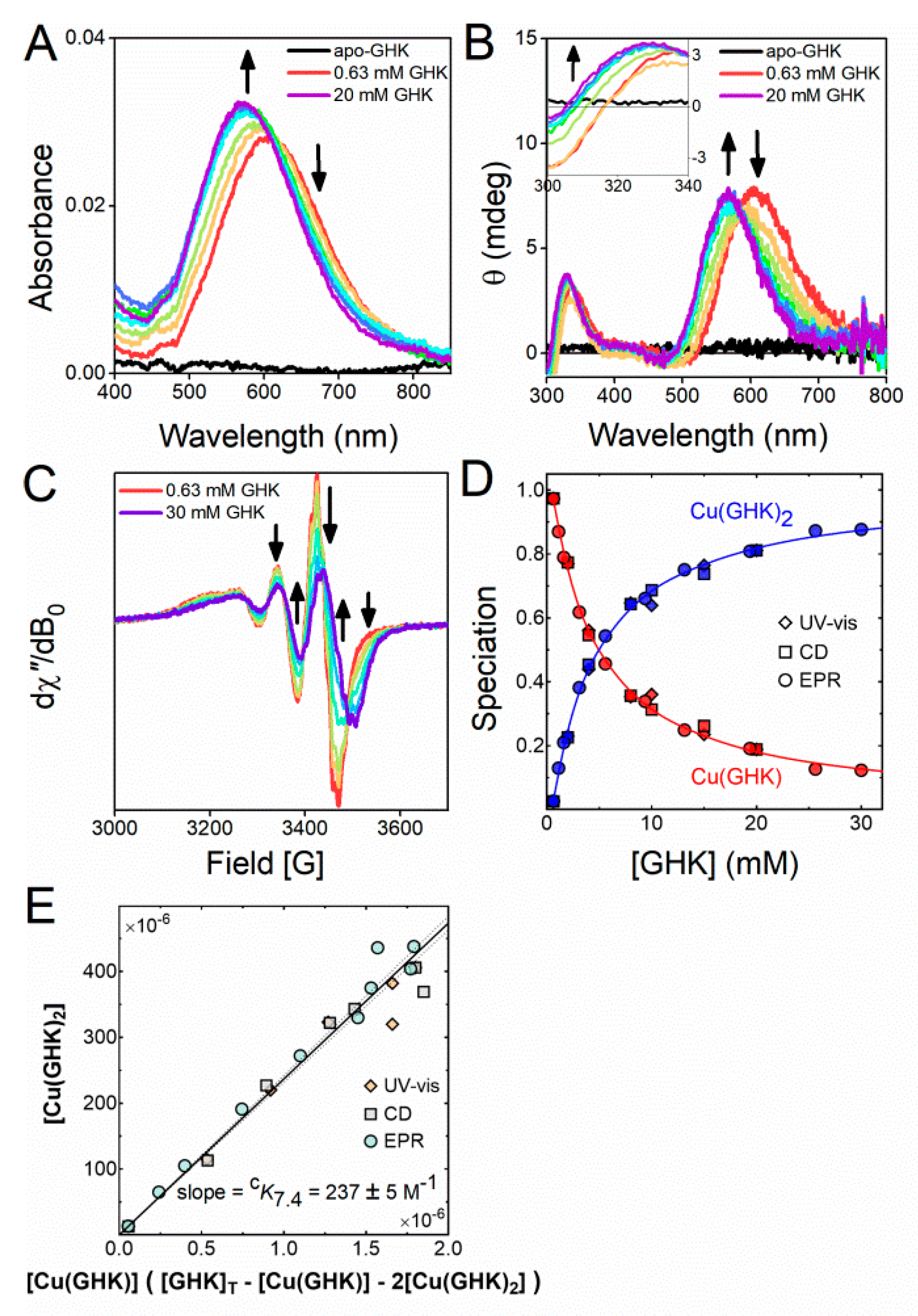

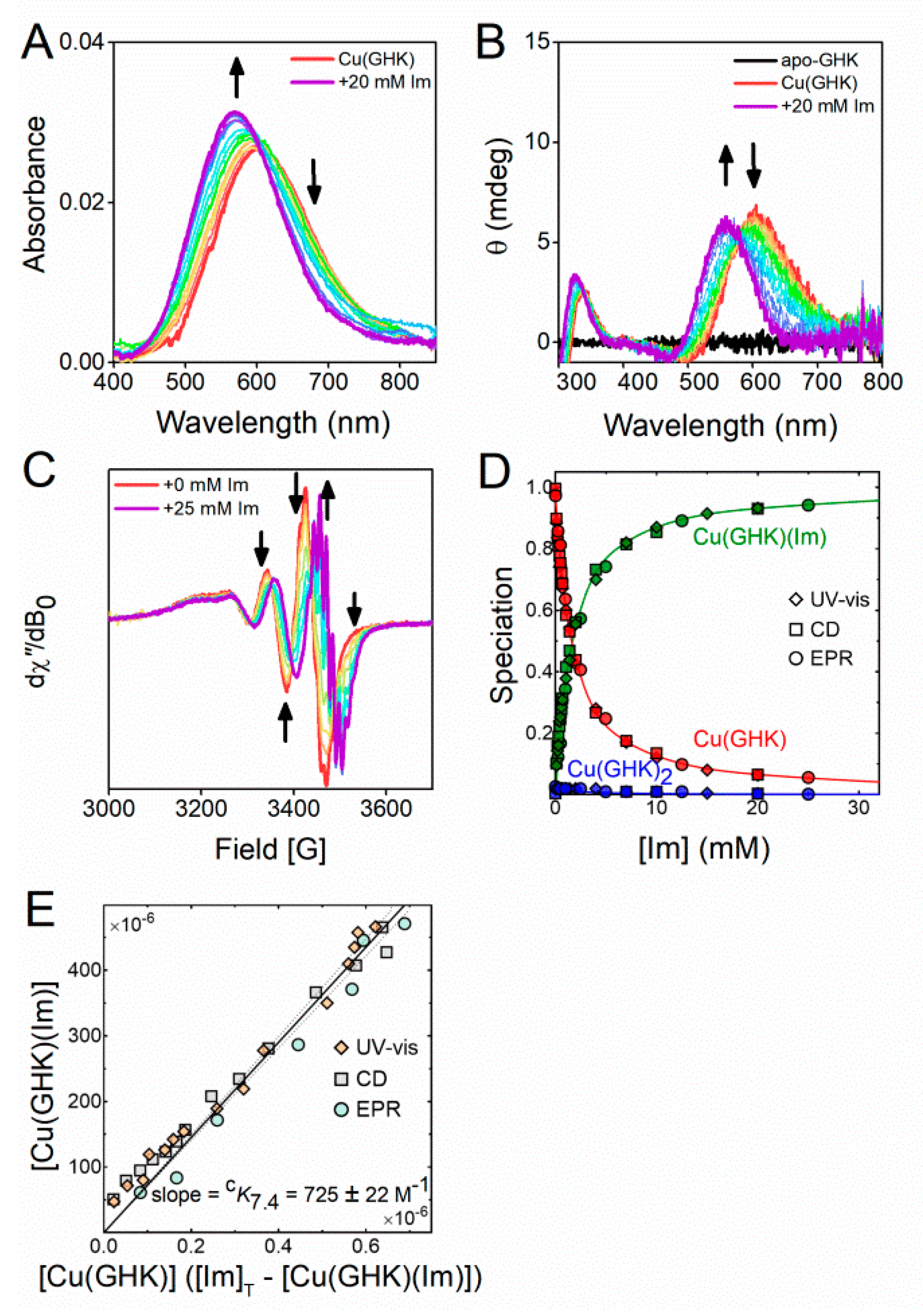


| Species | Logβ 1 | pK | Protonation Event |
|---|---|---|---|
| H2L | 9.541(6) | 2.80 | COOH |
| HL | 6.742(2) | 6.74 | NIm |
| CuL | 4.941(9) | COOH, NIm | |
| CuL2 | 8.76(1) | COOH, NIm |
| Equilibrium | cK6.5 (M−1) | cK7.4 (M−1) |
|---|---|---|
| 4.79 × 1010 1 | 4.17 × 1012 1 | |
| 3.8 × 104 1 | 7.94 × 104 1 | |
| 237 ± 5 2 265 ± 46 3 | ||
| 725 ± 22 2 532 ± 44 3 | ||
| 345 ± 26 4 | 540 ± 17 4 | |
| 186 ± 10 4 | 200 ± 10 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossak-Ahmad, K.; Wiśniewska, M.D.; Bal, W.; Drew, S.C.; Frączyk, T. Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate. Int. J. Mol. Sci. 2020, 21, 6190. https://doi.org/10.3390/ijms21176190
Bossak-Ahmad K, Wiśniewska MD, Bal W, Drew SC, Frączyk T. Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate. International Journal of Molecular Sciences. 2020; 21(17):6190. https://doi.org/10.3390/ijms21176190
Chicago/Turabian StyleBossak-Ahmad, Karolina, Marta D. Wiśniewska, Wojciech Bal, Simon C. Drew, and Tomasz Frączyk. 2020. "Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate" International Journal of Molecular Sciences 21, no. 17: 6190. https://doi.org/10.3390/ijms21176190
APA StyleBossak-Ahmad, K., Wiśniewska, M. D., Bal, W., Drew, S. C., & Frączyk, T. (2020). Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate. International Journal of Molecular Sciences, 21(17), 6190. https://doi.org/10.3390/ijms21176190