Novel Therapeutic Application of Self-Assembly Peptides Targeting the Mitochondria in In Vitro and In Vivo Experimental Models of Gastric Cancer
Abstract
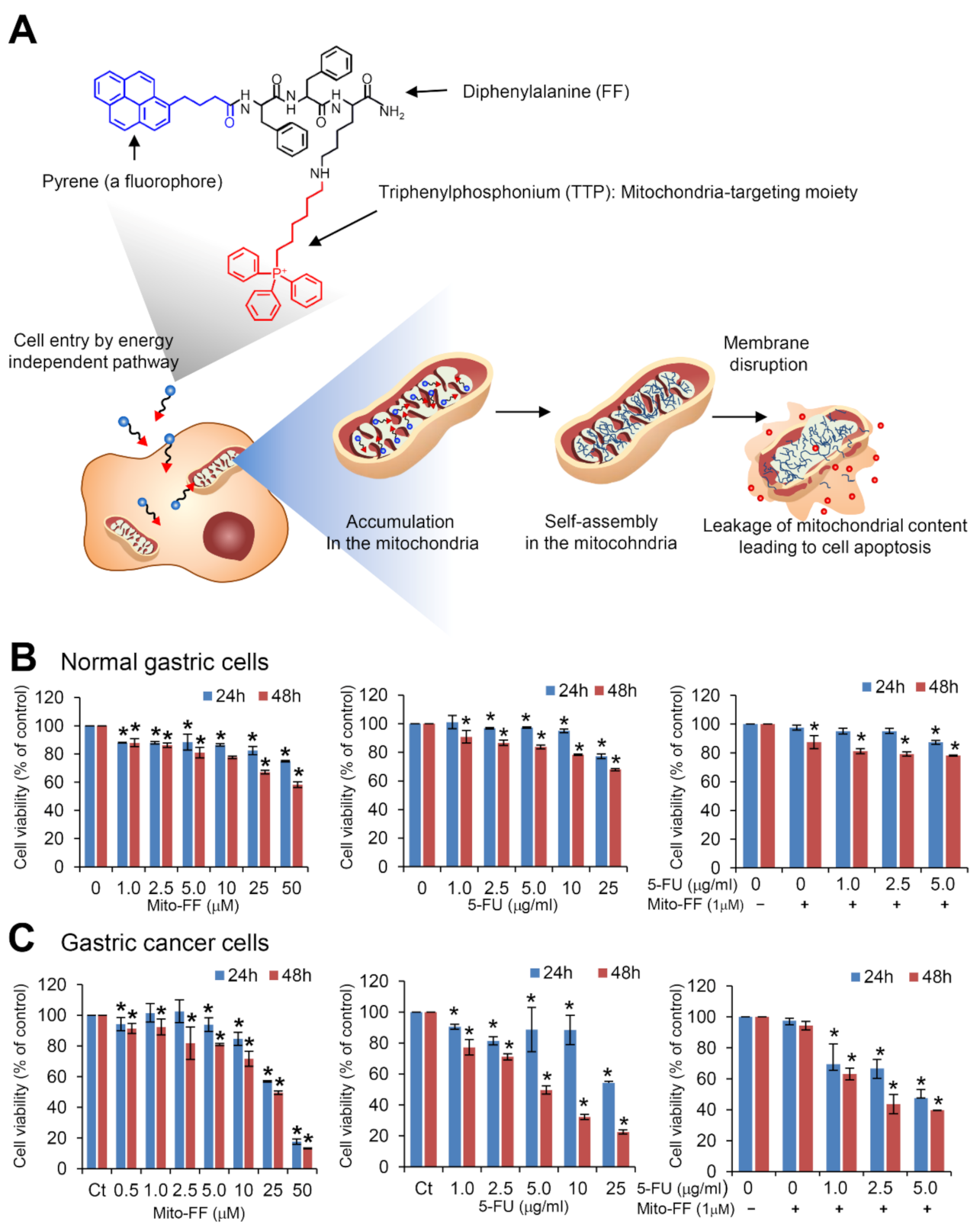
1. Introduction
2. Results
2.1. Cell Viability Assay in Normal Gastric Cells and Gastric Cancer Cells
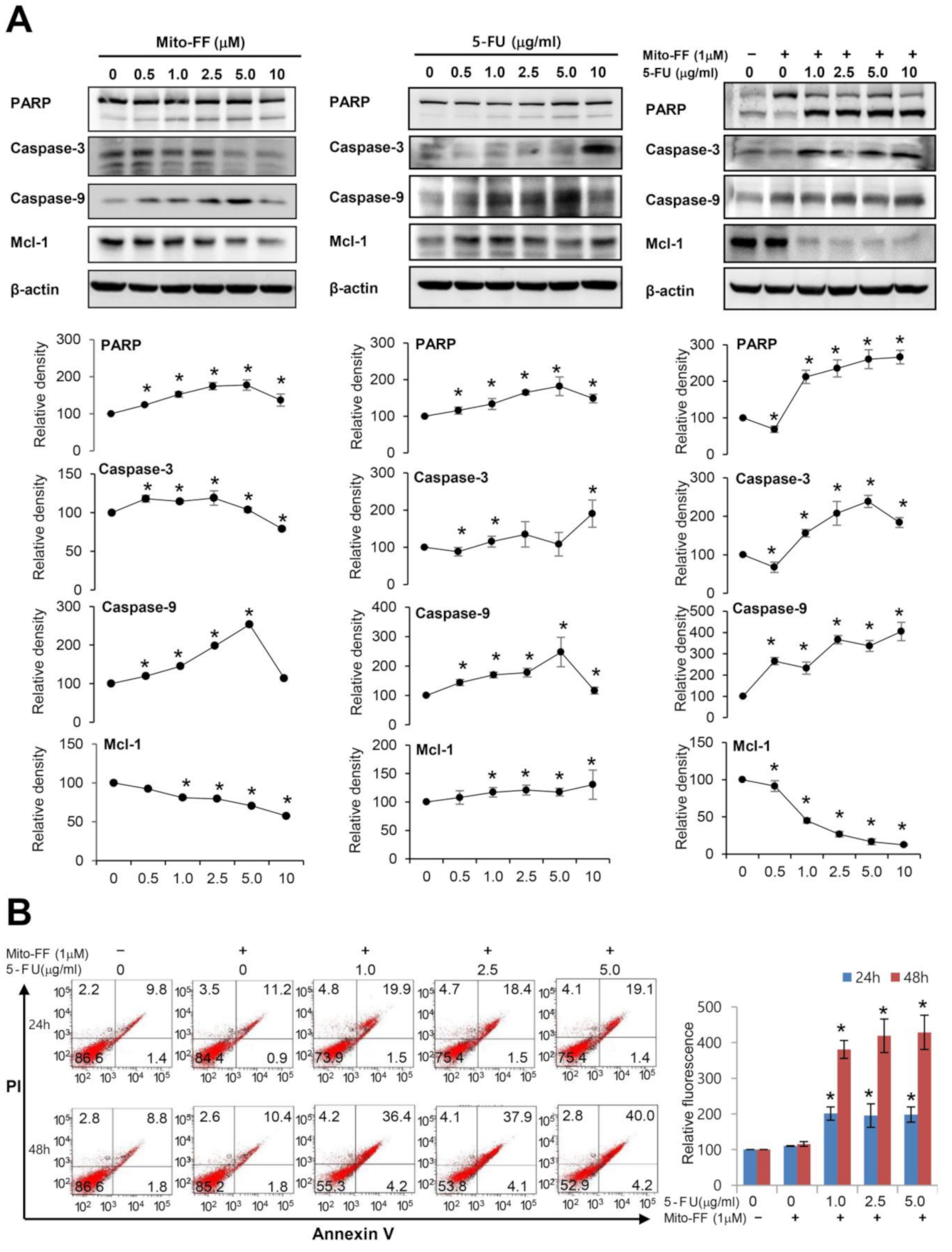
2.2. Cell Apoptosis Assay in Gastric Cancer Cells
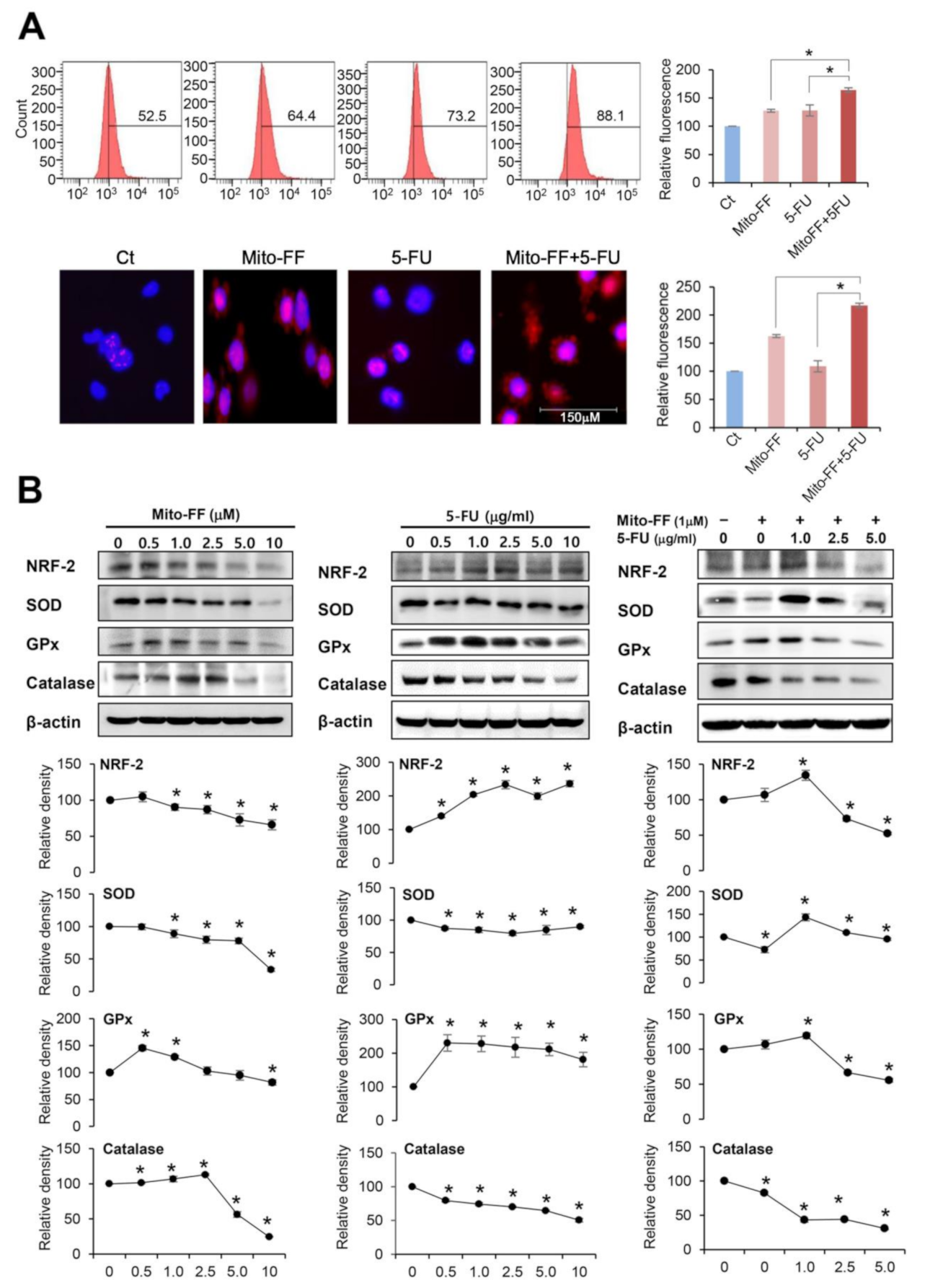
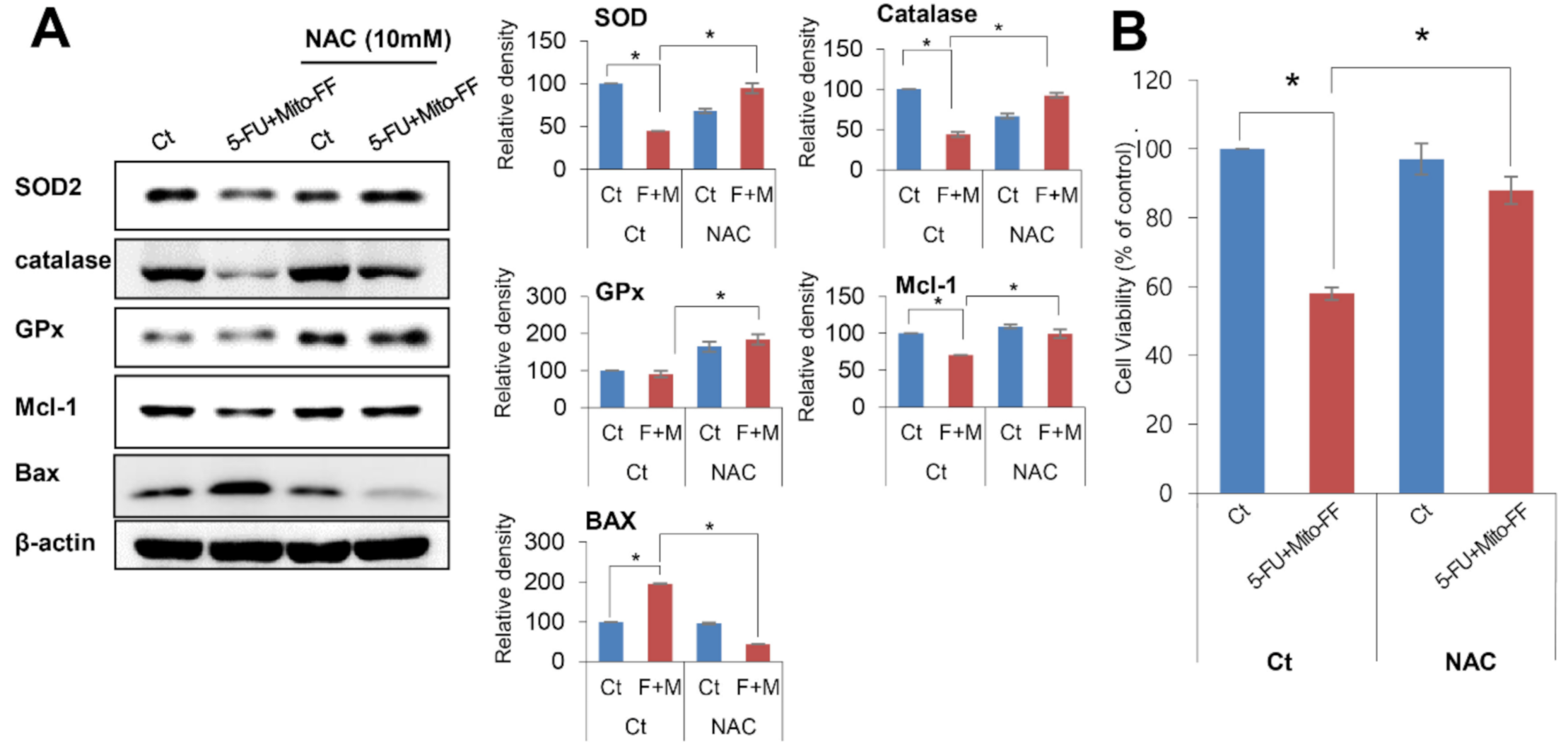
2.3. Anticancer Activities of Each Treatment in Relation with Oxidative Stress
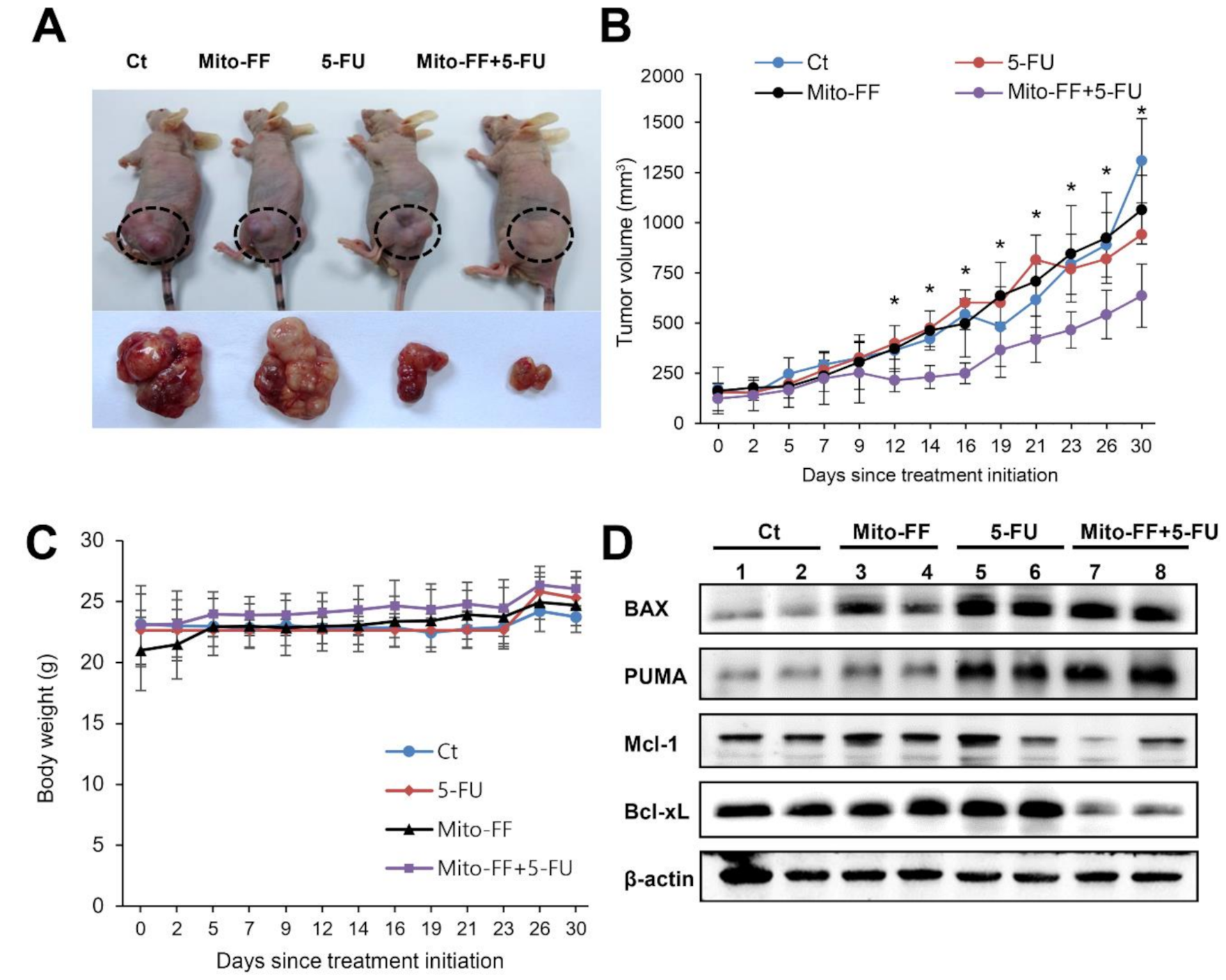
2.4. Effects of Combination Therapy on AGS Cell Growth Xenografted in Nude Mice
2.5. Determination of Antioxidant Activities of Individual Treatment in the Xenografted AGS Cells
2.6. Inhibition Test for the Determination of the Action of Combination Therapy
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Culture and Cell Viability Assay
4.3. Western Blot Analysis
4.4. MitoSOX Staining
4.5. Quantification of Reactive Oxygen Species (ROS) and Apoptosis
4.6. Real-Time Quantitative PCR
4.7. In Vivo Xenograft Model
4.8. Immunohistochemical Analyses
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Mito-FF | Mitochondria-accumulating phenylalanine dipeptide with triphenyl phosphonium |
| ROS | Reactive oxygen species |
| 5-FU | 5-Flurouracil |
| NAC | N-acetyl-L-cysteine |
| AGS | Adeonocarcinoma gastric cell |
| NRF-2 | Nuclear factor erythroid 2-related factor 2 |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| c-PARP | Cleaved poly-ADP (adenosine diphosphate)-ribose polymerase; c-caspase-3: cleaved caspase-3 |
| c-caspase-9 | cleaved caspase-9 |
| Mcl-1 | myeloid cell leukemia-1 |
| PUMA | nuclear factor erythroid 2-related factor 2 |
| BAX | bcl-2-like protein 4 |
| Bcl-XL | B-cell lymphoma-extra large |
| DMSO | dimethyl sulfoxide |
| HO-1 | heme oxygenase 1 |
References
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Jeena, M.T.; Palanikumar, L.; Go, E.M.; Kim, I.; Kang, M.G.; Lee, S.; Park, S.; Choi, H.; Kim, C.; Jin, S.M.; et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat. Commun. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhu, P.; Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Frantz, M.C.; Wipf, P. Mitochondria as a target in treatment. Environ. Mol. Mutagen. 2010, 51, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi. Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Seitz, L.C.; Abramczyk, A.M.; Chan, C. Synergistic effect of cAMP and palmitate in promoting altered mitochondrial function and cell death in HepG2 cells. Exp. Cell Res. 2010, 316, 716–727. [Google Scholar] [CrossRef]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef]
- Lehn, J.M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, F.; Xu, H.; Yaseen, M.; Shan, H.; Hauser, C.A.; Zhang, S.; Lu, J.R. Molecular self-assembly and applications of designer peptide amphiphiles. Chem. Soc. Rev. 2010, 39, 3480–3498. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Fratzl, P. Biological and Biomimetic Materials. Adv. Mater. 2009, 21, 387–388. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Duan, L.; Qi, W.; Wang, K.W.; Cui, Y.; Yan, X.H.; Li, J.B. Microcapsules containing a biomolecular motor for ATP biosynthesis. Adv. Mater. 2008, 20, 2933–2937. [Google Scholar] [CrossRef]
- Nie, Z.H.; Kumacheva, E. Patterning surfaces with functional polymers. Nat. Mater. 2008, 7, 277–290. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired by Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Sanchez, C.; Arribart, H.; Guille, M.M.G. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef]
- Cui, H.G.; Webber, M.J.; Stupp, S.I. Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. Biopolymers 2010, 94, 1–18. [Google Scholar] [CrossRef]
- Iscen, A.; Schatz, G.C. Peptide amphiphile self-assembly. Epl-Europhys. Lett. 2017, 119, 38002. [Google Scholar] [CrossRef]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe Motif for Peptide Self-Assembly in Nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Vaks, L.; Carny, O.; Trudler, D.; Magno, A.; Caflisch, A.; Frenkel, D.; Gazit, E. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat. Chem. Biol. 2012, 8, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Chandel, N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015, 11, 9–15. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Durak, I.; Karaayvaz, M.; Kavutcu, M.; Cimen, M.Y.B.; Kacmaz, M.; Buyukkocak, S.; Ozturk, H.S. Reduced antioxidant defense capacity in myocardial tissue from guinea pigs treated with 5-fluorouracil. J. Toxicol. Environ. Health A 2000, 59, 585–589. [Google Scholar]
- Lamberti, M.; Porto, S.; Marra, M.; Zappavigna, S.; Grimaldi, A.; Feola, D.; Pesce, D.; Naviglio, S.; Spina, A.; Sannolo, N.; et al. 5-Fluorouracil induces apoptosis in rat cardiocytes through intracellular oxidative stress. J. Exp. Clin. Canc Res. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, S.C.; Kim, O.H.; Kim, K.H.; Song, K.Y.; Lee, S.K.; Choi, B.J.; Jeong, W.; Kim, S.J. HSP90 inhibitor 17-DMAG exerts anticancer effects against gastric cancer cells principally by altering oxidant-antioxidant balance. Oncotarget 2017, 8, 56473–56489. [Google Scholar] [CrossRef][Green Version]
- Maurya, S.K.; Mishra, R. Pax6 Binds to Promoter Sequence Elements Associated with Immunological Surveillance and Energy Homeostasis in Brain of Aging Mice. Ann. Neurosci. 2017, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Panayiotou, E.; Papacharalambous, R.; Antoniou, A.; Christophides, G.; Papageorgiou, L.; Fella, E.; Malas, S.; Kyriakides, T. Genetic background modifies amyloidosis in a mouse model of ATTR neuropathy. Biochem. Biophys. Rep. 2016, 8, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, T.; Jin, Y.; Fu, Z. Effects of age and jet lag on D-galactose induced aging process. Biogerontology 2009, 10, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Jo, M.J.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jeong, Y.A.; Kim, B.G.; et al. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, Y.; Cheng, M.; Zeng, C.Y.; Yang, Z.; Zhou, X.D.; Chen, J.; Lu, N.H. Establishment and Characterization of a Nude Mouse Model of Subcutaneously Implanted Tumors and Abdominal Metastasis in Gastric Cancer. Gastroenterol. Res. Pract. 2017, 2017, 6856107. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Kang, S.A.; Chang, J.W.; Liu, Q.S.; Zhang, J.M.; Gao, Y.; Reichling, L.J.; Sim, T.B.; Sabatini, D.M.; Gray, N.S. An ATP-competitive Mammalian Target of Rapamycin Inhibitor Reveals Rapamycin-resistant Functions of mTORC1. J. Biol. Chem. 2009, 284, 8023–8032. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.J.; Jeena, M.T.; Kim, O.-H.; Hong, H.-E.; Seo, H.; Ryu, J.-H.; Kim, S.-J. Novel Therapeutic Application of Self-Assembly Peptides Targeting the Mitochondria in In Vitro and In Vivo Experimental Models of Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 6126. https://doi.org/10.3390/ijms21176126
Kim DJ, Jeena MT, Kim O-H, Hong H-E, Seo H, Ryu J-H, Kim S-J. Novel Therapeutic Application of Self-Assembly Peptides Targeting the Mitochondria in In Vitro and In Vivo Experimental Models of Gastric Cancer. International Journal of Molecular Sciences. 2020; 21(17):6126. https://doi.org/10.3390/ijms21176126
Chicago/Turabian StyleKim, Dong Jin, M. T. Jeena, Ok-Hee Kim, Ha-Eun Hong, Haeyeon Seo, Ja-Hyoung Ryu, and Say-June Kim. 2020. "Novel Therapeutic Application of Self-Assembly Peptides Targeting the Mitochondria in In Vitro and In Vivo Experimental Models of Gastric Cancer" International Journal of Molecular Sciences 21, no. 17: 6126. https://doi.org/10.3390/ijms21176126
APA StyleKim, D. J., Jeena, M. T., Kim, O.-H., Hong, H.-E., Seo, H., Ryu, J.-H., & Kim, S.-J. (2020). Novel Therapeutic Application of Self-Assembly Peptides Targeting the Mitochondria in In Vitro and In Vivo Experimental Models of Gastric Cancer. International Journal of Molecular Sciences, 21(17), 6126. https://doi.org/10.3390/ijms21176126






