Abstract
Bile acids are commonly known as digestive agents for lipids. The mechanisms of bile acids in the gastrointestinal track during normal physiological conditions as well as hepatic and cholestatic diseases have been well studied. Bile acids additionally serve as ligands for signaling molecules such as nuclear receptor Farnesoid X receptor and membrane-bound receptors, Takeda G-protein-coupled bile acid receptor and sphingosine-1-phosphate receptor 2. Recent studies have shown that bile acid signaling may also have a prevalent role in the central nervous system. Some bile acids, such as tauroursodeoxycholic acid and ursodeoxycholic acid, have shown neuroprotective potential in experimental animal models and clinical studies of many neurological conditions. Alterations in bile acid metabolism have been discovered as potential biomarkers for prognosis tools as well as the expression of various bile acid receptors in multiple neurological ailments. This review explores the findings of recent studies highlighting bile acid-mediated therapies and bile acid-mediated signaling and the roles they play in neurodegenerative and neurological diseases.
1. Introduction
Bile acids are amphipathic molecules synthesized in the liver, stored in the gallbladder and released into the intestinal lumen in response to food intake as a digestion mechanism. Their primary function is to serve as detergents in the solubilization of dietary lipids and fat-soluble vitamins. The majority of bile acids are passively or actively recovered throughout the intestinal tract and then returned to the liver for recycling via enterohepatic circulation with a small percentage of bile acids excreted as waste. Bile acids maintain secondary functions as steroid hormones and influence metabolic processes as potent signaling molecules via membrane-bound receptors such as sphingosine-1-phosphate receptor 2 (S1PR2) and Takeda G-protein-coupled bile acid receptor 5 (TGR5) and nuclear receptors such as Farnesoid X receptor (FXR) [1]. Surprisingly, emerging evidence suggests that bile acid signaling may also play a role in the physiology and pathophysiology of the brain. Furthermore, the usage of the bile acids ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) may possess therapeutic benefits in neurological ailments due to their neuroprotective properties, lack of cytotoxicity and permeability across the blood brain barrier (BBB), shown with UDCA in clinical studies [2] and TUDCA in animal models [3]. For some neurodegenerative diseases clinical trials implementing bile acid treatments may offer therapeutic potential, from a phrase III trial with UDCA [4] and to follow-up tracking of long term chenodeoxycholic acid (CDCA) efficacy [5]. Furthermore, in neurological disorders, there have been an increased amount of published studies implementing the use of bile acids or specifically targeting bile acid signaling. In this review, we focused on the signaling pathways of bile acids relevant to the CNS and their direct influence in the pathologies of neurological and neurodegenerative diseases.
2. Bile Acids Synthesis, Metabolism and Enterohepatic Circulation
Each day roughly 500mg of cholesterol is converted into bile acids in the adult human liver. There are two major bile acid synthetic pathways: the classic (or neutral) pathway that occurs in the liver and the alternate (or acidic) pathway found in peripheral tissues and the liver. A pathway for cholesterol regulation in the brain, the neural cholesterol clearance pathway, was discovered more recently and will be discussed at length below. In humans, cholic acid (CA) and CDCA are the only primary bile acids synthesized [6]. For rodents, their bile acid pool composition consists of primary bile acids CA, CDCA and the creation of α-muricholic acid (MCA) and β-muricholic (β-MCA) acid from CDCA [7]. Studies have reported sex differences in bile acid metabolism in healthy humans, with men displaying a higher percentage of fasting plasma concentrations of individual bile acids and total bile acids, increased by 111% and 51%, respectively [8]. Serum comparisons for bile acid profiles even showed significantly lower amounts of primary bile acids CA and CDCA when comparing women to men [9]. The variance of these findings with circulating bile acids may be useful when therapeutic drugs are being implemented for clinical trials.
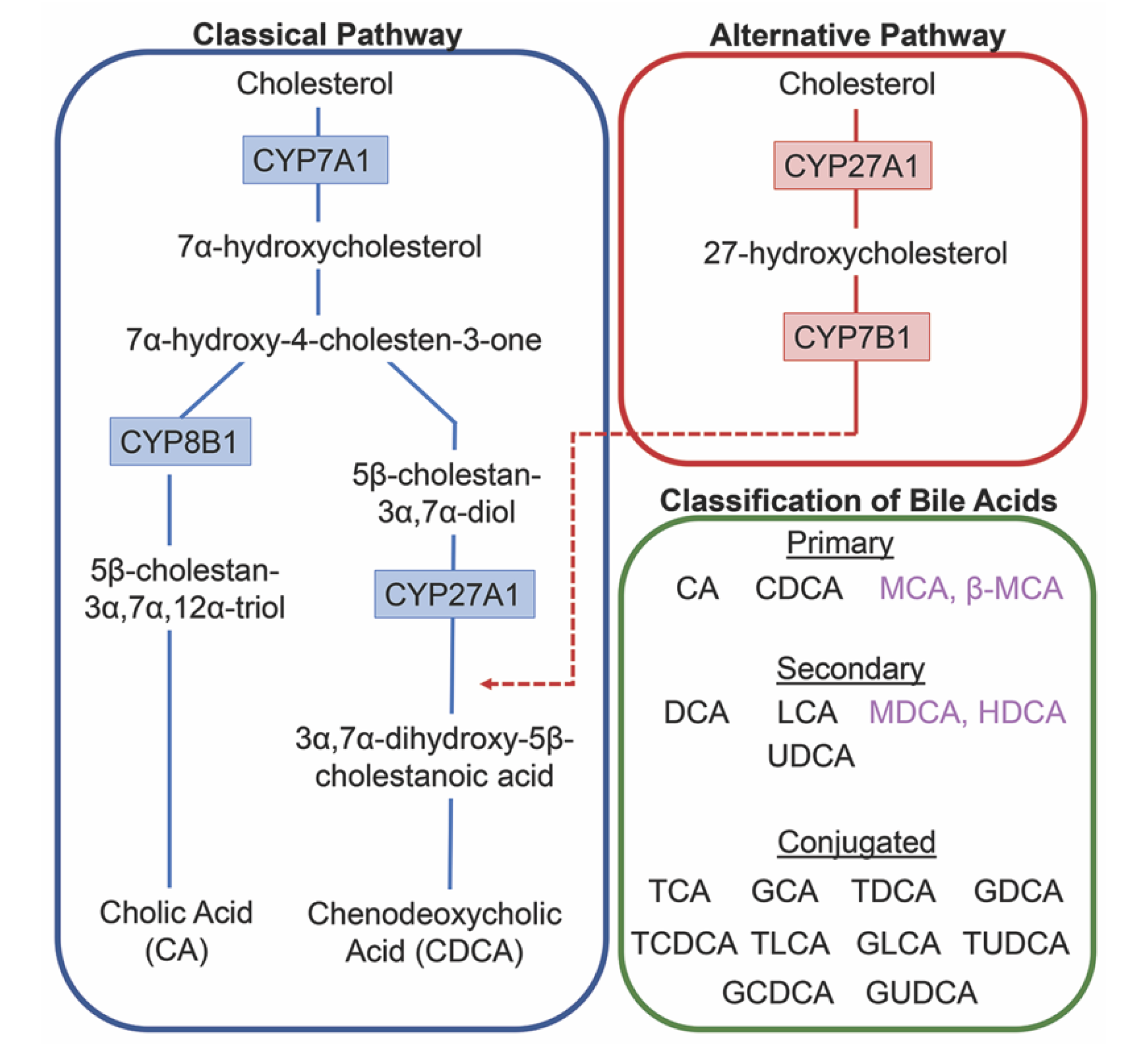
The conversion of cholesterol to primary bile acids is facilitated by a family of unique cytochrome P450 enzymes that are located in the cytosol, endoplasmic reticulum, mitochondria, and peroxisomes. Expressed solely in the hepatocytes, the classic bile acid synthesis pathway is initiated via 7α-hydroxylase (CYP7A1) converting cholesterol into 7α-hydroxycholesterol with the resulting metabolic products of primary bile acids synthesized to CA via sterol 12α-hydroxylase (CYP8B1) or CDCA by sterol 27-hydroxylase (CYP27A1) [10]. In contrast, the alternative pathway catabolizes cholesterol in all tissues; cholesterol is metabolized via mitochondrial CYP27A1, converting it into 27-hydroxycholesterol. For further conversion, these midpoint metabolites are transported from peripheral tissues back to the liver to be converted to primary bile acids CA and CDCA [11]. The classic pathway is the primary route for bile acid synthesis regulated by CYP7A1, the only rate-limited enzyme in all bile acid synthesis. More than 90% of total bile acid production in humans is sourced from this pathway, with less than 10% of the total bile acids coming from the alternative pathway during routine physiological conditions [7]. In contrast, in healthy wild type mice, only 60% of their total bile composition is sourced from the classical pathway due to their bile acid pool, including the addition of MCA and β-MCA that are not present in healthy humans [12]. Enterohepatic circulation allows the total bile salt pool to undergo 4–12 cycles a day, an efficient method of reabsorption and recycling that ensures minimal bile acid loss via urinary or fecal excretion [13]. A summary of the bile acid synthesis pathways can be seen in Figure 1.
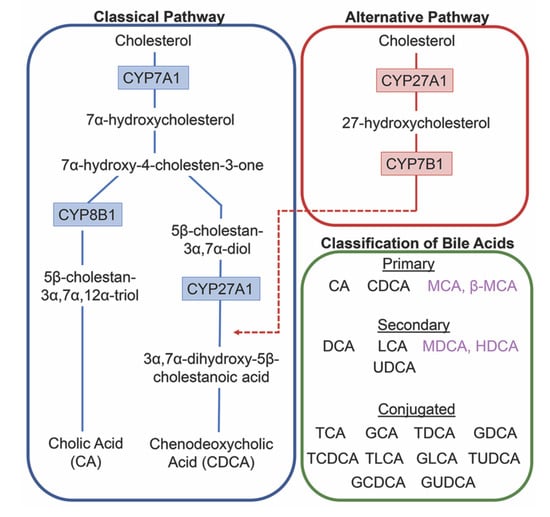
Figure 1.
Bile acid synthesis pathways. The classic pathway for bile acid synthesis occurs in the hepatocytes of the liver via 7α-hydroxylase (CYP7A1) converting cholesterol into 7α-hydroxycholesterol. Primary bile acid cholic acid (CA) is formed after subsequent conversions from sterol 12α-hydroxylase (CYP8B1) and chenodeoxycholic acid from sterol 27-hydroxylase (CYP27A1). In the alternative or acidic pathway, mitochondrial CYP27A1 in peripheral tissues convert cholesterol into 27-hydroxycholesterol. Oxysterol 7α-hydroxylase (CYP7B1) is an additional assisting enzyme in this pathway and the resulting products feed back into the liver, indicated by the red arrow feeding into the classical pathway under CYP27A1. Primary and secondary bile acids specific to rodents are listed in purple. Bile acids can become conjugated with glycine or taurine after interactions with gut flora.
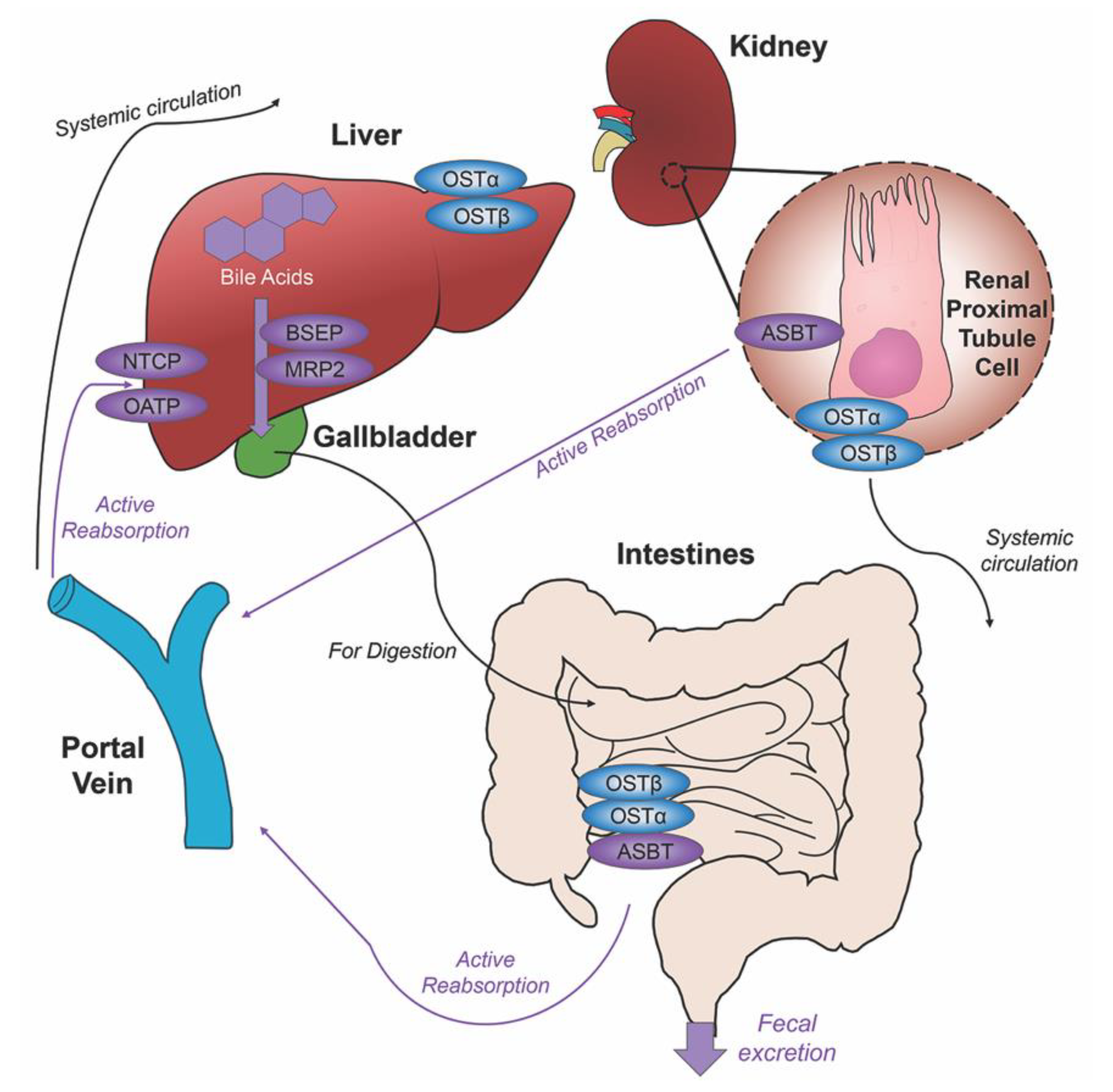
Before de novo primary bile acids CA and CDCA are released from the liver, some are conjugated with either glycine (in humans) or taurine (in mice), granting increased water solubility and reduced cytotoxicity to fulfill their dietary roles of lipid emulsification throughout the intestines. Taurocholic acid (TCA) and glycocholic acid (GCA) are synthesized from CA, taurochenodeoxycholic acid (TCDCA) and glycochenodeoxycholic acid (GCDCA) are synthesized from CDCA. Along with CA and CDCA, these newly synthesized conjugated bile acids are transported from hepatocytes to the bile canaliculus via the bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2) for storage in the gallbladder awaiting their release into the intestinal lumen with the intake of food. Once nutrients enter the stomach, they trigger the gallbladder to release bile acids into the duodenum where they contribute to the digestion of lipids and fat-soluble vitamins. As bile acids continue through to the ileum, unconjugated and some glycine-conjugated bile acids will be reabsorbed via passive diffusion in the jejunum and colon with the majority of conjugated bile acids requiring active reabsorption via the apical sodium dependent bile acid transporter (ASBT) in the ileum. Other active membrane transporters sodium taurocholate cotransporting polypeptide (NTCP) and organic anion transport polypeptide (OATP) in hepatocytes mediate in bile acid reuptake once they’ve entered portal venous circulation [14].
Unconjugated primary bile acids not passively reabsorbed will interact with the intestinal bacteria flora present in the colon, creating secondary bile acids deoxycholic acid (DCA) from CA and lithocholic acid (LCA) and UDCA from CDCA for humans [15], with the secondary bile acids of murideoxycholic acid (MDCA) and hyodeoxycholic acid (HDCA) for mice [16]. When these secondary bile acids are recirculated back to the liver, conjugation with glycine or taurine can further differentiate them, such as the addition of taurine to UDCA forms TUDCA [17]. The enterohepatic circulation efficiently reclaims approximately 95% of bile acids and minimizes fecal and urinary bile acid expulsion with the help of a collective transporter process. Located on the membranes of ileocytes, proximal renal tubule cells and cholangiocytes, ASBT facilitates the absorption of the majority of bile acids lacking passive diffusion qualifications or reclaims bile acids in systemic circulation for portal venous distribution back to the liver, minimizing excretion in urine. The heteromeric organic solute transporter (OST) α and β located in the cytosol of renal proximal tubule cells, ileocytes and hepatocytes direct bile acids to systemic circulation [18], a process which when malfunctioning could exacerbate elevated systemic bile acids levels affecting the blood brain barrier [19,20]. A graphic depiction of the enterohepatic circulation of bile acids is shown in Figure 2.
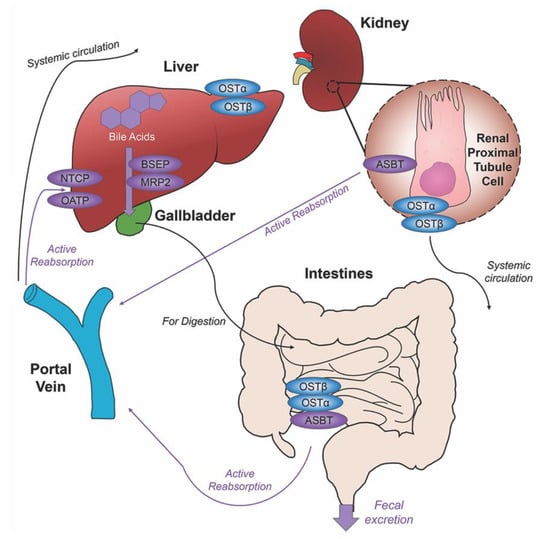
Figure 2.
Enterohepatic circulation of bile acids. After primary bile acids are synthesized in the liver, the bile acid transporters bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2) facilitate their storage in the gallbladder, indicated via thick purple arrow, to be released in the intestines to aid in the digestion of food. Following food intake, bile acids are released into the duodenum for the digestion of lipids and fat-soluble vitamins, bile acid movement indicated via black arrow. Some bile acids can be reabsorbed through passive diffusion in the jejunum and colon throughout the journey, while the majority of conjugated bile acids can interact with the apical sodium dependent bile acid transporter (ASBT) in the ileum for active reabsorption, indicated by multiple purple arrows. Other bile acid transporters sodium taurocholate cotransporting polypeptide (NTCP) and organic anion transport polypeptide (OATP) expressed in hepatocytes mediate active reabsorption back to the liver. Bile acids in systemic circulation will be reabsorbed by ASBT in the renal proximal tubule cells of the kidney and directed back to the liver via the portal vein. Heteromeric organic solute transporter (OST) α and β in renal proximal tubule cells, ileocytes and hepatocytes direct bile acids into systemic circulation. The efficiency of this system recycles and minimizes fecal and urinary bile acid loss by excretion.
Bile acids can undergo an additional elimination pathway consisting of glucuronidation, a process that converts hydrophobic bile acids into excretable metabolites. Uridine 5′-diphosphate-glucuronosyltransferase (UDP-glucuronosyltransferase, UGT) are multigenic enzymes that catalyze the glucuronidation reaction, conjugating glucuronic acid with exogenous and endogenous molecules. Aiding in bile acid detoxification, the resultant hydrophilic glucuronide products possess increased ability for urinary excrement [21]. Glucuronidation of bile acids leads to the important introduction of a negative charge to the molecule, allowing transport by conjugate-transporters that can facilitate bile acid-glucuronide secretion. Multidrug resistance-associated protein 1 (MRP1) and 3 (MRP3) expressed across the basolateral hepatocyte membrane aid in the efflux of glucuronides [22]. Bile acid glucuronides are present in hepatic dysfunction, with increased concentrations of glucuronidated bile acids CDCA and LCA in the plasma of patients with hepatobiliary diseases [23]. In biliary obstruction patients whose bile flow had been restored via stenting, the urinary composition of bile acid glucuronides was increased [24].
Several UGT genes have been identified in human, mouse, rat and other mammalian species. The gene superfamily consists of four UGT families, UGT1, UGT2, UGT3 and UGT8, with enzymes of UGT1 and UGT2 families the most efficient at glucuronic acid transfer [25]. Of the 18 UGT enzymes, three enzymes can be attributed to glucuronidation of bile acids: UGT2B4 for bile acids such as HDCA, UGT2B7 for primary, secondary and hydroxylated bile acids, and lastly UGT1A3 for bile acids such as CDCA, LCA, and HDCA [26]. While many UGT isoforms are predominately expressed in the liver, these enzymes are also expressed in a variety of extrahepatic tissues including the small intestines, colon, bladder, kidney, ovaries, uterus, testis, and stomach [27]. UGT expression levels and glucuronidation activity have been detected in all nine regions of the rat brain [28] and are present in the human brain [29]. Lastly, UGT has been identified in several neural cell types: neurons [30], astrocytes [30,31] and microglia [32].
3. Bile Acids in the Brain
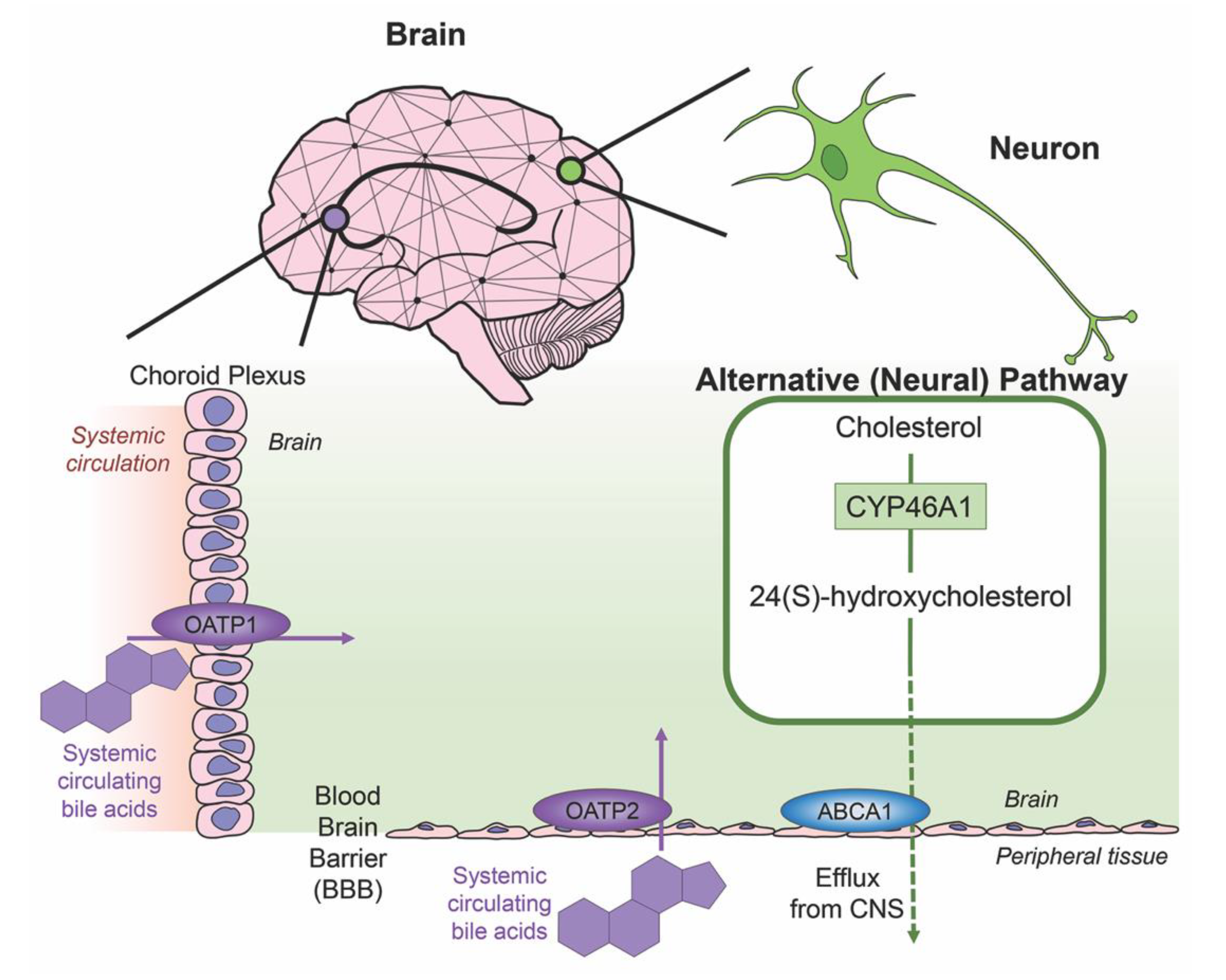
Cholesterol is an essential component of neural development and in the composition of neurons and neuroglia, with nearly 25% of the total body cholesterol found in the brain [33]. It is a major component of the lipid molecules in the membranes of neuron and glial cells, a large fraction in the myelination performed by oligodendrocytes and is involved in the synthesis of steroid hormones [34]. While local cholesterol biosynthesis is observed at higher rates in glial cells than neurons [35], neurons solely possess the ability for cholesterol clearance. The last alternative pathway for bile acid synthesis is in the brain (shown in Figure 3), catalyzing cholesterol by neuron-specific sterol 24-hydroxylase (CYP46A1) and converting this into 24(S)-hydroxycholesterol; the increased solubility of this intermediate allows for efflux from neural tissue via the BBB through lipoprotein transport ATP-binding cassette transporter 1 (ABCA1) [36]. Specific bile acid transporters allowing the influx of bile acids from the periphery into the CNS also exist. For example, OATPs, with rat OATP1 expressed in the choroid plexus and rat OATP2 highly expressed at the BBB allow for the influx of bile salts and a variety of other amphipathic organic compounds into the CNS [37]. Similarly, subpopulations of neurons, particularly in the hypothalamus express the transported ASBT which facilitates the internalization of bile acids into neurons where they have been shown to influence the activity of the hypothalamic–pituitary–adrenal (HPA) axis [38,39].
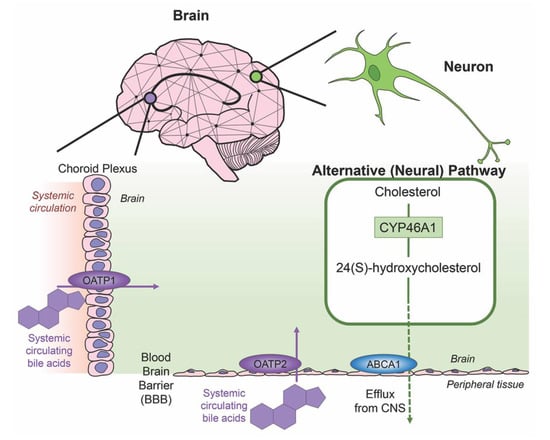
Figure 3.
Neural cholesterol clearance pathway and bile acid transport into the CNS. Cholesterol is catalyzed in the brain via sterol 24-hydroxylase (CYP46A1), an enzyme expressed only in neurons. It is converted to 24(S)-hydroxycholesterol and is able to be removed from the CNS through the blood brain barrier (BBB) via the transporter ATP-binding cassette transporter 1 (ABCA1), indicated via green arrow. Other transporters mediate systemic circulating bile acids into the CNS. Organic anion transporter polypeptide 1 (OATP1) expressed in the choroid plexus and organic anion transporter polypeptide 2 (OATP2) expressed at the BBB both mediate the transport of bile acids, both processes indicated by purple arrows.
Bile acid functionality increases when acting as ligands for nuclear receptors farnesoid X receptor (FXR), the pregnane X receptor (PXR), the vitamin D receptor (VDR), and membrane receptors Takeda G-protein-coupled receptor 5 (TGR5; a G-protein-coupled receptor also called G-protein-coupled bile acid receptor 1, GPBAR-1), sphingosine-1-phosphate receptor 2 (S1PR2) and α5β1 integrin. These receptors are highly expressed in the liver and the intestines but also display activity in a variety of tissues throughout the body including the brain [40]. Bile acids can act as signaling molecules to modulate their own homeostasis. Among the bile acid receptors, FXR plays many important roles in the regulation mechanisms of bile acid synthesis and transport. FXR activation via bile acids can induce the expression of BSEP [41,42], regulating the canalicular secretion of bile acids into bile. Key players in bile acid synthesis, CYP7A1 and CYP27A1, can be repressed by FXR [22] and human UGT2B4, involved in the conversion of hydrophobic bile acids to their less toxic glucuronide derivatives, can be upregulated by FXR [43].
The affinities of primary, secondary and conjugated bile acids with individual bile acid receptors vary. Bile acids can serve as weak activators of the glucocorticoid receptor (GR) in the brain to influence the HPA axis [39]. LCA, a hydrophobic and cytotoxic bile acid, has been shown as a weak ligand for FXR with the ability to decrease BSEP expression [44]. This same bile acid is the most potent bile acid for TGR5 [45], displaying anti-tumor effects in human neuroblastoma cell cultures [46] as well as pro-apoptotic effects in breast cancer cells [47]. The primary bile acid CDCA is the most potent activator for FXR, with CA and secondary bile acids DCA and LCA showing less activation [48]. Both nuclear receptors PXR [49] and VDR [50] can be activated by secondary bile acid LCA. There are even conjugated bile acids with selective activity for receptors, such as TUDCA for α5β1 integrin [51] and TCA for S1P2R [52]. Other reviews have eloquently covered the liver and intestinal focused signaling of these receptors [11,53,54,55]. Given these points, Table 1 lists bile acid-mediated receptors that are relevant in the CNS.

Table 1.
Bile Acid Receptors in the CNS.
4. Bile Acids in Neurodegenerative Diseases
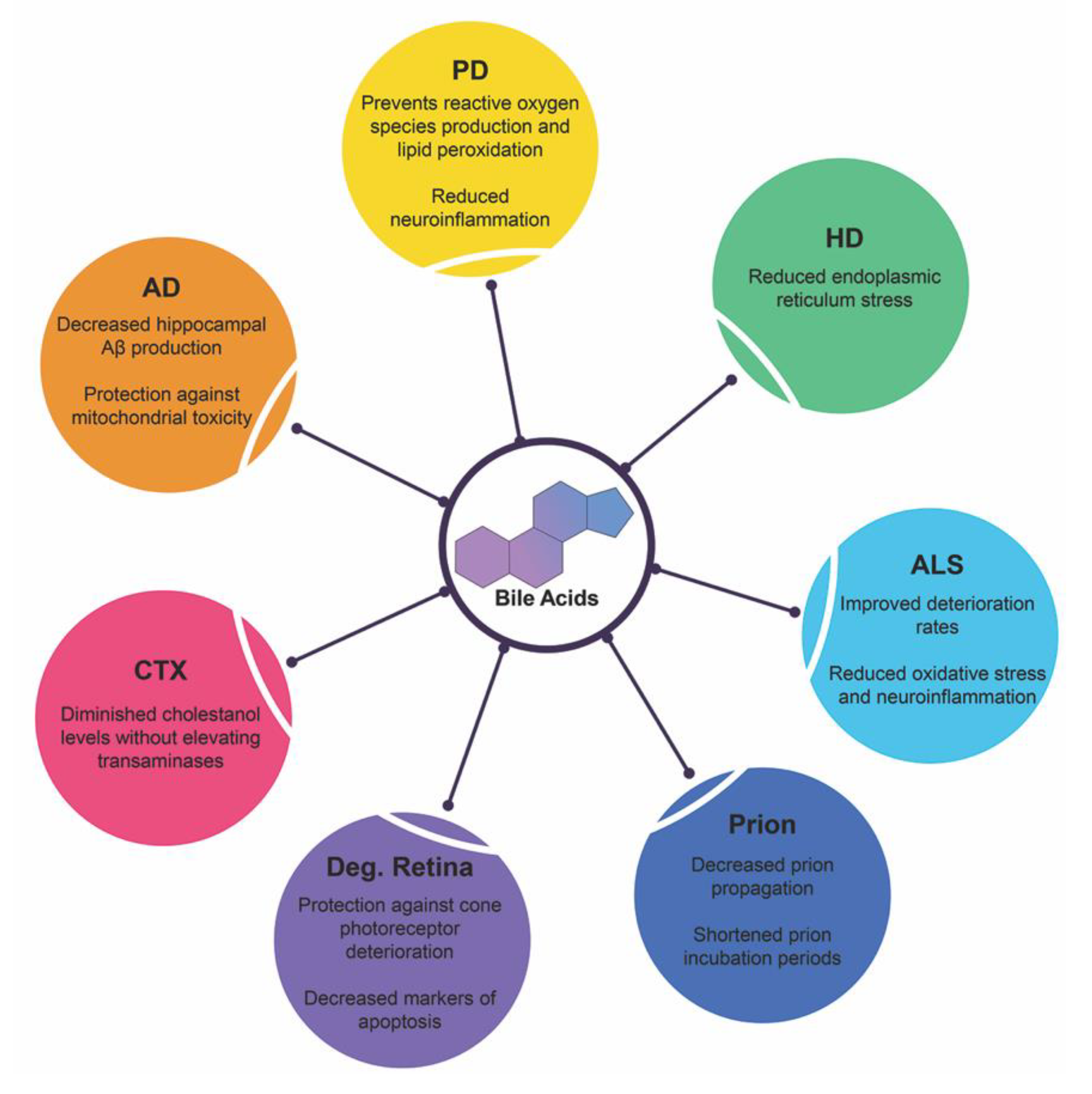
Affecting millions worldwide, neurodegenerative diseases stem from a variety of factors. The exact mechanism underlying the route of pathogenesis for each disease state varies but commonality exists between them all: accumulation of misfolded/mutated protein and aberrant pathways of endoplasmic reticulum stress leading to increased dysfunction, widespread neuronal loss and cerebral atrophy. Below is recent research highlighting bile acid signaling and its therapeutic potentials in these debilitating diseases and is summarized in Figure 4.
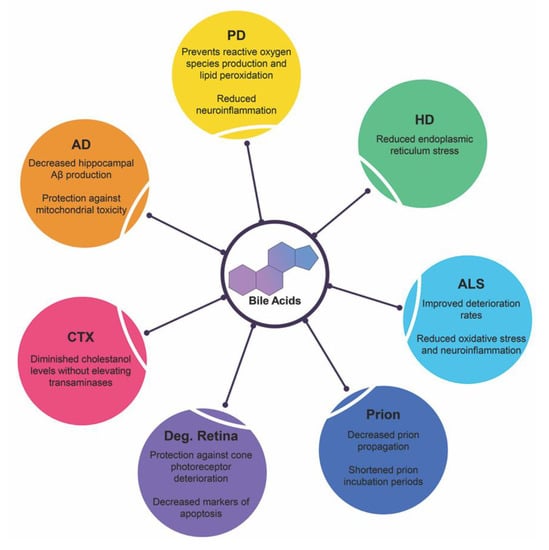
Figure 4.
Neuroprotective functions of bile acids in neurodegenerative diseases. Recent clinical trials and experimental animal studies have shown the protective qualities of therapeutic bile acids in these disease states. Abbreviations: Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), prion diseases (Prion), degenerative retina diseases (Deg. Retina), Cerebrotendinous xanthomatosis (CTX).
4.1. Alzheimer’s Disease
The most common cause of dementia, Alzheimer’s disease (AD) is an aggressive and fatal degenerative disease with etiologies stemming from the combination of aggregated beta-amyloid plaques and tau tangles, neuroinflammation, massive neuronal demise, and cerebral atrophy [73]. The burden of this disease is magnified by the progressive decline in cognitive and motor functions, marked by subjective cognitive decline (the worsening memory loss and inability to remember common routine tasks), confusion with time/location and distinct changes in mood and personality [74]. Different length Aβ are produced by the amyloid precursor protein (APP). The terminal which results from subsequent proteolytic cleavages yields various Aβs, with the Aβ42 peptide as the most linked to disease development due to hydrophobicity and liability of aggregation. Animal models utilized to understand AD pathogenesis focus on mutations of human genes APP, PSEN1 and PSEN2 that modulate amyloid β peptides (Aβ) via the γ-secretase complex and lipid metabolism via apolipoprotein E (ApoE) which focuses on Aβ clearance mechanisms [75].
The presence of bile acids in AD is an emerging topic of research, with altered bile acid compositions providing novel insight. A recent clinical study took plasma from 30 healthy controls, 20 subjects with mild cognitive impairment and 30 subjects with clinical AD and performed widespread bile acid testing. Levels of LCA were significantly increased in AD patients compared to the controls whereas levels of glycochenodeoxycholic acid, glycodeoxycholic acid and glycolithocholic acid were significantly elevated in AD patients compared to mild cognitive impairment patients. The presence of LCA and these glycine-conjugated bile acids demonstrate helpful biomarker qualities for diagnostic purposes [76], although how these bile acids may be contributing to the pathogenesis of AD is unknown.
In pre-clinical studies, targeting bile acid signaling has also been identified as a potential experimental therapy to alleviate various aspects of AD. Using a surgical model of AD, Aβ toxicity induced via single intracerebral ventricular injection of Aβ1-42, treatment with INT-777 (6α-ethyl-23(S)-methylcholic acid, a TGR5 agonist) significantly attenuated the cognitive impairment and decreased neuroinflammation, as measured by decreased proinflammatory tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) cytokine production and microglia activation [77]. In contrast, an in vitro study using an SH-SY5Y treated Aβ1-42 AD cell model noted FXR overexpression triggered neuronal apoptosis via activation of the cAMP-response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF) pathway [78]. Furthermore, the addition of 6α-ethyl-chenodeoxycholic acid (6ECDCA), an FXR agonist, aggravated the Aβ-induced apoptosis, whereas knockdown of FXR in these cells, both basal and Aβ-induced, inhibited neuronal apoptosis [78]. Together these data would suggest opposing actions of the bile acid receptors TGR5 and FXR in the pathogenesis of AD.
Other studies utilized bile acids to alter different mechanisms of AD pathogenesis, from impeding Aβ production to improving mitochondrial function. A rat model of AD neurotoxicity using intraperitoneal injections of AlCl3 for six weeks noted that daily injections of CDCA significantly attenuated AlCl3-induced cognitive and spatial deficits markedly similar to the control and decreased hippocampal Aβ production via Aβ42 levels. Hematoxylin and eosin staining morphologically indicate a CDCA neuroprotective effect on the control and CDCA + AlCl3 groups when compared to the severe neuronal degradation in the AlCl3-treated group [79]. Lastly, mitochondrial damage and morphological abnormalities are implicated in patients with sporadic and familial AD and factors such as dynamin-related protein 1 (Drp1), are known to protect against AD-related mitochondrial toxicities [80,81]. Treatment with the bile acid UDCA exerts a neuroprotective effect on mitochondrial membrane potential and morphology of primary fibroblasts through fission and fusion modulator Drp1 [82]. Taken together, while data to suggest that bile acids and bile acid signaling is involved in the pathogenesis of AD are sparse, the use of bile acids as therapeutic options for the treatment of AD is promising.
4.2. Parkinson’s Disease
After AD, Parkinson’s disease (PD) is the second most common neurodegenerative disease marked by progressive motor deterioration. Dopaminergic neuronal death and α-synuclein-containing Lewy bodies in the substantia nigra are two known characteristics although the majority of PD cases are of sporadic origin [83]. Animal models replicate this pathology using neurotoxins, genetic mutations or combinations of the two [84]. Phenotypically, clinical diagnosis of PD is more recognizable at later stages, when motor deficits are apparent due to the misfolded α-synuclein proteins spreading to additional parts of the brain and subsequently affecting the substantia nigra. However therapeutic options starting prior to the onset of motor symptoms (prodromal phase), would be the most beneficial in slowing the disease progression thus highlighting the importance of identifying key biomarkers for successful diagnosis [85].
PD research utilizing surgical rodent models of PD observed bile acid metabolism alterations and potential bile acid markers. Using a prodromal PD mouse model created by injecting human α-synuclein fibrils and human α-synuclein monomers (as a control) via stereotactic unilateral injection, serum and brain tissue from the mice was analyzed for metabolomics. Metabolite pathway analysis in the brain tissue of the α-synuclein fibrils treated mice yielded significant alterations of four biochemical pathways: taurine and hypotaurine metabolism, bile acid biosynthesis, glycine, serine and threonine metabolism and the citric acid cycle. The taurine and hypotaurine metabolism pathway that was disrupted includes taurine which has the crucial role in the conjugation of neuroprotective TUDCA and UDCA [86]. An adeno-associated virus-α-synuclein injected bilaterally into the substantia nigra of rats noted that overexpression of α-synuclein, which additionally is expressed in enteric neurons, altered their gut microbiome. Along with diversifying the gut microbiome, this overexpression significantly increased the level of free bile acids and primary bile acids (CA, total MCA and β-MCA), and additionally increased secondary bile acids (taurodeoxycholic acid, taurohyodeoxycholic acid and DCA) irrespective of influence from exercise [87]. Another study further clarified the presence of bile acids using a surgical mouse prodromal PD model, with three being found significantly decreased in the serum of the α-synuclein-fibrils-treated group: omega-muricholic acid, TUDCA and UDCA. UDCA and TUDCA, both neuroprotective secondary bile acids that can pass the BBB, were markedly affected with a 17- and 14-fold decrease from the control group [88]. These surgical rodent model studies, replicating aspects of PD, shows increased research in this field will assist in therapeutic changes.
Other recent PD research has used anti-inflammatory secondary bile acids TUDCA and UDCA in experimental therapy studies. Decreased mitochondrial activity has been implicated in PD; the mitochondrial inhibitor 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) replicating glial activation and the pro-inflammatory cytokine cascade of PD. A series of TUDCA injections were introduced prior to and after the MPTP-injection in a mouse model of PD. Motor capabilities improved in the MPTP-treated + TUDCA groups in comparison to MPTP-treated mice along with the ability to initiate movements and amend tremors. Parkin levels, an E3 ubiquitin ligase associated with mitochondrial biogenesis, were decreased in MPTP-treated mice and were attenuated in mice treated with TUDCA prior to MPTP [89]. This same group looked into dopaminergic cell death, oxidative stress and reactive oxygen species (ROS), using the same MPTP-induced PD mouse model. SH-SY5Y cells were treated with 1-methyl-4-phenylpyridinium (MPP+) or doxycycline for two in vitro PD models, displaying TUDCA’s antioxidant qualities in both by preventing ROS production and lipid peroxidation through increased nuclear factor erythroid 2 related factor 2 (Nrf2) expression. TUDCA’s neuroprotective potential was replicated in vivo with the MPTP-induced mouse model, reverting ROS production caused by MPTP and increasing the expression of Nrf2 and Nrf2 downstream cytoprotective enzymes, glutathione peroxidase and heme oxygenase-1 [90]. Lastly, a rotenone-induced PD model using rats with daily intraperitoneal injections of UDCA resolved striatal dopamine content close to the control group level and significantly downregulated nuclear factor-κB (NF-κB), BCL2 associated X apoptosis regulator (Bax) and caspase-9 mRNA levels. Striatal TNF-α and IL-1β levels that were significantly increased in the rotenone-treated group were attenuated in the UDCA administered group. Additionally, this UDCA treatment reduced rotenone-induced alterations of striatal neuron mitochondrial and increased striatal ATP to 2-fold above the control values [91]. PD research implementing bile acid-mediated therapeutics has attenuated several harmful cellular mechanisms of this disease state.
4.3. Huntington’s Disease
Huntington’s disease (HD) is an inherited autosomal-dominant neurodegenerative disease classified by progressive motor degeneration, cognitive disorder and neuropsychiatric decline. The mutant gene huntingtin, HTT, on chromosome four induces neuronal loss in the striatum and causes multiple irregularities such as cellular proteostasis, mitochondrial and synaptic dysfunction through mutant 7–35 cytosine-adenine-guanine (CAG) repeats. The end product of multiple CAG repeats lengthens glutamine residues on the mutant huntingtin protein leading to accumulation and toxicity [92].
Clinical and rodent-focused animal research in HD lacked consideration of the direct effects of bile acid signaling but rather focused on noteworthy alterations found in pathways related to the enzymes in bile acid synthesis. One study noted a link between brain cholesterol homeostasis and a reduction of CYP46A1, the enzyme initiating cholesterol clearance in neural tissue, levels in the striatum of post-mortem patients of HD, transgenic R6/2 mice (a rodent HD model) and a striatal neuron progenitor line expressing mutant HTT. Gene therapy using a stereotaxic injection of adeno-associated virus (AAV)rh10 viral constructs for GFP or human CYP46A1 restored CYP46A1 levels in striatal neurons of R6/2 mice and increasing neuronal survival through production of sterols laneosterol and desmosterol, metabolites of CYP46A1 processing, and reestablishment of normal cholesterol levels [93]. Sphingosine-1-phosphate metabolism in HD patients and two rodent models has shown aberrant signaling of intermediates and metabolizing enzymes, with increased expression of sphingosine-1-phosphate lyase and decreased expression of sphingosine kinase 1/2 in the striatum of post-mortem humans and HD transgenic models, both in early and late stages of the HD rodent model [94]. Decreasing the bioavailability of sphingosine-1-phosphate could dismantle downstream signaling from G-protein coupled receptors sphingospine-1-phosphate receptors 1–5 [95] of which S1PR2 has been shown to have expression in neurons [62].
Other HD research delved into alternative experimental methods for creative solutions to HD’s characteristic protein accumulation. Aggregation of mutant huntingtin protein, the trademark of the disease, and the association of ER stress mechanisms has shown causative pathogenesis conditions in HD [96]. One study observed low molecular weight chemical chaperones to reduce protein accumulation and misfolding due to their ability to pass through the BBB. TUDCA showed an initial significant reduction in thapsigargin-induced ER stress comparable to other chaperones of the study (4-phenylbutyrate and docasahexaenoic acid), but the dosage response had diminished efficacy even after higher concentrations were administered [97]. Extending beyond solely implementing bile acids therapeutics, this recent HD research has shown that looking at indirect changes involving pathways related to bile acid synthesis pathways can be progressive prognosis tools.
4.4. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease marked by deterioration of the upper and lower motor neurons of the brain stem and spinal cord resulting in muscular atrophy, paralysis and a patient survival prognosis of 2–5 years. Mutations of chromosome 9 open reading frame 72 (C9orf72), fused in sarcoma (FUS), superoxide dismutase (SOD)1, and transactive response DNA-binding protein 43 (TARDBP/TDP-43) genes are commonly associated with ALS pathogenesis [98]. Animal models include glial cells along with neurons among affected cell types, with ER stress, autophagy and RNA metabolism dysfunction. Rodent ALS models are primarily transgenic knockouts of SOD1 and TDP-43 variants, with SOD1 mutant mice the only rodent model with a phenotype similar to ALS in humans [99].
The efficacy of targeting bile acid signaling as a therapeutic strategy for the treatment of ALS has been assessed in a Phase II clinical trial. A double-blind placebo controlled clinical trial was performed with a 54-week TUDCA daily oral treatment in 34 ALS patients currently taking riluzole as an add-on regimen. The treatment was well tolerated in all patients without any severe adverse effects beyond common gastrointestinal symptoms. TUDCA treatment for 1 year has potential neuroprotective effects with slowed deterioration of function in ALS patients, with a 15% increase in ALS functional rating scale (ALSFRS-R) scoring [100]. Due to the aggressive nature of ALS, studies that improve deterioration rates or increase neuronal growth show a promising future for ALS research.
Other recent ALS research focused on experimental studies with bile acid therapies instead of targeting specific bile acid signaling. An in vitro ALS model using motor neuron-like NSC-34 cells expressing wild type or G93A mutation of human SOD1 and treated with glycoursodeoxycholic acid (GUDCA). Treatment with GUDCA diminished caspase-9 levels and the amount of apoptotic nuclei present, regardless of treatment occurring at the beginning or after cell differentiation of NSC-34 cells transfected with mutated G93A. Oxidative stress and neuroinflammatory mediators of nitric oxide production and metalloproteinase-9 (MMP-9) were attenuated by GUDCA therapy, but extracellular ATP levels remained depleted [101]. Another study combined both in vitro and in vivo experiments. Human G93A mutated motor neuron cultures determined that, amongst several others, prior treatment of bile acids TCA, TUDCA and taurine-glycine-conjugated cholic acid 45 min prior to cyclopiazonic acid (CPA) addition, a mycotoxin that inhibits calcium ATPase in the ER and selectively targets motor neurons over other cell types to induce ER stress, rescued 50% of neurite growth. TUDCA displayed strong neurite outgrowth-promoting effects but insignificant motor neuron survival or relevant ER stress-related gene expression. In a smaller study with mice expressing mutated G93A, human SOD1 were used for early disease state ALS during a presymptomatic period in the hind limb muscle. Subcutaneous injections of TUDCA every 3 days for 21 days yielded a significant increase in neuromuscular junction innervation when compared to vehicle-treated animals, attenuating one of the earliest phenotypes observed in ALS mouse models [102]. With the maximum patient survival prognosis of 5 years due to the rapid deterioration of this disease, this bile acid-centric research promoting neurogenesis is a step in the right direction.
4.5. Prion Diseases
Structured around the deviant aggregation of membrane-bound prion protein PrPC found on human gene PRNP, prion diseases are incurable neurodegenerative diseases derived from sporadic (Creutzfeldt–Jakob disease, CJD), genetic (familial CJD, fatal familial insomnia and Gerstmann-Straussler-Scheinker disease) or acquired (kuru and iatrogenic CJD) origin. The pathogenic conformation PrPSc, composed of approximately 47% β-sheet in relation its benign counterpart, induces neurotoxicity and a variety of rapid manifestations of neuronal degeneration [103]. Due to the highly protease-resistant and seeding properties of PrPSc, research targeting rapid detection is crucial for therapeutic manipulations to address primary and secondary nucleation. Various rodent models of prion diseases aid in therapeutic progression with accurate expressions of the disease phenotype; direct intracerebral inoculation of PRNP or mutated PRNP transgenic mice lines have all been produced [104]. Mechanisms inducing the phosphorylation of eukaryotic translation initiation factor, eIF2α, are linked to ER stress, unfolded protein response (UPR) activation and neurodegeneration [105].
Novel prion disease research conducted has implemented experimental bile acid treatment to strictly target and delay protein aggregation plaguing prion diseases. One study looked at a series of anti-prion compounds and their effects of formation kinetics with TUDCA among them. TUDCA treatment resulted in a delay in prion fibril formation and blocked seeding in a shaking-induced conversion model for prion conversion. However, additional analysis for time-dependent prion oligomer and fibril formation yielded no anti-prion effects [106]. Another aggregation study of different mouse prion strains (RML, 22L and ME7) with TUDCA produced inhibition of lag phases and prevented exponential growth when compared to groups without TUDCA implementation. Nontoxic treatments of TUDCA and UDCA in RML-infected mouse neuroblastoma cells diminished preliminary PrPSc levels after the second passage but neither fully cleared proteinase resistant prions even after six passages. The neuroprotective elements of TUDCA and UDCA against prion propagation could additionally be observed in prion-infected cerebellar slice cultures: treatment with either bile acid at day 14 maintained levels of granule and Purkinje cells for 49 days when compared to RML-treated slices; starting treatment at 21 days post-infection yielded less beneficial effects [107].
Other studies utilized rodent prion disease models and implemented TUDCA treatments to explore dysfunctional cellular mechanisms attributed to the disease state. Another recent study compared different secondary bile acid-mediated therapies and the effects on ER stress, a relevant cellular mechanism of the disease state. A gender-difference rodent model of prion disease implemented treatment trials of TUDCA and UDCA and generated several results. Treatment of 0.4% TUDCA in chow 7 days post inoculation increased incubation periods and significantly increased phosphorylated eIF2α levels in TUDCA-treated infected male mice when compared to control infected male mice. Increased dosage to 1% TUDCA with the same experimental manipulations yielded no statistical difference in incubation periods or levels of prion protein, aggregation, ER stress markers (binding immunoglobulin protein (BiP), phosphorylated-eIF2α) or neuronal loss (neuronal nuclei, NeuN)/synaptic activity postsynaptic density protein 95, PSD95). Interestingly, treatment with 1% UDCA 100 days post inoculation, to replicate later-stage disease relevance, significantly shortened the incubation period in both mice genders yet provided a diminished survival effect—levels of PSD95 and BiP were significantly increased in UDCA-treated infected mice when compared to untreated infected female mice. Further immunohistological examinations of mice with shorter survival rates displayed symptoms consistent with prion disease and not due to UDCA toxicity [108]. While prion disease remains a fatal affliction, the increase in research implementing bile acid therapy to delaying and subsequently diminishing prion-specific protein accumulation will prove fruitful to understanding this disease state.
4.6. Degenerative Retina Diseases
Retinal degeneration diseases involve the deterioration, dysfunction and death of light-sensitive neurons, called photoreceptor cells, that leads to incurable blindness. Layered retinal cytoarchitecture combines distinguished layers of rod and cone bipolar (outer plexiform layer), amacrine (inner nuclear layer) and ganglion (inner plexiform layer) cells that establish and transfer synaptic information through to the brain via the optic nerve [109]. Combinations of genetic mutations (i.e., mutant variants of genes Peripherin or retinal pigment epithelium (RPE)65), morphological changes of the retinal pigment epithelium (RPE) and photoreceptor dysfunction (i.e., photoreceptor-specific transcription factor CRX) contribute to multiple degenerative retina diseases: glaucoma, retinitis pigmentosa (RP), age-related macular degeneration (AMD) and inherited retinal degeneration (RD). Commonly used animal models consist of zebrafish for ocular development studies, primates and other large animals with macula for human disease comprehension, and multiple transgenic mice strains with retinal and photoreceptor degeneration and cone-rod dystrophy phenotypes [110].
Recent research has focused on rodent models of experimental bile acid therapies to study the origins of photoreceptor degeneration in retina diseases. One study highlights TUDCA ability to interact with rhodopsin via a spectroscopic assay measuring the stability of rhodopsin’s photoactivated form, metarhodopsin II. Three different confirmation models of TUDCA computer docking to the binding site on metarhodopsin II gave plausible options due to the energy minimum [111]. An RP transgenic rodent line, the rd1 mouse, detected the effect of daily TUDCA intraperitoneal injections on morphological photoreceptor deterioration. TUDCA provided protective effects to cone photoreceptor function at P21 and preserved the outer nuclear layer and significant quantities of photoreceptor nuclei in the retinas of TUDCA-treated mice when compared to vehicle-treated mice [112]. Another study observed the apoptotic and oxidative stress hallmarks in RD’s photoreceptor degeneration via a chemically-induced model from administration of N-methyl-N-nitrosourea with subcutaneous treatments of TUDCA. Along with preserving retinal thickness and cone photoreceptors, expressions of apoptotic markers Caspase-3, Calpain-2 and Bax were significantly downregulated when compared to RD-induced mice. TUDCA additionally alleviated oxidative stress and increased expression of endogenous antioxidant superoxide dismutase (SOD) in TUDCA-treated mice [113].
Other studies observed degenerative retina diseases in rodent models to monitor gene expression and the effects of various bile acid-mediated treatments. An retinitis pigmentosa GTPase regulator (RPGR) conditional knockout mouse model of RP with weekly TUDCA treatments was utilized to determine signaling-transduction mechanisms of rhodopsin. Mutations of RPGR are the common cause of RP and the RPGR protein complex a regulator of protein trafficking in the retina. RPGR knockout mice displayed varied locations of rhodopsin, opsin and transducing when compared to wild type mice. Expression of nephrocystin-4 (NPHP4), a component of the RPGR unit, was also absent in connecting cilium along with scaffold protein NOD-like receptor family pyrin domain containing receptor 3 (NLRP3) colocalization with microglial neuroinflammation marker ionized calcium binding adaptor molecule 1 (IBA1) in early stages of RPGR knockout mice morphology, a potential contributor to degeneration. Treatment with TUDCA significantly attenuated photoreceptor loss when compared to untreated knockout mice and significantly reduced microglia activation, mimicking morphological similarities to the wild type group [114]. Lastly, an in vitro model of AMD using retinal pigment epithelial and choroid endothelial cells; TCA treatment maintained tight junction structure and function affected by AMD-induced oxidative stress and inhibited choroidal angiogenesis [115]. Despite the varied mechanisms and disease states, bile acid therapies have shown protective qualities in multiple aspects of degenerative retina diseases.
4.7. Cerebrotendinous Xanthomatosis
Cerebrotendinous xanthomatosis (CTX) is an autosomal recessive lipid storage disorder caused by mutations in the CYP27A1 gene creating dysfunctional bile acid metabolism, afflicting patients with a progressive disarray of symptoms including but not limited to: ataxia, dementia, epilepsy, tendon xanthomas and cataracts. The mutated gene leads to a reduction in the formation of CDCA and an upregulation of cholesterol 7α-hydroxylase, elevating levels of 7α-hydroxy-4-cholesten-3-one and subsequently serum and urine levels of cholestanol and bile alcohol. Administration of bile acids for replacement therapy improves the symptoms CTX patients face, with CDCA the predominant choice for treating both neurological and non-neurological symptoms [116]. CYP27A1 transgenic mice do not form xanthomas in the tendons or the brain, with the level of accumulated cholestanol not replicating that of CTX patients. These mice are useful for mechanistic evaluations of CTX but still not an ideal animal model for the disease [117].
Clinical studies for CTX are abundant in this field, targeting alternative therapies for reconstructing the aberrant bile acid synthesis pathways or identifying potential disease biomarkers. One study highlighted the efficacy of using CA, instead of the standard CDCA, with significantly diminished cholestanol levels without elevated transaminases. The adverse effect of altered transaminases and subsequently cholestasis has been shown in the traditional CDCA treatment in a neonatal CTX case [118]. Another group differentiated indirect bile acid synthesis biomarkers in an extensive long-term study, with significant elevation of plasma cholestanol and serum lathosterol in untreated patients. However, notably, serum 27-hydroxycholesterol (a bile acid metabolite) was very low or absent in all CTX patients despite CDCA treatment, highlighting its efficacy as an overall prognosis marker at any stage in the disease [119]. Different methods of bile acid replacement therapy and identifying possible significant metabolites will aid in improving CTX patients’ quality of life.
5. Neurological Disorders and Bile Acids
The presence of bile acids continues to have increased relevance in maladies of the CNS beyond neurodegenerative diseases. With aspects of neuroinflammation, neural cell injury and overall deterioration playing a role in these disease states, studies implementing therapeutic techniques or targeting bile acid signaling have shown neuroprotective value. Current research highlights these findings and the mechanisms behind them.
5.1. Multiple Sclerosis
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS characterized by demyelinating processes, immune-mediated neuroinflammation and axonal dysfunction leading to motor, sensory and cognitive deterioration. There are several phenotypes: relapsing-remitting MS (RRMS), primary progressive MS (PMS), and secondary progressive MS (SPMS) [120]. Due to the symptoms of MS varying by the area of the CNS that is affect, there are several animal models utilized to study clinical manifestations: experimental autoimmune encephalomyelitis, viral-induced encephalomyelitis, or chemical/toxin-induced demyelination [121].
Identification of bile acid synthesis pathway intermediates, metabolites and bile acid receptors in MS has been shown in clinical studies. Several metabolomic analyses yielded differences in bile acid metabolism of patients afflicted with MS when compared to controls. One study indicates a significant increase in (25R)26-hydroxycholesterol, a precursor in the alternative pathway in bile acid synthesis, in the plasma of patients with RRMS [122]. Another showed significant increases in the ratio of DCA metabolites to CA metabolites in the plasma of patients with PMS. Furthermore, the increased expression of receptors FXR and TGR5 were identified in positive-stained white matter lesions of PMS autopsy tissue. Using the experimental autoimmune encephalomyelitis mouse model, TUDCA treatment reduced the severity of demyelination and astrocytosis through the effects of TGR5 signaling [123].
Other studies have focused on the expression of FXR in MS and the downstream effects of FXR signaling. One group noted the expression of FXR was downregulated in MS patients, more so in PPMS than RRMS patients and an in vivo treatment with FXR agonist GW4064 yielded significant diminished inflammatory markers when compared to vehicle-treated mice. Myeloid cell-mediated FXR activation increased levels of anti-inflammatory IL-10; making FXR a novel regulator for autoimmunity and mediating CNS inflammation of MS [124]. Another study looked into FXR activity in autoimmunity of MS via orally active synthetic FXR agonist obeticholic acid. Toxin-induced MS in FXR knockout mice treated with the synthetic agonist diminishes active and passive MS pathology more than CDCA treatment, but significantly increased proinflammatory IL-6. FXR agonists possess a causative role in influencing T-cell and B-cell expression as well as markers of apoptosis in activated T-cells [125]. Taken all together, the increased presence of bile acid metabolites and moderating FXR expression for MS therapies will increase our knowledge of this disease state.
5.2. Hepatic Encephalopathy
Hepatic encephalopathy (HE) is a neuropsychiatric disorder that creates neurological, cognitive and functional deterioration caused by multifactorial elements of three routes of liver impairment: acute liver failure (type A HE), portal-systemic shunting (type B HE) and cirrhotic liver damage (type C HE). Clinical stages of HE progress from minimal HE (some alterations without clear mental change), covert HE (mild abnormalities of awareness, cognitive abilities and sleep fluctuations) to overt HE (ataxia, asterixis, confusion and bizarre behavior and progression to coma) [126]. Associated with the cognitive deficits is hyperammonemia and the presence of neuroinflammation that are thought to co-ordinately contribute to the pathogenesis of HE [127]. Several well-characterized animal models for HE exist depending on each disease phenotype, toxin-induced or via surgical means, for the varied aspects of HE pathogenesis [128].
Studies have shown fluctuations of bile acid levels, underlying the significance of BBB permeability in this disease state and how this could affect neurological dysfunctions. Metabolomic analysis of 14 patients with overt HE displayed increased bile acid levels when compared to the levels of controls. Cerebrospinal fluid levels of bile acids possessed a 93-fold and 241-fold increase for GUDCA and GCA, respectively, highlighting BBB permeability in HE [129]. Furthermore, serum analysis of cirrhotic patients with and without HE, displaying a significant increase in total and conjugated bile acids as well as levels of GCA, GCDCA, TCA and TCDCA. Using animal models of Type A HE, increased total bile acid content in brain tissue has been demonstrated [19], and is associated with an increase in the bile acid TCA specifically [62], although the individual species of other bile acids contributing to this tissue specific increase is unknown.
The involvement of aberrant bile acid signaling in the pathogenesis of HE has been demonstrated in preclinical studies. Strategies to reduce the increased serum bile acids that occur during liver damage, either by feeding mice the bile acid sequestrant cholestyramine [19], using Cyp7A1 knockout mice with a reduced bile acid pool [19], or inhibiting ASBT activity in the intestine to prevent bile acid reabsorption into the blood stream [20], attenuated the cognitive deficits observed in a various models of Type A HE. Furthermore, it has been demonstrated that the increase in serum bile acid during liver damage/failure may be contributing to the hyperpermeability of the blood brain barrier [130] that is observed during HE.
Other HE studies specifically target bile acid signaling and their contributions to HE pathogenesis with in vitro and rodent HE models, the majority of which focus on FXR signaling. Specifically, in a model of Type A HE, FXR expression has been shown to increase in neurons of the frontal cortex [19] and that intracranial infusion of an FXR-specific vivo morpholino to knockdown the expression of FXR attenuated the cognitive deficits observed in this HE model [19], suggesting that aberrant FXR signaling maybe contributing to the pathogenesis of HE. It is thought that the consequences of aberrant neuronal FXR activation during HE may be a dysregulation of cholesterol homeostasis [58]. Specifically, a decrease in the expression of Cyp46A1, a component of the neural cholesterol clearance pathway, was observed which resulted in a concomitant increase in cholesterol content in the brains of mice with Type A HE [58]. Strategies to inhibit FXR receptor activity attenuated the dysregulation of cholesterol homeostasis in the brain during HE [58]. Neural bile acid signaling during HE also has implications for the neuroinflammatory processes and signals. For example, an in vitro neuroinflammation model using LPS and primary microglia showed decreased proinflammatory cytokines IL-1β and IL-6 when co-treated with taurolithocholic acid (TLCA) [131]. Furthermore, S1PR2 expression can be seen in neurons, directly increasing the expression of proinflammatory cytokine chemokine ligand 2 (CCL2) upon interacting with bile acid TCA [62]. CCL2, has been shown as a key regulatory chemokine regulating the activation of microglia during HE [132]. Conversely, TGR5, expressed in neurons and microglia, was found to be upregulated in the cortex of a type A rodent HE model, whereas activating this receptor suppressed CCL2 neuronal paracrine signaling and reduced microglial-induced inflammation [60]. Taken together, these studies highlight the involvement of bile acid signaling in the pathogenesis of HE and may prove an effective therapeutic target for the development of strategies to manage this complex disease.
5.3. Miscellaneous Neurological Disorders
Other neuropathies induced by physical damage or genetic mutations also have been studied through experimental studies involving bile acid-mediated therapy.
Traumatic brain injury (TBI) is a heterogenous trauma-based disorder caused by physical injury to the head. Axonal and neuronal cell injury, neuroinflammation and neurodegeneration responses in acute, subacute and chronic TBI stages are potential protein biomarkers for disease tracking [133]. One study looked into therapeutic roles of TUDCA in mechanism of TBI’s disease state. The ratio of protein kinase B (Akt)/p-Akt was shown to be decreased in a rodent model of TBI along with the expression of 78-kDa glucose-regulated protein (GRP78), an indicator of ER stress. The neuroprotective role of TUDCA solely influenced the Akt/p-Akt pathway by decreasing ER stress and improved disease conditions by decreasing neuronal apoptosis, improving secondary brain injury factors of TBI [134]. Another group displayed significant bile acid signaling in a TBI animal model. A significant decrease in ASBT-expressing neurons was observed in the hypothalamus of a rodent model of TBI, which could adversely contribute to inflammatory responses [135].
X-linked adrenoleukodystrophy (XALD) is a neurometabolic disorder caused by mutations in the ABCD1 gene that leads to progressive axonopathy of ascending and descending sensory and motor spinal cord tracts. Mitochondrial dysfunction, accumulated very long chain fatty acids, cerebral inflammation and demyelination contribute to the pathophysiology of the disease [136]. Clinical studies showing markers of irregular bile acid metabolism have given useful XALD insight. Metabolic screening of XALD patients found significant decreases in plasma bile acid levels, with unconjugated CA and CDCA showing the most significant reduction. Lathosterol, a marker of cholesterol synthesis reflective of bile acid malabsorption, was significantly reduced as well when compared to control subjects [137]. Another study focused experimental therapy to look into ER stress, an XALD disease mechanism. Diet supplementation of TUDCA in genetic mouse models of XALD reduced UPR activation (significantly increasing levels of GRP94, GRP78 and significantly decreasing protein disulphide isomerase (PDI) levels) and attenuated axonal degeneration (significantly decreasing markers of synaptophysin and APP in spinal cord axons) [138].
While TBI and XALD vary greatly in terms of the origin of their pathology, both have been included in studies with experimental methods driven by neuroprotective bile acids and targeting ER stress mechanisms that affect both disease states.
6. Conclusions
In summary, the therapeutic implementation of bile acids and targeting bile acid-mediated signaling in neurodegenerative and neurological diseases cannot be overstated. Research that includes bile acids and their specific receptors in neurology is continually expanding due to multiple factors: bile acids have neuroprotective properties that can be seen replicated in treatment regimens (both in clinical and experimental animal models), bile acid receptors have been located in the brain and specific several neural cell types, and bile acids possess the ability to permeate through the BBB thus offering simple and feasible pharmacological options. The prevalence of bile acids in neurological research is a growing field and will continue to expand beyond the scope of hepatic and cholestatic diseases, provide novel discoveries and strategies for controlling these debilitating diseases.
Author Contributions
S.M.G. and S.D. equally contributed to the conceptualization of the review, S.M.G. wrote the initial draft and illustrated the figures, S.D. and S.M.G. critically reviewed, edited and approved the final version for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by NIH R01 awards (DK082435 and DK112803) and a VA Merit award (BX002638) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service to DeMorrow.
Acknowledgments
This work was completed with support from the Veterans Health Administration and with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Abbreviations
| 6ECDCA | 6α-ethyl-chenodeoxycholic acid |
| AAV | Associated virus |
| Aβ | Amyloid β peptides |
| ABCA1 | ATP-binding cassette transporter 1 |
| AD | Alzheimer’s Disease |
| Akt | Protein kinase B |
| ALS | Amyotrophic Lateral Sclerosis |
| AMD | Age-related macular degeneration |
| ApoE | Apolipoprotein E |
| APP | Amyloid precursor protein |
| ASBT | Apical sodium-dependent bile acid transporter |
| Bax | BCL2 associated X apoptosis regulator |
| BBB | Blood brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BiP | Binding immunoglobulin protein |
| BSEP | Bile salt export pump |
| C9orf72 | Chromosome 9 open reading frame 72 |
| CA | Cholic acid |
| CAG | Cytosine-adenine-guanine |
| CDCA | Chenodeoxycholic acid |
| CJD | Creutzfeldt-Jakob disease |
| CCL2 | Chemokine ligand 2 |
| CPA | Cyclopiazonic acid |
| CREB | cAMP-response element-binding protein |
| CTX | Cerebrotendinous Xanthomatosis |
| CYP | Cytochrome P450 |
| DCA | Deoxycholic acid |
| Drp1 | Dynamin-related protein 1 |
| eIF2α | Eukaryotic translation initiation factor |
| FUS | Fused in sarcoma |
| FXR | Farnesoid X receptor |
| GCA | Glycocholic acid |
| GCDCA | Glycochenodeoxycholic acid |
| GR | Glucocorticoid receptor |
| GRP78 | 78-kDa glucose-regulated protein |
| GUDCA | Glycoursodeoxycholic acid |
| HD | Huntington’s disease |
| HDCA | Hyodeoxycholic acid |
| HE | Hepatic Encephalopathy |
| HPA | Hypothalamic pituitary adrenal |
| IBA1 | Ionized calcium binding adaptor molecule 1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| LCA | Lithocholic acid |
| MCA | α-Muricholic acid |
| β-MCA | β-Muricholic acid |
| MDCA | Murideoxycholic acid |
| MMP-9 | Metalloproteinase-9 |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRP1 | Multidrug resistance-associated protein 1 |
| MRP2 | Multidrug resistance-associated protein 2 |
| MRP3 | Multidrug resistance-associated protein 3 |
| MS | Multiple Sclerosis |
| NeuN | Neuronal nuclei |
| NF-κB | Nuclear factor-κB |
| NLRP3 | NOD-like receptor family pyrin domain containing receptor 3 |
| NPHP4 | Nephrocystin-4 |
| Nrf2 | Nuclear factor erythroid 2 related factor 2 |
| NTCP | Sodium taurocholate cotransporting polypeptide |
| OATP | Organic anion transport polypeptide |
| OST | Organic solute transporter |
| PD | Parkinson’s Disease |
| PDI | Protein disulphide isomerase |
| PMS | Primary progressive Multiple Sclerosis |
| PSD95 | Postsynaptic density protein 95 |
| PXR | Pregnane X receptor |
| RD | Retinal degeneration |
| RRMS | Relapsing-remitting Multiple Sclerosis |
| ROS | Reactive oxygen species |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| RPGR | Retinitis pigmentosa GTPase regulator |
| S1PR2 | Sphingosine-1-phospphate receptor |
| SOD | Superoxide dismutase |
| SPMS | Secondary progressive Multiple Sclerosis |
| TBI | Traumatic brain injury |
| TCA | Taurocholic acid |
| TCDCA | Taurochenodeoxycholic acid |
| TDP-43 | Transactive response DNA-binding protein 43 |
| TGR5 | Takeda G-protein-coupled bile acid receptor 5 |
| TLCA | Taurolithocholic acid |
| TNF-α | Tumor necrosis factor-α |
| TUDCA | Tauroursodeoxycholic acid |
| UDCA | Ursodeoxycholic acid |
| UDP | Uridine 5′-disphosphate |
| UGT | Uridine 5′-disphoasphate-glucuronosyltransferase |
| UPR | Unfolded protein response |
| VDR | Vitamin D receptor |
| XALD | X-linked adrenoleukodystrophy |
References
- Zwicker, B.L.; Agellon, L.B. Transport and biological activities of bile acids. Int. J. Biochem. Cell Biol. 2013, 45, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Parry, G.J.; Rodrigues, C.M.; Aranha, M.M.; Hilbert, S.J.; Davey, C.; Kelkar, P.; Low, W.C.; Steer, C.J. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol. 2010, 33, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; Sola, S.; Nan, Z.; Castro, R.E.; Ribeiro, P.S.; Low, W.C.; Steer, C.J. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc. Natl. Acad. Sci. USA 2003, 100, 6087–6092. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Hong, Y.H.; Sung, J.J.; Kim, S.M.; Lee, J.B.; Lee, K.W. Oral solubilized ursodeoxycholic acid therapy in amyotrophic lateral sclerosis: A randomized cross-over trial. J. Korean Med. Sci. 2012, 27, 200–206. [Google Scholar] [CrossRef]
- Mondelli, M.; Sicurelli, F.; Scarpini, C.; Dotti, M.T.; Federico, A. Cerebrotendinous xanthomatosis: 11-Year treatment with chenodeoxycholic acid in five patients. An electrophysiological study. J. Neurol. Sci. 2001, 190, 29–33. [Google Scholar] [CrossRef]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef]
- Xiang, X.; Backman, J.T.; Neuvonen, P.J.; Niemi, M. Gender, but not CYP7A1 or SLCO1B1 polymorphism, affects the fasting plasma concentrations of bile acids in human beings. Basic Clin. Pharmacol. Toxicol. 2012, 110, 245–252. [Google Scholar] [CrossRef]
- Trottier, J.; Caron, P.; Straka, R.J.; Barbier, O. Profile of serum bile acids in noncholestatic volunteers: Gender-related differences in response to fenofibrate. Clin. Pharmacol. Ther. 2011, 90, 279–286. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid metabolism in liver pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Phelps, T.; Snyder, E.; Rodriguez, E.; Child, H.; Harvey, P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol. Sex Differ. 2019, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2018, 16, 4–14. [Google Scholar] [CrossRef]
- Dawson, P.A. Role of the Intestinal Bile acid Transporters in Bile Acid and Drug Disposition. In Drug Tranporters; Handbook of Experimental Pharmacology, Vol. 201; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–203. [Google Scholar] [CrossRef]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef]
- Chen, I.; Cassaro, S. Physiology, Bile Acids; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Quinn, M.; Ashfaq, S.; de los Santos 3rd, M.; Grant, S.; DeMorrow, S. Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure. Am. J. Pathol. 2016, 186, 312–323. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Jiang, R.; Zhao, A.; Yan, J.; Zheng, X.; Huang, F.; Liu, X.; Panee, J.; Rajani, C.; et al. Dysregulated bile acid signaling contributes to the neurological impairment in murine models of acute and chronic liver failure. EBioMedicine 2018, 37, 294–306. [Google Scholar] [CrossRef]
- Barbier, O.; Trottier, J.; Kaeding, J.; Caron, P.; Verreault, M. Lipid-activated transcription factors control bile acid glucuronidation. Mol. Cell. Biochem. 2009, 326, 3–8. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Meier, P.J. Hepatocellular transporters and cholestasis. J. Clin. Gastroenterol. 2005, 39, S103–S110. [Google Scholar] [CrossRef]
- Takikawa, H.; Otsuka, H.; Beppu, T.; Seyama, Y.; Yamakawa, T. Serum concentrations of bile acid glucuronides in hepatobiliary diseases. Digestion 1983, 27, 189–195. [Google Scholar] [CrossRef]
- Perreault, M.; Bialek, A.; Trottier, J.; Verreault, M.; Caron, P.; Milkiewicz, P.; Barbier, O. Role of glucuronidation for hepatic detoxification and urinary elimination of toxic bile acids during biliary obstruction. PLoS ONE 2013, 8, e80994. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.I.; Bock, K.W.; Burchell, B.; Guillemette, C.; Ikushiro, S.; Iyanagi, T.; Miners, J.O.; Owens, I.S.; Nebert, D.W. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet. Genom. 2005, 15, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Trottier, J.; Milkiewicz, P.; Kaeding, J.; Verreault, M.; Barbier, O. Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol. Pharm. 2006, 3, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Nakajima, M.; Yamanaka, H.; Fujiwara, R.; Yokoi, T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab. Dispos. 2008, 36, 1461–1464. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Katoh, M.; Imai, K.; Kondo, Y.; Asai, Y.; Ikushiro, S.; Nadai, M. Expression of UGT1A subfamily in rat brain. Biopharm. Drug Dispos. 2016, 37, 314–319. [Google Scholar] [CrossRef]
- King, C.D.; Rios, G.R.; Assouline, J.A.; Tephly, T.R. Expression of UDP-glucuronosyltransferases (UGTs) 2B7 and 1A6 in the human brain and identification of 5-hydroxytryptamine as a substrate. Arch. Biochem. Biophys. 1999, 365, 156–162. [Google Scholar] [CrossRef]
- Suleman, F.G.; Abid, A.; Gradinaru, D.; Daval, J.L.; Magdalou, J.; Minn, A. Identification of the uridine diphosphate glucuronosyltransferase isoform UGT1A6 in rat brain and in primary cultures of neurons and astrocytes. Arch. Biochem. Biophys. 1998, 358, 63–67. [Google Scholar] [CrossRef]
- Gradinaru, D.; Minn, A.L.; Artur, Y.; Minn, A.; Heydel, J.M. Effect of oxidative stress on UDP-glucuronosyltransferases in rat astrocytes. Toxicol. Lett. 2012, 213, 316–324. [Google Scholar] [CrossRef]
- Togna, A.R.; Antonilli, L.; Dovizio, M.; Salemme, A.; De Carolis, L.; Togna, G.I.; Patrignani, P.; Nencini, P. In vitro morphine metabolism by rat microglia. Neuropharmacology 2013, 75, 391–398. [Google Scholar] [CrossRef]
- Bjorkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 2003, 60, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Nieweg, K.; Schaller, H.; Pfrieger, F.W. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J. Neurochem. 2009, 109, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M. Central nervous system: Cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 2009, 390, 287–293. [Google Scholar] [CrossRef]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar]
- McNeilly, A.D.; Macfarlane, D.P.; O’Flaherty, E.; Livingstone, D.E.; Mitic, T.; McConnell, K.M.; McKenzie, S.M.; Davies, E.; Reynolds, R.M.; Thiesson, H.C.; et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J. Hepatol. 2010, 52, 705–711. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Quinn, M.; Divan, A.; Grant, S.; Patel, N.; Newell-Rogers, K.; DeMorrow, S. Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Mol. Endocrinol. 2015, 29, 1720–1730. [Google Scholar] [CrossRef]
- Copple, B.L.; Li, T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef]
- Ananthanarayanan, M.; Balasubramanian, N.; Makishima, M.; Mangelsdorf, D.J.; Suchy, F.J. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 2001, 276, 28857–28865. [Google Scholar] [CrossRef]
- Plass, J.R.; Mol, O.; Heegsma, J.; Geuken, M.; Faber, K.N.; Jansen, P.L.; Muller, M. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology 2002, 35, 589–596. [Google Scholar] [CrossRef]
- Barbier, O.; Torra, I.P.; Sirvent, A.; Claudel, T.; Blanquart, C.; Duran-Sandoval, D.; Kuipers, F.; Kosykh, V.; Fruchart, J.C.; Staels, B. FXR induces the UGT2B4 enzyme in hepatocytes: A potential mechanism of negative feedback control of FXR activity. Gastroenterology 2003, 124, 1926–1940. [Google Scholar] [CrossRef]
- Yu, J.; Lo, J.L.; Huang, L.; Zhao, A.; Metzger, E.; Adams, A.; Meinke, P.T.; Wright, S.D.; Cui, J. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J. Biol. Chem. 2002, 277, 31441–31447. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef]
- Goldberg, A.A.; Beach, A.; Davies, G.F.; Harkness, T.A.; Leblanc, A.; Titorenko, V.I. Lithocholic bile acid selectively kills neuroblastoma cells, while sparing normal neuronal cells. Oncotarget 2011, 2, 761–782. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.H.; Bard, J.M.; Carbonnelle, D.; Chaillou, C.; Huvelin, J.M.; Bobin-Dubigeon, C.; Nazih, H. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell. Oncol. 2018, 41, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Staudinger, J.L.; Goodwin, B.; Jones, S.A.; Hawkins-Brown, D.; MacKenzie, K.I.; LaTour, A.; Liu, Y.; Klaassen, C.D.; Brown, K.K.; Reinhard, J.; et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 3369–3374. [Google Scholar] [CrossRef]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D receptor as an intestinal bile acid sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef]
- Gohlke, H.; Schmitz, B.; Sommerfeld, A.; Reinehr, R.; Haussinger, D. α5β1-integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology 2013, 57, 1117–1129. [Google Scholar] [CrossRef]
- Studer, E.; Zhou, X.; Zhao, R.; Wang, Y.; Takabe, K.; Nagahashi, M.; Pandak, W.M.; Dent, P.; Spiegel, S.; Shi, R.; et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 2012, 55, 267–276. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y. Nuclear receptors in bile acid metabolism. Drug Metab. Rev. 2013, 45, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, J.; Hu, W.; Wang, C.; Lu, X.; Tong, L.; Wu, F.; Zhang, W. Identification of functional farnesoid X receptors in brain neurons. FEBS Lett. 2016, 590, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, T.; Lan, Y.; Yang, L.; Pan, W.; Zhu, Y.; Lv, B.; Wei, Y.; Shi, H.; Wu, H.; et al. Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front. Behav. Neurosci. 2015, 9, 70. [Google Scholar] [CrossRef]
- McMillin, M.; Grant, S.; Frampton, G.; Petrescu, A.D.; Kain, J.; Williams, E.; Haines, R.; Canady, L.; DeMorrow, S. FXR-Mediated cortical cholesterol accumulation contributes to the pathogenesis of type A hepatic encephalopathy. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 47–63. [Google Scholar] [CrossRef]
- Keitel, V.; Gorg, B.; Bidmon, H.J.; Zemtsova, I.; Spomer, L.; Zilles, K.; Haussinger, D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 2010, 58, 1794–1805. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Tobin, R.; Dusio, G.; Smith, J.; Shin, H.; Newell-Rogers, K.; Grant, S.; DeMorrow, S. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J. Neurochem. 2015, 135, 565–576. [Google Scholar] [CrossRef]
- Kempf, A.; Tews, B.; Arzt, M.E.; Weinmann, O.; Obermair, F.J.; Pernet, V.; Zagrebelsky, M.; Delekate, A.; Iobbi, C.; Zemmar, A.; et al. The sphingolipid receptor S1PR2 is a receptor for Nogo-a repressing synaptic plasticity. PLoS Biol. 2014, 12, e1001763. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Grant, S.; Khan, S.; Diocares, J.; Petrescu, A.; Wyatt, A.; Kain, J.; Jefferson, B.; DeMorrow, S. Bile Acid-Mediated Sphingosine-1-Phosphate Receptor 2 Signaling Promotes Neuroinflammation during Hepatic Encephalopathy in Mice. Front. Cell. Neurosci. 2017, 11, 191. [Google Scholar] [CrossRef]
- Lemmen, J.; Tozakidis, I.E.; Galla, H.J. Pregnane X receptor upregulates ABC-transporter Abcg2 and Abcb1 at the blood-brain barrier. Brain Res. 2013, 1491, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Litwa, E.; Rzemieniec, J.; Wnuk, A.; Lason, W.; Krzeptowski, W.; Kajta, M. RXRα, PXR and CAR xenobiotic receptors mediate the apoptotic and neurotoxic actions of nonylphenol in mouse hippocampal cells. J. Steroid Biochem. Mol. Biol. 2016, 156, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Liu, P.Y.; Josh, P.; Cui, X. Intracellular distribution of the vitamin D receptor in the brain: Comparison with classic target tissues and redistribution with development. Neuroscience 2014, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bonus, M.; Sommerfeld, A.; Qvartskhava, N.; Gorg, B.; Ludwig, B.S.; Kessler, H.; Gohlke, H.; Haussinger, D. Evidence for functional selectivity in TUDC- and norUDCA-induced signal transduction via α5β1 integrin towards choleresis. Sci. Rep. 2020, 10, 5795. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Escuin, S.; van der Flier, A.; De Arcangelis, A.; Hynes, R.O.; Georges-Labouesse, E. Integrin α5β1 is necessary for regulation of radial migration of cortical neurons during mouse brain development. Eur. J. Neurosci. 2010, 31, 399–409. [Google Scholar] [CrossRef]
- Roberts, J.; de Hoog, L.; Bix, G.J. Mice deficient in endothelial α5 integrin are profoundly resistant to experimental ischemic stroke. J. Cereb. Blood Flow Metab. 2017, 37, 85–96. [Google Scholar] [CrossRef]
- Miura, T.; Ouchida, R.; Yoshikawa, N.; Okamoto, K.; Makino, Y.; Nakamura, T.; Morimoto, C.; Makino, I.; Tanaka, H. Functional modulation of the glucocorticoid receptor and suppression of NF-κB-dependent transcription by ursodeoxycholic acid. J. Biol. Chem. 2001, 276, 47371–47378. [Google Scholar] [CrossRef]
- Sun, X.C.; Ren, X.F.; Chen, L.; Gao, X.Q.; Xie, J.X.; Chen, W.F. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J. Steroid. Biochem. Mol. Biol. 2016, 155, 94–103. [Google Scholar] [CrossRef]
- Sola, S.; Amaral, J.D.; Borralho, P.M.; Ramalho, R.M.; Castro, R.E.; Aranha, M.M.; Steer, C.J.; Rodrigues, C.M. Functional modulation of nuclear steroid receptors by tauroursodeoxycholic acid reduces amyloid β-peptide-induced apoptosis. Mol. Endocrinol. 2006, 20, 2292–2303. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Alzheimer’s, Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Guell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Marksteiner, J.; Blasko, I.; Kemmler, G.; Koal, T.; Humpel, C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics 2018, 14, 1. [Google Scholar] [CrossRef]
- Wu, X.; Lv, Y.G.; Du, Y.F.; Chen, F.; Reed, M.N.; Hu, M.; Suppiramaniam, V.; Tang, S.S.; Hong, H. Neuroprotective effects of INT-777 against Aβ1–42-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Brain Behav. Immun. 2018, 73, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, H.; Guo, X.; Liu, J.; Gui, T.; Gai, Z. Farnesoid X Receptor (FXR) Aggravates Amyloid-β-Triggered Apoptosis by Modulating the cAMP-Response Element-Binding Protein (CREB)/Brain-Derived Neurotrophic Factor (BDNF) Pathway In Vitro. Med. Sci. Monit. 2019, 25, 9335–9345. [Google Scholar] [CrossRef] [PubMed]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Chenodeoxycholic acid ameliorates AlCl3-induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules 2019, 24, 1992. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Kandimalla, R.; Fry, D.; Sesaki, H.; Reddy, P.H. Protective effects of reduced dynamin-related protein 1 against amyloid β-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum. Mol. Genet. 2016, 25, 5148–5166. [Google Scholar] [CrossRef]
- Kandimalla, R.; Manczak, M.; Fry, D.; Suneetha, Y.; Sesaki, H.; Reddy, P.H. Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum. Mol. Genet. 2016, 25, 4881–4897. [Google Scholar] [CrossRef]
- Bell, S.M.; Barnes, K.; Clemmens, H.; Al-Rafiah, A.R.; Al-Ofi, E.A.; Leech, V.; Bandmann, O.; Shaw, P.J.; Blackburn, D.J.; Ferraiuolo, L.; et al. Ursodeoxycholic acid improves mitochondrial function and redistributes drp1 in fibroblasts from patients with either sporadic or familial Alzheimer’s disease. J. Mol. Biol. 2018, 430, 3942–3953. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal models for Parkinson’s disease research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Seppi, K.; Poewe, W. The concept of prodromal Parkinson’s disease. J. Parkinsons Dis. 2015, 5, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.F.; Rey, N.L.; Yilmaz, A.; Kumar, P.; Madaj, Z.; Maddens, M.; Bahado-Singh, R.O.; Becker, K.; Schulz, E.; Meyerdirk, L.K.; et al. Biochemical profiling of the brain and blood metabolome in a mouse model of prodromal Parkinson’s disease reveals distinct metabolic profiles. J. Proteome Res. 2018, 17, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.M.; Crowley, E.K.; Brown, J.R.; O’Sullivan, O.; O’Leary, O.F.; Timmons, S.; Nolan, Y.M.; Clarke, D.J.; Hyland, N.P.; Joyce, S.A.; et al. Nigral overexpression of α-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol. Motil. 2020, 32, e13726. [Google Scholar] [CrossRef]
- Graham, S.F.; Rey, N.L.; Ugur, Z.; Yilmaz, A.; Sherman, E.; Maddens, M.; Bahado-Singh, R.O.; Becker, K.; Schulz, E.; Meyerdirk, L.K.; et al. Metabolomic profiling of bile acids in an experimental model of prodromal Parkinson’s disease. Metabolites 2018, 8, 71. [Google Scholar] [CrossRef]
- Rosa, A.I.; Duarte-Silva, S.; Silva-Fernandes, A.; Nunes, M.J.; Carvalho, A.N.; Rodrigues, E.; Gama, M.J.; Rodrigues, C.M.P.; Maciel, P.; Castro-Caldas, M. Tauroursodeoxycholic acid improves motor symptoms in a mouse model of Parkinson’s disease. Mol. Neurobiol. 2018, 55, 9139–9155. [Google Scholar] [CrossRef]
- Moreira, S.; Fonseca, I.; Nunes, M.J.; Rosa, A.; Lemos, L.; Rodrigues, E.; Carvalho, A.N.; Outeiro, T.F.; Rodrigues, C.M.P.; Gama, M.J.; et al. Nrf2 activation by tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Exp. Neurol. 2017, 295, 77–87. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; Safar, M.M.; Salem, H.A. Ursodeoxycholic acid ameliorates apoptotic cascade in the Rotenone model of Parkinson’s disease: Modulation of mitochondrial perturbations. Mol. Neurobiol. 2016, 53, 810–817. [Google Scholar] [CrossRef]
- Jodeiri Farshbaf, M.; Ghaedi, K. Huntington’s Disease and Mitochondria. Neurotox. Res. 2017, 32, 518–529. [Google Scholar] [CrossRef]
- Boussicault, L.; Alves, S.; Lamaziere, A.; Planques, A.; Heck, N.; Moumne, L.; Despres, G.; Bolte, S.; Hu, A.; Pages, C.; et al. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain 2016, 139, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Di Pardo, A.; Amico, E.; Basit, A.; Armirotti, A.; Joshi, P.; Neely, M.D.; Vuono, R.; Castaldo, S.; Digilio, A.F.; Scalabri, F.; et al. Defective Sphingosine-1-phosphate metabolism is a druggable target in Huntington’s disease. Sci. Rep. 2017, 7, 5280. [Google Scholar] [CrossRef] [PubMed]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, G.; Kaplan, A.; Gaschler, M.M.; Zhang, X.; Hou, Z.; Jiang, M.; Zott, R.; Cremers, S.; Stockwell, B.R.; et al. Small molecule modulator of protein disulfide isomerase attenuates mutant huntingtin toxicity and inhibits endoplasmic reticulum stress in a mouse model of Huntington’s disease. Hum. Mol. Genet. 2018, 27, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Upagupta, C.; Carlisle, R.E.; Dickhout, J.G. Analysis of the potency of various low molecular weight chemical chaperones to prevent protein aggregation. Biochem. Biophys. Res. Commun. 2017, 486, 163–170. [Google Scholar] [CrossRef]
- Hulisz, D. Amyotrophic lateral sclerosis: Disease state overview. Am. J. Manag. Care 2018, 24, S320–S326. [Google Scholar]
- Philips, T.; Rothstein, J.D. Rodent models of amyotrophic lateral sclerosis. Curr. Protoc. Pharmacol. 2015, 69, 5.67.1–5.67.21. [Google Scholar] [CrossRef]
- Elia, A.E.; Lalli, S.; Monsurro, M.R.; Sagnelli, A.; Taiello, A.C.; Reggiori, B.; La Bella, V.; Tedeschi, G.; Albanese, A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2016, 23, 45–52. [Google Scholar] [CrossRef]
- Vaz, A.R.; Cunha, C.; Gomes, C.; Schmucki, N.; Barbosa, M.; Brites, D. Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol. Neurobiol. 2015, 51, 864–877. [Google Scholar] [CrossRef]
- Thams, S.; Lowry, E.R.; Larraufie, M.H.; Spiller, K.J.; Li, H.; Williams, D.J.; Hoang, P.; Jiang, E.; Williams, L.A.; Sandoe, J.; et al. A stem cell-based screening platform identifies compounds that desensitize motor neurons to endoplasmic reticulum stress. Mol. Ther. 2019, 27, 87–101. [Google Scholar] [CrossRef]
- Sigurdson, C.J.; Bartz, J.C.; Glatzel, M. Cellular and molecular mechanisms of prion disease. Annu. Rev. Pathol. 2019, 14, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Diack, A.B.; Alibhai, J.D.; Manson, J.C. Gene targeted transgenic mouse models in prion research. Prog. Mol. Biol. Transl. Sci. 2017, 150, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Radford, H.; Peretti, D.; Steinert, J.R.; Verity, N.; Martin, M.G.; Halliday, M.; Morgan, J.; Dinsdale, D.; Ortori, C.A.; et al. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature 2012, 485, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Ladner-Keay, C.L.; Ross, L.; Perez-Pineiro, R.; Zhang, L.; Bjorndahl, T.C.; Cashman, N.; Wishart, D.S. A simple in vitro assay for assessing the efficacy, mechanisms and kinetics of anti-prion fibril compounds. Prion 2018, 12, 280–300. [Google Scholar] [CrossRef]
- Cortez, L.M.; Campeau, J.; Norman, G.; Kalayil, M.; Van der Merwe, J.; McKenzie, D.; Sim, V.L. Bile acids reduce prion conversion, reduce neuronal loss, and prolong male survival in models of prion disease. J. Virol. 2015, 89, 7660–7672. [Google Scholar] [CrossRef]
- Norman, G.; Campeau, J.; Sim, V.L. High dose and delayed treatment with bile acids ineffective in RML prion-infected mice. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Veleri, S.; Lazar, C.H.; Chang, B.; Sieving, P.A.; Banin, E.; Swaroop, A. Biology and therapy of inherited retinal degenerative disease: Insights from mouse models. Dis. Model. Mech. 2015, 8, 109–129. [Google Scholar] [CrossRef]
- Lobysheva, E.; Taylor, C.M.; Marshall, G.R.; Kisselev, O.G. Tauroursodeoxycholic acid binds to the G-protein site on light activated rhodopsin. Exp. Eye Res. 2018, 170, 51–57. [Google Scholar] [CrossRef]
- Lawson, E.C.; Bhatia, S.K.; Han, M.K.; Aung, M.H.; Ciavatta, V.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic Acid Protects Retinal Function and Structure in rd1 Mice. Adv Exp Med Biol. 2016, 854, 431–436. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, X.; Lu, X.; Qu, Y.; Wang, C.; Peng, G.; Zhang, J. Subcutaneous delivery of tauroursodeoxycholic acid rescues the cone photoreceptors in degenerative retina: A promising therapeutic molecule for retinopathy. Biomed. Pharmacother. 2019, 117, 109021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shahani, U.; Reilly, J.; Shu, X. Disease mechanisms and neuroprotection by tauroursodeoxycholic acid in Rpgr knockout mice. J. Cell. Physiol. 2019, 234, 18801–18812. [Google Scholar] [CrossRef] [PubMed]
- Warden, C.; Barnett, J.M.; Brantley, M.A., Jr. Taurocholic acid inhibits features of age-related macular degeneration in vitro. Exp. Eye Res. 2020, 193, 107974. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Chen, G.; Cao, X.; Zhang, Y. Cerebrotendinous xanthomatosis: A comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet. J. Rare Dis. 2014, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Bavner, A.; Shafaati, M.; Hansson, M.; Olin, M.; Shpitzen, S.; Meiner, V.; Leitersdorf, E.; Bjorkhem, I. On the mechanism of accumulation of cholestanol in the brain of mice with a disruption of sterol 27-hydroxylase. J. Lipid Res. 2010, 51, 2722–2730. [Google Scholar] [CrossRef]
- Mandia, D.; Chaussenot, A.; Besson, G.; Lamari, F.; Castelnovo, G.; Curot, J.; Duval, F.; Giral, P.; Lecerf, J.M.; Roland, D.; et al. Cholic acid as a treatment for cerebrotendinous xanthomatosis in adults. J. Neurol. 2019, 266, 2043–2050. [Google Scholar] [CrossRef]
- Mignarri, A.; Magni, A.; Del Puppo, M.; Gallus, G.N.; Bjorkhem, I.; Federico, A.; Dotti, M.T. Evaluation of cholesterol metabolism in cerebrotendinous xanthomatosis. J. Inherit. Metab. Dis. 2016, 39, 75–83. [Google Scholar] [CrossRef]
- Diaz, C.; Zarco, L.A.; Rivera, D.M. Highly active multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2019, 30, 215–224. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Pucino, V.; Formisano, L.; Matarese, G. Animal models of Multiple Sclerosis. Eur. J. Pharmacol. 2015, 759, 182–191. [Google Scholar] [CrossRef]
- Crick, P.J.; Griffiths, W.J.; Zhang, J.; Beibel, M.; Abdel-Khalik, J.; Kuhle, J.; Sailer, A.W.; Wang, Y. Reduced plasma levels of 25-hydroxycholesterol and increased cerebrospinal fluid levels of bile acid precursors in multiple sclerosis patients. Mol. Neurobiol. 2017, 54, 8009–8020. [Google Scholar] [CrossRef]
- Bhargava, P.; Smith, M.D.; Mische, L.; Harrington, E.; Fitzgerald, K.C.; Martin, K.; Kim, S.; Reyes, A.A.; Gonzalez-Cardona, J.; Volsko, C.; et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J. Clin. Investig. 2020, 130, 3467–3482. [Google Scholar] [CrossRef] [PubMed]
- Hucke, S.; Herold, M.; Liebmann, M.; Freise, N.; Lindner, M.; Fleck, A.K.; Zenker, S.; Thiebes, S.; Fernandez-Orth, J.; Buck, D.; et al. The farnesoid-X-receptor in myeloid cells controls CNS autoimmunity in an IL-10-dependent fashion. Acta Neuropathol. 2016, 132, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.P.; Steinman, L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2016, 113, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, P.A.; Kumar, G. Hepatic encephalopathy—The old and the new. Crit. Care Clin. 2016, 32, 311–329. [Google Scholar] [CrossRef]
- Liere, V.; Sandhu, G.; DeMorrow, S. Recent advances in hepatic encephalopathy. F1000Research 2017, 6, 1637. [Google Scholar] [CrossRef]
- Butterworth, R.F.; Norenberg, M.D.; Felipo, V.; Ferenci, P.; Albrecht, J.; Blei, A.T.; Members of the ISHEN Commission on Experimental Models of HE. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009, 29, 783–788. [Google Scholar] [CrossRef]
- Weiss, N.; Barbier Saint Hilaire, P.; Colsch, B.; Isnard, F.; Attala, S.; Schaefer, A.; Amador, M.D.; Rudler, M.; Lamari, F.; Sedel, F.; et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J. Hepatol. 2016, 65, 1120–1130. [Google Scholar] [CrossRef]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef]
- Karababa, A.; Groos-Sahr, K.; Albrecht, U.; Keitel, V.; Shafigullina, A.; Gorg, B.; Haussinger, D. Ammonia attenuates LPS-induced upregulation of pro-inflammatory cytokine mRNA in co-cultured astrocytes and microglia. Neurochem. Res. 2017, 42, 737–749. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Thompson, M.; Galindo, C.; Standeford, H.; Whittington, E.; Alpini, G.; DeMorrow, S. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J. Neuroinflamm. 2014, 11, 121. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gu, G.; Wang, J.; Chai, Y.; Fan, Y.; Yang, M.; Xu, X.; Gao, W.; Li, F.; Yin, D.; et al. Administration of tauroursodeoxycholic acid attenuates early brain injury via akt pathway activation. Front. Cell. Neurosci. 2017, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinov, D.; DeMorrow, S.; McMillin, M.; Kain, J.; Mukherjee, S.; Zeitouni, S.; Frampton, G.; Bricker, P.C.; Hurst, J.; Shapiro, L.A. Hepatic alterations are accompanied by changes to bile acid transporter-expressing neurons in the hypothalamus after traumatic brain injury. Sci. Rep. 2017, 7, 40112. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Forss-Petter, S.; Eichler, F.S. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 2014, 98, 135–142. [Google Scholar] [CrossRef]
- Platek, T.; Orso, E.; Zapala, B.; Polus, A.; Kiec-Wilk, B.; Piwowar, M.; Chojnacka, M.; Cialowicz, U.; Malczewska-Malec, M.; Schmitz, G.; et al. Case report of dysregulation of primary bile acid synthesis in a family with X-linked adrenoleukodystrophy. Medicine. 2018, 97, e13353. [Google Scholar] [CrossRef]
- Launay, N.; Ruiz, M.; Grau, L.; Ortega, F.J.; Ilieva, E.V.; Martinez, J.J.; Galea, E.; Ferrer, I.; Knecht, E.; Pujol, A.; et al. Tauroursodeoxycholic bile acid arrests axonal degeneration by inhibiting the unfolded protein response in X-linked adrenoleukodystrophy. Acta Neuropathol. 2017, 133, 283–301. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).