Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration
Abstract
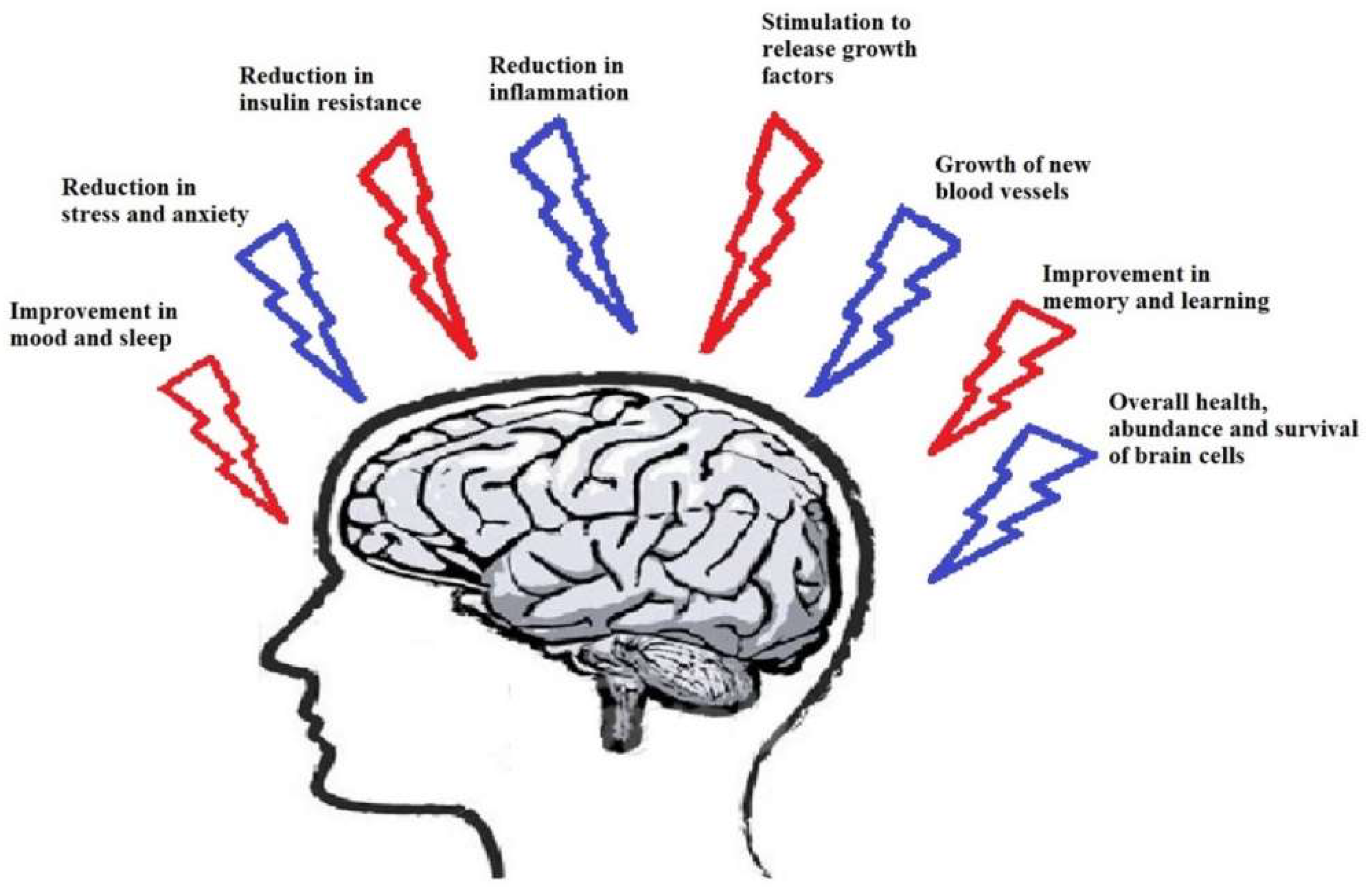
1. Introduction
2. Physical Exercise and Neurodegenerative Disease
3. The Role of Exercise in Neurological Diseases and Involved Mechanisms
3.1. Neuroendocrine Regulation by Physical Exercise
3.2. Neurotransmitter Regulation by PE
3.3. Exercise-Enhanced Neural Insulin Signaling
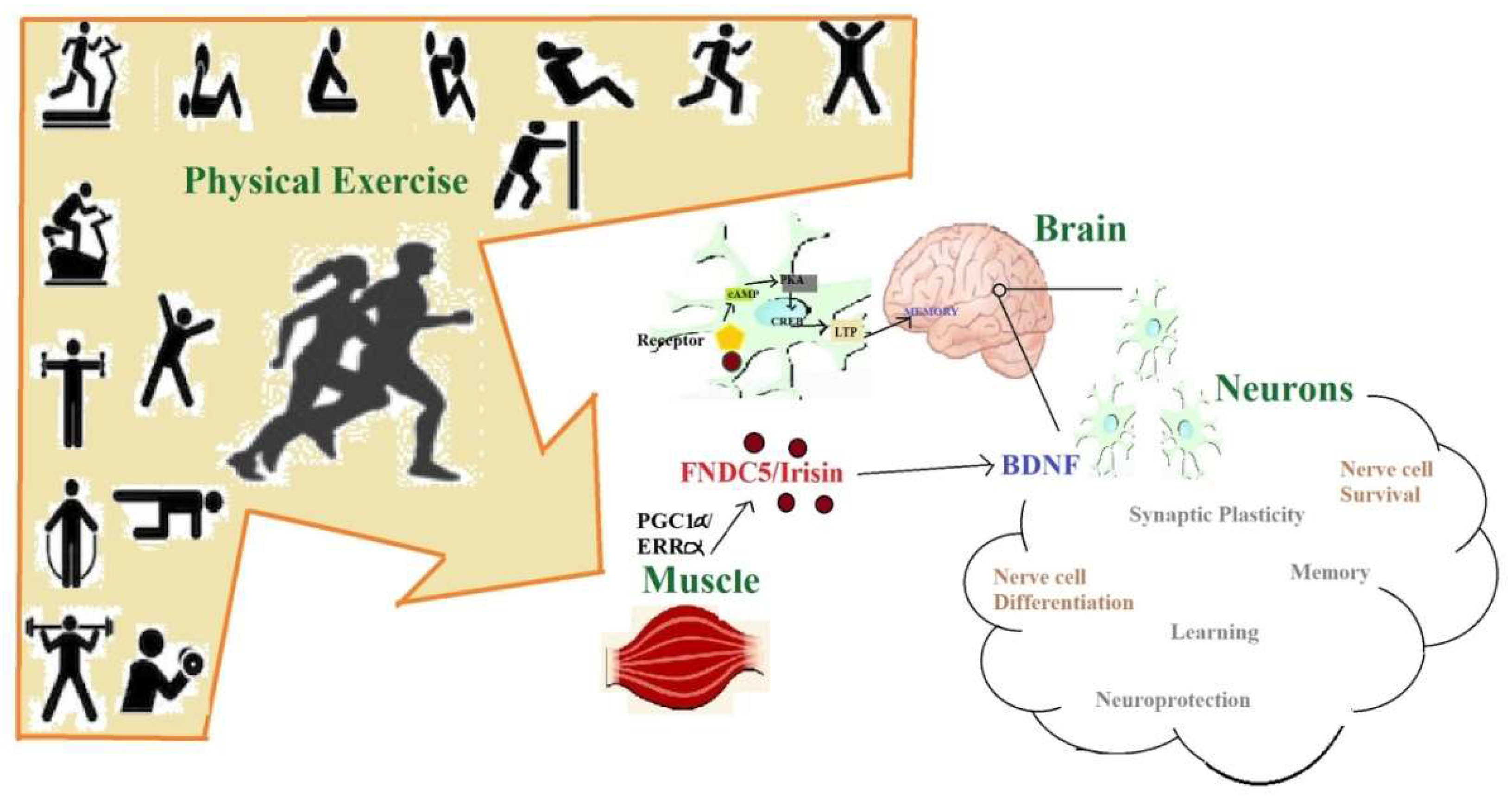
3.4. Exercise-Enhanced Brain-Derived Neurotrophic Factor BDNF-Signaling
3.5. Irisin Production and Secretion
3.6. Anti-Neural-Inflammatory and Anti-Neural-Oxidative Responses of PE
3.7. Neural Pro-Survival and Anti-Apoptotsis Effects of PE
3.8. Autophagy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Majid, A. Neuroprotection in stroke: Past, present, and future. ISRN Neurol. 2014, 2014, 515716. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.; Croteau, D.; Bohr, V. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15. [Google Scholar] [CrossRef]
- Brown, B.; Shah, T.M. The link between exercise and mediation of alzheimer’s disease and neurodegenerative diseases. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119356752.ch13 (accessed on 28 July 2020).
- 2018 Alzheimer’s disease facts and figures. Alzheimer Dement. 2018, 14, 367–429. [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Sobol, N.A.; Frederiksen, K.S.; Beyer, N.; Vogel, A.; Vestergaard, K.; Brændgaard, H.; Gottrup, H.; Lolk, A.; Wermuth, L.; et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s Disease: A randomized controlled trial. J. Alzheimer Dis. JAD 2016, 50, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.H.M.; van Berckel, B.N.M.; Scheltens, P.; Scherder, E.J.A.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef]
- Frederiksen, K.S.; Gjerum, L.; Waldemar, G.; Hasselbalch, S.G. Effects of physical exercise on Alzheimer’s Disease biomarkers: A systematic review of intervention studies. J. Alzheimer Dis. JAD 2018, 61, 359–372. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Tucker, D.; Wang, R.; Ahmed, M.E.; Brann, D.; Zhang, Q. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer’s Disease. J. Alzheimer Dis. 2017, 56, 1469–1484. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Zheng, M.; Chen, J.; Chen, H.; Liu, N. Effects of treadmill exercise on cerebral angiogenesis and MT1-MMP expression after cerebral ischemia in rats. Brain Behav. 2018, 8, e01079. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.S.; Ntekim, O.; Johnson, S.P.; Ngwa, J.S.; Bond, V.; Pinder, D.; Gillum, R.F.; Fungwe, T.V.; Kwagyan, J.; Obisesan, T.O. APOEε4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly African Americans: A pilot study. Exp. Gerontol. 2017, 87, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Martin, B.; Maudsley, S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, T.; Chu, J.M.; Chen, Y.; Dunnett, S.; Ho, Y.S.; Wong, G.T.; Chang, R.C. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig. 2019, 99, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T.; Rolland, Y.; de Souto Barreto, P. Protective effects of physical exercise in Alzheimer’s Disease and Parkinson’s Disease: A narrative review. J. Clin. Neurol. 2015, 11, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Taubert, M.; Muller, N.G. Physical exercise as personalized medicine for dementia prevention? Front. Physiol. 2019, 10, 672. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Slot, M.I.E.; Lee, E.H.M.; Scheltens, P.; Sommer, I.E.C. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2019. (Online ahead of print). [Google Scholar] [CrossRef]
- Chang, M.L.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Saczynski, J.S.; Aspelund, T.; Eiriksdottir, G.; Jonsdottir, M.K.; Lopez, O.L.; Harris, T.B.; et al. The effect of midlife physical activity on cognitive function among older adults: AGES-Reykjavik study. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2010, 65, 1369–1374. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle modulators of neuroplasticity: How physical activity, mental engagement, and diet promote cognitive health during aging. Neural. Plast. 2017, 2017, 1–22. [Google Scholar] [CrossRef]
- Bass, R.W.; Brown, D.D.; Laurson, K.R.; Coleman, M.M. Physical fitness and academic performance in middle school students. Acta Paediatr. 2013, 102, 832–837. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Crysdale, N.Y.; Chapman, T.R.; Ahrendsen, J.T.; Day, H.E.; Campeau, S.; Watkins, L.R.; Patterson, S.L.; Maier, S.F. Little exercise, big effects: Reversing aging and infection-induced memory deficits, and underlying processes. J. Neurosci. 2011, 31, 11578–11586. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Deuster, P.A. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus 2014, 4. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic fitness reduces brain tissue loss in aging humans. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2003, 58, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Khalil, D.; Gould, E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 2007, 17, 1017–1022. [Google Scholar] [CrossRef]
- Phillips, C.; Baktir, M.A.; Das, D.; Lin, B.; Salehi, A. The link between physical activity and cognitive dysfunction in Alzheimer Disease. Phys. Ther. 2015, 95, 1046–1060. [Google Scholar] [CrossRef]
- Goodwin, V.A.; Richards, S.H.; Taylor, R.S.; Taylor, A.H.; Campbell, J.L. The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2008, 23, 631–640. [Google Scholar] [CrossRef]
- Monteiro, R.S.; Cevada, T.; Oliveira, B.R.R.; Lattari, E.; Portugal, E.M.M.; Carvalho, A.; Deslandes, A.C. We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med. Hypotheses 2015, 85, 537–541. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef]
- Erickson, K.I.; Gildengers, A.G.; Butters, M.A. Physical activity and brain plasticity in late adulthood. Dialogues Clin. Neurosci. 2013, 15, 99–108. [Google Scholar] [PubMed]
- Moreira, O.C.; Estebanez, B.; Martinez-Florez, S.; de Paz, J.A.; Cuevas, M.J.; Gonzalez-Gallego, J. Mitochondrial function and mitophagy in the elderly: Effects of exercise. Oxidative Med. Cell. Longev. 2017, 2017, 2012798. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical activity and brain health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. AMS 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.; Nho, K.; Saykin, A.J. Deep learning in Alzheimer’s Disease: Diagnostic classification and prognostic prediction using neuroimaging data. Front. Aging Neurosci. 2019, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef]
- Taylor, M.K.; Swerdlow, R.H.; Sullivan, D.K. Dietary neuroketotherapeutics for Alzheimer’s Disease: An evidence update and the potential role for diet quality. Nutrients 2019, 11, 1910. [Google Scholar] [CrossRef]
- Li, B.; Liang, F.; Ding, X.; Yan, Q.; Zhao, Y.; Zhang, X.; Bai, Y.; Huang, T.; Xu, B. Interval and continuous exercise overcome memory deficits related to beta-Amyloid accumulation through modulating mitochondrial dynamics. Behav. Brain Res. 2019, 376, 112171. [Google Scholar] [CrossRef]
- Kouloutbani, K.; Karteroliotis, K.; Politis, A. The effect of physical activity on dementia. Psychiatriki 2019, 30, 142–155. [Google Scholar] [CrossRef]
- Lavie, C.J.; Church, T.S.; Milani, R.V.; Earnest, C.P. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J. Cardiopulm. Rehabil. Prev. 2011, 31, 137–145. [Google Scholar] [CrossRef]
- Alomari, M.A.; Khabour, O.F.; Alzoubi, K.H.; Alzubi, M.A. Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behav. Brain Res. 2013, 247, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J.; Sukoff Rizzo, S.J.; Howell, G.R. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef] [PubMed]
- McGurran, H.; Glenn, J.M.; Madero, E.N.; Bott, N.T. Prevention and treatment of Alzheimer’s Disease: Biological mechanisms of exercise. J. Alzheimer Dis. JAD 2019, 69, 311–338. [Google Scholar] [CrossRef]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef] [PubMed]
- Cucarian, J.D.; Berrio, J.P.; Rodrigues, C.; Zancan, M.; Wink, M.R.; de Oliveira, A. Physical exercise and human adipose-derived mesenchymal stem cells ameliorate motor disturbances in a male rat model of Parkinson’s disease. J. Neurosci. Res. 2019, 97, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Uc, E.Y.; Doerschug, K.C.; Magnotta, V.; Dawson, J.D.; Thomsen, T.R.; Kline, J.N.; Rizzo, M.; Newman, S.R.; Mehta, S.; Grabowski, T.J.; et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 2014, 83, 413–425. [Google Scholar] [CrossRef]
- Cancino, M.G.; Vasquez, E.R.; Pavez-Adasme, G.; Hernandez-Mosqueira, C. Multicomponent physical training in patients with Parkinson disease. Rev. Med. Chile 2019, 147, 465–469. [Google Scholar]
- Zhou, W.; Barkow, J.C.; Freed, C.R. Running wheel exercise reduces alpha-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson’s disease. PLoS ONE 2017, 12, e0190160. [Google Scholar] [CrossRef]
- Goudy, L.S.; Rigby, B.R.; Silliman-French, L.; Becker, K.A. Effects of simulated horseback riding on balance, postural sway, and quality of life in older adults with Parkinson’s Disease. Adapt. Phys. Act. Q. 2019, 36, 1–18. [Google Scholar] [CrossRef]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical exercise inhibits inflammation and microglial activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Hamzehloei, L.; Rezvani, M.E.; Rajaei, Z. Effects of carvacrol and physical exercise on motor and memory impairments associated with Parkinson’s disease. Arq. De Neuro-Psiquiatr. 2019, 77, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Combs-Miller, S.A.; Dugan, E.L.; Beachy, A.; Derby, B.B.; Hosinski, A.L.; Robbins, K. Physiological complexity of gait between regular and non-exercisers with Parkinson’s disease. Clin. Biomech. 2019, 68, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hu, C.; Wu, Z.; Chen, Y.; Li, Z.; Zhang, M. Effects of coordination and manipulation therapy for patients with Parkinson disease. Int. J. Neurosci. 2017, 127, 762–769. [Google Scholar] [CrossRef]
- Aaseth, J.; Dusek, P.; Roos, P.M. Prevention of progression in Parkinson’s disease. Biometals 2018, 31, 737–747. [Google Scholar] [CrossRef]
- Minakaki, G.; Canneva, F.; Chevessier, F.; Bode, F.; Menges, S.; Timotius, I.K.; Kalinichenko, L.S.; Meixner, H.; Muller, C.P.; Eskofier, B.M.; et al. Treadmill exercise intervention improves gait and postural control in alpha-synuclein mouse models without inducing cerebral autophagy. Behav. Brain Res. 2019, 363, 199–215. [Google Scholar] [CrossRef]
- Hackney, A.C. Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Expert Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef]
- Viru, A. Plasma hormones and physical exercise. Int. J. Sports Med. 1992, 13, 201–209. [Google Scholar] [CrossRef]
- Leal-Cerro, A.; Gippini, A.; Amaya, M.J.; Lage, M.; Mato, J.A.; Dieguez, C.; Casanueva, F.F. Mechanisms underlying the neuroendocrine response to physical exercise. J. Endocrinol. Investig. 2003, 26, 879–885. [Google Scholar] [CrossRef]
- Meeusen, R.; De Meirleir, K. Exercise and brain neurotransmission. Sports Med. 1995, 20, 160–188. [Google Scholar] [CrossRef]
- Lin, T.W.; Kuo, Y.M. Exercise benefits brain function: The monoamine connection. Brain Sci. 2013, 3, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Sutoo, D.; Akiyama, K. Regulation of brain function by exercise. Neurobiol. Dis. 2003, 13, 1–14. [Google Scholar] [CrossRef]
- MacRae, P.G.; Spirduso, W.W.; Cartee, G.D.; Farrar, R.P.; Wilcox, R.E. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neurosci. Lett. 1987, 79, 138–144. [Google Scholar] [CrossRef]
- MacRae, P.G.; Spirduso, W.W.; Walters, T.J.; Farrar, R.P.; Wilcox, R.E. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology 1987, 92, 236–240. [Google Scholar] [CrossRef]
- Greenwood, B.N.; Kennedy, S.; Smith, T.P.; Campeau, S.; Day, H.E.; Fleshner, M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience 2003, 120, 269–281. [Google Scholar] [CrossRef]
- Sciolino, N.R.; Holmes, P.V. Exercise offers anxiolytic potential: A role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev. 2012, 36, 1965–1984. [Google Scholar] [CrossRef]
- Pieribone, V.A.; Xu, Z.Q.; Zhang, X.; Grillner, S.; Bartfai, T.; Hokfelt, T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience 1995, 64, 861–874. [Google Scholar] [CrossRef]
- Murchison, C.F.; Zhang, X.Y.; Zhang, W.P.; Ouyang, M.; Lee, A.; Thomas, S.A. A distinct role for norepinephrine in memory retrieval. Cell 2004, 117, 131–143. [Google Scholar] [CrossRef]
- Dunn, A.L.; Reigle, T.G.; Youngstedt, S.D.; Armstrong, R.B.; Dishman, R.K. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med. Sci. Sports Exerc. 1996, 28, 204–209. [Google Scholar] [CrossRef]
- Chen, H.I.; Lin, L.C.; Yu, L.; Liu, Y.F.; Kuo, Y.M.; Huang, A.M.; Chuang, J.I.; Wu, F.S.; Liao, P.C.; Jen, C.J. Treadmill exercise enhances passive avoidance learning in rats: The role of down-regulated serotonin system in the limbic system. Neurobiol. Learn. Mem. 2008, 89, 489–496. [Google Scholar] [CrossRef]
- Chennaoui, M.; Grimaldi, B.; Fillion, M.P.; Bonnin, A.; Drogou, C.; Fillion, G.; Guezennec, C.Y. Effects of physical training on functional activity of 5-HT1B receptors in rat central nervous system: Role of 5-HT-moduline. Naunyn-Schmiedeberg Arch. Pharmacol. 2000, 361, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, S.S.; Kim, C.J.; Shin, M.S.; Kim, T.W. Exercise alleviates cognitive functions by enhancing hippocampal insulin signaling and neuroplasticity in high-fat diet-induced obesity. Nutrients 2019, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.T.; Tiganis, T. Insulin action in the brain: Roles in energy and glucose homeostasis. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef]
- Freychet, P. Insulin receptors and insulin actions in the nervous system. Diabetes Metab. Res. Rev. 2000, 16, 390–392. [Google Scholar] [CrossRef]
- Ketterer, C.; Tschritter, O.; Preissl, H.; Heni, M.; Haring, H.U.; Fritsche, A. Insulin sensitivity of the human brain. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. 1), S47–S51. [Google Scholar] [CrossRef]
- McNay, E.C.; Ong, C.T.; McCrimmon, R.J.; Cresswell, J.; Bogan, J.S.; Sherwin, R.S. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010, 93, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Kuga, G.K.; Botezelli, J.D.; Gaspar, R.C.; Gomes, R.J.; Pauli, J.R.; Leme, J.A.C.d.A. Hippocampal insulin signaling and neuroprotection mediated by physical exercise in Alzheimer´s Disease. Motiv. J. Phys. Educ. 2017, 23. [Google Scholar] [CrossRef]
- Lovatel, G.A.; Elsner, V.R.; Bertoldi, K.; Vanzella, C.; Moyses Fdos, S.; Vizuete, A.; Spindler, C.; Cechinel, L.R.; Netto, C.A.; Muotri, A.R.; et al. Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol. Learn. Mem. 2013, 101, 94–102. [Google Scholar] [CrossRef]
- Pauli, J.R.; Cintra, D.E.; Souza, C.T.d.; Ropelle, E.R. Novos mecanismos pelos quais o exercício físico melhora a resistência à insulina no músculo esquelético. Arq. Bras. Endocrinol. Metabol. 2009, 53, 399–408. [Google Scholar] [CrossRef][Green Version]
- Intlekofer, K.A.; Cotman, C.W. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol. Dis. 2013, 57, 47–55. [Google Scholar] [CrossRef]
- Pauli, J.R.; Ropelle, E.R.; Cintra, D.E.; Souza, C.T.D. Efeitos do exercà cio fà sico na expressão e atividade da AMPK± em ratos obesos induzidos por dieta rica em gordura. Rev. Bras. Med. Esporte 2009, 15, 98–103. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Diegues, J.C.; Pauli, J.R.; Luciano, E.; de Almeida Leme, J.A.; de Moura, L.P.; Dalia, R.A.; de Araujo, M.B.; Sibuya, C.Y.; de Mello, M.A.; Gomes, R.J. Spatial memory in sedentary and trained diabetic rats: Molecular mechanisms. Hippocampus 2014, 24, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef] [PubMed]
- Van der Borght, K.; Kobor-Nyakas, D.E.; Klauke, K.; Eggen, B.J.L.; Nyakas, C.; Van der Zee, E.A.; Meerlo, P. Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus 2009, 19, 928–936. [Google Scholar] [CrossRef]
- Paillard, T. Preventive effects of regular physical exercise against cognitive decline and the risk of dementia with age advancement. Sports Med. Open 2015, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Gligoroska, J.P.; Manchevska, S. The effect of physical activity on cognition-physiological mechanisms. Mater. Socio Medica 2012, 24, 198–202. [Google Scholar] [CrossRef] [PubMed]
- von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018, 373, 729–741. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Cammarota, M.; Izquierdo, I.; Medina, J.H. BDNF and memory formation and storage. Neuroscientist 2008, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Gordon, A.D. Relationship between exercise capacity and brain size in mammals. PLoS ONE 2011, 6, e20601. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Evolutionary aspects of human exercise-Born to run purposefully. Ageing Res. Rev. 2012, 11, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Arbat-Plana, A.; Cobianchi, S.; Herrando-Grabulosa, M.; Navarro, X.; Udina, E. Endogenous modulation of TrkB signaling by treadmill exercise after peripheral nerve injury. Neuroscience 2017, 340, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Shih, Y.H.; Chen, S.J.; Lien, C.H.; Chang, C.Y.; Huang, T.Y.; Chen, S.H.; Jen, C.J.; Kuo, Y.M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef]
- Fahimi, A.; Baktir, M.A.; Moghadam, S.; Mojabi, F.S.; Sumanth, K.; McNerney, M.W.; Ponnusamy, R.; Salehi, A. Physical exercise induces structural alterations in the hippocampal astrocytes: Exploring the role of BDNF-TrkB signaling. Brain Struct. Funct. 2017, 222, 1797–1808. [Google Scholar] [CrossRef]
- Zsuga, J.; Tajti, G.; Papp, C.; Juhasz, B.; Gesztelyi, R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med. Hypotheses 2016, 90, 23–28. [Google Scholar] [CrossRef]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Deng, X.; Huang, W.; Yu, J.H.; Wang, J.X.; Wang, J.P.; Yang, S.B.; Liu, X.; Wang, L.; Zhang, Y.; et al. Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol. Immunol. 2017, 91, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Dameni, S.; Janzadeh, A.; Yousefifard, M.; Nasirinezhad, F. The effect of intrathecal injection of irisin on pain threshold and expression rate of GABAB receptors in peripheral neuropathic pain model. J. Chem. Neuroanat. 2018, 91, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Li, H.Y.; Wang, H.X.; Wang, J.H.; Song, F.; Sun, Y. Irisin exerts neuroprotective effects on cultured neurons by regulating astrocytes. Mediat. Inflamm. 2018, 2018, 9070341. [Google Scholar] [CrossRef]
- Mastorakos, G.; Pavlatou, M.; Diamanti-Kandarakis, E.; Chrousos, G.P. Exercise and the stress system. Hormones 2005, 4, 73–89. [Google Scholar]
- Kurgan, N.; Noaman, N.; Pergande, M.R.; Cologna, S.M.; Coorssen, J.R.; Klentrou, P. Changes to the human serum proteome in response to high intensity interval exercise: A sequential top-down proteomic analysis. Front. Physiol. 2019, 10, 362. [Google Scholar] [CrossRef]
- Gil-Martinez, A.L.; Cuenca, L.; Sanchez, C.; Estrada, C.; Fernandez-Villalba, E.; Herrero, M.T. Effect of NAC treatment and physical activity on neuroinflammation in subchronic Parkinsonism; is physical activity essential? J. Neuroinflammation 2018, 15, 328. [Google Scholar] [CrossRef]
- Krause, M.; Rodrigues-Krause Jda, C. Extracellular heat shock proteins (eHSP70) in exercise: Possible targets outside the immune system and their role for neurodegenerative disorders treatment. Med. Hypotheses 2011, 76, 286–290. [Google Scholar] [CrossRef]
- Koester-Hegmann, C.; Bengoetxea, H.; Kosenkov, D.; Thiersch, M.; Haider, T.; Gassmann, M.; Gasser, E.M.S. High-altitude cognitive impairment is prevented by enriched environment including exercise via VEGF signaling. Front. Cell. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- Serra, F.T.; Carvalho, A.D.; Araujo, B.H.S.; Torres, L.B.; Cardoso, F.D.S.; Henrique, J.S.; Placencia, E.V.D.; Lent, R.; Gomez-Pinilla, F.; Arida, R.M.; et al. Early exercise induces long-lasting morphological changes in cortical and hippocampal neurons throughout of a sedentary period of rats. Sci. Rep. 2019, 9, 13684. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef]
- Henrique, J.S.; Franca, E.F.; Cardoso, F.D.S.; Serra, F.T.; de Almeida, A.A.; Fernandes, J.; Arida, R.M.; Gomes da Silva, S. Cortical and hippocampal expression of inflammatory and intracellular signaling proteins in aged rats submitted to aerobic and resistance physical training. Exp. Gerontol 2018, 110, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.; Jiang, L.; Zhang, Y.; Zhou, C.; Zhang, L.; Tang, J.; Liang, X.; Qi, Y.; Zhu, Y.; Ma, J.; et al. Stereological investigation of the effects of treadmill running exercise on the hippocampal neurons in middle-aged APP/PS1 transgenic mice. J. Alzheimer Dis. JAD 2018, 63, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.B.; Kwon, I.S.; Koo, J.H.; Kim, E.J.; Kim, C.H.; Lee, J.; Yang, C.H.; Lee, Y.I.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death and inflammation during Abeta-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis 2013, 18, 1332–1347. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Li, J.M.; Chen, C.X.; Li, S.X.; Xue, C.J. Effect on intensity of treadmill running on learning, memory and expressions of cell cycle-related proteins in rats with cerebral ischemia. Oncotarget 2017, 8, 40633–40642. [Google Scholar] [CrossRef] [PubMed]
- Mastrorilli, V.; Scopa, C.; Saraulli, D.; Costanzi, M.; Scardigli, R.; Rouault, J.P.; Farioli-Vecchioli, S.; Tirone, F. Physical exercise rescues defective neural stem cells and neurogenesis in the adult subventricular zone of Btg1 knockout mice. Brain Struct. Funct. 2017, 222, 2855–2876. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Galic, S.; van Wijngaarden, P.; Trounce, I.A.; Steinberg, G.R.; Crowston, J.G. Exercise reverses age-related vulnerability of the retina to injury by preventing complement-mediated synapse elimination via a BDNF-dependent pathway. Aging Cell 2016, 15, 1082–1091. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef]
- Mattson, M.P. Interventions that improve body and brain bioenergetics for Parkinson’s Disease risk reduction and therapy. J. Parkinson Dis. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. biol. Med. 2017, 102, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Maruzs, T.; Simon-Vecsei, Z.; Kiss, V.; Csizmadia, T.; Juhasz, G. On the fly: Recent progress on autophagy and aging in drosophila. Front. Cell Dev. Biol. 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Yamamoto, S.; Ting, T.; Fan, Y.; Sadleir, K.; Wang, Y.; Zhang, W.; Huang, S.; Levine, B.; Vassar, R.; et al. A becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. PLoS Genet. 2017, 13, e1006962. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahalakshmi, B.; Maurya, N.; Lee, S.-D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. https://doi.org/10.3390/ijms21165895
Mahalakshmi B, Maurya N, Lee S-D, Bharath Kumar V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. International Journal of Molecular Sciences. 2020; 21(16):5895. https://doi.org/10.3390/ijms21165895
Chicago/Turabian StyleMahalakshmi, B., Nancy Maurya, Shin-Da Lee, and V. Bharath Kumar. 2020. "Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration" International Journal of Molecular Sciences 21, no. 16: 5895. https://doi.org/10.3390/ijms21165895
APA StyleMahalakshmi, B., Maurya, N., Lee, S.-D., & Bharath Kumar, V. (2020). Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. International Journal of Molecular Sciences, 21(16), 5895. https://doi.org/10.3390/ijms21165895





