Proteome-Wide Analyses Provide New Insights into the Compatible Interaction of Rice with the Root-Knot Nematode Meloidogyne graminicola
Abstract
1. Introduction
2. Results
2.1. Proteome Measurement of the Roots of Rice Cultivar NPB with or without Infection of M. graminicola
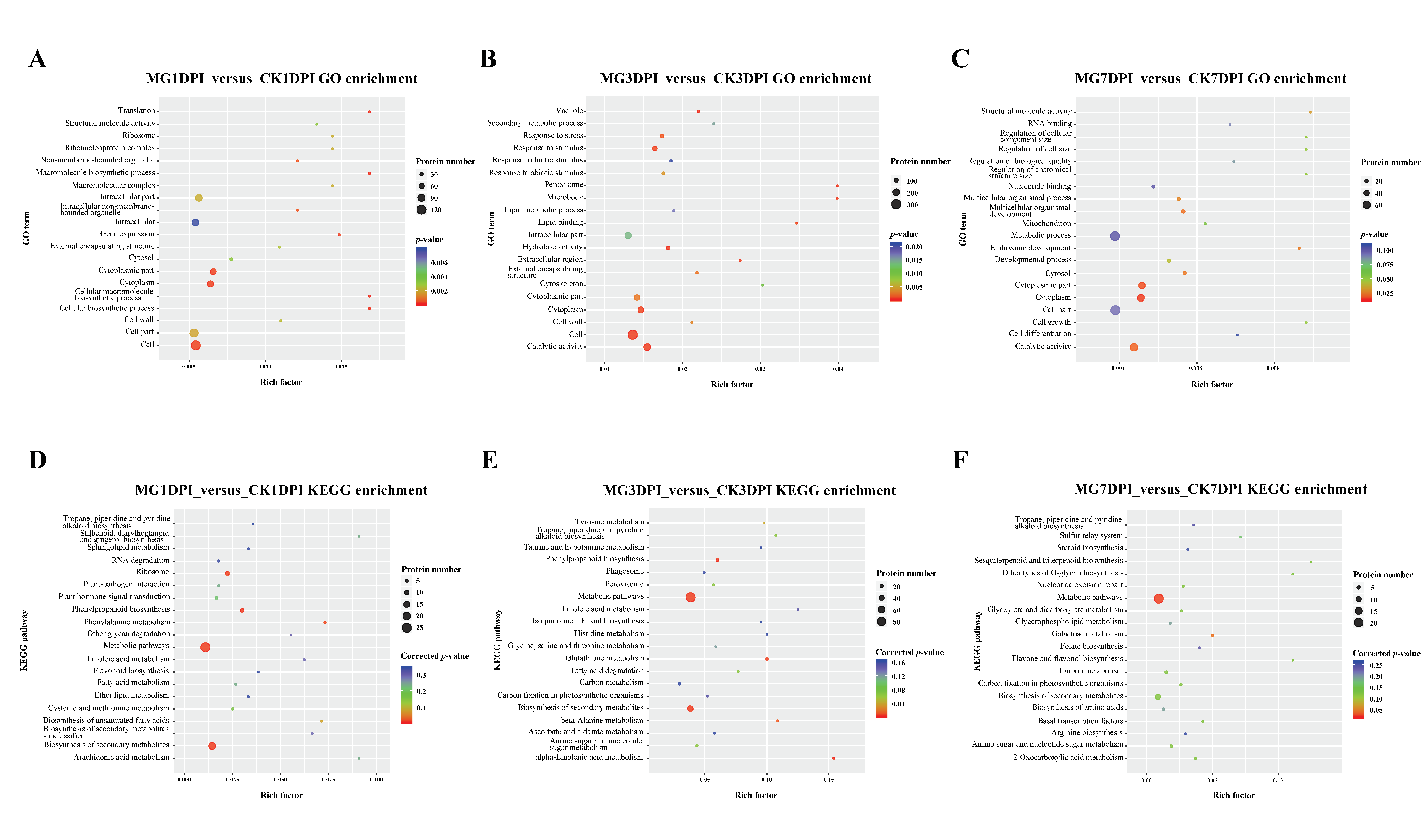
2.2. Proteome-wide Analyses of the Roots of NPB with Infection of M. graminicola
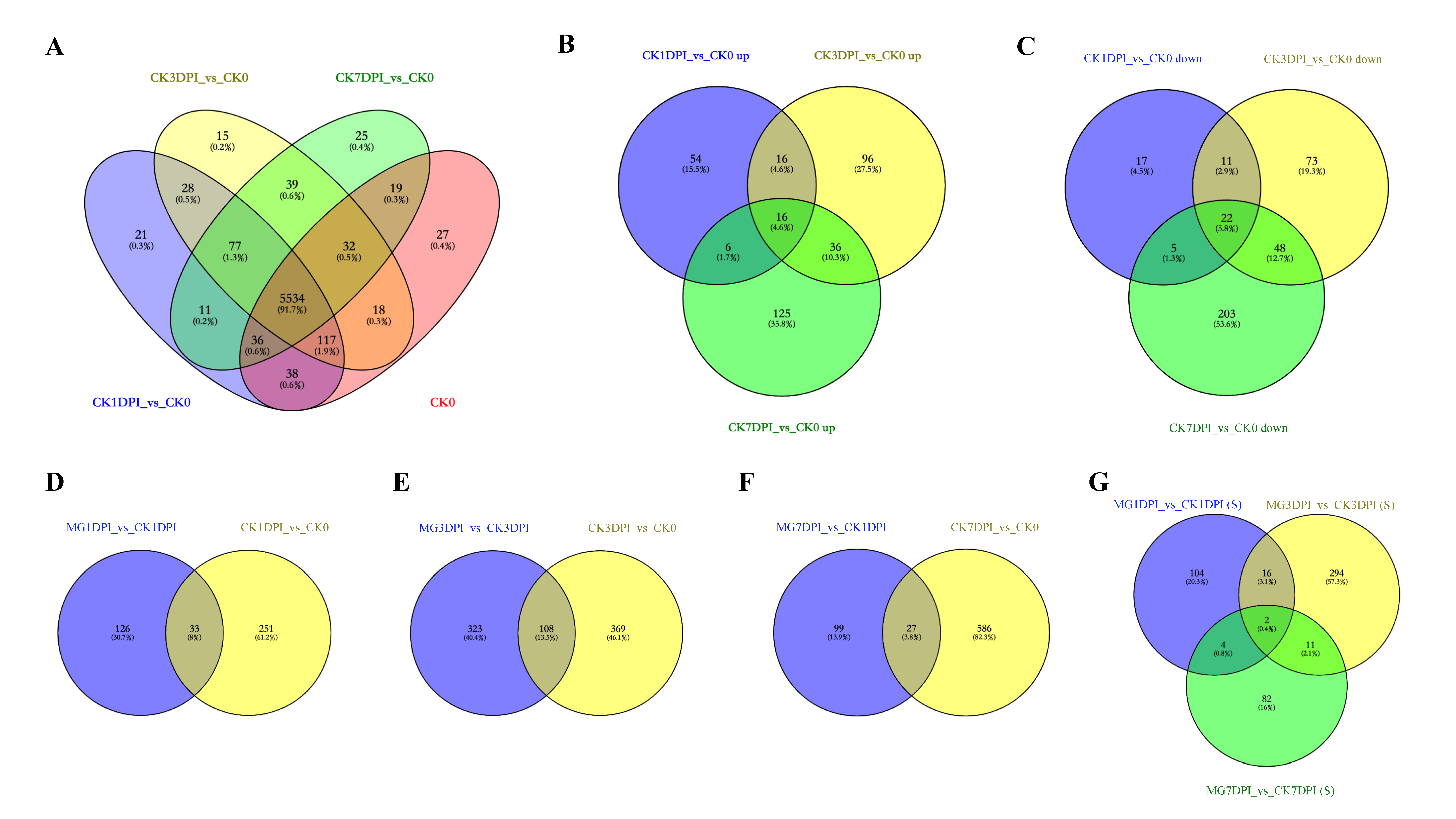
2.3. Proteins in the Roots of NPB Uniquely Caused by Infection of M. graminicola
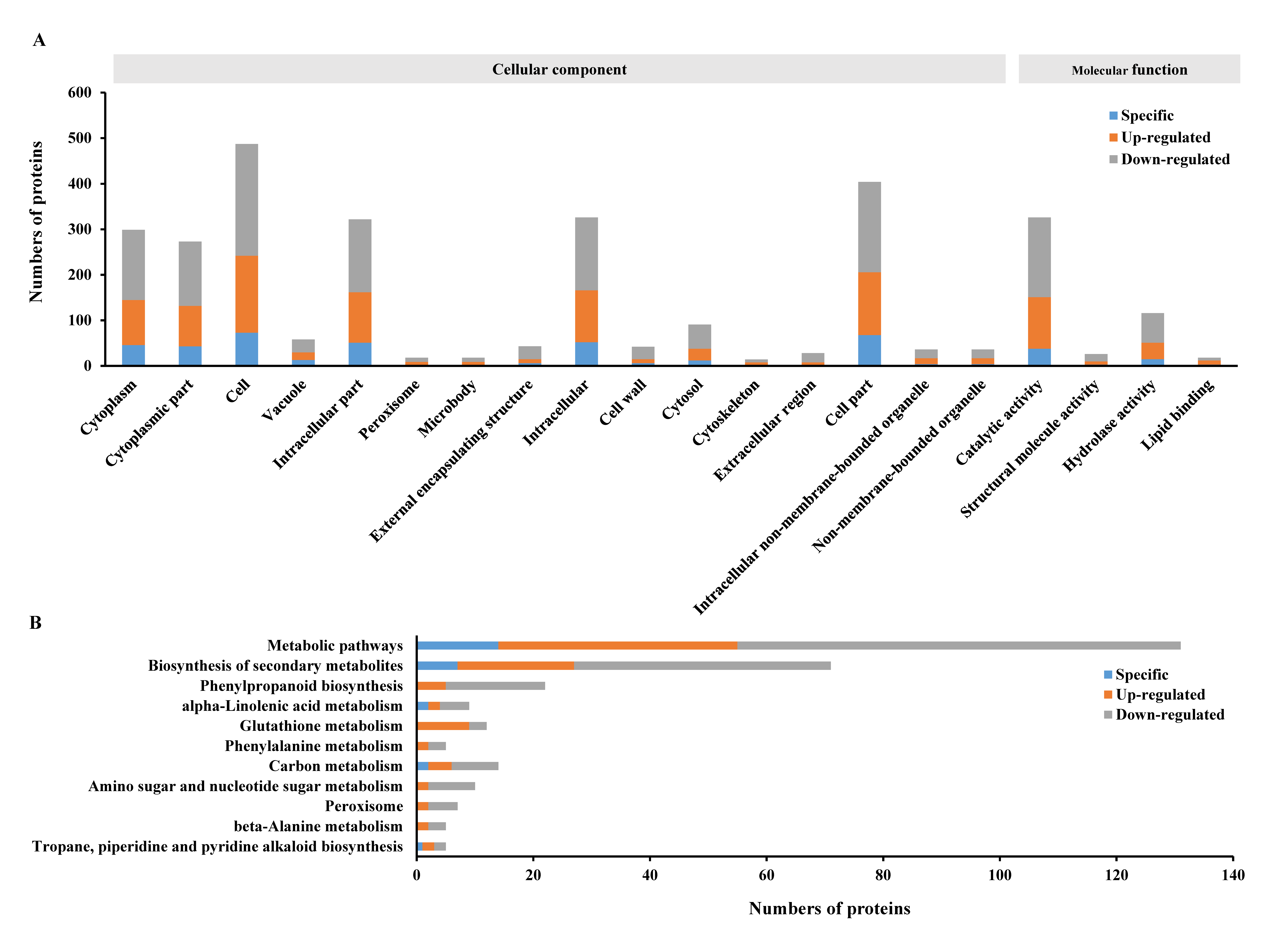
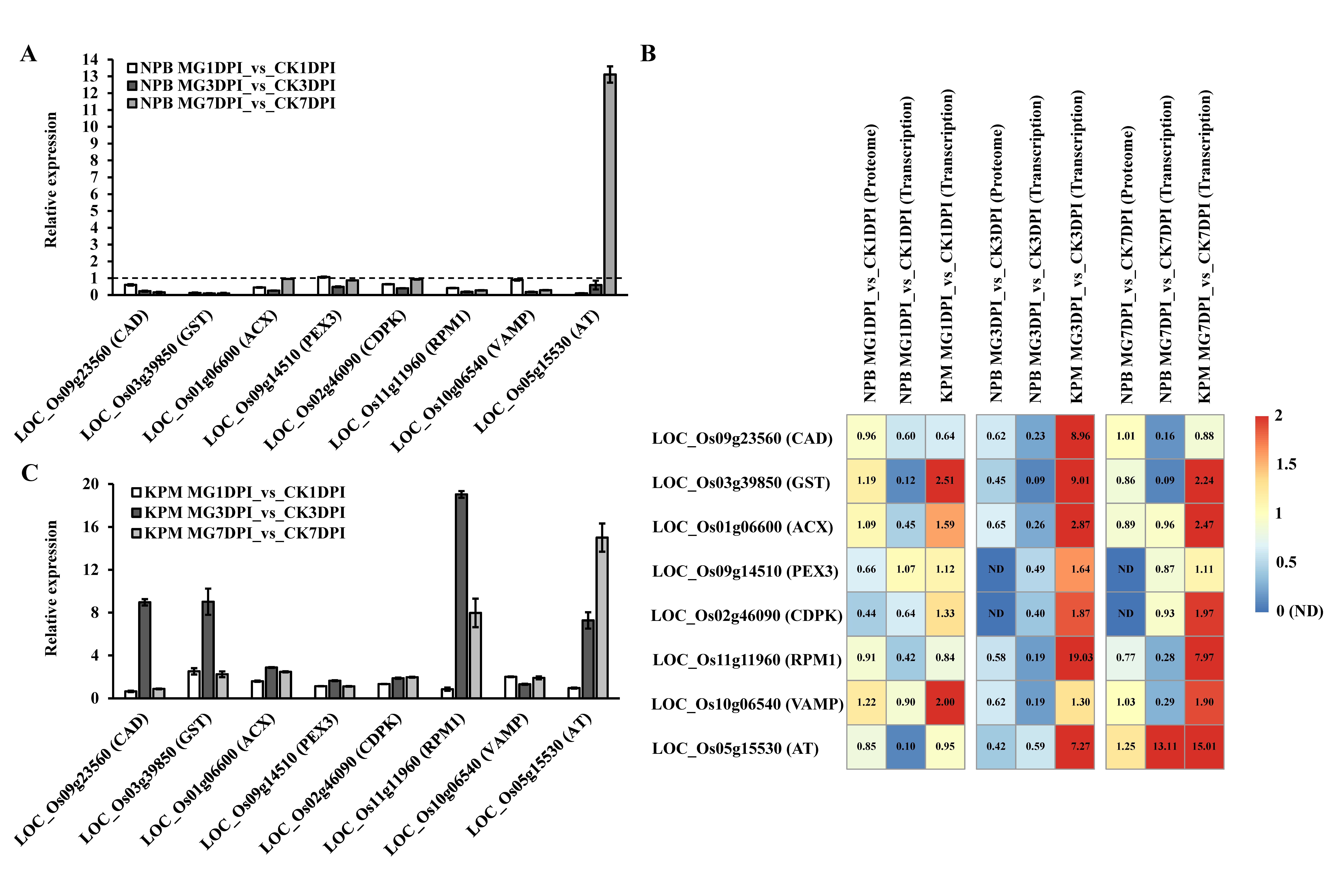
2.4. Important Proteins in the Roots Associated with the Compatible Interaction of NPB with M. graminicola
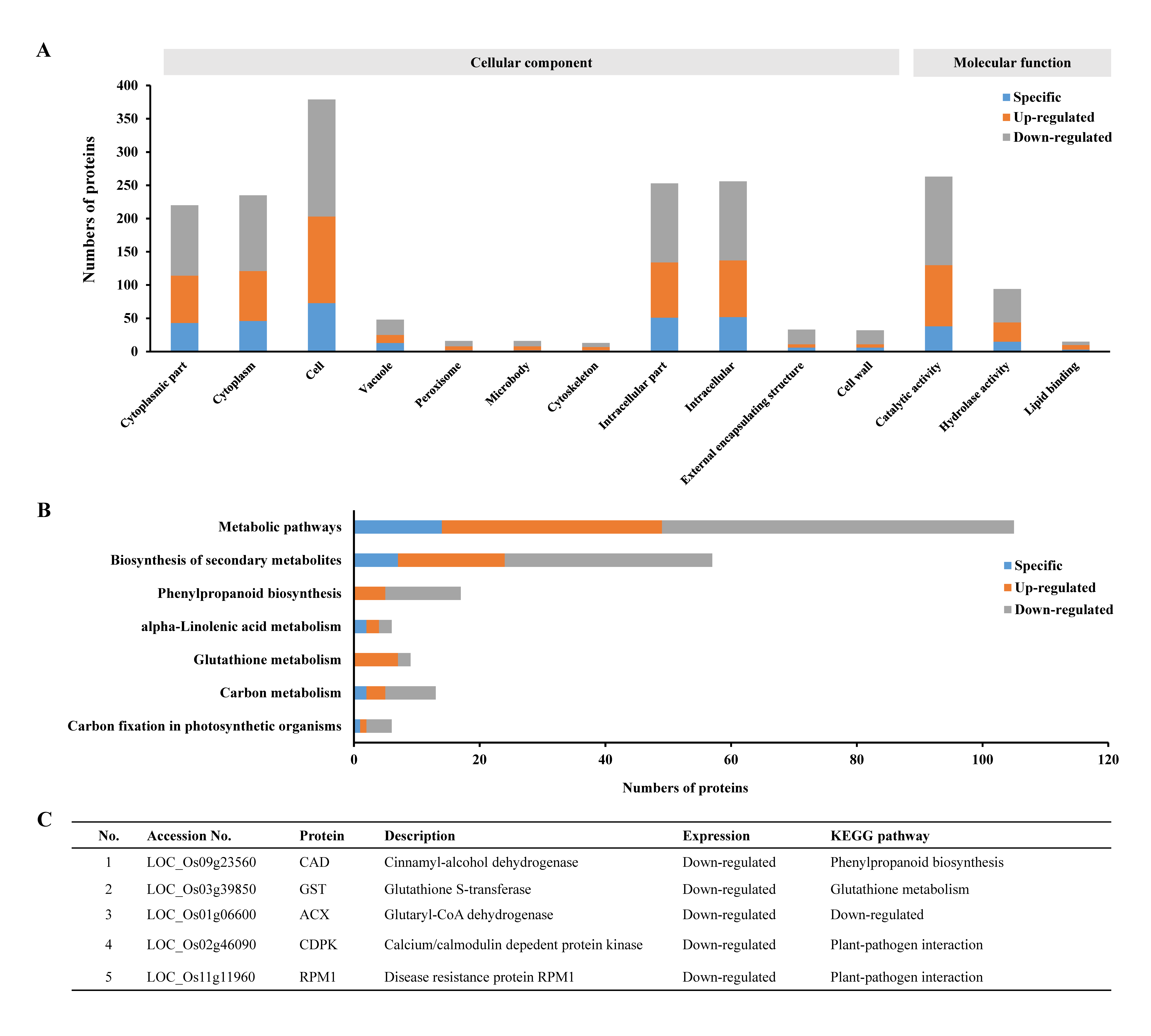
2.5. Proteins with Opposite Expression Patterns in Susceptible and Resistant Rice Lines in Response to the Infection of M. graminicola
3. Discussion
4. Materials and Methods
4.1. Rice Planting, Nematode Inoculation and Resistance Evaluation
4.2. Sample Collection
4.3. Protein Extraction and Digestion
4.4. High Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC–MS/MS)
4.5. Sequence Database Search and Data Analyses
4.6. Volcano Plot and Hierarchical Clustering Analyses
4.7. Bioinformatic Analysis
4.8. RNA Extraction and qRT-PCR
4.9. Statistical Analyses of Data
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- De Waele, D.; Elsen, A. Challenges in tropical plant nematology. Annu. Rev. Phytopathol. 2007, 45, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Z.; Peng, D.L.; Liu, S.M.; Kong, L.A.; Peng, H.; Xiang, C.; Li, Z.C.; Huang, W.K. Evaluation of the biocontrol potential of Aspergillus welwitschiae against the root-knot nematode Meloidogyne graminicola in rice (Oryza sativa L.). J. Integr. Agric. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, Y.; Liu, S.M.; Huang, Y.F.; Kong, L.A.; Peng, H.; Liu, M.Y.; Liu, J.; Peng, D.L.; Huang, W.K. αβ-Dehydrocurvularin isolated from the fungus Aspergillus welwitschiae effectively inhibited the behaviour and development of the root-knot nematode Meloidogyne graminicola in rice roots. BMC Microbiol. 2020, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Netscher, C.; Erlan, X. A root-knot nematode, Meloidogyne graminicola, parasitic on rice in Indonesia. Afro-Asia J. Nematol. 1993, 3, 90–95. [Google Scholar]
- Mantelin, S.; Bellafiore, S.; Kyndt, T. Meloidogyne graminicola: A major threat to rice agriculture. Mol. Plant Pathol. 2017, 18, 3–15. [Google Scholar] [CrossRef]
- Nguyễn, P.V.; Bellafiore, S.; Petitot, A.S.; Haidar, R.; Bak, A.; Abed, A.; Gantet, P.; Mezzalira, I.; Engler, J.A.; Fernandez, D. Meloidogyne incognita-rice (Oryza sativa) interaction: A new model system to study plant-root-knot nematode interactions in monocotyledons. Rice 2014, 7, 23. [Google Scholar] [CrossRef]
- Peng, D.L.; Gaur, S.H.; Bridge, J. Nematode parasites of rice. In Plant-Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI Publishing: Wallingford, UK, 2018; pp. 120–162. ISBN 1786391244. [Google Scholar]
- Galeng-Lawilao, J.; Kumar, A.; De Waele, D. QTL mapping for resistance to and tolerance for the rice root-knot nematode, Meloidogyne graminicola. BMC Genet. 2018, 19, 53. [Google Scholar] [CrossRef]
- Kyndt, T.; Denil, S.; Haegeman, A.; Trooskens, G.; Bauters, L.; Criekinge, W.V.; De Meyer, T.; Gheysen, G. Transcriptional reprogramming by root knot and migratory nematode infection in rice. New Phytol. 2012, 196, 887–900. [Google Scholar] [CrossRef]
- Ji, H.; Gheysen, G.; Denil, S.; Lindsey, K.; Topping, J.F.; Nahar, K.; Haegeman, A.; De Vos, W.H.; Trooskens, G.; Criekinge, W.V.; et al. Transcriptional analysis through RNA sequencing of giant cells induced by Meloidogyne graminicola in rice roots. J. Exp. Bot. 2013, 64, 3885–3898. [Google Scholar] [CrossRef]
- Petitot, A.S.; Kyndt, T.; Haidar, R.; Dereeper, A.; Collin, M.; de Almeida Engler, J.; Gheysen, G.; Fernandez, D. Transcriptomic and histological responses of African rice (Oryza glaberrima) to Meloidogyne graminicola provide new insights into root-knot nematode resistance in monocots. Ann. Bot-London 2017, 119, 885–899. [Google Scholar] [CrossRef]
- Xu, G.; Greene, H.H.; Yoo, H.; Liu, L.; Marqués, J.; Motley, J.; Dong, X. Global translational reprogramming is a fundamental layer of immune regulation in plants. Nature 2017, 545, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Yu, L.; Chen, Z.; Zhang, S.; Shi, J.; Zhao, X.; Yang, Y.; Hu, D.; Song, B. Label-free quantitative proteomic analysis of chitosan oligosaccharide-treated rice infected with southern rice black-streaked dwarf virus. Viruses 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zeng, Y.; Liang, Y.; Qian, Q.; Yang, C. Proteomic dissection of the rice-Fusarium fujikuroi interaction and the correlation between the proteome and transcriptome under disease stress. BMC Genom. 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, L.; Zhao, M.; Gu, S.; Wang, C.; Zhao, J.; Tang, Z.; Gao, H.; Zhang, L.; Fu, L.; et al. iTRAQ proteomics reveals the regulatory response to Magnaporthe oryzae in durable resistant vs. susceptible rice genotypes. PLoS ONE 2020, 15, e0227470. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.B.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V.M. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 1998, 10, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Ammiraju, J.; Veremis, J.; Huang, X.; Roberts, P.; Kaloshian, I. The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome. Theor. App. Genet. 2003, 106, 478–484. [Google Scholar] [CrossRef]
- Tzortzakakis, E.A.; Adam, M.A.M.; Blok, V.C.; Paraskevopoulos, C.; Bourtzis, K. Occurrence of resistance-breaking populations of root-knot nematodes on tomato in Greece. Eur. J. Plant. Pathol. 2005, 113, 101–105. [Google Scholar] [CrossRef]
- Jablonska, B.; Ammiraju, J.S.; Bhattarai, K.K.; Mantelin, S.; Martinez, I.O.; Roberts, P.A.; Kaloshian, I. The Mi-9 gene from Solanum arcanum conferring heat-stable resistance to root-knot nematodes is a homolog of Mi-1. Plant Physiol. 2007, 143, 1044–1054. [Google Scholar] [CrossRef]
- Claverie, M.; Dirlewanger, E.; Bosselut, N.; Ghelder, C.V.; Voisin, R.; Kleinhentz, M.; Lafargue, B.; Abad, P.; Rosso, M.N.; Chalhoub, B.; et al. The Ma gene for complete-spectrum resistance to Meloidogyne species in Prunus is a TNL with a huge repeated C-terminal post-LRR region. Plant Physiol. 2011, 156, 779–792. [Google Scholar] [CrossRef]
- Dimkpa, S.O.N.; Lahari, Z.; Shrestha, R.; Douglas, A.; Gheysen, G.; Price, A.H. A genome-wide association study of a global rice panel reveals resistance in Oryza sativa to root-knot nematodes. J. Exp. Bot. 2016, 67, 1191–1200. [Google Scholar] [CrossRef]
- Cook, D.E.; Lee, T.G.; Guo, X.; Melito, S.; Wang, K.; Bayless, A.M.; Wang, J.; Hughes, T.J.; Willis, D.K.; Clemente, T.E.; et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Sci. 2012, 338, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kandoth, P.K.; Warren, S.D.; Yeckel, G.; Heinz, R.; Alden, J.; Yang, C.; Jamai, A.; El-Mellouki, T.; Juvale, P.S.; et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 2012, 492, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kandoth, P.K.; Lakhssassi, N.; Kang, J.; Colantonio, V.; Heinz, R.; Yeckel, G.; Zhou, Z.; Bekal, S.; Dapprich, J.; et al. The soybean GmSNAP18 gene underlies two types of resistance to soybean cyst nematode. Nat. Commun. 2017, 8, 14822. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Del Río, L.A.; Palma, J.M. Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J. Integrat. Plant Biol. 2019, 61, 803–816. [Google Scholar]
- Wang, X.; Cheng, C.; Li, Q.; Zhang, K.; Lou, Q.; Li, J.; Chen, J. Multi-omics analysis revealed that MAPK signaling and flavonoid metabolic pathway contributed to resistance against Meloidogyne incognita in the introgression line cucumber. J. Proteom. 2020, 220, 103675. [Google Scholar] [CrossRef]
- Kyndt, T.; Fernandez, D.; Gheysen, G. Plant-parasitic nematode infection in rice: Molecular and cellular insights. Annu. Rev. Phytopathol. 2014, 52, 7–19. [Google Scholar] [CrossRef]
- Hatzade, B.; Singh, D.; Phani, V.; Kumbhar, S.; Rao, U. Profiling of defense responsive pathway regulatory genes in Asian rice (Oryza sativa) against infection of Meloidogyne graminicola (Nematoda: Meloidogynidae). 3 Biotech 2020, 10, 60. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Bredow, M.; Monaghan, J. Regulation of plant immune signaling by calcium-dependent protein kinases. Mol. Plant Microb. Interact. 2019, 32, 6–19. [Google Scholar] [CrossRef]
- Grant, M.R.; Godiard, L.; Straube, E.; Ashfield, T.; Dangl, J.L. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 1995, 269, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.P.; Peng, D.L.; Wang, X.L.; Kong, L.A.; Peng, H.; Liu, S.M.; Liu, Y.; Huang, W.K. Priming effect of root-applied silicon on the enhancement of induced resistance to the root-knot nematode Meloidogyne graminicola in rice. BMC Plant Biol. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Reversat, G.; Boyer, J.; Sannier, C.; Pando-Bahuon, A. Use of a mixture of sand and water-absorbent synthetic polymer as substrate for the xenic culturing of plant-parasitic nematodes in the laboratory. Nematology 1999, 1, 209–212. [Google Scholar] [CrossRef]
- Huang, W.K.; Ji, H.L.; Gheysen, G.; Debode, J.; Kyndt, T. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Bio. 2015, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.P.; Ding, Z.; Peng, D.L.; Peng, H.; Kong, L.A.; Liu, S.M.; Liu, Y.; Li, Z.C.; Huang, W.K. Evaluation of Chinese rice varieties resistant to the root-knot nematode Meloidogyne graminicola. J. Integr. Agric. 2018, 17, 621–630. [Google Scholar] [CrossRef]
- Bybd, D.W.; Kirkpatrick, T.; Barker, K.R. An improved technique for clearing and staining plant tissues for detection of nematodes. J. Nematol. 1983, 15, 142. [Google Scholar]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Mann, M. Combination of FASP and stagetip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J. Proteome Res. 2009, 8, 5674–5678. [Google Scholar] [CrossRef]
- Kao, S.H.; Wong, H.K.; Chiang, C.Y.; Chen, H.M. Evaluating the compatibility of three colorimetric protein assays for two-dimensional electrophoresis experiments. Proteomics 2008, 8, 2178–2184. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendricjson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Yan, H.Y.; You, Q.; Yi, X.; Du, Z.; Xu, W.Y.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020, 29, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△ct method. Methods 2000, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Samples | Repeat 1 | Repeat 2 | Repeat 3 | Total | Common in All 3 Repeats | Common Only in 2 Repeats |
|---|---|---|---|---|---|---|
| CK0 | 5801 | 5819 | 5683 | 5821 | 5473 | 348 |
| CK1DPI | 5784 | 5838 | 5824 | 5862 | 5568 | 294 |
| CK3DPI | 5861 | 5774 | 5771 | 5860 | 5503 | 357 |
| CK7DPI | 5702 | 5763 | 5692 | 5773 | 5379 | 394 |
| MG1DPI | 5795 | 5760 | 5797 | 5822 | 5538 | 284 |
| MG3DPI | 5821 | 5769 | 5772 | 5844 | 5511 | 333 |
| MG7DPI | 5734 | 5757 | 5744 | 5784 | 5482 | 302 |
| Average | 5824 | |||||
| Total | 6072 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, C.; Yang, X.; Peng, D.; Kang, H.; Liu, M.; Li, W.; Huang, W.; Liu, S. Proteome-Wide Analyses Provide New Insights into the Compatible Interaction of Rice with the Root-Knot Nematode Meloidogyne graminicola. Int. J. Mol. Sci. 2020, 21, 5640. https://doi.org/10.3390/ijms21165640
Xiang C, Yang X, Peng D, Kang H, Liu M, Li W, Huang W, Liu S. Proteome-Wide Analyses Provide New Insights into the Compatible Interaction of Rice with the Root-Knot Nematode Meloidogyne graminicola. International Journal of Molecular Sciences. 2020; 21(16):5640. https://doi.org/10.3390/ijms21165640
Chicago/Turabian StyleXiang, Chao, Xiaoping Yang, Deliang Peng, Houxiang Kang, Maoyan Liu, Wei Li, Wenkun Huang, and Shiming Liu. 2020. "Proteome-Wide Analyses Provide New Insights into the Compatible Interaction of Rice with the Root-Knot Nematode Meloidogyne graminicola" International Journal of Molecular Sciences 21, no. 16: 5640. https://doi.org/10.3390/ijms21165640
APA StyleXiang, C., Yang, X., Peng, D., Kang, H., Liu, M., Li, W., Huang, W., & Liu, S. (2020). Proteome-Wide Analyses Provide New Insights into the Compatible Interaction of Rice with the Root-Knot Nematode Meloidogyne graminicola. International Journal of Molecular Sciences, 21(16), 5640. https://doi.org/10.3390/ijms21165640








