l-Carnitine Supplementation during In Vitro Maturation and In Vitro Culture Does not Affect the Survival Rates after Vitrification and Warming but Alters Inf-T and ptgs2 Gene Expression
Abstract
1. Introduction
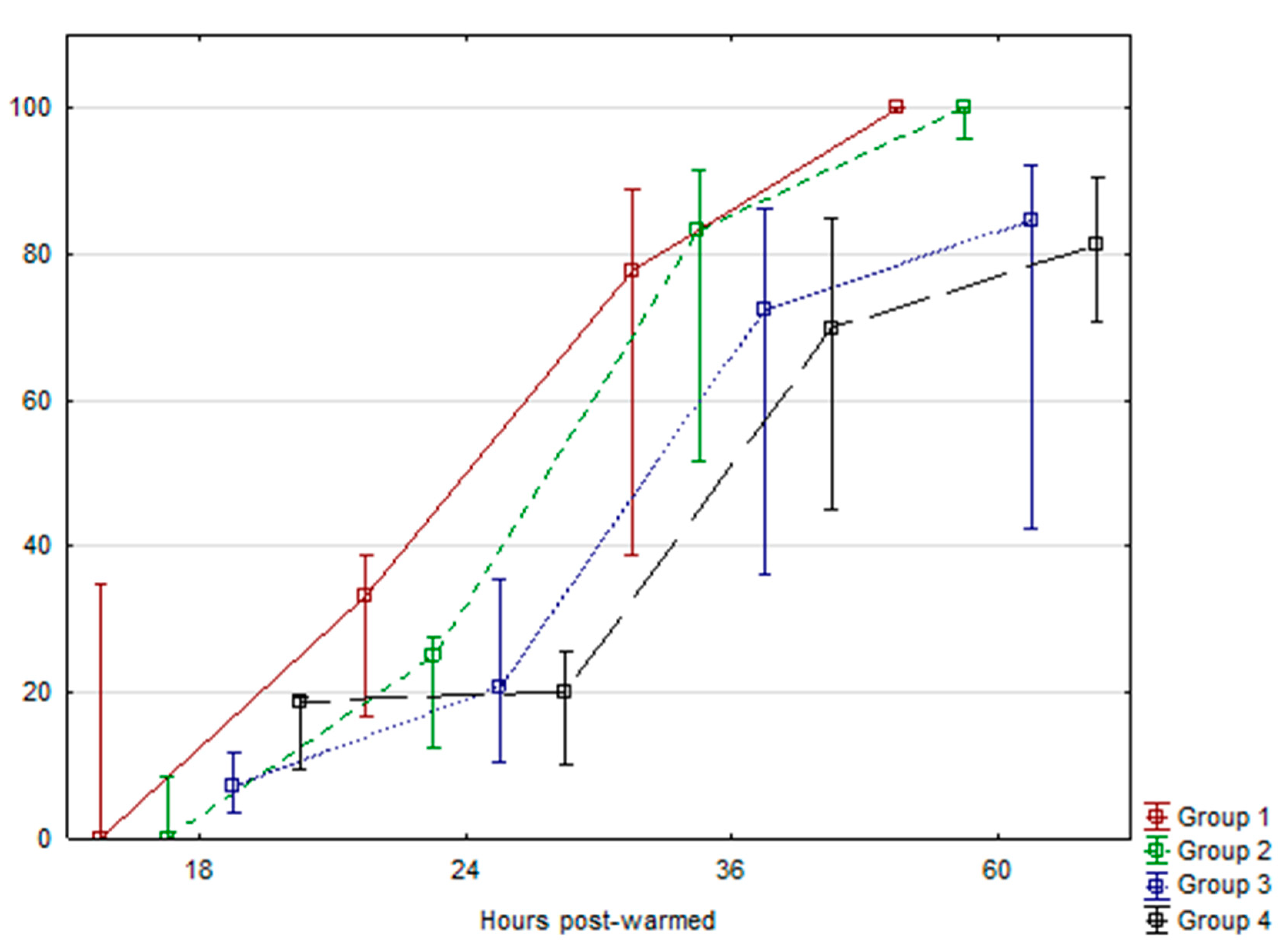
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Oocyte Collection
4.3. Oocyte Maturation
4.4. Oocyte Fertilization
4.5. Assessment of Embryo Culture and Development
4.6. Determination of Mitochondrial Activity in Embryos Cultured in the Presence of l-Carnitine
4.7. Determination of Lipid Droplets in Embryos Cultured in the Presence of l-Carnitine
4.8. Quantification of Cellularity in Bovine Embryos Cultured in the Presence of l-Carnitine
4.9. Vitrification, Warming, and Subsequent Embryo Culture
4.10. RNA Extraction, Reverse Transcription, and Quantification of mRNA Transcript Abundance
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donnay, I.; Partridge, R.J.; Leese, H.J. Can embryo metabolism be used for selecting bovine embryos before transfer? Reprod. Nutr. Dev. 1999, 39, 523–533. [Google Scholar] [CrossRef]
- Simintiras, C.A.; Fröhlich, T.; Sathyapalan, T.; Arnold, G.J.; Ulbrich, S.E.; Leese, H.J.; Sturmey, R.G. Modelling aspects of oviduct fluid formation in vitro. Reproduction 2017, 153, 23–33. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.; Feil, D.; Robker, R.L.; Thompson, J.G.; Dunning, K.R. Utilization of endogenous fatty acid stores for energy production in bovine preimplantation embryos. Theriogenology 2012, 77, 1632–1641. [Google Scholar] [CrossRef]
- de Souza, D.K.; Salles, L.P.; Rosa e Silva, A.A.M. Aspects of energetic substrate metabolism of in vitro and in vivo bovine embryos. Braz. J. Med. Biol. Res. 2015, 48, 191–197. [Google Scholar] [CrossRef]
- McKeegan, P.J. Metabolic Regulation during Early Embryo Development. Ph.D. Thesis, University of York, Hull York Medical School, York, UK, 2015. [Google Scholar]
- Knitlova, D.; Hulinska, P.; Jeseta, M.; Hanzalova, K.; Kempisty, B.; Machatkova, M. Supplementation of l-carnitine during in vitro maturation improves embryo development from less competent bovine oocytes. Theriogenology 2017, 102, 16–22. [Google Scholar] [CrossRef]
- Sprícigo, J.F.; Morató, R.; Arcarons, N.; Yeste, M.; Dode, M.A.; López-Bejar, M.; Mogas, T. Assessment of the effect of adding l-carnitine and/or resveratrol to maturation medium before vitrification on in vitro -matured calf oocytes. Theriogenology 2017, 89, 47–57. [Google Scholar] [CrossRef]
- Ghanem, N.; Salilew-Wondim, D.; Gad, A.; Tesfaye, D.; Phatsara, C.; Tholen, E.; Looft, C.; Schellander, K.; Hoelker, M. Bovine blastocysts with developmental competence to term share similar expression of developmentally important genes although derived from different culture environments. Reproduction 2011, 142, 551–564. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Hochi, S. Recent Progress in Cryopreservation of Bovine Oocytes. Biomed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Takahashi, T.; Inaba, Y.; Somfai, T.; Kaneda, M.; Geshi, M.; Nagai, T.; Manabe, N. Supplementation of culture medium with l-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod. Fertil. Dev. 2013, 25, 589–599. [Google Scholar] [CrossRef]
- Carrillo-Gonzalez, D.; Rodríguez-Osorio, N.; Long, C.; Vásquez-Araque, N.; Maldonado-Estrada, J. Effect of l-carnitine supplementation during in vitro maturation and in vitro culture on oocyte quality and embryonic development rate of bovines. Asian Pac. J. Reprod. 2019, 8, 289. [Google Scholar] [CrossRef]
- Somfai, T.; Kaneda, M.; Akagi, S.; Watanabe, S.; Haraguchi, S.; Mizutani, E.; Dang-Nguyen, T.Q.; Geshi, M.; Kikuchi, K.; Nagai, T. Enhancement of lipid metabolism with l-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reprod. Fertil. Dev. 2011, 23, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.L.; Bartolac, L.K.; Bathgate, R.; Grupen, C.G. Supplementation of culture medium with l-carnitine improves the development and cryotolerance of in vitro-produced porcine embryos. Reprod. Fertil. Dev. 2017, 29, 2357. [Google Scholar] [CrossRef] [PubMed]
- Phongnimitr, T.; Liang, Y.; Srirattana, K.; Panyawai, K.; Sripunya, N.; Treetampinich, C.; Parnpai, R. Effect of l-carnitine on maturation, cryo-tolerance and embryo developmental competence of bovine oocytes: l-carnitine on in vitro Maturation and Fertilization in Bovines. Anim. Sci. J. 2013, 84, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, N. l-carnitine improved bovine blastocyst rate and quality when supplemented at different preimplantation stages. Egypt J. Anim. Prod. 2015, 52, 82–89. [Google Scholar]
- Held-Hoelker, E.; Klein, S.; Rings, F.; Salilew-Wondim, D.; Saeed-Zidane, M.; Neuhoff, C.; Tesfaye, D.; Schellander, K.; Hoelker, M. Cryosurvival of in vitro produced bovine embryos supplemented with l-Carnitine and concurrent reduction of fatty acids. Theriogenology 2017, 96, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zolini, A.M.; Carrascal-Triana, E.; de King, A.R.; Hansen, P.J.; Torres, C.A.A.; Block, J. Effect of addition of l-carnitine to media for oocyte maturation and embryo culture on development and cryotolerance of bovine embryos produced in vitro. Theriogenology 2019, 133, 135–143. [Google Scholar] [CrossRef]
- Baldoceda, L.; Gagné, D.; Ferreira, C.R.; Robert, C. Genetic influence on the reduction in bovine embryo lipid content by l-carnitine. Reprod. Fertil. Dev. 2016, 28, 1172–1184. [Google Scholar] [CrossRef]
- Absalón-Medina, V.A.; Butler, W.R.; Gilbert, R.O. Preimplantation embryo metabolism and culture systems: Experience from domestic animals and clinical implications. J. Assist. Reprod. Genet. 2014, 31, 393–409. [Google Scholar] [CrossRef]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef]
- Sudano, M.J.; Paschoal, D.M.; Rascado, T.D.S.; Magalhães, L.C.O.; Crocomo, L.F.; De Lima-Neto, J.F.; Landim-Alvarenga, F.D.C. Lipid content and apoptosis of in vitro-produced bovine embryos as determinants of susceptibility to vitrification. Theriogenology 2011, 75, 1211–1220. [Google Scholar] [CrossRef]
- Mishra, A.; Reddy, I.; Gupta, P.; Mondal, S. Developmental regulation and modulation of apoptotic genes expression in sheep oocytes and embryos cultured in vitro with l-carnitine. Reprod. Domest. Anim. 2016, 51, 1020–1029. [Google Scholar] [CrossRef]
- Ghanem, N.; Ha, A.-N.; Fakruzzaman, M.; Bang, J.-I.; Lee, S.-C.; Kong, I.-K. Differential expression of selected candidate genes in bovine embryos produced in vitro and cultured with chemicals modulating lipid metabolism. Theriogenology 2014, 82, 238–250. [Google Scholar] [CrossRef]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef]
- Barceló-Fimbres, M.; Seidel, G.E. Effects of fetal calf serum, phenazine ethosulfate and either glucose or fructose during in vitro culture of bovine embryos on embryonic development after cryopreservation. Mol. Reprod. Dev. 2007, 74, 1395–1405. [Google Scholar] [CrossRef]
- del Collado, M.; Saraiva, N.Z.; Lopes, F.L.; Gaspar, R.C.; Padilha, L.C.; Costa, R.R.; Rossi, G.F.; Vantini, R.; Garcia, J.M. Influence of bovine serum albumin and fetal bovine serum supplementation during in vitro maturation on lipid and mitochondrial behaviour in oocytes and lipid accumulation in bovine embryos. Reprod. Fertil. Dev. 2016, 28, 1721–1732. [Google Scholar] [CrossRef]
- Rizos, D.; Gutiérrez-Adán, A.; Pérez-Garnelo, S.; de la Fuente, J.; Boland, M.P.; Lonergan, P. Bovine Embryo Culture in the Presence or Absence of Serum: Implications for Blastocyst Development, Cryotolerance, and Messenger RNA Expression1. Biol. Reprod. 2003, 68, 236–243. [Google Scholar] [CrossRef]
- Saraiva, H.F.; Batista, R.I.; Alfradique, V.A.; Pinto, P.H.; Ribeiro, L.S.; Oliveira, C.S.; Souza-Fabjan, J.M.; Camargo, L.S.; Fonseca, J.F.; Brandão, F.Z. l-carnitine supplementation during vitrification or warming of in vivo -produced ovine embryos does not affect embryonic survival rates, but alters CrAT and PRDX1 expression. Theriogenology 2018, 105, 150–157. [Google Scholar] [CrossRef]
- Chankitisakul, V.; Somfai, T.; Inaba, Y.; Techakumphu, M.; Nagai, T. Supplementation of maturation medium with l-carnitine improves cryo-tolerance of bovine in vitro matured oocytes. Theriogenology 2013, 79, 590–598. [Google Scholar] [CrossRef]
- Verma, M.; Pandey, S.; Bhat, I.A.; Mukesh, B.; Anand, J.; Chandra, V.; Sharma, G. Impact of l-carnitine on lipid content and post thaw survivability of buffalo embryos produced in vitro. Cryobiology 2018, 82, 99–105. [Google Scholar] [CrossRef]
- You, J.; Lee, J.; Hyun, S.-H.; Lee, E. l-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology 2012, 78, 235–243. [Google Scholar] [CrossRef]
- Betts, D.H.; Bain, N.T.; Madan, P. The p66Shc Adaptor Protein Controls Oxidative Stress Response in Early Bovine Embryos. PLoS ONE 2014, 9, e86978. [Google Scholar] [CrossRef]
- Walker, A.M.; Kimura, K.; Roberts, R.M. Expression of bovine interferon-tau variants according to sex and age of conceptuses. Theriogenology 2009, 72, 44–53. [Google Scholar] [CrossRef][Green Version]
- Kiyma, Z.; Kose, M.; Atli, M.O.; Ozel, C.; Hitit, M.; Sen, G.; Kaya, M.; Kaya, M.S.; Kurar, E.; Kayis, S.A.; et al. Investigation of interferon-tau stimulated genes (ISGs) simultaneously in the endometrium, corpus luteum (CL) and peripheral blood leukocytes (PBLs) in the preluteolytic stage of early pregnancy in ewes. Small Rumin. Res. 2016, 140, 1–6. [Google Scholar] [CrossRef]
- Ding, N.-S.; Dong, R.R.; Guo, Y.-M.; Ren., J.; Yan., Y.; Ma, J.-W.; Chen, K.-F.; Hunng, L.-S. Genetic Variation of Porcine Prostaglandin-endoperoxide Synthase 2 (PTGS2) Gene and Its Association with Reproduc Traits in an Erhualian × Duroc F2 Population. Acta Genet. Sin. 2006, 33, 213–219. [Google Scholar] [CrossRef]
- Leroy, J.; Van Hoeck, V.; Clemente, M.; Rizos, D.; Gutierrez-Adan, A.; Van Soom, A.; Uytterhoeven, M.; Bols, P. The effect of nutritionally induced hyperlipidaemia on in vitro bovine embryo quality. Hum. Reprod. 2010, 25, 768–778. [Google Scholar] [CrossRef]
- Gómez, E.; Caamaño, J.N.; Bermejo-Álvarez, P.; Díez, C.; Muñoz, M.; Martín, D.; Carrocera, S.; Gutierrez-Adan, A. Gene Expression in Early Expanded Parthenogenetic and In Vitro Fertilized Bovine Blastocysts. J. Reprod. Dev. 2009, 55, 607–614. [Google Scholar] [CrossRef]
- Carrillo-González, D.F.; Long, C.R.; Maldonado-Estrada, J.G.; Hwang, J. A specific synthetic l-carnitine reduces the lipid contents by enhancing the mitochondrial activity in mouse adipocytes. Rev. Colomb. Cienc. Pecu. 2019, 32, 63. [Google Scholar]
- Calder, M.D.; Caveney, A.N.; Smith, L.C.; Watson, A.J. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reprod. Biol. Endocrinol. 2003, 1, 14. [Google Scholar] [CrossRef]
- Hawk, H.W.; Wall, R.J. Improved yields of bovine blastocysts from in vitro-produced oocytes. I. Selection of oocytes and zygotes. Theriogenology 1994, 41, 1571–1583. [Google Scholar] [CrossRef]
- Dode, M.A.N.; Rodovalho, N.C.; Ueno, V.G.; Fernandes, C.E. The effect of sperm preparation and co-incubation time on in vitro fertilization of bos indicus oocytes. Anim. Reprod. Sci. 2002, 69, 15–23. [Google Scholar] [CrossRef]
- Garcia, S.M.; Marinho, L.S.R.; Lunardelli, P.A.; Seneda, M.M.; Meirelles, F.V. Developmental Block and Programmed Cell Death in Bos indicus Embryos: Effects of Protein Supplementation Source and Developmental Kinetics. PLoS ONE 2015, 10, e0119463. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.B. Intracellular Lipids in Bos indicus and Box taurus oocytes. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2007. [Google Scholar]
- Barceló-Fimbres, M.; Seidel, G.E. Cross-validation of techniques for measuring lipid content of bovine oocytes and blastocysts. Theriogenology 2011, 75, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Romek, M.; Gajda, B.; Krzysztofowicz, E.; Kepczynski, M.; Smorag, Z. New technique to quantify the lipid composition of lipid droplets in porcine oocytes and pre-implantation embryos using Nile Red fluorescent probe. Theriogenology 2011, 75, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, S.M.; Herrera, A.L.; Betancur, G.R.; Urrego, R.; Zuluaga, J.J.E. Supplementation with resveratrol during culture improves the quality of in vitro produced bovine embryos. Livest. Sci. 2019, 221, 139–143. [Google Scholar] [CrossRef]
- Thouas, G.A.; Korfiatis, N.A.; French, A.J.; Jones, G.M.; Trounson, A.O. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod. Biomed. Online 2001, 3, 25–29. [Google Scholar] [CrossRef]
- Kuwayama, M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology 2007, 67, 73–80. [Google Scholar] [CrossRef]
- Zullo, G.; Albero, G.; Neglia, G.; De Canditiis, C.; Bifulco, G.; Campanile, G.; Gasparrini, B. l-ergothioneine supplementation during culture improves quality of bovine in vitro–produced embryos. Theriogenology 2016, 85, 688–697. [Google Scholar] [CrossRef]
- Bermejo-Álvarez, P.; Lonergan, P.; Rizos, D.; Gutiérrez-Adan, A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reprod. Biomed. Online 2010, 20, 341–349. [Google Scholar] [CrossRef]
- Lamas-Toranzo, I.; Pericuesta, E.; Bermejo-Álvarez, P. Mitochondrial and metabolic adjustments during the final phase of follicular development prior to IVM of bovine oocytes. Theriogenology 2018, 119, 156–162. [Google Scholar] [CrossRef]
- Goovaerts, I.G.F.; Leroy, J.; Rizos, D.; Bermejo-Álvarez, P.; Gutierrez-Adan, A.; Jorssen, E.; Bols, P. Single in vitro bovine embryo production: Coculture with autologous cumulus cells, developmental competence, embryo quality and gene expression profiles. Theriogenology 2011, 76, 1293–1303. [Google Scholar] [CrossRef]
- Lopera-Vásquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltrán-Breña, P.; Calle, A.; Redruello, A.; López-Martín, S.; Gutierrez-Adan, A.; Yáñez-Mó, M.; et al. Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| IVM Treatment | Total Oocytes Maturated | Cleavage Rate | 4-Cells Stage | ||
|---|---|---|---|---|---|
| n | Means ± SD | n | Means ± SD | ||
| Control | 431 | 363 | 84.09 ± 9.44 | 318 | 73.05 ± 12.05 |
| l-carnitine (3.8 mM) | 412 | 342 | 82.65 ± 4.60 | 270 | 65.34 ± 9.75 |
| Treatment | Blastocyst Rates | Mitochondrial Activity | Relative Lipid Content | TCN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Means | ±SEM | n | Means | ±SEM | n | Means | ±SEM | n | Means | ±SEM | |
| Group 1 | 42 | 26.60 | 5.86 | 37 | 100 a | 2.56 | 30 | 100 a | 3.79 | 18 | 133 | 8 |
| Group 2 | 54 | 26.51 | 4.09 | 36 | 87.30 b | 2.63 | 24 | 88.98 b,c | 4.25 | 17 | 124 | 8 |
| Group 3 | 73 | 27.41 | 3.08 | 28 | 88.22 b | 3.90 | 41 | 95.84 a,c | 2.38 | 28 | 132 | 6 |
| Group 4 | 62 | 29.47 | 2.82 | 42 | 82.61 b | 1.95 | 38 | 91.47 a,c | 2.56 | 26 | 138 | 4 |
| Gene | Primer Sequence (5′−3′) | Fragment Size (bp) | GenBank Access No. |
|---|---|---|---|
| slc27a4 | F: 5-ACTGTCAGGCGTGATATCTT-3 | 203 | NM_001075667 |
| R: 5-AGATCAGGGCTGTCTTGTC-3 | |||
| slc22a5 | F: 5-ACATCTACCTGTCCACCATC-3 | 173 | NM_001046502 |
| R: 5-CCTACAAGGAAAAACAGCAC-3 | |||
| tp53 | F: CTCAGTCCTCTGCCATACTA | 364 | NM_174201.2 |
| R: GGATCCAGGATAAGGTGAGC | |||
| bax | F: CTACTTTGCCAGCAAACTGG | 158 | NM_173894.1 |
| R: TCCCAAAGTAGGAGAGGA | |||
| shc1 shc | F: GGTTCGGACAAAGGATCACC | 335 | NM_001075305.1 (NM_001075305.2 updated) |
| R: GTGAGGTCTGGGGAGAAGC | |||
| ifn-t | F: 5-CTGGGAAATCATCAGAGTGGAG-3 | 279 | NM_001015511.3 |
| R: 5-TAAGGACTCATGCCCCTACAG -3 | |||
| ptgs2 | F: ATCTACCCGCCTCATGTTCCT | 187 | NM_174445.2 |
| R: GGATTAGCCTGCTTGTCTGGA | |||
| plac8 | F: CGGTGTTCCAGAGGTTTTTCC | 166 | NM_001025325.2 |
| R: AAGATGCCAGTCTGCCAGTCA | |||
| dgat1 | F: 5-TCCACTCCTGCCTGAACGC-3 | 165 | NM_174693 |
| R: 5-GCTGCCCACTTGCTGCTG-3 | |||
| dgat2 | F: 5-GCTTGACTGCAGGACTAAAC-3 | 151 | NM_205793 |
| R: 5-GCTCAGATTTCAGAGACTGG-3 | |||
| h2afz | F: 5-AGGACGACTAGCCATGGACGTGTG- 3 | 208 | NM_174809 |
| R: 5-CCACCACCAGCAATTGTAGCCTTG-3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-González, D.F.; Rodríguez-Osorio, N.; Long, C.R.; Vásquez-Araque, N.A.; Maldonado-Estrada, J.G. l-Carnitine Supplementation during In Vitro Maturation and In Vitro Culture Does not Affect the Survival Rates after Vitrification and Warming but Alters Inf-T and ptgs2 Gene Expression. Int. J. Mol. Sci. 2020, 21, 5601. https://doi.org/10.3390/ijms21165601
Carrillo-González DF, Rodríguez-Osorio N, Long CR, Vásquez-Araque NA, Maldonado-Estrada JG. l-Carnitine Supplementation during In Vitro Maturation and In Vitro Culture Does not Affect the Survival Rates after Vitrification and Warming but Alters Inf-T and ptgs2 Gene Expression. International Journal of Molecular Sciences. 2020; 21(16):5601. https://doi.org/10.3390/ijms21165601
Chicago/Turabian StyleCarrillo-González, Diego F., Nélida Rodríguez-Osorio, Charles R. Long, Neil A. Vásquez-Araque, and Juan G. Maldonado-Estrada. 2020. "l-Carnitine Supplementation during In Vitro Maturation and In Vitro Culture Does not Affect the Survival Rates after Vitrification and Warming but Alters Inf-T and ptgs2 Gene Expression" International Journal of Molecular Sciences 21, no. 16: 5601. https://doi.org/10.3390/ijms21165601
APA StyleCarrillo-González, D. F., Rodríguez-Osorio, N., Long, C. R., Vásquez-Araque, N. A., & Maldonado-Estrada, J. G. (2020). l-Carnitine Supplementation during In Vitro Maturation and In Vitro Culture Does not Affect the Survival Rates after Vitrification and Warming but Alters Inf-T and ptgs2 Gene Expression. International Journal of Molecular Sciences, 21(16), 5601. https://doi.org/10.3390/ijms21165601






