Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality
Abstract
1. Introduction
2. Results
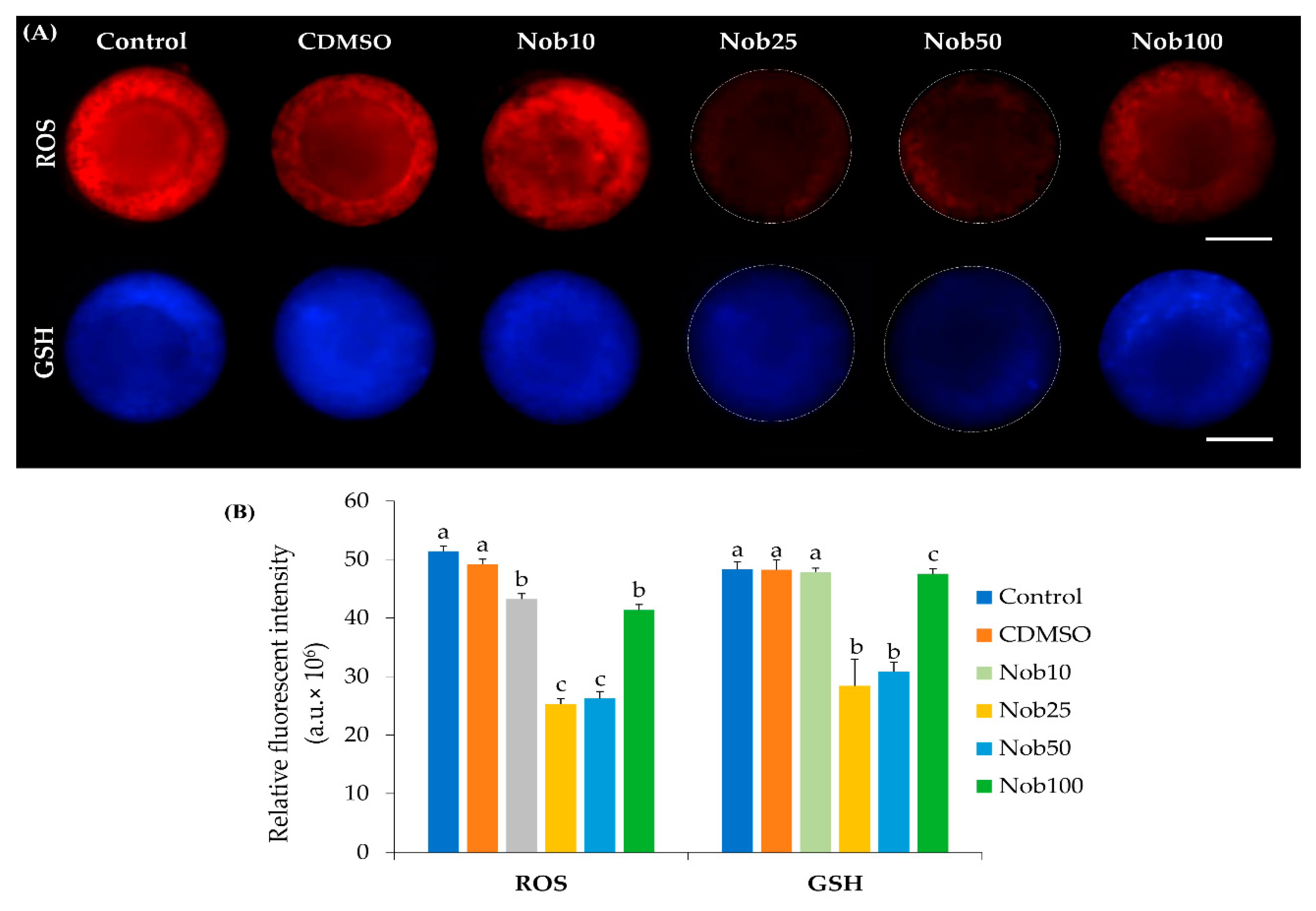
2.1. Nobiletin Enhances Oocyte In Vitro Maturation and Reduces Oxidative Stress
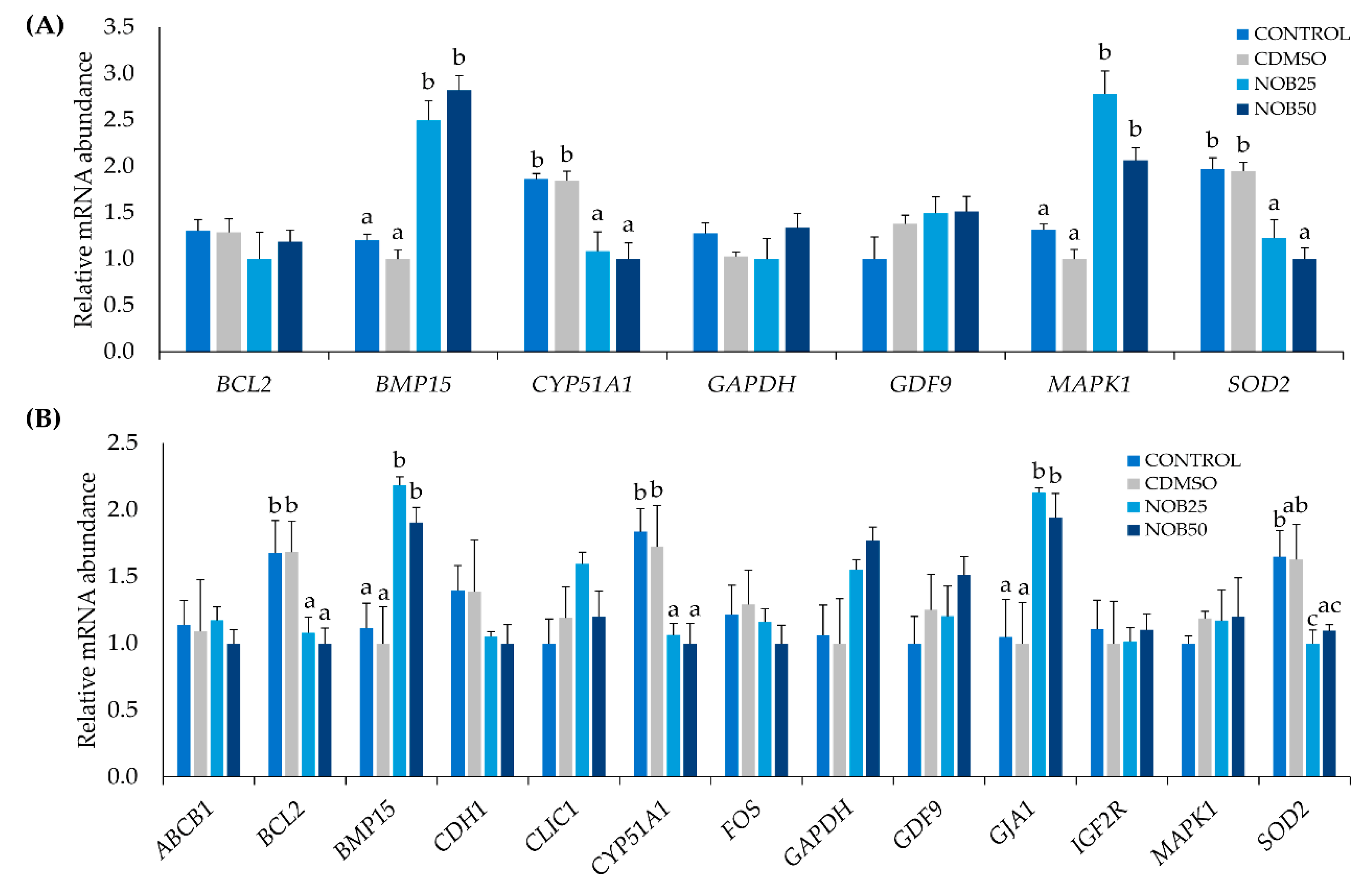
2.2. Nobiletin Increases Estradiol (E2) and Progesterone (P4) Production by Cumulus Cells
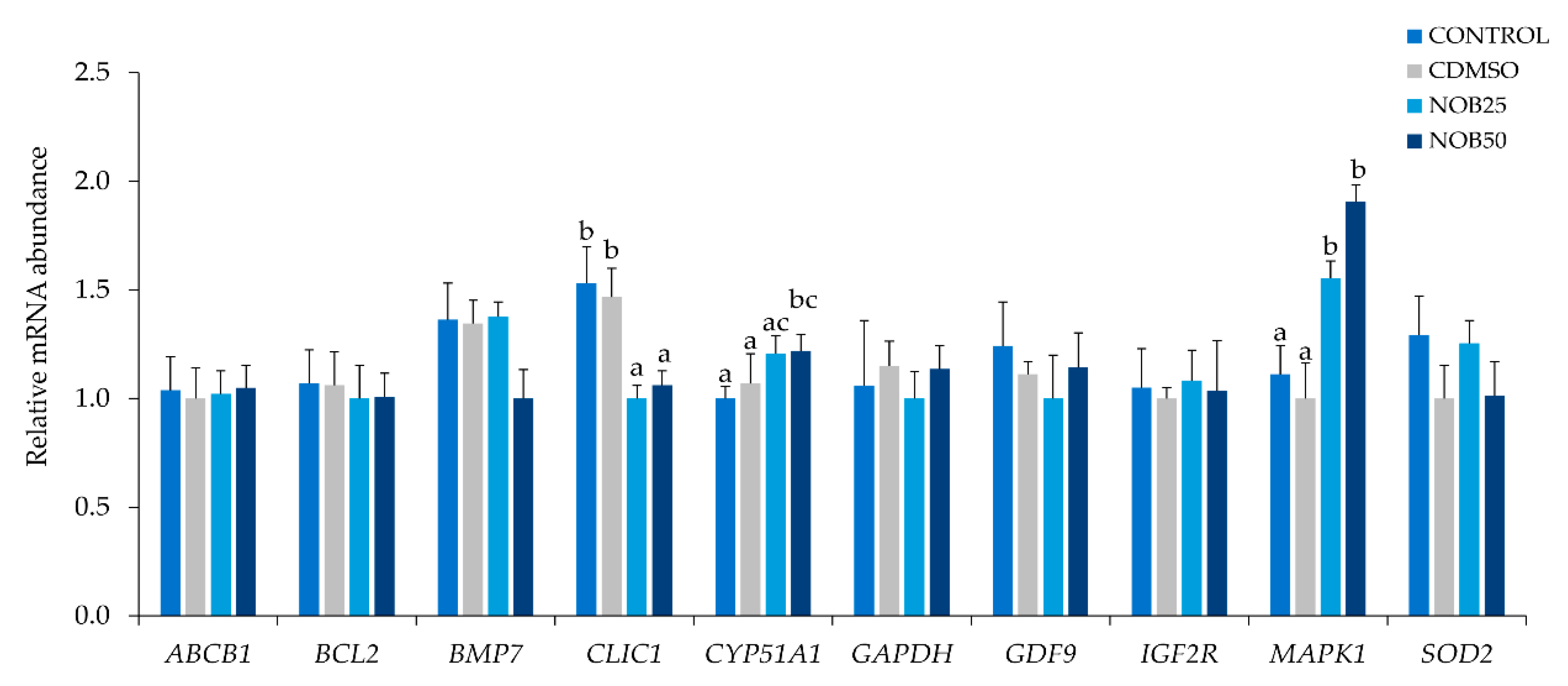
2.3. Nobiletin Increases Embryo Development and Quality
3. Discussion
4. Materials and Methods
4.1. Oocyte Collection and In Vitro Maturation
4.2. Cortical Granules (CG) Distribution Patterns
4.3. Mitochondrial Distribution Patterns and Quantification of Mitochondrial Activity
4.4. Assessment of Oocyte Nuclear Maturation
4.5. Levels of Reactive Oxygen Species (ROS) and Glutathione (GSH)
4.6. Steroidogenic Production of Estradiol and Progesterone by CCs
4.7. Sperm Preparation and In Vitro Fertilization (IVF)
4.8. In Vitro Culture of Presumptive Zygotes
4.9. Gene Expression Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Fan, X.; Li, R.; Zhang, C.; Zhang, J. Effects of pre-incubation with C-type natriuretic peptide on nuclear maturation, mitochondrial behavior, and developmental competence of sheep oocytes. Biochem. Biophys. Res. Commun. 2018, 497, 200–206. [Google Scholar] [CrossRef]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Rocha-Frigoni, N.A.; Leão, B.C.D.S.; Dall’Acqua, P.C.; Mingoti, G. Improving the cytoplasmic maturation of bovine oocytes matured in vitro with intracellular and/or extracellular antioxidants is not associated with increased rates of embryo development. Theriogenology 2016, 86, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, M.; Dall’Acqua, P.C.; Rocha-Frigoni, N.; Leão, B.C.D.S.; Mingoti, G. Transporting bovine oocytes in a medium supplemented with different macromolecules and antioxidants: Effects on nuclear and cytoplasmic maturation and embryonic development in vitro. Reprod. Domest. Anim. 2017, 52, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Kere, M.; Siriboon, C.; Lo, N.-W.; Nguyen, N.T.; Ju, J.-C. Ascorbic Acid Improves the Developmental Competence of Porcine Oocytes After Parthenogenetic Activation and Somatic Cell Nuclear Transplantation. J. Reprod. Dev. 2012, 59, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sovernigo, T.; Adona, P.R.; Monzani, P.; Guemra, S.; Barros, F.; Lopes, F.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef] [PubMed]
- De Matos, D.; Furnus, C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development: Effect of β-mercaptoethanol, cysteine and cystine. Theriogenology 2000, 53, 761–771. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577.e1–586.e1. [Google Scholar] [CrossRef]
- Kimura, O.; Ohta, C.; Koga, N.; Haraguchi, K.; Kato, Y.; Endo, T. Carrier-mediated uptake of nobiletin, a citrus polymethoxyflavonoid, in human intestinal Caco-2 cells. Food Chem. 2014, 154, 145–150. [Google Scholar] [CrossRef]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its MetabolitesIn VivoandIn Vitro. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Liu, B.; Huang, J.; Zhang, B. Nobiletin protects against murine l -arginine-induced acute pancreatitis in association with downregulating p38MAPK and AKT. Biomed. Pharmacother. 2016, 81, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ono, M.; Takeshima, M.; Nakano, S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer. Res. 2014, 34, 1785–1792. [Google Scholar] [PubMed]
- Choi, S.-Y.; Hwang, J.-H.; Ko, H.-C.; Park, J.-G.; Kim, S.J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-κB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev. Boil. 1982, 89, 268–272. [Google Scholar] [CrossRef]
- Zuelke, K.A.; Brackett, B.G. Luteinizing hormone-enhanced in vitro maturation of bovine oocytes with and without protein supplementation. Boil. Reprod. 1990, 43, 784–787. [Google Scholar] [CrossRef]
- Mingoti, G.; Garcia, J.; Rosa-E-Silva, A. Steroidogenesis in cumulus cells of bovine cumulus–oocyte-complexes matured in vitro with BSA and different concentrations of steroids. Anim. Reprod. Sci. 2002, 69, 175–186. [Google Scholar] [CrossRef]
- Endo, M.; Kawahara-Miki, R.; Cao, F.; Kimura, K.; Kuwayama, T.; Monji, Y.; Iwata, H. Estradiol supports in vitro development of bovine early antral follicles. Reproduction 2012, 145, 85–96. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Tanida, T.; Abdel-Ghani, M.A.; Kanno, C.; Yanagawa, Y.; Katagiri, S.; Nagano, M. Relationship between the antral follicle count in bovine ovaries from a local abattoir and steroidogenesis of granulosa cells cultured as oocyte-cumulus-granulosa complexes. J. Reprod. Dev. 2018, 64, 503–510. [Google Scholar] [CrossRef]
- Horigome, S.; Maeda, M.; Ho, H.-J.; Shirakawa, H.; Komai, M. Effect of Kaempferia parviflora extract and its polymethoxyflavonoid components on testosterone production in mouse testis-derived tumour cells. J. Funct. Food. 2016, 26, 529–538. [Google Scholar] [CrossRef]
- Pang, Y.-W.; Zhao, S.; Sun, Y.; Jiang, X.; Hao, H.; Du, W.; Zhu, H. Protective effects of melatonin on the in vitro developmental competence of bovine oocytes. Anim. Sci. J. 2017, 89, 648–660. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Sun, Q.-Y. Involvement of Mitogen-Activated Protein Kinase Cascade During Oocyte Maturation and Fertilization in Mammals1. Boil. Reprod. 2004, 70, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Quetglas, M.D.; Adona, P.; De Bem, T.; Pires, P.; Leal, C.L.V. Effect of Cyclin-dependent Kinase (CDK) Inhibition on Expression, Localization and Activity of Maturation Promoting Factor (MPF) and Mitogen Activated Protein Kinase (MAPK) in Bovine Oocytes. Reprod. Domest. Anim. 2009, 45, 1074–1081. [Google Scholar] [CrossRef]
- Gordo, A.C.; He, C.L.; Smith, S.; Fissore, R. Mitogen activated protein kinase plays a significant role in metaphase II arrest, spindle morphology, and maintenance of maturation promoting factor activity in bovine oocytes. Mol. Reprod. Dev. 2001, 59, 106–114. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.A.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Wu, G.; Yuan, D.; Jia, B.; Liu, C.; Zhu, S.; Hou, Y. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2014, 81, 608–618. [Google Scholar] [CrossRef]
- Hosoe, M.; Shioya, Y. Distribution of cortical granules in bovine oocytes classified by cumulus complex. Zygote 1997, 5, 371–376. [Google Scholar] [CrossRef]
- Hoodbhoy, T.; Dandekar, P.; Calarco, P.; Talbot, P. p62/p56 are cortical granule proteins that contribute to formation of the cortical granule envelope and play a role in mammalian preimplantation development. Mol. Reprod. Dev. 2001, 59, 78–89. [Google Scholar] [CrossRef]
- Viana, K.; Caldas-Bussiere, M.C.; Matta, S.; Faes, M.; De Carvalho, C.P.; Quirino, C. Effect of sodium nitroprusside, a nitric oxide donor, on the in vitro maturation of bovine oocytes. Anim. Reprod. Sci. 2007, 102, 217–227. [Google Scholar] [CrossRef]
- Ferreira, E.; Vireque, A.A.; Adona, P.; Meirelles, F.; Ferriani, R.; Navarro, P. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Brand, M.D.; Orr, A.; Perevoshchikova, I.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169, 1–8. [Google Scholar] [CrossRef]
- Tarazona, A.; Rodriguez, J.; Restrepo, L.; Olivera-Ángel, M. Mitochondrial Activity, Distribution and Segregation in Bovine Oocytes and in Embryos Produced in Vitro. Reprod. Domest. Anim. 2006, 41, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Egerszegi, I.; Alm, H.; Rátky, J.; Heleil, B.; Brüssow, K.-P.; Torner, H. Meiotic progression, mitochondrial features and fertilisation characteristics of porcine oocytes with different G6PDH activities. Reprod. Fertil. Dev. 2010, 22, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; Bezerra, F.; Souza, G.; Matos, M.; Hurk, R.V.D.; Silva, J.R.V. Influence of interleukin 1 beta and tumour necrosis factor alpha on the in vitro growth, maturation and mitochondrial distribution of bovine oocytes from small antral follicles. Zygote 2018, 26, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-M.; Wang, N.; Hao, H.-S.; Li, C.-Y.; Zhao, Y.-H.; Yan, C.-L.; Wang, H.-Y.; Du, W.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2017, 64, e12445. [Google Scholar] [CrossRef]
- Barja, G. Mitochondrial Oxygen Consumption and Reactive Oxygen Species Production are Independently Modulated: Implications for Aging Studies. Rejuvenation Res. 2007, 10, 215–224. [Google Scholar] [CrossRef]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R843–R863. [Google Scholar] [CrossRef]
- Kang, J.-T.; Moon, J.H.; Choi, J.-Y.; Park, S.J.; Kim, S.J.; Saadeldin, I.M.; Lee, B.C. Effect of Antioxidant Flavonoids (Quercetin and Taxifolin) on In vitro Maturation of Porcine Oocytes. Asian-Australas. J. Anim. Sci. 2016, 29, 352–358. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Du, W.-H.; Wang, D.; Hao, H.-S.; Liu, Y.; Qin, T.; Zhu, H.-B. Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Mol. Reprod. Dev. 2011, 78, 942–950. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, Y.; Chen, L.; Zhu, Y.; Xiao, X.; Wang, D.; Zhu, Y. Nobiletin prevents cadmium-induced neuronal apoptosis by inhibiting reactive oxygen species and modulating JNK/ERK1/2 and Akt/mTOR networks in rats. Neurol. Res. 2018, 40, 211–220. [Google Scholar] [CrossRef]
- Torres, V.; Hamdi, M.; Maillo, V.; Urrego, R.; Echeverri, J.J.; López-Herrera, A.; Gutierrez-Adan, A.; Rizos, D.; Sánchez-Calabuig, M.J. Ascorbic acid-cyclodextrin complex alters the expression of genes associated with lipid metabolism in bovine in vitro produced embryos. Reprod. Domest. Anim. 2019, 54, 55–62. [Google Scholar] [CrossRef]
- Chowdhury, M.; Choi, B.-H.; Khan, I.; Lee, K.-L.; Mesalam, A.; Song, S.-H.; Xu, L.; Joo, M.-D.; Afrin, F.; Kong, I.-K. Supplementation of lycopene in maturation media improves bovine embryo quality in vitro. Theriogenology 2017, 103, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Reddy, I.; Gupta, P.; Mondal, S. l-carnitine Mediated Reduction in Oxidative Stress and Alteration in Transcript Level of Antioxidant Enzymes in Sheep Embryos Produced In Vitro. Reprod. Domest. Anim. 2016, 51, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Gulçin, I. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Goodchild, S.C.; Howell, M.W.; Cordina, N.M.; Littler, D.; Breit, S.N.; Curmi, P.M.G.; Brown, L.J. Oxidation promotes insertion of the CLIC1 chloride intracellular channel into the membrane. Eur. Biophys. J. 2009, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Averaimo, S.; Milton, R.H.; Duchen, M.R.; Mazzanti, M. Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett. 2010, 584, 2076–2084. [Google Scholar] [CrossRef]
- Hamdi, M.; Lopera-Vasquez, R.; Maillo, V.; Calabuig, M.-J.S.; Núnez, C.; Gutierrez-Adan, A.; Rizos, D. Bovine oviductal and uterine fluid support in vitro embryo development. Reprod. Fertil. Dev. 2018, 30, 935. [Google Scholar] [CrossRef]
- Salhab, M.; Dhorne-Pollet, S.; Auclair, S.; Guyader-Joly, C.; Brisard, D.; Dalbiès-Tran, R.; Dupont, J.; Ponsart, C.; Mermillod, P.; Uzbekova, S. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol. Reprod. Dev. 2013, 80, 166–182. [Google Scholar] [CrossRef]
- Li, S.-H.; Lin, M.-H.; Hwu, Y.-M.; Lu, C.-H.; Yeh, L.-Y.; Chen, Y.-J.; Lee, R.K.-K. Correlation of cumulus gene expression of GJA1, PRSS35, PTX3, and SERPINE2 with oocyte maturation, fertilization, and embryo development. Reprod. Boil. Endocrinol. 2015, 13, 93. [Google Scholar] [CrossRef]
- Nakamura, T.; Iwase, A.; Bayasula, B.; Nagatomo, Y.; Kondo, M.; Nakahara, T.; Takikawa, S.; Goto, M.; Kotani, T.; Kiyono, T.; et al. CYP51A1 Induced by Growth Differentiation Factor 9 and Follicle-Stimulating Hormone in Granulosa Cells Is a Possible Predictor for Unfertilization. Reprod. Sci. 2014, 22, 377–384. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Sugiura, K.; Eppig, J.J. Mouse Oocyte Control of Granulosa Cell Development and Function: Paracrine Regulation of Cumulus Cell Metabolism. Semin. Reprod. Med. 2009, 27, 32–42. [Google Scholar] [CrossRef]
- Noguchi, S.; Atsumi, H.; Iwao, Y.; Kan, T.; Itai, S. Nobiletin: A citrus flavonoid displaying potent physiological activity. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Hussein, T.S.; Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. Temporal effects of exogenous oocyte-secreted factors on bovine oocyte developmental competence during IVM. Reprod. Fertil. Dev. 2011, 23, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, E.S.; Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G.; Price, C.A.; Machado, M.F.; Lima, P.F.; Buratini, J. Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake, and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus–oocyte complexes. Reproduction 2013, 146, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, M.; Eswari, S.; Kumanan, V. Differential expression dynamics of Growth differentiation factor9 (GDF9) and Bone morphogenetic factor15 (BMP15) mRNA transcripts during in vitro maturation of buffalo (Bubalus bubalis) cumulus–oocyte complexes. SpringerPlus 2013, 2, 206. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; A Kim, G.; Bin Choi, Y.; Jo, Y.K.; Setyawan, E.M.N.; Lee, B.C. Oocyte maturation-related gene expression in the canine oviduct, cumulus cells, and oocytes and effect of co-culture with oviduct cells on in vitro maturation of oocytes. J. Assist. Reprod. Genet. 2017, 34, 929–938. [Google Scholar] [CrossRef]
- Inoue, M. Mitogen-activated protein kinase translocates into the germinal vesicle and induces germinal vesicle breakdown in porcine oocytes. Boil. Reprod. 1998, 58, 130–136. [Google Scholar] [CrossRef]
- Yoshimizu, N.; Otani, Y.; Saikawa, Y.; Kubota, T.; Yoshida, M.; Furukawa, T.; Kumai, K.; Kameyama, K.; Fujii, M.; Yano, M.; et al. Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: Antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment. Pharmacol. Ther. 2004, 20, 95–101. [Google Scholar] [CrossRef]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef]
- Brązert, M.; Kranc, W.; Nawrocki, M.J.; Sujka-Kordowska, P.; Konwerska, A.; Jankowski, M.; Kocherova, I.; Celichowski, P.; Jeseta, M.; Ożegowska, K.; et al. New markers for regulation of transcription and macromolecule metabolic process in porcine oocytes during in vitro maturation. Mol. Med. Rep. 2020, 21, 1537–1551. [Google Scholar] [CrossRef]
- Read, C.; Willhelm, G.; Dyce, P.W. Connexin 43 coupling in bovine cumulus cells, during the follicular growth phase, and its relationship to in vitro embryo outcomes. Mol. Reprod. Dev. 2018, 85, 579–589. [Google Scholar] [CrossRef]
- Boelhauve, M.; Sinowatz, F.; Wolf, E.; Paula-Lopes, F.F. Maturation of Bovine Oocytes in the Presence of Leptin Improves Development and Reduces Apoptosis of In Vitro-Produced Blastocysts1. Boil. Reprod. 2005, 73, 737–744. [Google Scholar] [CrossRef]
- Lam, K.H.; Alex, D.; Lam, I.K.; Tsui, S.K.; Yang, Z.F.; Lee, S.M. Nobiletin, a polymethoxylated flavonoid from citrus, shows anti-angiogenic activity in a zebrafish in vivo model and HUVEC in vitro model. J. Cell. Biochem. 2011, 112, 3313–3321. [Google Scholar] [CrossRef]
- Alvarez, M.A.; García-García, R.M.; Rebollar, P.; Nicodemus, N.; Revuelta, L.; Millan, P.; Lorenzo, P.L. Effects of a lignin-rich fibre diet on productive, reproductive and endocrine parameters in nulliparous rabbit does. Livest. Sci. 2009, 123, 107–115. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Gómez-Brunet, A.; Gonzalez-Bulnes, A.; Toledano, A.; Malpaux, B.; López-Sebastián, A. Differences in reproductive pattern between wild and domestic rams are not associated with inter-specific annual variations in plasma prolactin and melatonin concentrations. Domest. Anim. Endocrinol. 2005, 28, 416–429. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Lloreda, V.; Coy, P.; Gutierrez-Adan, A.; Bermejo-Álvarez, P.; Rizos, D. Effect of bovine oviductal fluid on development and quality of bovine embryos produced in vitro. Reprod. Fertil. Dev. 2017, 29, 621. [Google Scholar] [CrossRef]
- Holm, P.; Booth, P.; Schmidt, M.; Greve, T.; Callesen, H. High bovine blastocyst development in a static in vitro production system using sofaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999, 52, 683–700. [Google Scholar] [CrossRef]
- Bermejo-Alvarez, P.; Rizos, D.; Rath, D.; Lonergan, P.; Gutierrez-Adan, A. Epigenetic differences between male and female bovine blastocysts produced in vitro. Physiol. Genom. 2008, 32, 264–272. [Google Scholar] [CrossRef]
- Bermejo-Álvarez, P.; Rizos, D.; Rath, D.; Lonergan, P.; Gutierrez-Adan, A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2010, 107, 3394–3399. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]


| Parameters Evaluated | Control | CDMSO | Nob10 | Nob25 | Nob50 | Nob100 |
|---|---|---|---|---|---|---|
| Nuclear maturation n | 117 | 122 | 133 | 146 | 149 | 144 |
| Matured (M-II) n (%) | 84 (71.7 ± 0.8)b | 86 (70.5 ± 0.5)b | 97 (72.9 ± 0.4)b | 127 (87.0 ± 0.6)a | 133 (89.3 ± 0.4)a | 103 (71.5 ± 0.8)b |
| Immature n (%) | 33 (28.2 ± 0.7)a | 36 (29.5 ± 0.5)a | 36 (27.1 ± 0.4)a | 19 (12.9 ± 0.6)b | 16 (10.7 ± 0.4)b | 41 (28.4 ± 0.8)a |
| Cytoplasmic Maturation | ||||||
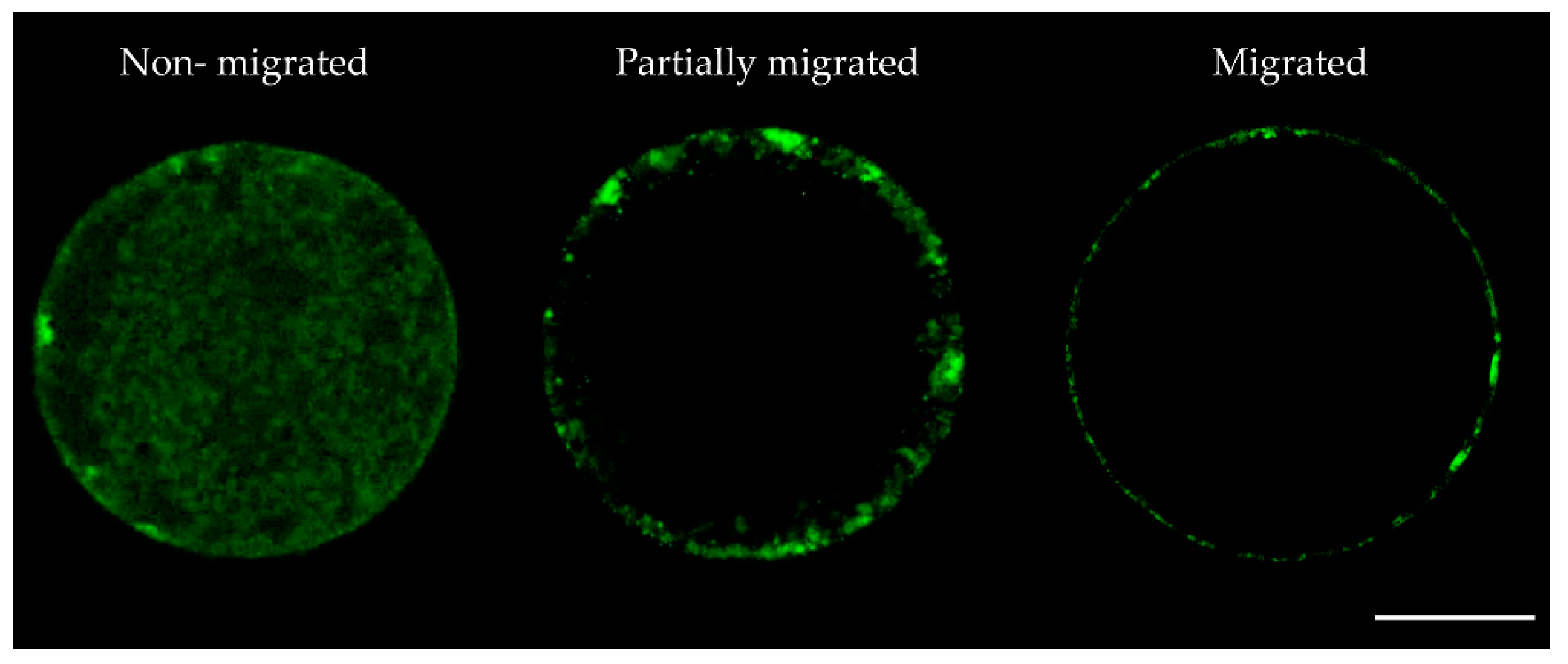
| Cortical Granules Distribution | ||||||
| n | 58 | 66 | 72 | 70 | 78 | 70 |
| Migrated n (%) | 40 (69.1 ± 1.1)b | 46 (69.6 ± 0.9)b | 52 (72.1 ± 1.0)b | 60 (85.7 ± 0.3)a | 70 (89.9 ± 2.2)a | 50 (71.2 ± 0.7)b |
| Partially migrated n (%) | 10 (17.2 ± 2.6)a | 12 (18.2 ± 1.7)a | 15 (20.9 ± 0.7)a | 7 (9.9 ± 1.6)b | 7 (8.8 ± 1.3)b | 15 (21.5 ± 0.6)a |
| Non-migrated n (%) | 8 (13.7 ± 1.9)a | 8 (12.2 ± 2.0)ac | 5 (6.9 ± 0.2)bc | 3 (4.4 ± 1.8)b | 1 (1.2 ± 1.2)b | 5 (7.3 ± 0.2)bc |
| Mitochondrial Distribution | ||||||
| n | 59 | 56 | 61 | 76 | 71 | 74 |
| Migrated n (%) | 42 (71.3 ± 1.5)b | 39 (69.7 ± 1.0)b | 45 (73.7 ± 1.0)b | 66 (86.7 ± 0.6)a | 63 (88.9 ± 1.2)a | 53 (71.6 ± 0.5)b |
| Partially migrated n (%) | 10 (17.0 ± 0.5)a | 11 (19.6 ± 1.1)a | 11 (17.9 ± 1.0)a | 5 (6.7 ± 0.3)b | 7 (9.8 ± 1.5) b | 13 (17.5 ± 1.5)a |
| Non-migrated n (%) | 7 (11.7 ± 1.8)a | 6 (10.8 ± 1.5)a | 5 (8.3 ± 0.4)a | 5 (6.6 ± 0.3)ab | 1 (1.3 ± 1.3)b | 8 (10.8 ± 1.7)a |
| Groups | Total No. Presumptive Zygotes in Culture | Cleavage Rate | Blastocyst Yield | |
|---|---|---|---|---|
| Day 7 | Day 8 | |||
| n (%) | n (%) | n (%) | ||
| Control | 359 | 267 (74.2 ± 0.4)b | 76 (21.1 ± 0.4)b | 92 (25.8 ± 0.5)b |
| CDMSO | 378 | 278 (73.6 ± 0.5)b | 78 (20.9 ± 0.4)b | 98 (26.1 ± 0.7)b |
| Nob10 | 397 | 300 (75.6 ±0.3)b | 75 (18.9 ± 0.4)b | 90 (23.1 ± 0.7)b |
| Nob25 | 372 | 335 (89.9 ± 0.4)a | 90 (24.4 ± 0.5)a | 119 (32.2 ± 0.8)a |
| Nob50 | 336 | 307 (91.3 ± 0.3)a | 86 (25.7 ± 0.6)a | 117 (35.3 ± 0.8)a |
| Nob100 | 414 | 306 (74.0 ± 0.6)b | 76 (18.9 ± 0.9)b | 100 (24.5 ± 1.0)b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cajas, Y.N.; Cañón-Beltrán, K.; Ladrón de Guevara, M.; Millán de la Blanca, M.G.; Ramos-Ibeas, P.; Gutiérrez-Adán, A.; Rizos, D.; González, E.M. Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality. Int. J. Mol. Sci. 2020, 21, 5340. https://doi.org/10.3390/ijms21155340
Cajas YN, Cañón-Beltrán K, Ladrón de Guevara M, Millán de la Blanca MG, Ramos-Ibeas P, Gutiérrez-Adán A, Rizos D, González EM. Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality. International Journal of Molecular Sciences. 2020; 21(15):5340. https://doi.org/10.3390/ijms21155340
Chicago/Turabian StyleCajas, Yulia N., Karina Cañón-Beltrán, Magdalena Ladrón de Guevara, María G. Millán de la Blanca, Priscila Ramos-Ibeas, Alfonso Gutiérrez-Adán, Dimitrios Rizos, and Encina M. González. 2020. "Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality" International Journal of Molecular Sciences 21, no. 15: 5340. https://doi.org/10.3390/ijms21155340
APA StyleCajas, Y. N., Cañón-Beltrán, K., Ladrón de Guevara, M., Millán de la Blanca, M. G., Ramos-Ibeas, P., Gutiérrez-Adán, A., Rizos, D., & González, E. M. (2020). Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality. International Journal of Molecular Sciences, 21(15), 5340. https://doi.org/10.3390/ijms21155340






