The Specific Molecular Composition and Structural Arrangement of Eleutherodactylus Coqui Gular Skin Tissue Provide Its High Mechanical Compliance
Abstract
1. Introduction
2. Results
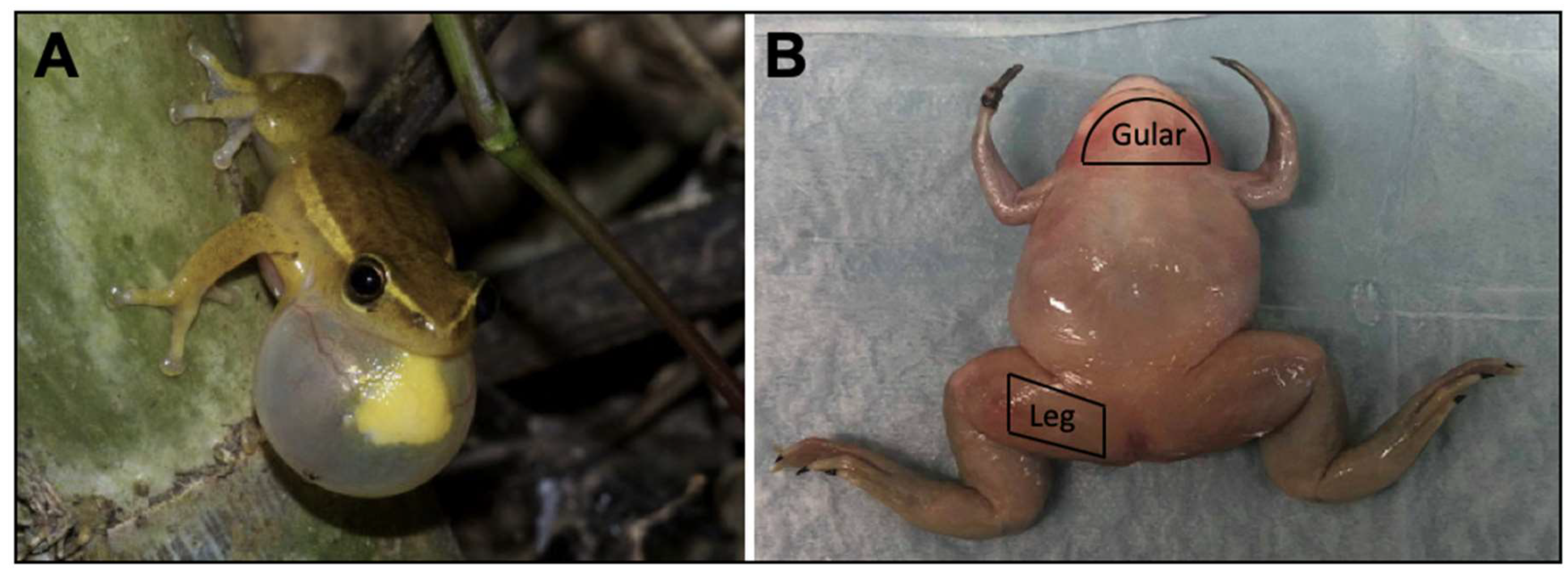
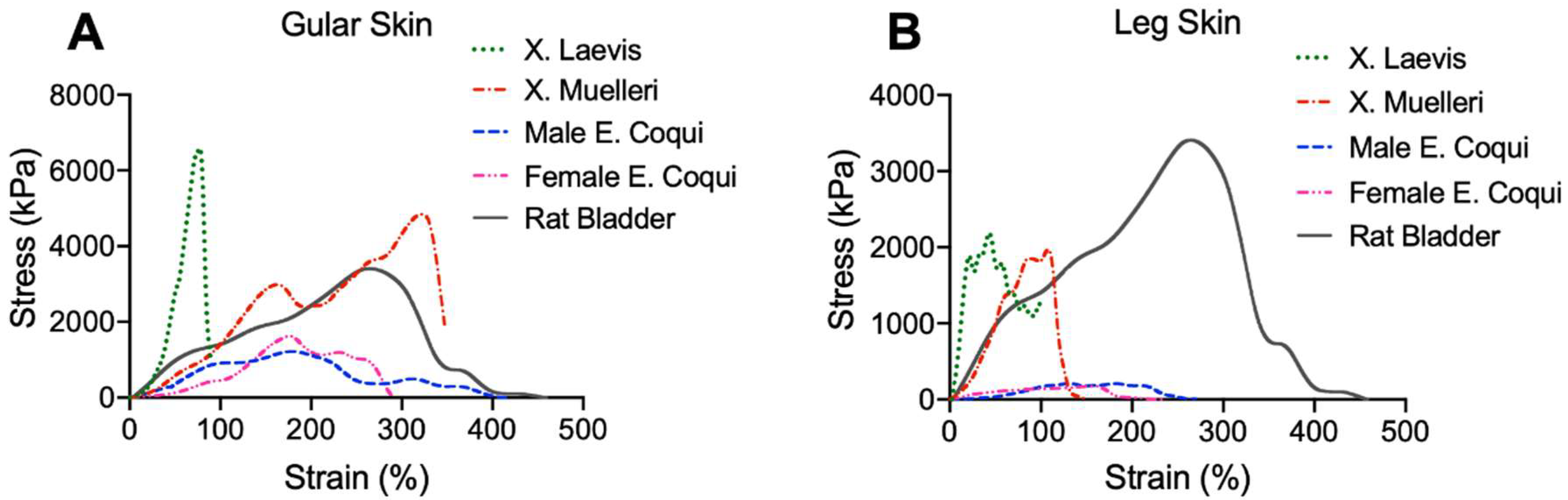
2.1. Mechanical Properties
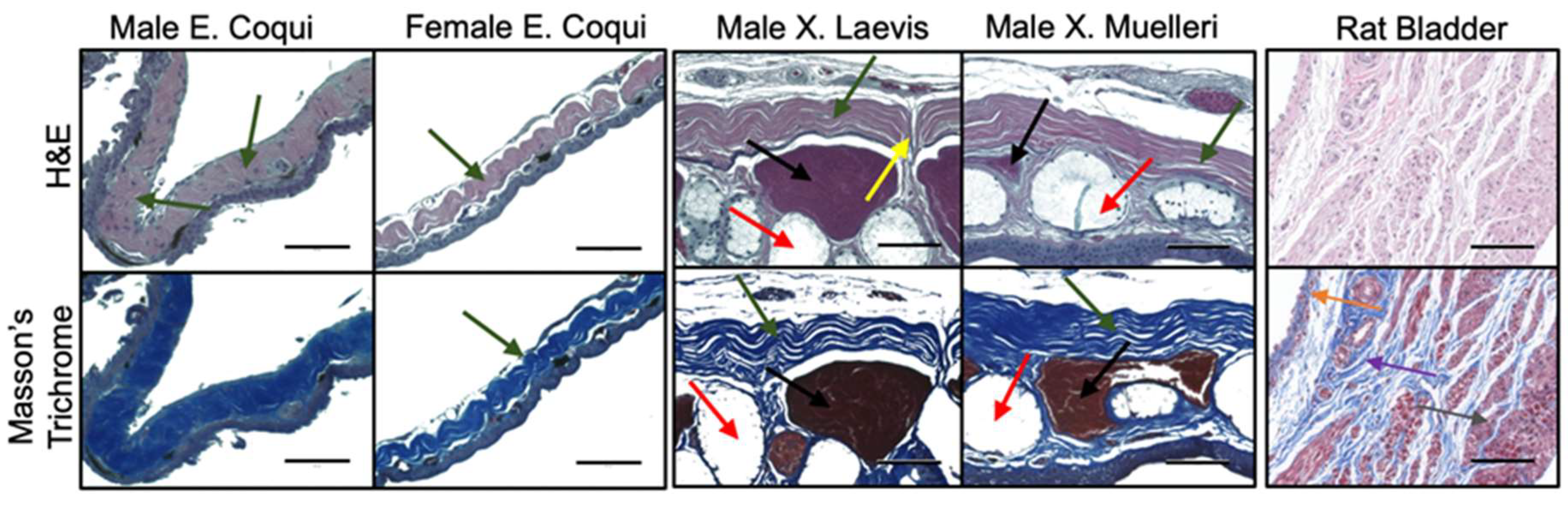
2.2. Tissue Morphology
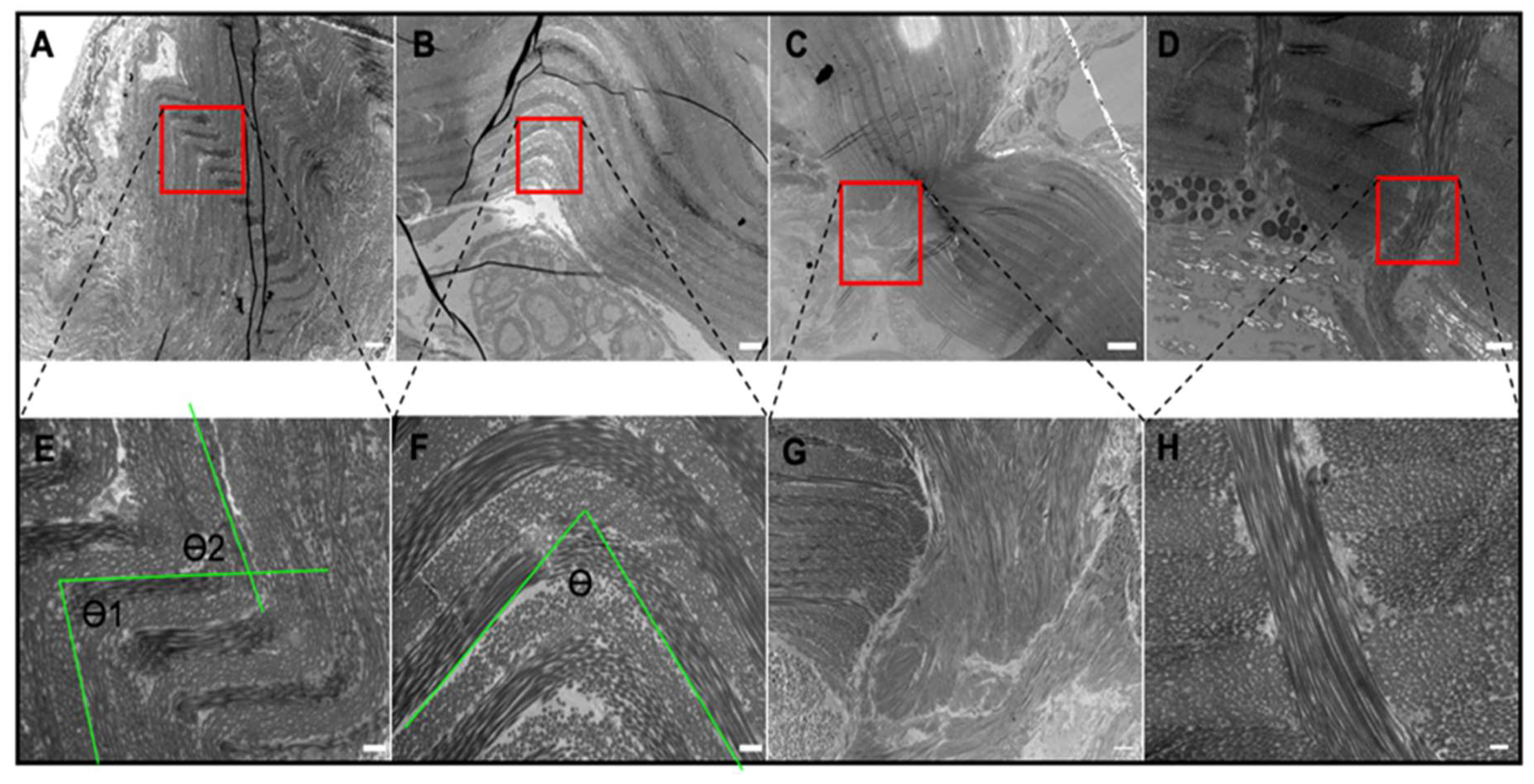
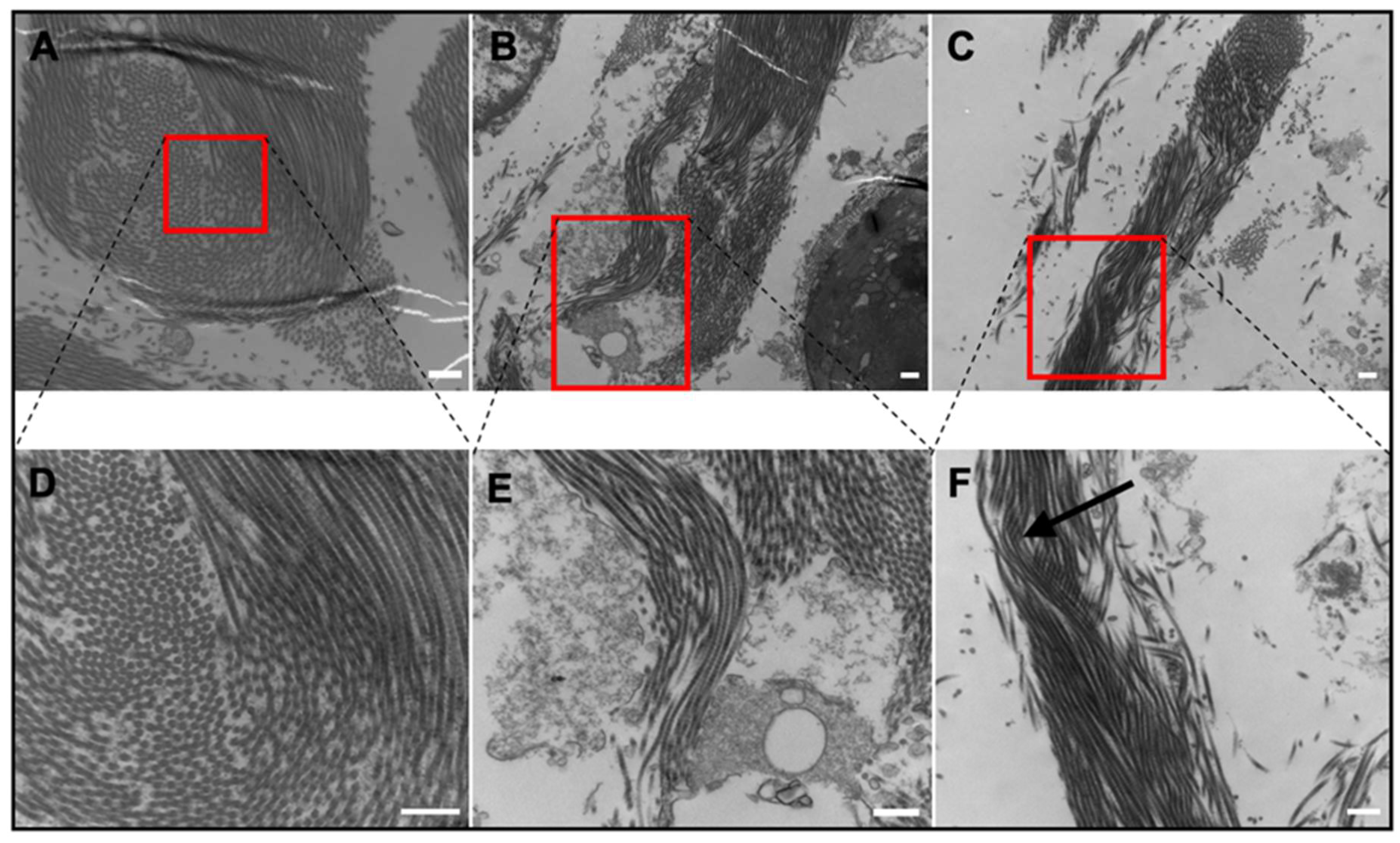
2.3. Tissue Ultrastructure
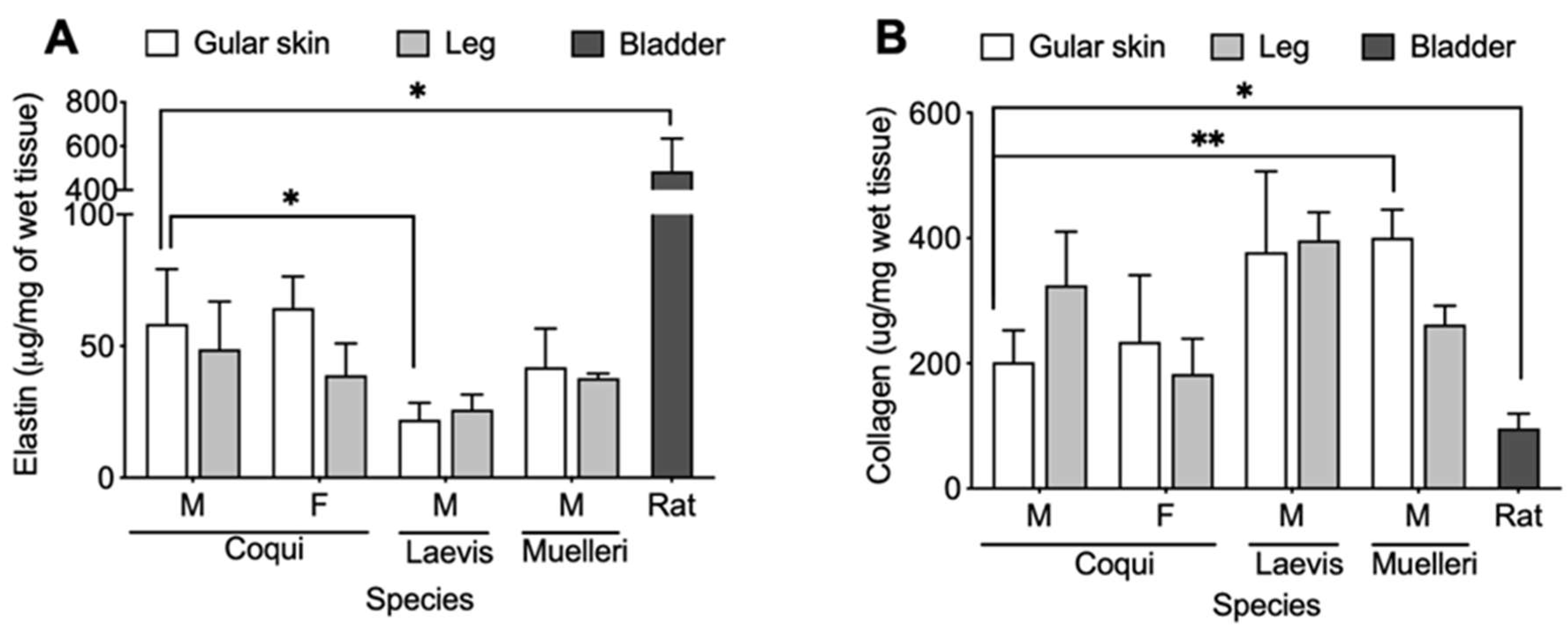
2.4. Biochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Specimen Collection
4.2. Uniaxial Tensile Test
4.3. Histology
4.4. Transmission Electron Microscopy
4.5. Crimp Angle Measurement
4.6. Collagen and Elastin Content
4.7. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UTS | Ultimate Tensile Strength |
| EC | Eleutherodactylus Coqui |
| XL | Xenopus Laevis (XL) |
| XM | Xenopus Muelleri |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| H&E | Haemotoxylin and Eosin |
References
- McCoy, D.E.; McCoy, V.E.; Mandsberg, N.K.; Shneidman, A.V.; Aizenberg, J.; Prum, R.O.; Haig, D. Structurally assisted super black in colourful peacock spiders. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190589. [Google Scholar] [CrossRef]
- Roberts, S.A.; Davidson, A.J.; Beynon, R.J.; Hurst, J.L. Female attraction to male scent and associative learning: The house mouse as a mammalian model. Anim. Behav. 2014, 97, 313–321. [Google Scholar] [CrossRef]
- Halfwerk, W.; Jones, P.L.; Taylor, R.C.; Ryan, M.J.; Page, R.A. Risky Ripples Allow Bats and Frogs to Eavesdrop on a Multisensory Sexual Display. Science 2014, 343, 413–416. [Google Scholar] [CrossRef]
- Solberg, E.J.; Saether, B.-E. Fluctuating asymmetry in the antlers of moose (Alces alces): Does it signal male quality? Proc. R. Soc. B Biol. Sci. 1993, 254, 251–255. [Google Scholar] [CrossRef]
- Starnberger, I.; Preininger, D.; Hödl, W. The anuran vocal sac: A tool for multimodal signalling. Anim. Behav. 2014, 97, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.P.; Krempels, D.M. Vocal Sac Variation among Frogs of the Genus Rana from Western North America. Copeia 1986, 1986, 927–936. [Google Scholar] [CrossRef]
- Elias-Costa, A.J.; Montesinos, R.; Grant, T.; Faivovich, J. The vocal sac of Hylodidae (Amphibia, Anura): Phylogenetic and functional implications of a unique morphology. J. Morphol. 2017, 278, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.B. Vocal communication in frogs. Curr. Opin. Neurobiol. 2004, 14, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Kirti; Khora, S.S. Mechanical properties of pufferfish (Lagocephalus gloveri) skin and its collagen arrangement. Mar. Freshw. Behav. Physiol. 2016, 49, 327–336. [Google Scholar] [CrossRef]
- Brainerd, E.L. Pufferfish inflation: Functional morphology of postcranial structures inDiodon holocanthus (Tetraodontiformes). J. Morphol. 1994, 220, 243–261. [Google Scholar] [CrossRef]
- Close, M.; Cundall, D. Snake lower jaw skin: Extension and recovery of a hyperextensible keratinized integument. J. Exp. Zoöl. Part A Ecol. Genet. Physiol. 2013, 321, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Madsen, V.; Balsby, T.J.S.; Dabelsteen, T.; Osorno, J.L. Bimodal Signaling of a Sexually Selected Trait: Gular Pouch Drumming in the Magnificent Frigatebird. Condor 2004, 106, 156–160. [Google Scholar] [CrossRef]
- Murakumo, M.; Ushiki, T.; Abe, K.; Matsumura, K.; Shinno, Y.; Koyanagi, T. Three-Dimensional Arrangement of Collagen and Elastin Fibers in the Human Urinary Bladder: A Scanning Electron Microscopic Study. J. Urol. 1995, 154, 251–256. [Google Scholar] [CrossRef]
- Viidik, A.; Danielson, C.C.; Oxlund, H.; Danielsen, C. On fundamental and phenomenological models, structure and mechanical properties of collagen, elastin and glycosaminoglycan complexes. Biorheol 1982, 19, 437–451. [Google Scholar] [CrossRef]
- Carter, F.J.; Frank, T.; Davies, P.J.; Cuschieri, A. Puncture forces of solid organ surfaces. Surg. Endosc. 2000, 14, 783–786. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; De Torre, I.G.; Ibañez-Fonseca, A.; Alonso, M.; Ibañez-Fonzeca, A. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018, 129, 118–133. [Google Scholar] [CrossRef]
- Croisier, E.; Liang, S.; Schweizer, T.; Balog, S.; Mionić, M.; Snellings, R.; Cugnoni, J.; Michaud, V.; Frauenrath, H. A toolbox of oligopeptide-modified polymers for tailored elastomers. Nat. Commun. 2014, 5, 4728. [Google Scholar] [CrossRef]
- Voorhaar, L.; Diaz, M.M.; Leroux, F.; Rogers, S.; Abakumov, A.M.; Van Tendeloo, G.; Van Assche, G.; Van Mele, B.; Hoogenboom, R. Supramolecular thermoplastics and thermoplastic elastomer materials with self-healing ability based on oligomeric charged triblock copolymers. NPG Asia Mater. 2017, 9, e385. [Google Scholar] [CrossRef]
- Urry, D.W.; Pattanaik, A.; Xu, J.; Woods, T.C.; McPherson, D.T.; Parker, T.M. Elastic protein-based polymers in soft tissue augmentation and generation. J. Biomater. Sci. Polym. Ed. 1998, 9, 1015–1048. [Google Scholar] [CrossRef]
- Ghafari, A.M.; Rajabi-Zeleti, S.; Naji, M.; Ghanian, M.H.; Baharvand, H. Mechanical reinforcement of urinary bladder matrix by electrospun polycaprolactone nanofibers. Sci. Iran. 2017, 24, 3476–3480. [Google Scholar] [CrossRef][Green Version]
- Feng, C.; Liu, C.; Liu, S.; Wang, Z.; Yu, K.; Zeng, X. Electrospun Nanofibers with Core–Shell Structure for Treatment of Bladder Regeneration. Tissue Eng. Part A 2019, 25, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Shen, J. Fabrication of Microropes via Bi-electrospinning with a Rotating Needle Collector. Macromol. Rapid Commun. 2010, 31, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical Applications of Layer-by-Layer Assembly: From Biomimetics to Tissue Engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Franchi, M.; Ottani, V.; Stagni, R.; Ruggeri, A. Tendon and ligament fibrillar crimps give rise to left-handed helices of collagen fibrils in both planar and helical crimps. J. Anat. 2010, 216, 301–309. [Google Scholar] [CrossRef]
- Shim, V.; Fernandez, J.; Besier, T.; Hunter, P. Investigation of the role of crimps in collagen fibers in tendon with a microstructually based finite element model. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 4871–4874. [Google Scholar] [CrossRef]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef]
- Jia, Z.; Yu, Y.; Wang, L. Learning from nature: Use material architecture to break the performance tradeoffs. Mater. Des. 2019, 168, 107650. [Google Scholar] [CrossRef]
- Naleway, S.E.; Porter, M.M.; McKittrick, J.; Meyers, M.A. Structural Design Elements in Biological Materials: Application to Bioinspiration. Adv. Mater. 2015, 27, 5455–5476. [Google Scholar] [CrossRef]
- Bouville, F.; Maire, E.; Meille, S.; Van De Moortèle, B.; Stevenson, A.; Deville, S. Strong, tough and stiff bioinspired ceramics from brittle constituents. Nat. Mater. 2014, 13, 508–514. [Google Scholar] [CrossRef]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2014, 14, 23–36. [Google Scholar] [CrossRef]
- Eilber, K.S.; Sukotjo, C.; Raz, S.; Nishimura, I. Alteration of collagen three-dimensional architecture in noncompliant human urinary bladder. Retinal Degener. Dis. 2003, 539, 791–801. [Google Scholar] [CrossRef]
- Chang, S.L.; Howard, P.S.; Koo, H.P.; Macarak, E.J. Role of type III collagen in bladder filling. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 1998, 17, 135–145. [Google Scholar] [CrossRef]
- Aitken, K.; Bägli, D.J. The bladder extracellular matrix. Part I: Architecture, development and disease. Nat. Rev. Urol. 2009, 6, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Misof, K.; Zizak, I.; Rapp, G.; Amenitsch, H.; Bernstorff, S. Fibrillar Structure and Mechanical Properties of Collagen. J. Struct. Biol. 1998, 122, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Landau, E.H.; Jayanthi, V.R.; Churchill, B.M.; Shapiro, E.; Gilmour, R.F.; Khoury, A.E.; Macarak, E.J.; McLorie, G.A.; Steckler, R.E.; Kogan, B.A. Loss of Elasticity in Dysfunctional Bladders: Urodynamic and Histochemical Correlation. J. Urol. 1994, 152, 702–705. [Google Scholar] [CrossRef]
- Majumdar, S.; Wang, X.; Sommerfeld, S.D.; Chae, J.J.; Athanasopoulou, E.; Shores, L.; Duan, X.; Amzel, L.M.; Stellacci, F.; Schein, O.; et al. Cyclodextrin Modulated Type I Collagen Self-Assembly to Engineer Biomimetic Cornea Implants. Adv. Funct. Mater. 2018, 28, 1804076. [Google Scholar] [CrossRef]
- Agrawal, A.; Rahbar, N.; Calvert, P. Strong fiber-reinforced hydrogel. Acta Biomater. 2013, 9, 5313–5318. [Google Scholar] [CrossRef]
- Eslami, M.; Vrana, N.E.; Zorlutuna, P.; Sant, S.; Jung, S.; Masoumi, N.; Khavari-Nejad, R.A.; Javadi, G.; Khademhosseini, A. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J. Biomater. Appl. 2014, 29, 399–410. [Google Scholar] [CrossRef]
- Iviglia, G.; Cassinelli, C.; Torre, E.; Baino, F.; Morra, M.; Vitale-Brovarone, C. Novel bioceramic-reinforced hydrogel for alveolar bone regeneration. Acta Biomater. 2016, 44, 97–109. [Google Scholar] [CrossRef]
- Nie, X.; Chuah, Y.J.; Zhu, W.; He, P.; Peck, Y.; Wang, D.-A. Decellularized tissue engineered hyaline cartilage graft for articular cartilage repair. Biomaterials 2020, 235, 119821. [Google Scholar] [CrossRef]
- Bejleri, D.; Davis, M.E. Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. Adv. Health Mater. 2019, 8, e1801217. [Google Scholar] [CrossRef]
- Dahms, S.E.; Piechota, H.J.; Dahiya, R.; Lue, T.F.; Tanagho, E.A. Composition and biomechanical properties of the bladder acellular matrix graft: Comparative analysis in rat, pig and human. BJU Int. 1998, 82, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lee, D.; Jeong, H.; Yu, C.; Li, J.; Fang, C.H.; Sabnekar, P.; Liu, X.; Yoshida, T.; Sopko, N.A.; et al. Tissue-Engineered Neo-Urinary Conduit from Decellularized Trachea. Tissue Eng. Part A 2018, 24, 1456–1467. [Google Scholar] [CrossRef] [PubMed]

| Specimen | Tissue | UTS (kPa) | Strain at Peak Stress (%) | Breaking Strain (%) | Secant Modulus (20% strain) (kPa) |
|---|---|---|---|---|---|
| Mean ± SD (p) | Mean ± SD (p) | Mean ± SD (p) | Mean ± SD (p) | ||
| Coqui (M) | Gular | 1263 ± 134 (0.188) | 187 ± 22 (0.999) | 398 ± 86 (0.999) | 373 ± 194 (0.808) |
| Leg | 193 ± 40 (0.006) | 83 ± 53 (0.999) | 348 ± 88 (0.999) | 318 ± 333 (0.762) | |
| Coqui (F) | Gular | 2142 ± 1789 (0.861) | 226 ± 43 (0.999) | 337 ± 83 (0.999) | 786 ± 1052 (0.991) |
| Leg | 936 ± 1265 (0.078) | 101 ± 59 (0.999) | 252 ± 39 (0.999) | 1475 ± 1823 (0.999) | |
| XL | Gular | 4461 ± 2215 (0.330) | 78 ± 12.5 (0.999) | 104 ± 17(0.999) | 3117 ± 1400 (0.143) |
| Leg | 2553 ± 1775 (0.996) | 61 ± 23 (0.999) | 108 ± 12 (0.999) | 4863 ± 2118 (0.003) | |
| XM | Gular | 4156 ± 1973 (0.581) | 271 ± 70 (0.999) | 350 ± 9 (0.999) | 807 ± 668 (0.993) |
| Leg | 2524 ± 560 (0.932) | 126 ± 16 (0.999) | 175 ± 28 (0.999) | 2877 ± 2265 (0.258) | |
| Rat | Bladder | 2985 ± 853 | 233 ± 28 | 412 ± 163 | 1288 ± 850 |
| Elastin (μg/mg Wet Tissue) | Collagen (μg/mg Wet Tissue) | Elastin/Collagen Ratio | ||
|---|---|---|---|---|
| Specimen | Tissue | Mean ± SD | Mean ± SD | |
| Female Coqui | Gular | 64.5 ± 9.8 | 234.5 ± 86.7 | 0.27 |
| Leg | 39.0 ± 9.8 | 183.1 ± 45.9 | 0.21 | |
| Male Coqui | Gular | 58.5 ± 16.9 | 202.0 ± 41.3 | 0.29 |
| Leg | 48.8 ± 14.8 | 325.0 ± 69.5 | 0.15 | |
| XL | Gular | 22.1 ± 5.2 | 377.8 ± 105.3 | 0.06 |
| Leg | 26.0 ± 4.6 | 396.7 ± 36.3 | 0.07 | |
| XM | Gular | 42.1 ± 11.9 | 400.8 ± 36.5 | 0.10 |
| Leg | 37.9 ± 1.4 | 262.1 ± 24.3 | 0.14 | |
| Rat | Bladder | 484.8 ± 121.9 | 96.1 ± 19.4 | 5.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, J.; Sharma, S.; Rajani, S.; Singh, A. The Specific Molecular Composition and Structural Arrangement of Eleutherodactylus Coqui Gular Skin Tissue Provide Its High Mechanical Compliance. Int. J. Mol. Sci. 2020, 21, 5593. https://doi.org/10.3390/ijms21165593
Hui J, Sharma S, Rajani S, Singh A. The Specific Molecular Composition and Structural Arrangement of Eleutherodactylus Coqui Gular Skin Tissue Provide Its High Mechanical Compliance. International Journal of Molecular Sciences. 2020; 21(16):5593. https://doi.org/10.3390/ijms21165593
Chicago/Turabian StyleHui, Justin, Shivang Sharma, Sarah Rajani, and Anirudha Singh. 2020. "The Specific Molecular Composition and Structural Arrangement of Eleutherodactylus Coqui Gular Skin Tissue Provide Its High Mechanical Compliance" International Journal of Molecular Sciences 21, no. 16: 5593. https://doi.org/10.3390/ijms21165593
APA StyleHui, J., Sharma, S., Rajani, S., & Singh, A. (2020). The Specific Molecular Composition and Structural Arrangement of Eleutherodactylus Coqui Gular Skin Tissue Provide Its High Mechanical Compliance. International Journal of Molecular Sciences, 21(16), 5593. https://doi.org/10.3390/ijms21165593






