Social Fear Memory Requires Two Stages of Protein Synthesis in Mice
Abstract
1. Introduction
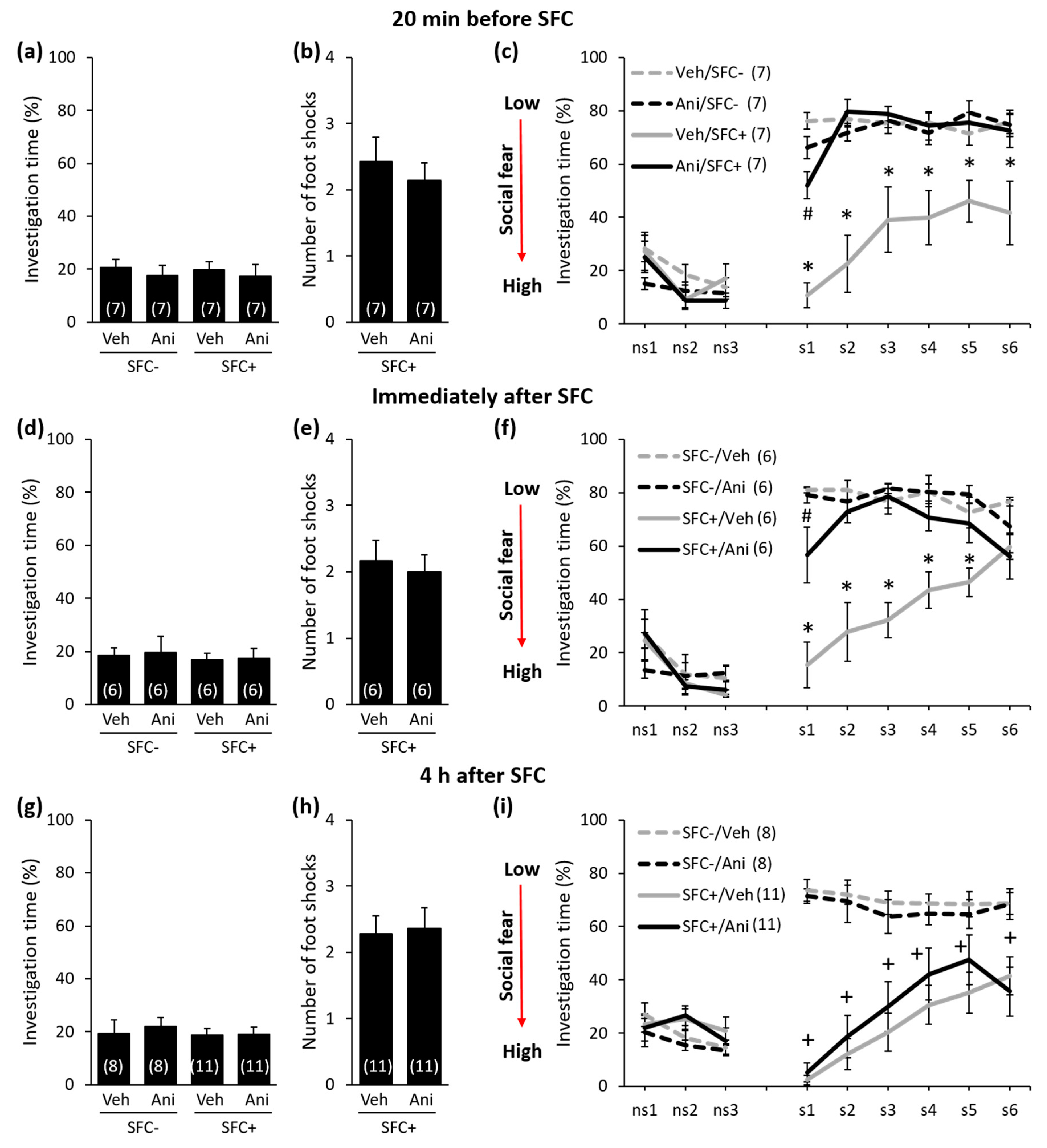
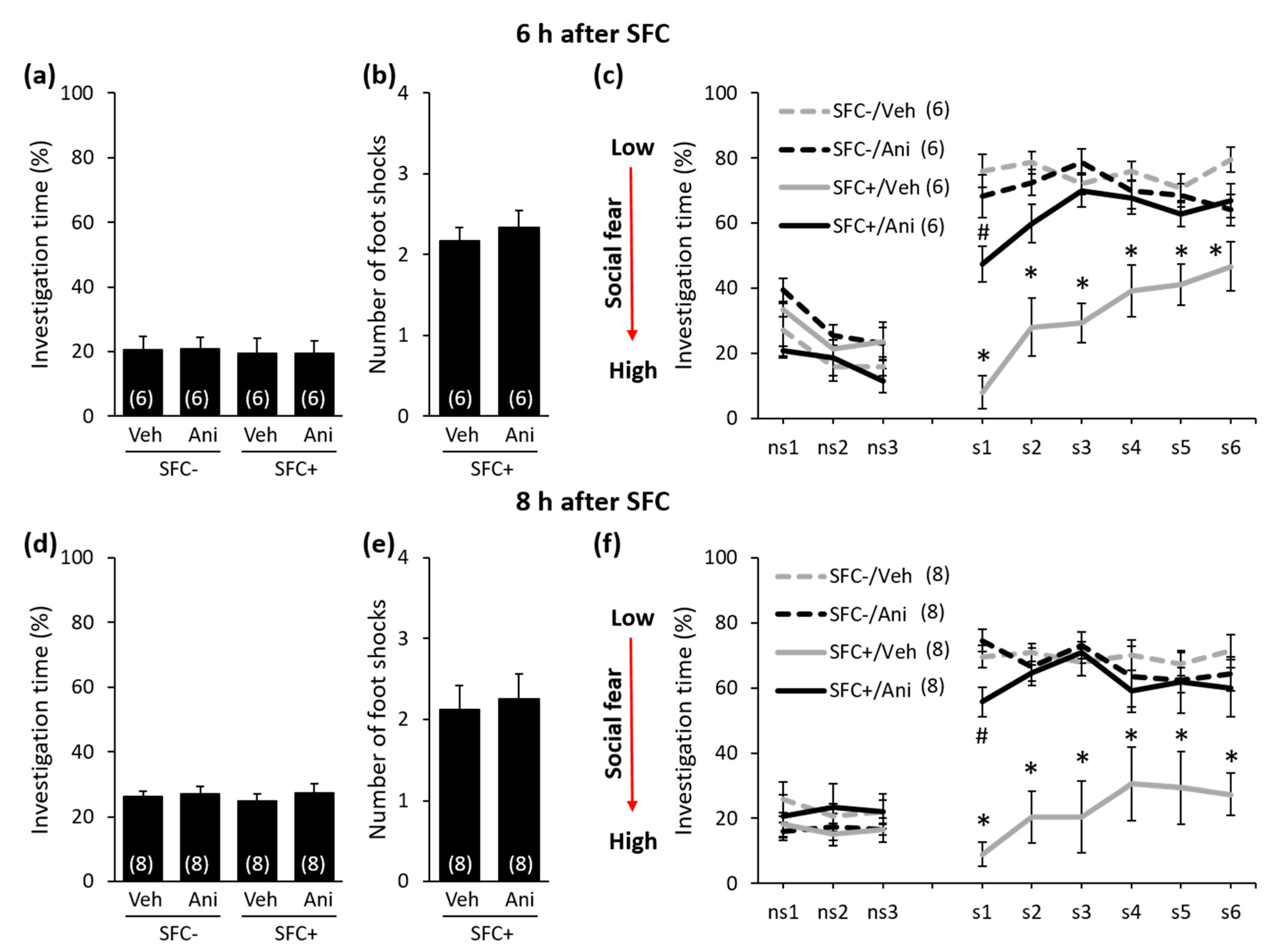
2. Results
Anisomycin Impairs the Consolidation of Social Fear Memory in a Time-Point-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Social Fear Conditioning (SFC) Paradigm
4.3. Blockade of Protein Synthesis
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abel, T.; Lattal, K.M. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 2001, 11, 180–187. [Google Scholar] [CrossRef]
- Bailey, C.H.; Bartsch, D.; Kandel, E.R. Toward a molecular definition of long-term memory storage. Proc. Natl. Acad. Sci. USA 1996, 93, 13445–13452. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.P.; Squire, L.R. Protein synthesis and memory: A review. Psychol. Bull. 1984, 96, 518–559. [Google Scholar] [CrossRef]
- Rossato, J.I.; Bevilaqua, L.R.; Myskiw, J.C.; Medina, J.H.; Izquierdo, I.; Cammarota, M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn. Mem. 2007, 14, 36–46. [Google Scholar] [CrossRef]
- Artinian, J.; McGauran, A.M.; De Jaeger, X.; Mouledous, L.; Frances, B.; Roullet, P. Protein degradation, as with protein synthesis, is required during not only long-term spatial memory consolidation but also reconsolidation. Eur. J. Neurosci. 2008, 27, 3009–3019. [Google Scholar] [CrossRef]
- Richter, K.; Wolf, G.; Engelmann, M. Social recognition memory requires two stages of protein synthesis in mice. Learn. Mem. 2005, 12, 407–413. [Google Scholar] [CrossRef]
- Wanisch, K.; Wotjak, C.T.; Engelmann, M. Long-lasting second stage of recognition memory consolidation in mice. Behav. Brain Res. 2008, 186, 191–196. [Google Scholar] [CrossRef]
- Freeman, F.M.; Rose, S.P.; Scholey, A.B. Two time windows of anisomycin-induced amnesia for passive avoidance training in the day-old chick. Neurobiol. Learn. Mem. 1995, 63, 291–295. [Google Scholar] [CrossRef][Green Version]
- Bourtchouladze, R.; Abel, T.; Berman, N.; Gordon, R.; Lapidus, K.; Kandel, E.R. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn. Mem. 1998, 5, 365–374. [Google Scholar] [PubMed]
- Quevedo, J.; Vianna, M.R.; Roesler, R.; de-Paris, F.; Izquierdo, I.; Rose, S.P. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: Protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn. Mem. 1999, 6, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Jha, S.K. Influence of cued-fear conditioning and its impairment on NREM sleep. Neurobiol. Learn. Mem. 2017, 144, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, A.B.; Ng, K.H.; Freeman, J.H. Memory consolidation within the central amygdala is not necessary for modulation of cerebellar learning. Learn. Mem. 2017, 24, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Raven, F.; Bolsius, Y.G.; van Renssen, L.V.; Meijer, E.L.; van der Zee, E.A.; Meerlo, P.; Havekes, R. Elucidating the role of protein synthesis in hippocampus-dependent memory consolidation across the day and night. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Grecksch, G.; Matthies, H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol. Biochem. Behav. 1980, 12, 663–665. [Google Scholar] [CrossRef]
- Igaz, L.M.; Vianna, M.R.; Medina, J.H.; Izquierdo, I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J. Neurosci. 2002, 22, 6781–6789. [Google Scholar] [CrossRef]
- Markham, C.M.; Luckett, C.A.; Huhman, K.L. The medial prefrontal cortex is both necessary and sufficient for the acquisition of conditioned defeat. Neuropharmacology 2012, 62, 933–939. [Google Scholar] [CrossRef]
- Markham, C.M.; Taylor, S.L.; Huhman, K.L. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn. Mem. 2010, 17, 109–116. [Google Scholar] [CrossRef][Green Version]
- McDonald, M.M.; Markham, C.M.; Norvelle, A.; Albers, H.E.; Huhman, K.L. GABAA receptor activation in the lateral septum reduces the expression of conditioned defeat and increases aggression in Syrian hamsters. Brain Res. 2012, 1439, 27–33. [Google Scholar] [CrossRef]
- Markham, C.M.; Huhman, K.L. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn. Mem. 2008, 15, 6–12. [Google Scholar] [CrossRef]
- Wanisch, K.; Wotjak, C.T. Time course and efficiency of protein synthesis inhibition following intracerebral and systemic anisomycin treatment. Neurobiol. Learn. Mem. 2008, 90, 485–494. [Google Scholar] [CrossRef]
- Toth, I.; Neumann, I.D.; Slattery, D.A. Social fear conditioning: A novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology 2012, 37, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Neumann, I.D.; Slattery, D.A. Social fear conditioning as an animal model of social anxiety disorder. Curr. Protoc. Neurosci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Slattery, D.A.; Neumann, I.D. Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum. Neuropsychopharmacology 2014, 39, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Grund, T.; Zoicas, I.; Althammer, F.; Fiedler, D.; Biermeier, V.; Bosch, O.J.; Hiraoka, Y.; Nishimori, K.; Eliava, M.; et al. Oxytocin Signaling in the Lateral Septum Prevents Social Fear during Lactation. Curr. Biol. 2018, 28, 1066–1078.e6. [Google Scholar] [CrossRef] [PubMed]
- Flood, J.F.; Rosenzweig, M.R.; Bennett, E.L.; Orme, A.E. The influence of duration of protein synthesis inhibition on memory. Physiol. Behav. 1973, 10, 555–562. [Google Scholar] [CrossRef]
- Peng, J.Y.; Li, B.M. Protein synthesis is essential not only for consolidation but also for maintenance and post-retrieval reconsolidation of acrobatic motor skill in rats. Mol. Brain 2009, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.P. Glycoproteins and memory formation. Behav. Brain Res. 1995, 66, 73–78. [Google Scholar] [CrossRef]
- Kandel, E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef]
- Bernabeu, R.; Bevilaqua, L.; Ardenghi, P.; Bromberg, E.; Schmitz, P.; Bianchin, M.; Izquierdo, I.; Medina, J.H. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. USA 1997, 94, 7041–7046. [Google Scholar] [CrossRef]
- Trifilieff, P.; Herry, C.; Vanhoutte, P.; Caboche, J.; Desmedt, A.; Riedel, G.; Mons, N.; Micheau, J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn. Mem. 2006, 13, 349–358. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, J.H.; Min, B.; Lee, Y.H. The translation inhibitor anisomycin induces Elk-1-mediated transcriptional activation of egr-1 through multiple mitogen-activated protein kinase pathways. Exp. Mol. Med. 2006, 38, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, N.; Majlessi, N.; Bozorgmehr, T. The effects of anisomycin (a protein synthesis inhibitor) on spatial learning and memory in CA1 region of rats hippocampus. Behav. Brain Res. 2003, 139, 69–73. [Google Scholar] [CrossRef]
- Gibbs, M.E.; Bowser, D.N.; Hutchinson, D.S.; Loiacono, R.E.; Summers, R.J. Memory processing in the avian hippocampus involves interactions between beta-adrenoceptors, glutamate receptors, and metabolism. Neuropsychopharmacology 2008, 33, 2831–2846. [Google Scholar] [CrossRef] [PubMed]
- Zinck, R.; Cahill, M.A.; Kracht, M.; Sachsenmaier, C.; Hipskind, R.A.; Nordheim, A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol. Cell. Biol. 1995, 15, 4930–4938. [Google Scholar] [CrossRef]
- Kornhuber, J.; Huber, S.E.; Zoicas, I. Effects of conditioned social fear on ethanol drinking and vice-versa in male mice. Psychopharmacology 2019, 236, 2059–2067. [Google Scholar] [CrossRef]
- Kornhuber, J.; Zoicas, I. Neuropeptide Y reduces expression of social fear via simultaneous activation of Y1 and Y2 receptors. J. Psychopharmacol. 2019, 33, 1533–1539. [Google Scholar] [CrossRef]
- Zoicas, I.; Menon, R.; Neumann, I.D. Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 2016, 108, 284–291. [Google Scholar] [CrossRef]
- Zoicas, I.; Neumann, I.D. Maternal separation facilitates extinction of social fear in adult male mice. Behav. Brain Res. 2016, 297, 323–328. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornhuber, J.; Zoicas, I. Social Fear Memory Requires Two Stages of Protein Synthesis in Mice. Int. J. Mol. Sci. 2020, 21, 5537. https://doi.org/10.3390/ijms21155537
Kornhuber J, Zoicas I. Social Fear Memory Requires Two Stages of Protein Synthesis in Mice. International Journal of Molecular Sciences. 2020; 21(15):5537. https://doi.org/10.3390/ijms21155537
Chicago/Turabian StyleKornhuber, Johannes, and Iulia Zoicas. 2020. "Social Fear Memory Requires Two Stages of Protein Synthesis in Mice" International Journal of Molecular Sciences 21, no. 15: 5537. https://doi.org/10.3390/ijms21155537
APA StyleKornhuber, J., & Zoicas, I. (2020). Social Fear Memory Requires Two Stages of Protein Synthesis in Mice. International Journal of Molecular Sciences, 21(15), 5537. https://doi.org/10.3390/ijms21155537




