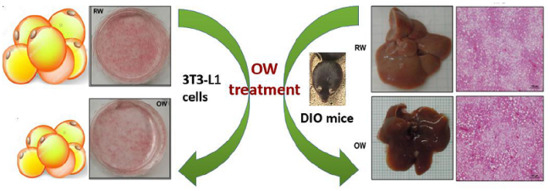
Oxygenated Water Inhibits Adipogenesis and Attenuates Hepatic Steatosis in High-Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Results
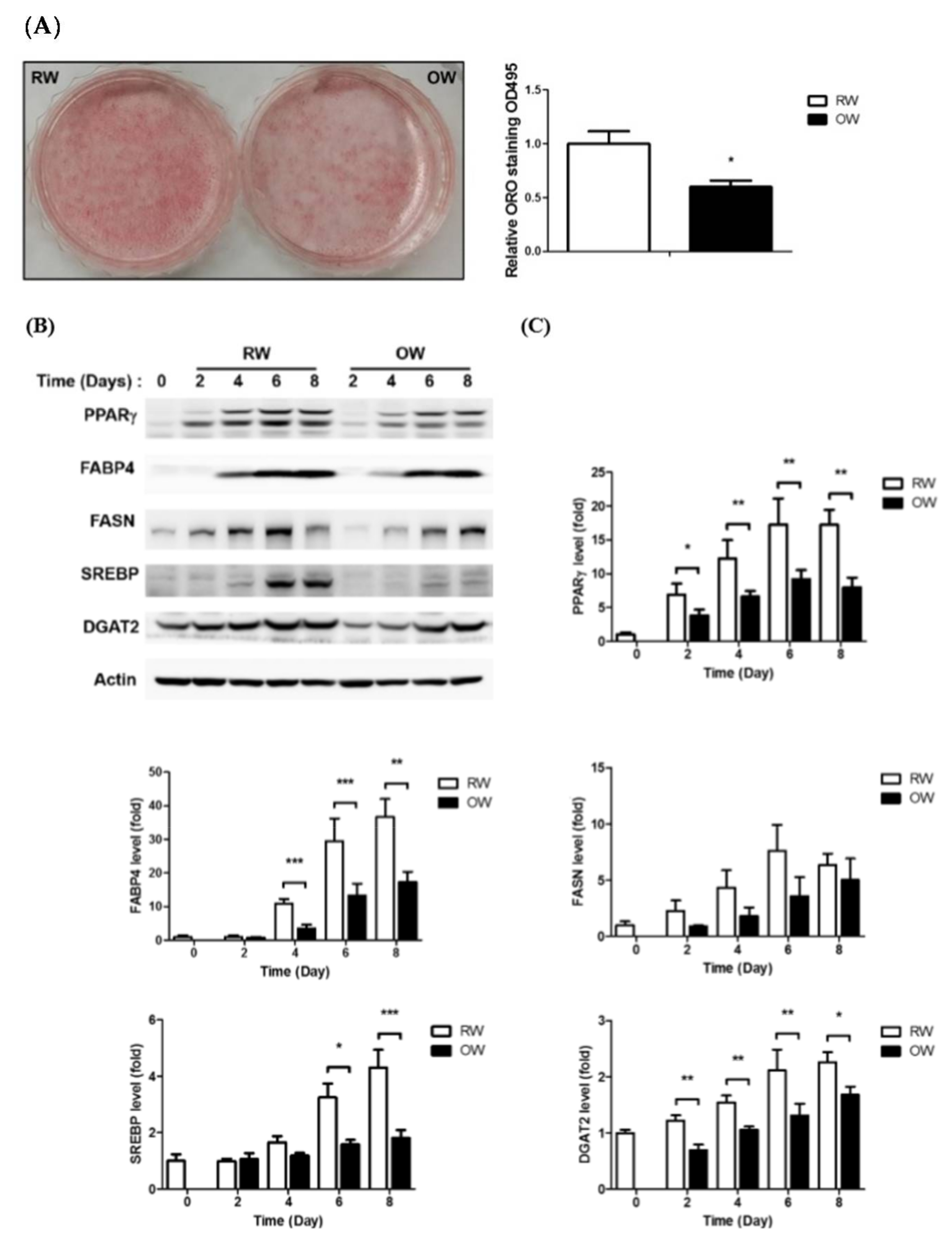
2.1. Oxygenated Water Inhibits Adipogenesis
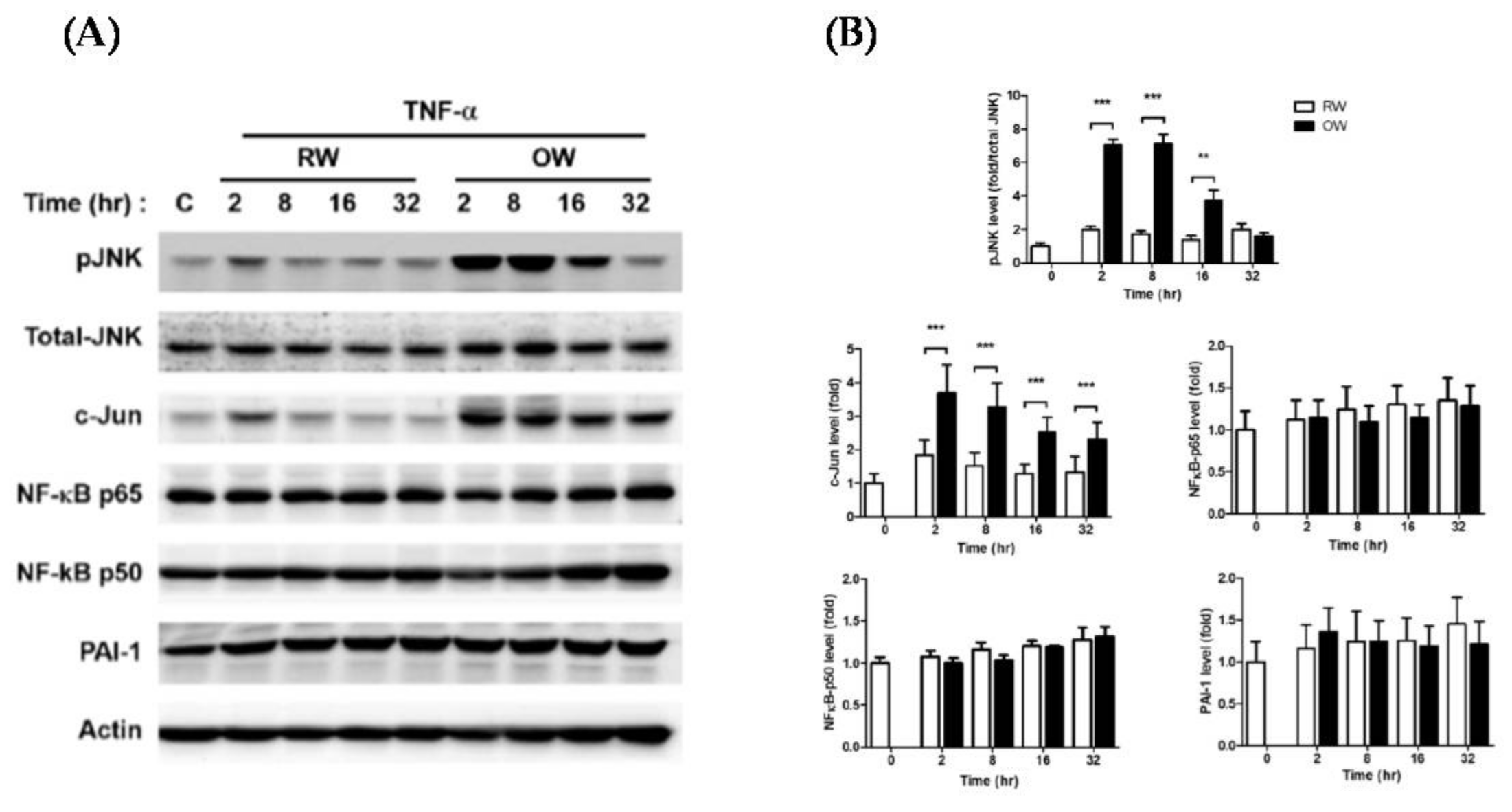
2.2. Effects of Oxygenated Water on Inflammatory Signaling in Mature Adipocytes
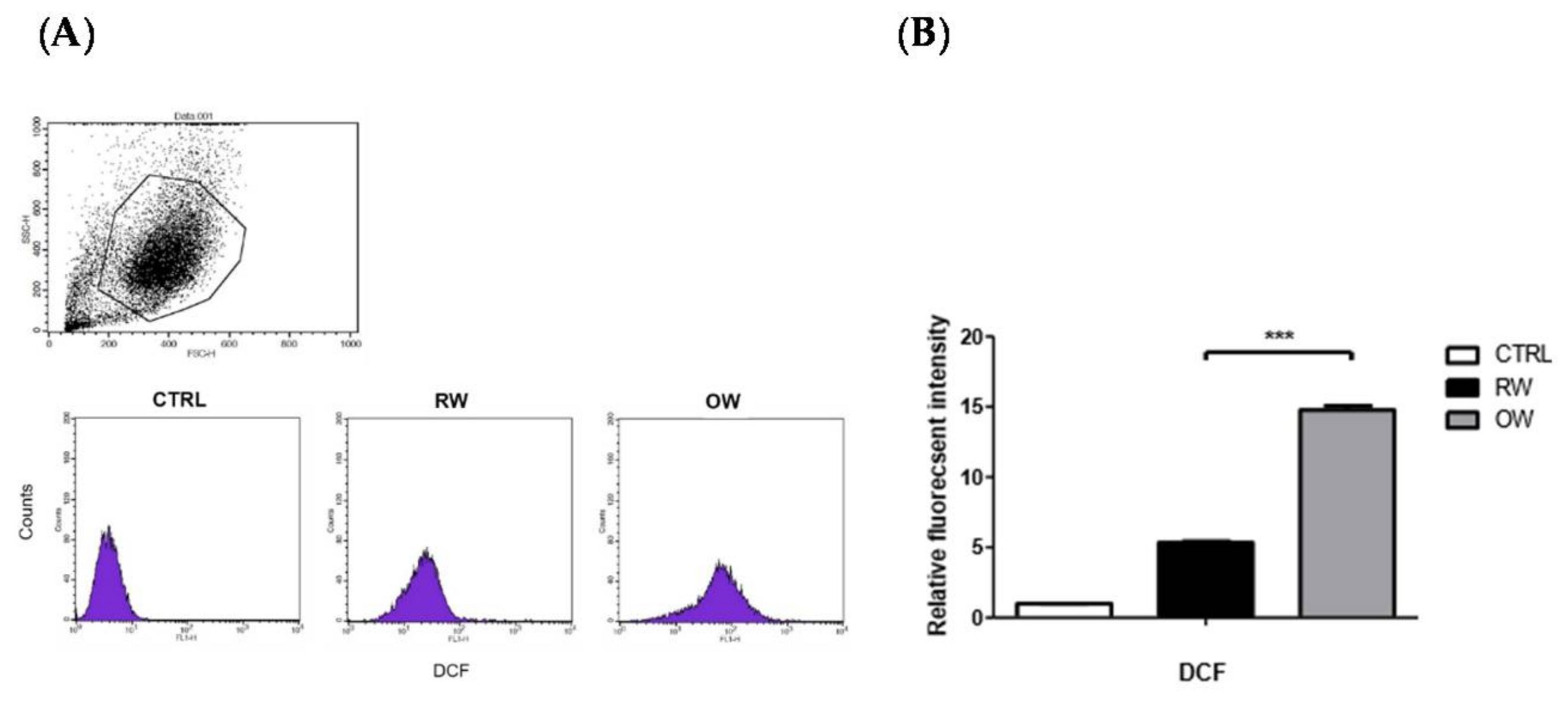
2.3. Effects of Oxygenated Water on HIF-1α and Oxidative Stress
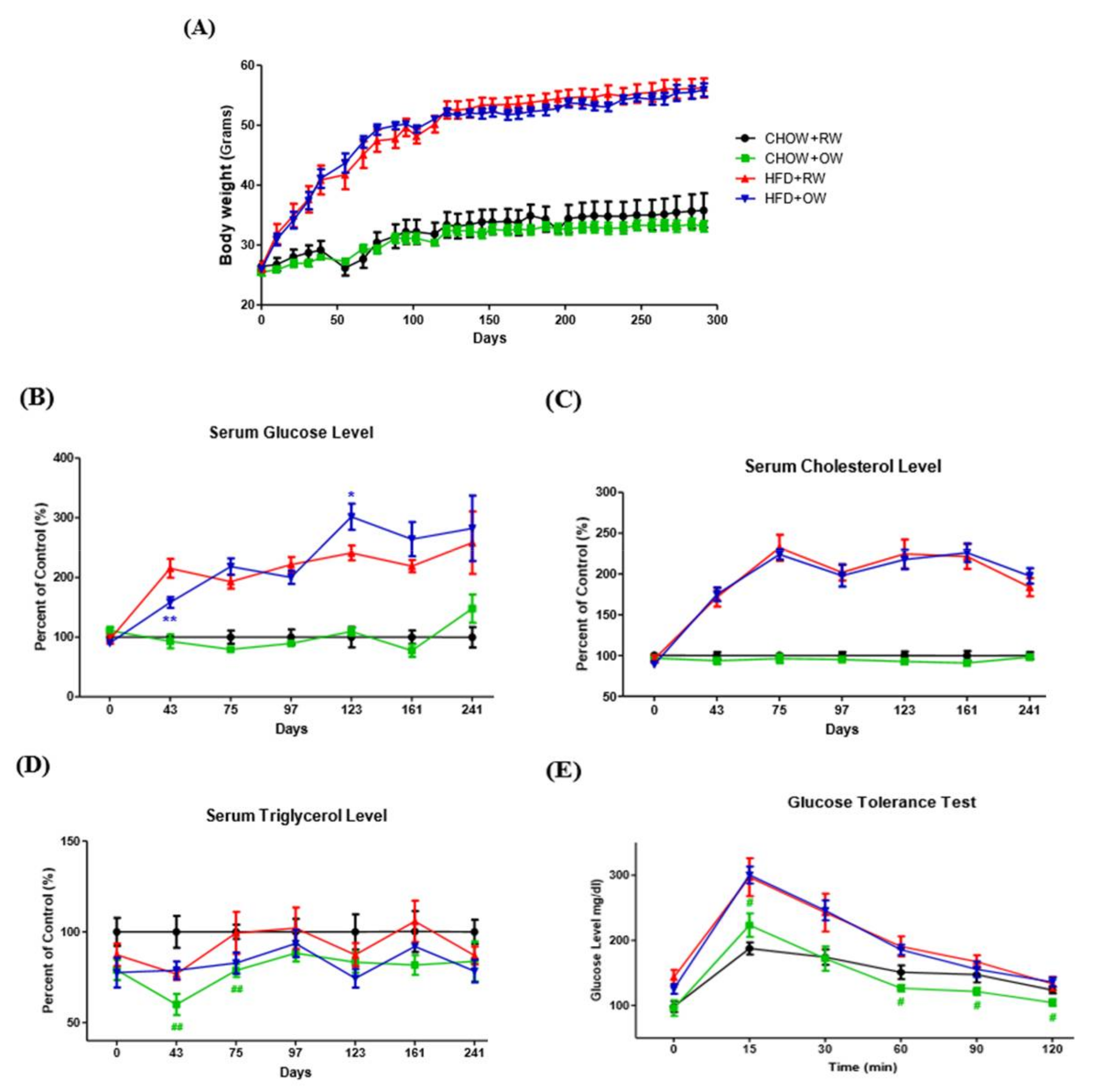
2.4. Effects of Long-Term Oxygenated Water Consumption on Biochemical Parameters of Diet-Induced Obesity
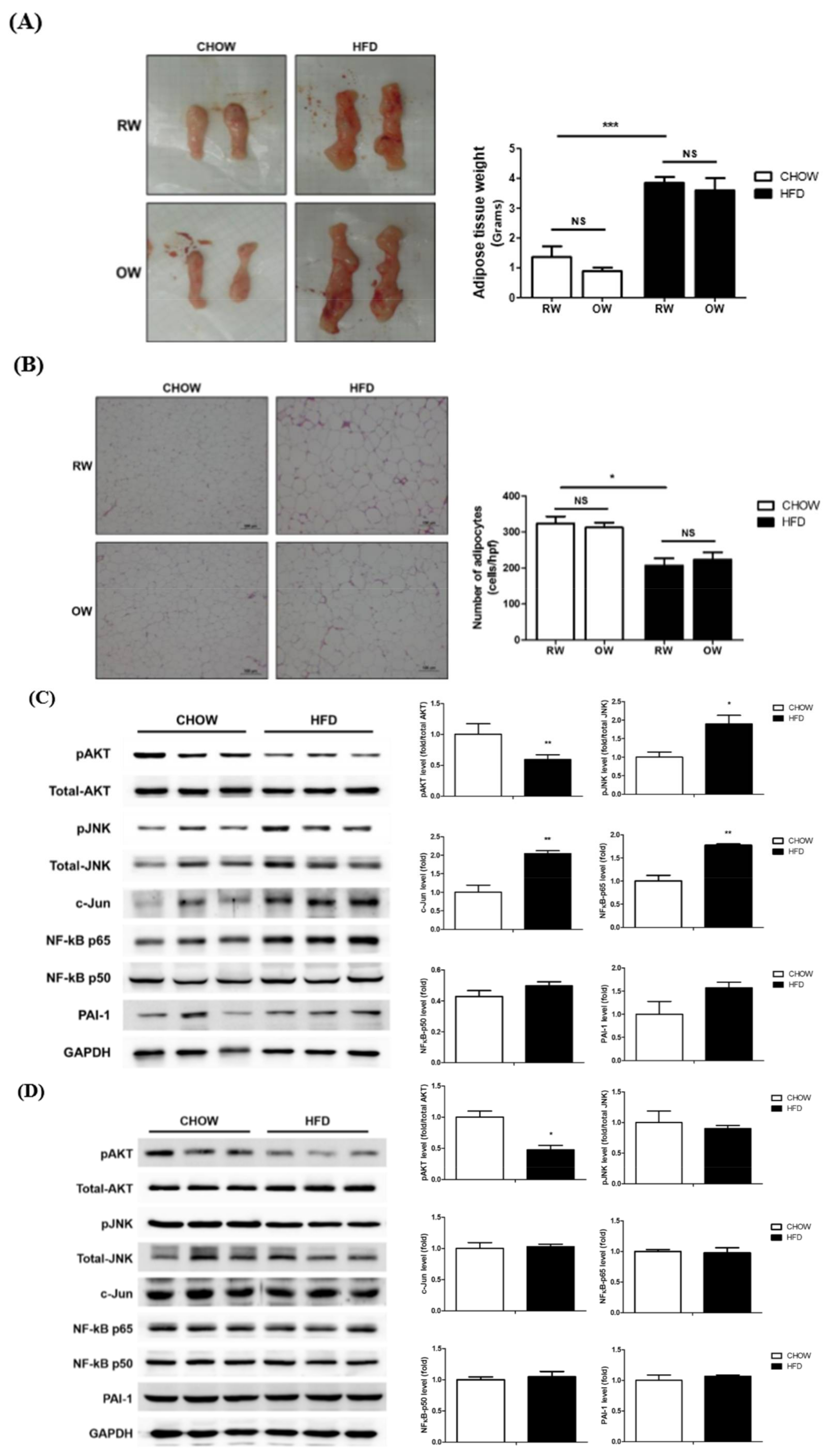
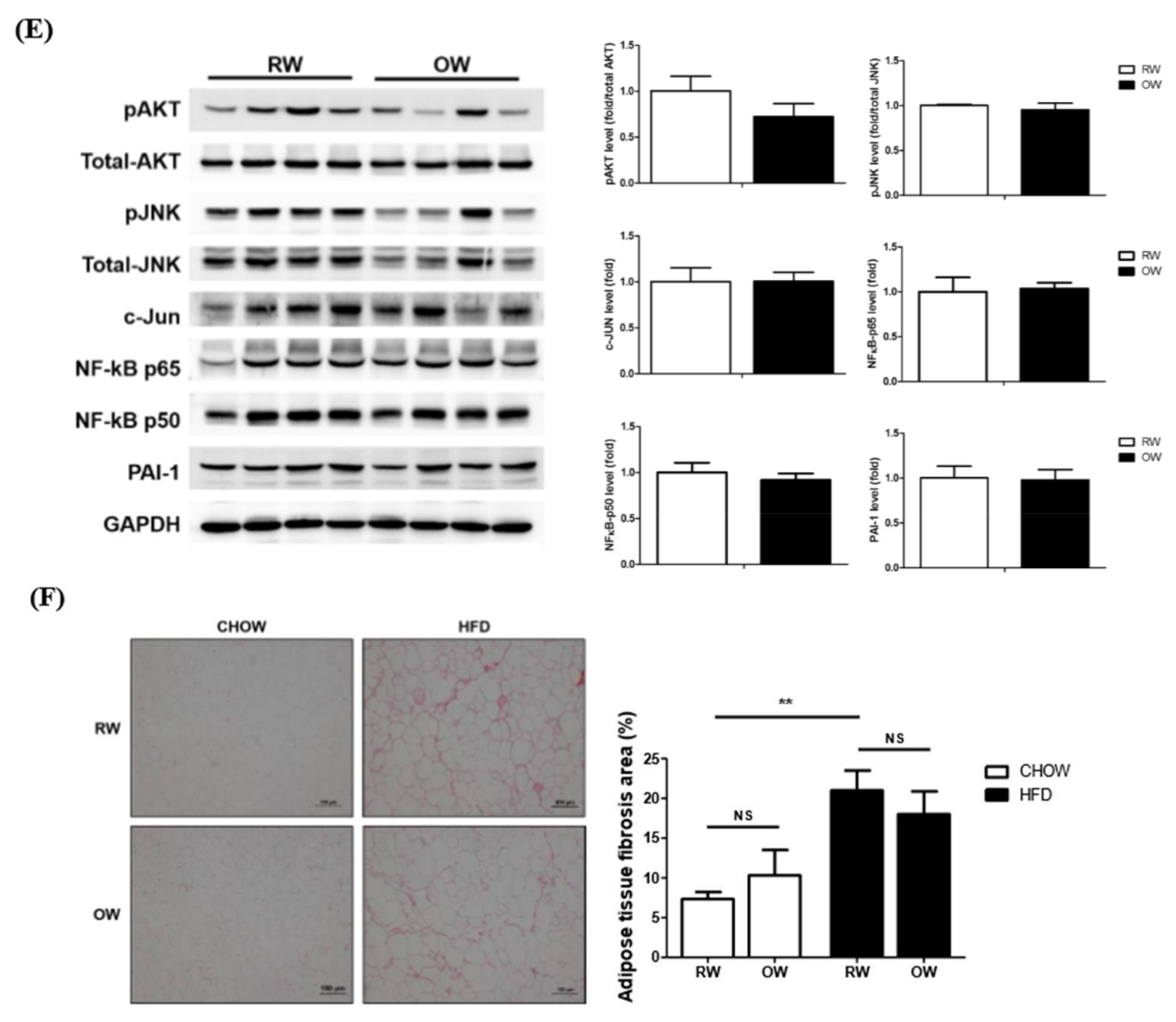
2.5. Effects of Long-Term Oxygenated Water Consumption on Adipose Tissue
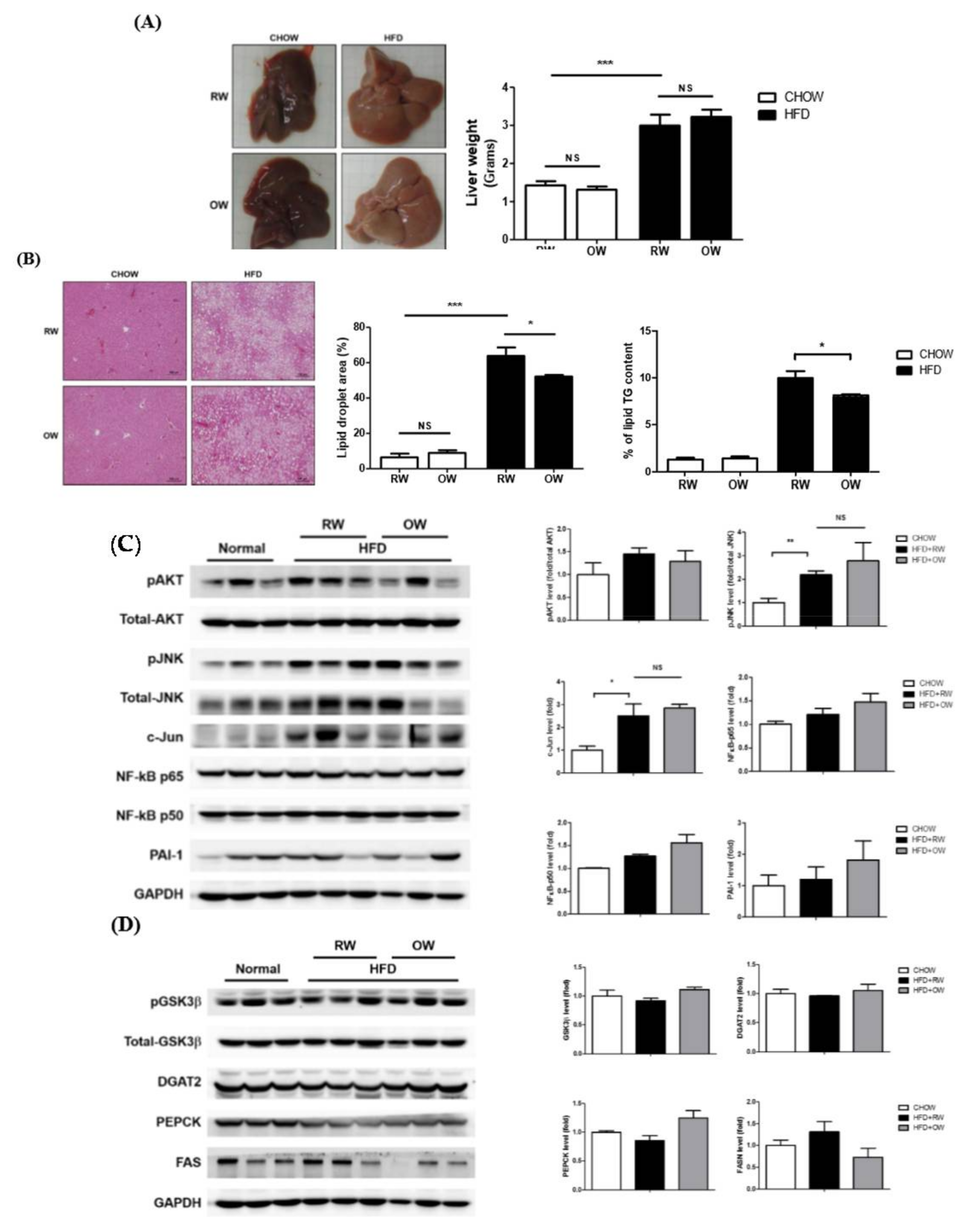
2.6. Long-Term Oxygenated Water Consumption Ameliorates Hepatic Steatosis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Saturated Oxygenated Water
4.3. T3-L1 Cell Culture, Adipogenesis and Oil-Red O Staining
4.4. Western Blot Analysis
4.5. RNA Extraction and RT-PCR
4.6. Detection of Intracellular Reactive Oxygen Species (ROS)
4.7. Animal Experiments
4.8. Intraperitoneal Glucose Tolerance Test (IGTT)
4.9. Histological Analysis
4.10. Hepatic Lipid Contents and Triglyceride Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Dex | dexamethasone |
| DGAT2 | diacylglycerol O-acyltransferase 2 |
| FABP4 | Fatty Acid-Binding Protein 4 |
| FASN | fatty acid synthase |
| GLU | glucose |
| GLUT | glucose transporter |
| HFD | high fat diet |
| HIFs | hypoxia-inducible factors |
| IBMX | 3-isobuty-methylxanthine |
| IL- | interleukin- |
| OW | oxygenated water |
| PEPCK | phosphoenolpyruvate carboxykinase |
| PPARγ | peroxisome proliferator-activated receptor-gamma |
| p- | phosphorylated |
| RW | regular water |
| SREBP | sterol regulatory element-binding transcription factor 1 |
| siRNA | small interfering RNA |
| T2DM | type 2 diabetes mellitus |
| TCHO | total cholesterol |
| TG | triacylglycerol |
| TNF-α | tumor necrosis factor-alpha |
| VEGFA | vascular endothelial growth factor A |
| VHL | von Hippel-Lindau |
References
- Negrao, M.R.; Monteiro, R.; Calhau, C.; Soares, R.; Azevedo, I. Comment on: Hosogai et al. (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911, 2007. Diabetes 2008, 57, e15. [Google Scholar] [CrossRef][Green Version]
- Lolmede, K.; Durand de Saint Front, V.; Galitzky, J.; Lafontan, M.; Bouloumie, A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lam, K.S.; Wang, Y.; Wu, D.; Lam, M.C.; Shen, J.; Wong, L.; Hoo, R.L.; Zhang, J.; Xu, A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem. Biophys. Res. Commun. 2006, 341, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Taube, A.; Schlich, R.; Sell, H.; Eckardt, K.; Eckel, J. Inflammation and metabolic dysfunction: Links to cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2148–H2165. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wood, I.S.; Trayhurn, P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Eur. J. Physiol. 2007, 455, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Pouyssegur, J. Oxygen, a source of life and stress. FEBS Lett. 2007, 581, 3582–3591. [Google Scholar] [CrossRef]
- Rocha, S. Gene regulation under low oxygen: Holding your breath for transcription. Trends Biochem. Sci. 2007, 32, 389–397. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFα targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wang, B.; Wood, I.S. Comment on: Hosagai et al. (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation: Diabetes 56:901–911. Diabetes 2007, 56, e14. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Qu, A.; Matsubara, T.; Chanturiya, T.; Jou, W.; Gavrilova, O.; Shah, Y.M.; Gonzalez, F.J. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 2011, 60, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, S.; Nakada, K.; Kuge, Y.; Tamaki, N.; Okada, F.; Wang, J.; Shindo, M.; Higashino, F.; Takeda, K.; et al. Dominant-negative hypoxia-inducible factor-1α reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am. J. Pathol. 2003, 162, 1283–1291. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta. Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Mueller, E. Understanding the variegation of fat: Novel regulators of adipocyte differentiation and fat tissue biology. Biochim. Biophys. Acta 2014, 1842, 352–357. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Ventre, J.; Doebber, T.; Wu, M.; MacNaul, K.; Stevens, K.; Pasparakis, M.; Kollias, G.; Moller, D.E. Targeted disruption of the tumor necrosis factor-α gene: Metabolic consequences in obese and nonobese mice. Diabetes 1997, 46, 1526–1531. [Google Scholar] [CrossRef]
- Sun, K.; Halberg, N.; Khan, M.; Magalang, U.J.; Scherer, P.E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 2013, 33, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef] [PubMed]
- Funaki, M. Saturated fatty acids and insulin resistance. J. Med. Investig. 2009, 56, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Batchelor, B. Adipose tissue cellularity in human obesity. Clin. Endocrinol. Metab. 1976, 5, 299–311. [Google Scholar] [CrossRef]
- Rudich, A.; Kanety, H.; Bashan, N. Adipose stress-sensing kinases: Linking obesity to malfunction. Trends Endocrinol. Metab. 2007, 18, 291–299. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Wood, I.S.; de Heredia, F.P.; Wang, B.; Trayhurn, P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc. Nutr. Soc. 2009, 68, 370–377. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Fukuda, K.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.M.; Kaczorowski, D.J.; Sugimoto, R.; Yang, R.; Wang, Y.; Billiar, T.R.; McCurry, K.R.; Bauer, A.J.; Nakao, A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant. 2008, 8, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakashima-Kamimura, N.; Mikami, T.; Ohsawa, I.; Ohta, S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology 2009, 34, 501–508. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol. 2009, 64, 753–761. [Google Scholar] [CrossRef]
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109. [Google Scholar] [CrossRef]
- Fujita, K.; Seike, T.; Yutsudo, N.; Ohno, M.; Yamada, H.; Yamaguchi, H.; Sakumi, K.; Yamakawa, Y.; Kido, M.A.; Takaki, A.; et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS ONE 2009, 4, e7247. [Google Scholar] [CrossRef]
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Suzuki, Y.; Maesawa, S.; Kajita, Y.; Hirayama, M.; et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009, 453, 81–85. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogenrich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef]
- Gruber, R.; Axmann, S.; Schoenberg, M.H. The influence of oxygenated water on the immune status, liver enzymes, and the generation of oxygen radicals: A prospective, randomised, blinded clinical study. Clin. Nutr. 2005, 24, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Wing-Gaia, S.L.; Subudhi, A.W.; Askew, E.W. Effects of purified oxygenated water on exercise performance during acute hypoxic exposure. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.H.; Hierl, T.C.; Zhao, J.; Wohlgemuth, N.; Nilsson, U.A. The generation of oxygen radicals after drinking of oxygenated water. Eur. J. Med. Res. 2002, 7, 109–116. [Google Scholar] [PubMed]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative stress in diabetes and Alzheimer’s disease. J. Alzheimer Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Fearon, I.M.; Faux, S.P. Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight. J. Mol. Cell. Cardiol. 2009, 47, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.H.; Chung, K.K. Oxidative and nitrosative stress in Parkinson’s disease. Biochim. Biophys. Acta 2009, 1792, 643–650. [Google Scholar] [CrossRef]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef]
- Nakao, A.; Kaczorowski, D.J.; Wang, Y.; Cardinal, J.S.; Buchholz, B.M.; Sugimoto, R.; Tobita, K.; Lee, S.; Toyoda, Y.; Billiar, T.R.; et al. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. J. Heart Lung Transplant. 2010, 29, 544–553. [Google Scholar] [CrossRef]
- Hu, Q.; Liang, X.; Chen, D.; Chen, Y.; Doycheva, D.; Tang, J.; Zhang, J.H. Delayed hyperbaric oxygen therapy promotes neurogenesis through reactive oxygen species/hypoxia-inducible factor-1α/β-catenin pathway in middle cerebral artery occlusion rats. Stroke 2014, 45, 1807–1814. [Google Scholar] [CrossRef]
- Peng, Z.; Ren, P.; Kang, Z.; Du, J.; Lian, Q.; Liu, Y.; Zhang, J.H.; Sun, X. Up-regulated HIF-1α is involved in the hypoxic tolerance induced by hyperbaric oxygen preconditioning. Brain Res. 2008, 1212, 71–78. [Google Scholar] [CrossRef]
- Salhanick, S.D.; Belikoff, B.; Orlow, D.; Holt, D.; Reenstra, W.; Buras, J.A. Hyperbaric oxygen reduces acetaminophen toxicity and increases HIF1α expression. Acad. Emerg. Med. 2006, 13, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, J.; Adler, I.; Manaenko, A.; Schwarz, S.C.; Walkinshaw, G.; Arend, M.; Flippin, L.A.; Storch, A.; Schwarz, J. Non-hypoxic stabilization of hypoxia-inducible factor α (HIF-α): Relevance in neural progenitor/stem cells. Neurotox. Res. 2009, 15, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Baiguera, S.; Guidolin, D.; Furlan, C.; Menti, A.M.; Vigolo, S.; Belloni, A.S.; Parnigotto, P.P.; Nussdorfer, G.G. Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3T3/J2 cell line. J. Investig. Med. 2003, 51, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Tsai, J.N.; Chang, S.W.; Hsu, W.T.; Yang, C.P.; Hsiao, C.W.; Shiau, M.Y. Regulation of adipogenesis and lipid deposits by collapsin response mediator protein 2. Int. J. Mol. Sci. 2020, 21, 2172. [Google Scholar] [CrossRef] [PubMed]
- Shiau, M.Y.; Lu, H.F.; Chang, Y.H.; Chiu, Y.C.; Shih, Y.L. Characterization of proteins regulated by interleukin-4 in 3T3-L1 adipocytes. SpringerPlus 2015, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Shiau, M.Y.; Lee, P.S.; Huang, Y.J.; Yang, C.P.; Hsiao, C.W.; Chang, K.Y.; Chen, H.W.; Chang, Y.H. Role of PARL-PINK1-Parkin pathway in adipocyte differentiation. Metabolism 2017, 72, 1–17. [Google Scholar] [CrossRef]
- Tsao, C.H.; Chuang, P.H.; Chang, Y.H.; Hwang, J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 2014, 55, 385–397. [Google Scholar] [CrossRef]
- Shiau, M.Y.; Chuang, P.H.; Yang, C.P.; Hsiao, C.W.; Chang, S.W.; Chang, K.Y.; Liu, T.M.; Chen, H.W.; Chuang, C.C.; Yuan, S.Y.; et al. Mechanism of interleukin-4 reducing lipid deposit by regulating hormone-sensitive lipase. Sci. Rep. 2019, 9, 11974. [Google Scholar] [CrossRef]
- Yang, C.P.; Shiau, M.Y.; Lai, Y.R.; Ho, K.T.; Hsiao, C.W.; Chen, C.J.; Lo, Y.L.; Chang, Y.H. Anti-inflammatory cytokine interleukin-4 boosts insulin-induced energy deposits by enhancing glucose uptake and lipogenesis in hepatocytes. Oxid. Med. Cel. Longev. 2018, 2018, 6923187. [Google Scholar]
- Chang, Y.H.; Ho, K.T.; Lu, S.H.; Huang, C.N.; Shiau, M.Y. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int. J. Obes. 2012, 36, 993–998. [Google Scholar] [CrossRef]
- Chang, Y.H.; Tsai, J.N.; Chen, T.Z.; Ho, K.T.; Cheng, H.Y.; Hsiao, C.W.; Shiau, M.Y. Interleukin-4 promotes myogenesis and boosts myocyte insulin efficacy. Mediators Inflam. 2019, 2019, 4182015. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Yang, C.P.; Wang, Y.Y.; Hsiao, C.W.; Chen, W.Y.; Liao, S.L.; Lo, Y.L.; Chang, Y.H.; Hone, C.J.; Chen, C.J. Interleukin-4 improves metabolic abnormalities in leptin-deficient and high-fat diet mice. Int. J. Mol. Sci. 2020, 21, 4451. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-J.; Liu, C.-C.; Chu, F.-Y.; Yang, C.-P.; Hsiao, C.-W.; Chuang, C.-W.; Shiau, M.-Y.; Lee, H.-T.; Tsai, J.-N.; Chang, Y.-H. Oxygenated Water Inhibits Adipogenesis and Attenuates Hepatic Steatosis in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2020, 21, 5493. https://doi.org/10.3390/ijms21155493
Cheng Y-J, Liu C-C, Chu F-Y, Yang C-P, Hsiao C-W, Chuang C-W, Shiau M-Y, Lee H-T, Tsai J-N, Chang Y-H. Oxygenated Water Inhibits Adipogenesis and Attenuates Hepatic Steatosis in High-Fat Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2020; 21(15):5493. https://doi.org/10.3390/ijms21155493
Chicago/Turabian StyleCheng, Yuh-Jen, Chao-Chi Liu, Fang-Yeh Chu, Ching-Ping Yang, Chiao-Wan Hsiao, Cheng-Wei Chuang, Ming-Yuh Shiau, Hsueh-Te Lee, Jen-Ning Tsai, and Yih-Hsin Chang. 2020. "Oxygenated Water Inhibits Adipogenesis and Attenuates Hepatic Steatosis in High-Fat Diet-Induced Obese Mice" International Journal of Molecular Sciences 21, no. 15: 5493. https://doi.org/10.3390/ijms21155493
APA StyleCheng, Y.-J., Liu, C.-C., Chu, F.-Y., Yang, C.-P., Hsiao, C.-W., Chuang, C.-W., Shiau, M.-Y., Lee, H.-T., Tsai, J.-N., & Chang, Y.-H. (2020). Oxygenated Water Inhibits Adipogenesis and Attenuates Hepatic Steatosis in High-Fat Diet-Induced Obese Mice. International Journal of Molecular Sciences, 21(15), 5493. https://doi.org/10.3390/ijms21155493