Recent Advances on Biomarkers of Early and Late Kidney Graft Dysfunction
Abstract
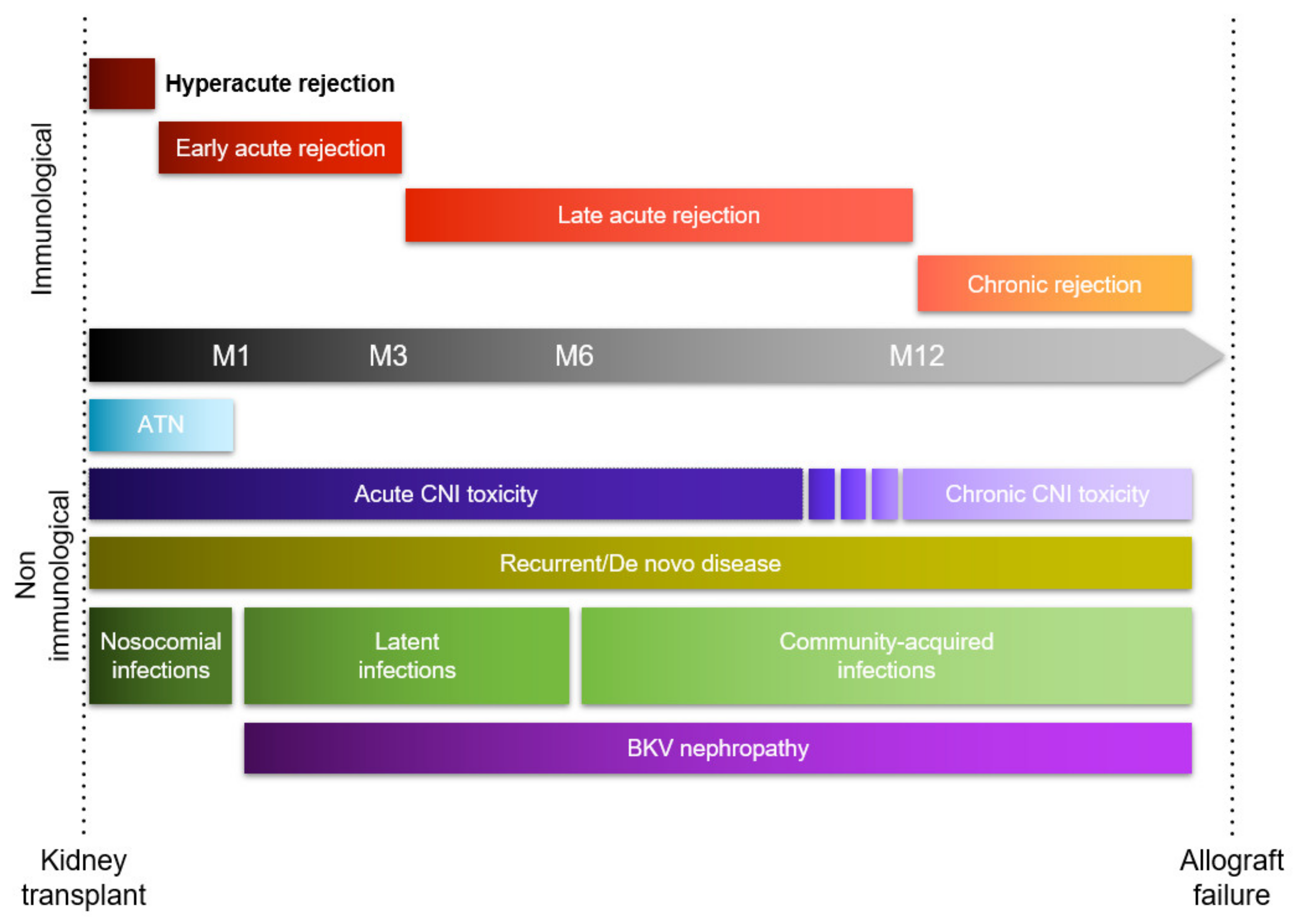
1. Introduction
General Features and Meaning of a Biomarker
2. IRI and DGF
2.1. Donor-Related Biomarkers
2.1.1. Donor Biological Fluids
2.1.2. Graft Preservation Fluid
2.1.3. Perfusate of Machine-Perfused Kidneys
2.2. Recipient-Related Biomarkers
2.2.1. Furosemide Stress Test
2.2.2. miRNAs
2.2.3. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Other Biomarkers
2.2.4. BioMarkers of EndMT
2.2.5. EVs
| Type of EV | Main Features | Author |
|---|---|---|
| Plasma Endothelial EVs | EVs level and their procoagulant activity progressively decrease after KTx, paralleling renal function recovery | Al-Massarani G et al. [44,45] |
| Plasma Endothelial and platelet EVs | Endothelial and platelet EVs size and level progressively decrease after KTx, paralleling renal function recovery | Martins S et al. [46] |
| Urinary EVs | NGAL expression in urinary EVs correlated with DGF | Alvarez S et al. [47] |
| Urinary CD 133+ EVs | Decreased level in recipients with DGF and vascular damage | Dimuccio V et al. [48] |
| Acquaporin-1 containing EVs | Decreased urinary Acquaporin-1-containing EVs in DGF | Sonoda H et al. [49] Asvapromtada S et al. [50] |
3. AR
3.1. Transcriptomic Studies
3.1.1. Urine and Peripheral Blood Transcriptomics
3.1.2. Renal Tissue Transcriptomics
3.2. Complement-Related Biomarkers
3.3. Urinary and Serum Chemokines
3.4. Other Potential Urinary Biomarkers
3.5. dd-cfDNA
3.6. Allogenic Circulating B-Cell and T-Cell Assays
3.7. Peripheral Blood miRNAs
3.8. Immune Cells Biomarkers
3.9. Non-HLA DSA
| Biomarker | Main Features | Author |
|---|---|---|
| Anti-AT1R | Pre-transplant levels associated with, acute and chronic ABMR, severity of microvascular inflammation, graft dysfunction, and graft loss | Dragun D et al. [119] Philogene MC Hum Imm 2019 [120] Sas-Strozik et al. [121] Shinae Y et al. [122] DF Pinelli et al. [123] MA Lim et al. [125] |
| Anti-ETAR | Pre-transplant levels associated with acute and chronic ABMR graft dysfunction and graft loss | Philogene MC et al. Hum Imm 2019 [120] Shinae Y et al. [122] DF Pinelli et al. [123] Jackson AM et al. [131] |
| Anti-Vimentin | Pre-transplant levels associated with graft dysfunction | Dyvanian T et al. [126] |
| Anti-Perlecan | Highly prevalent in hypersensitized patients. Pre-transplant levels associated with increased risk of DGF, acute ABMR, and reduced long-term function | Dieudè M et al. [127] Riesco L et al. [128] Padet L et al. [129] Yang B et al. [130] |
| AECA | They include a variety of antibodies against endothelial antigens (Endoglin, FLT-3, EDIL-3, ICAM-4, KTR-1) and correlate with increased risk of ABMR | Jackson AM et al. [131] Guo X et al. [132] Sanchez Zapardiel E et al. [133] |
| Anti-FN and Col-IV | De novo development increases risk of AR (PKT) and transplant glomerulopathy (KTx) | Angaswamy N et al. [135] Gunasekeran M et al. [136] |
3.10. Other Biomarkers
3.11. EVs
| Type of EV | Type of Rejection | Main Features | Author |
|---|---|---|---|
| Plasma C4d+CD144+ endothelial EVs | ABMR | Levels correlate with ABMR presence and severity and decrease after successful treatment | Tower C et al. [99] |
| Plasma endothelial EVs | ABMR | A combination score based on 4 mRNA transcripts overexpressed in EVs of patients with ABMR predicts imminent rejection in HLA- sensitized patients | Zhang H et al. [142] |
| Plasma endothelial EVs | ABMR | Levels increase in ABMR and decrease after treatment in the early post-transplant; however, they are also influenced by renal function recovery | Qamri Z et al. [143] |
| Urinary EVs | TCMR | A total of 11 protein enriched in urinary EV in patients with TCMR | Sigdel T et al. [144] |
| Urinary EVs | TCMR | A total of 17 protein enriched in urinary EV in patients with TCMR; Tetraspanin-1 and Hemopexin proposed as biomarkers | Lim J et al. [145] |
| Urinary EVs | TCMR | High levels of CD3 + EVs released by T-cell in urine are strongly associated with TCMR | Park J et al. [146] |
4. Chronic Allograft Dysfunction (CAD)
4.1. Chronic Rejection and IFTA
4.1.1. Transcriptomic Studies
4.1.2. miRNAs
4.1.3. Biomarkers of EMT and EndMT
- (a)
- Biomarkers of EMT
- Histological biomarkers
- Urinary biomarkers
- (b)
- Biomarkers of EndMT
4.2. Chronic CNI Nephrotoxicity
| Biomarker | Main Features | Author |
|---|---|---|
| Urinary symmetric dimethylarginine and serine | Highly accurate for CNI nephrotoxicity (AUC of 0.95 and 0.81, respectively) | Xia T et al. [177] |
| uNGAL | It correlates with duration of CsA therapy in children with CNI nephrotoxicity | Gacka E et al. [178] |
| Genetic polymorphism of FK-506-binding protein, rs6041749 C variant | It enhances FKBP1A gene transcription and is associated with an increased risk of CAD in TAC-treated KTx recipients | Wu Z et al. [179] |
| Increased urinary TNAα, LIM-1, FN Osteopontin, and TGF-β | These markers correlate with different stages of CsA nephrotoxicity in rat models | Carlos C et al. [180] |
| Decreased renal expression of Slc12a3 and KS-WNK1 | These markers correlate with different stages of CNI nephrotoxicity in rat models | Cui Y et al. [181] |
4.3. PVAN
5. Current Limits and Perspectives of Biomarkers in Renal Transplant
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABMR | antibody-mediated rejection |
| cABMR | chronic antibody-mediated rejection |
| AI | artificial intelligence |
| AR | acute rejection |
| ATN | acute tubular necrosis |
| AT1R | Angiotensin 2 receptor 1 |
| AUC | area under the curve |
| CAD | chronic allograft dysfunction |
| CNI | calcineurin inhibitor |
| Col-IV | Collagen type IV |
| CsA | Cyclosporin A |
| DCD | donation after circulatory death |
| DBD | donation after brain death |
| DGF | delayed graft function |
| DSA | donor-specific antibodies |
| ECD | extended criteria donor |
| EDIL-3 | EGF-like repeats and discoidin I-like domains 3 |
| EM | extracellular matrix |
| EMT | epithelial-to-mesenchymal transition |
| ENDATs | endothelial associated transcripts |
| EndMT | endothelial-to-mesenchymal transition |
| ETAR | endothelin type A receptor |
| EVs | extracellular vesicles |
| FLT3-L | Fms-like tyrosine kinase-3 ligand |
| FN | Fibronectin |
| GFR | glomerular filtration rate |
| GST | glutathione S-transferase |
| HLA | human leukocyte antigen |
| HSP | heat shock protein |
| ICAM-4 | intercellular adhesion molecule 4 |
| IFTA | interstitial fibrosis tubular atrophy |
| iIFTA | inflammatory interstitial fibrosis tubular atrophy |
| IRI | ischemia-reperfusion injury |
| kSORT | kidney solid organ response test |
| KTx | kidney transplant |
| KTR-3 | Keratin-3 |
| FST | furosemide stress test |
| LD | living-donor |
| MFI | mean fluorescence intensity |
| MMP-2 | matrix metalloprotein-2 |
| miRNA | microRNA |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NPP | negative predictive power |
| PBTs | pathogenesis-based transcript sets |
| pEMT | partial epithelial-to-mesenchymal transition |
| POSTN | Periostin |
| PVAN | Polyomavirus-associated nephropathy |
| PPP | Positive predictive power |
| ROC | receiver operating characteristic |
| SNP | single nucleotide polymorphism |
| TAC | Tacrolimus |
| TCMR | T-cell mediated rejection |
| tCRM | tissue common rejection module |
| TIMP-2 | tissue inhibitor of metalloproteinases-2 |
| TLR-4 | Toll-like receptor 4 |
| VIM | Vimentin |
References
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. Maywood NJ 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Tsalouchos, A. Biomarkers in renal transplantation: An updated review. World J. Transplant. 2017, 7, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Sarwal, M.; Chua, M.-S.; Kambham, N.; Hsieh, S.-C.; Satterwhite, T.; Masek, M.; Salvatierra, O.J. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N. Engl. J. Med. 2003, 349, 125–138. [Google Scholar] [CrossRef]
- Stapleton, C.P.; Conlon, P.J.; Phelan, P.J. Using omics to explore complications of kidney transplantation. Transpl. Int. 2018, 31, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Anglicheau, D. Precision Transplant Medicine: Biomarkers to the Rescue. J. Am. Soc. Nephrol. 2018, 29, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Erlich, J.; Au, A.Y.M.; Endre, Z.H. Advances in Detection of Kidney Transplant Injury. Mol. Diagn. Ther. 2019, 23, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Nashan, B.; Abbud-Filho, M.; Citterio, F. Prediction, prevention, and management of delayed graft function: Where are we now? Clin. Transplant. 2016, 30, 1198–1208. [Google Scholar] [CrossRef]
- Noble, J.; Jouve, T.; Malvezzi, P.; Süsal, C.; Rostaing, L. Transplantation of Marginal Organs: Immunological Aspects and Therapeutic Perspectives in Kidney Transplantation. Front. Immunol. 2019, 10, 3142. [Google Scholar] [CrossRef]
- Caulfield, T.; Murdoch, B.; Sapir-Pichhadze, R.; Keown, P. Policy Challenges for Organ Allocation in an Era of “Precision Medicine”. Can. J. Kidney Health Dis. 2020, 7, 2054358120912655. [Google Scholar] [CrossRef]
- Han, F.; Wan, S.; Sun, Q.; Chen, N.; Li, H.; Zheng, L.; Zhang, N.; Huang, Z.; Hong, L.; Sun, Q. Donor Plasma Mitochondrial DNA Is Correlated with Posttransplant Renal Allograft Function. Transplantation 2019, 103, 2347–2358. [Google Scholar] [CrossRef]
- Schröppel, B.; Heeger, P.S.; Thiessen-Philbrook, H.; Hall, I.E.; Doshi, M.D.; Weng, F.L.; Reese, P.P.; Parikh, C.R. Donor Urinary C5a Levels Independently Correlate with Posttransplant Delayed Graft Function. Transplantation 2019, 103, e29–e35. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Dos-Santos, V.; Ramos-Muñoz, E.; García-Bermejo, M.L.; Ruiz-Hernández, M.; Rodríguez-Serrano, E.M.; Saiz-González, A.; Martínez-Perez, A.; Burgos-Revilla, F.J. MicroRNAs in Kidney Machine Perfusion Fluid as Novel Biomarkers for Graft Function. Normalization Methods for miRNAs Profile Analysis. Transplant. Proc. 2019, 51, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Roest, H.P.; Ooms, L.S.S.; Gillis, A.J.M.; IJzermans, J.N.M.; Looijenga, L.H.J.; Dorssers, L.C.J.; Dor, F.J.M.F.; van der Laan, L.J.W. Cell-free MicroRNA miR-505-3p in Graft Preservation Fluid Is an Independent Predictor of Delayed Graft Function After Kidney Transplantation. Transplantation 2019, 103, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.A.J.; Sawicka, K.; Arcand, S.; O’Brien, P.; Luke, P.; Beck, G.; Sawicka, J.; Cohen, A.; Sawicki, G. Proteomic Analysis of Perfusate from Machine Cold Perfusion of Transplant Kidneys: Insights into Protection from Injury. Ann. Transplant. 2017, 22, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Cappuccilli, M.; Capelli, I.; Comai, G.; Cianciolo, G.; La Manna, G. Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Allograft Function after Renal Transplantation: Evaluation of the Current Status and Future Insights. Artif. Organs 2018, 42, 8–14. [Google Scholar] [CrossRef]
- Hall, I.E.; Bhangoo, R.S.; Reese, P.P.; Doshi, M.D.; Weng, F.L.; Hong, K.; Lin, H.; Han, G.; Hasz, R.D.; Goldstein, M.J.; et al. Glutathione S-transferase iso-enzymes in perfusate from pumped kidneys are associated with delayed graft function. Am. J. Transplant. 2014, 14, 886–896. [Google Scholar] [CrossRef]
- Udomkarnjananun, S.; Townamchai, N.; Iampenkhae, K.; Petchlorlian, A.; Srisawat, N.; Katavetin, P.; Sutherasan, M.; Santingamkun, A.; Praditpornsilpa, K.; Eiam-Ong, S.; et al. Furosemide Stress Test as a Predicting Biomarker for Delayed Graft Function in Kidney Transplantation. Nephron 2019, 141, 236–248. [Google Scholar] [CrossRef]
- Wilflingseder, J.; Sunzenauer, J.; Toronyi, E.; Heinzel, A.; Kainz, A.; Mayer, B.; Perco, P.; Telkes, G.; Langer, R.M.; Oberbauer, R. Molecular pathogenesis of post-transplant acute kidney injury: Assessment of whole-genome mRNA and miRNA profiles. PLoS ONE 2014, 9, e104164. [Google Scholar] [CrossRef]
- Milhoransa, P.; Montanari, C.C.; Montenegro, R.; Manfro, R.C. Micro RNA 146a-5p expression in Kidney transplant recipients with delayed graft function. Braz. J. Nephrol. 2019, 41, 242–251. [Google Scholar] [CrossRef]
- Khalid, U.; Newbury, L.J.; Simpson, K.; Jenkins, R.H.; Bowen, T.; Bates, L.; Sheerin, N.S.; Chavez, R.; Fraser, D.J. A urinary microRNA panel that is an early predictive biomarker of delayed graft function following kidney transplantation. Sci. Rep. 2019, 9, 3584. [Google Scholar] [CrossRef]
- Maier, H.T.; Ashraf, M.I.; Denecke, C.; Weiss, S.; Augustin, F.; Messner, F.; Vallant, N.; Böcklein, M.; Margreiter, C.; Göbel, G.; et al. Prediction of delayed graft function and long-term graft survival by serum and urinary neutrophil gelatinase-associated lipocalin during the early postoperative phase after kidney transplantation. PLoS ONE 2018, 13, e0189932. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sandoval, J.C.; Herrington, W.; Morales-Buenrostro, L.E. Neutrophil gelatinase-associated lipocalin in kidney transplantation: A review. Transplant. Rev. 2015, 29, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Li, Y.; Yan, L.; Wang, H.; Wu, X.J.; Tang, J.T.; Wang, L.L.; Shi, Y.Y. Comparison of urine and blood NGAL for early prediction of delayed graft function in adult kidney transplant recipients: A meta-analysis of observational studies. BMC Nephrol. 2019, 20, 291. [Google Scholar] [CrossRef] [PubMed]
- Sahraei, Z.; Mehdizadeh, M.; Salamzadeh, J.; Nafar, M.; Eshraghi, A. Association between Delayed Graft Function (DGF) Biomarkers and Long-term Outcomes after Living Donor Kidney Transplantation. Rev. Recent Clin. Trials 2018, 13, 312–318. [Google Scholar] [CrossRef]
- Hu, X.; Su, M.; Lin, J.; Zhang, L.; Sun, W.; Zhang, J.; Tian, Y.; Qiu, W. Corin Is Downregulated in Renal Ischemia/Reperfusion Injury and Is Associated with Delayed Graft Function after Kidney Transplantation. Dis. Markers 2019, 2019, 9429323. [Google Scholar] [CrossRef]
- Zmonarski, S.; Madziarska, K.; Banasik, M.; Mazanowska, O.; Magott-Procelewska, M.; Hap, K.; Krajewska, M. Expression of PBMC TLR4 in Renal Graft Recipients Who Experienced Delayed Graft Function Reflects Dynamic Balance Between Blood and Tissue Compartments and Helps Select a Problematic Patient. Transplant. Proc. 2018, 50, 1744–1749. [Google Scholar] [CrossRef]
- Comai, G.; Baraldi, O.; Cuna, V.; Corradetti, V.; Angeletti, A.; Brunilda, S.; Capelli, I.; Cappuccilli, M.; LA Manna, G. Increase in Serum Amylase and Resistive Index after Kidney Transplant Are Biomarkers of Delayed Graft Function. Vivo Athens Greece 2018, 32, 397–402. [Google Scholar] [CrossRef]
- Xu-Dubois, Y.-C.; Ahmadpoor, P.; Brocheriou, I.; Louis, K.; Snanoudj, N.A.; Rouvier, P.; Taupin, J.-L.; Corchia, A.; Galichon, P.; Barrou, B.; et al. Microvasculature partial endothelial mesenchymal transition in early posttransplant biopsy with acute tubular necrosis identifies poor recovery renal allografts. Am. J. Transplant. 2020. [Google Scholar] [CrossRef]
- Castellano, G.; Franzin, R.; Stasi, A.; Divella, C.; Sallustio, F.; Pontrelli, P.; Lucarelli, G.; Battaglia, M.; Staffieri, F.; Crovace, A.; et al. Complement Activation during Ischemia/Reperfusion Injury Induces Pericyte-to-Myofibroblast Transdifferentiation Regulating Peritubular Capillary Lumen Reduction Through pERK Signaling. Front. Immunol. 2018, 9, 1002. [Google Scholar] [CrossRef]
- Castellano, G.; Franzin, R.; Sallustio, F.; Stasi, A.; Banelli, B.; Romani, M.; De Palma, G.; Lucarelli, G.; Divella, C.; Battaglia, M.; et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury. Aging 2019, 11, 4382–4406. [Google Scholar] [CrossRef]
- Curci, C.; Castellano, G.; Stasi, A.; Divella, C.; Loverre, A.; Gigante, M.; Simone, S.; Cariello, M.; Montinaro, V.; Lucarelli, G.; et al. Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol. Dial. Transplant. 2014, 29, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Gremmels, H.; de Jong, O.G.; Toorop, R.J.; Michielsen, L.; van Zuilen, A.D.; Vlassov, A.V.; Verhaar, M.C.; van Balkom, B.W.M. The Small RNA Repertoire of Small Extracellular Vesicles Isolated from Donor Kidney Preservation Fluid Provides a Source for Biomarker Discovery for Organ Quality and Posttransplantation Graft Function. Transplant. Direct 2019, 5, e484. [Google Scholar] [CrossRef] [PubMed]
- Franco-Acevedo, A.; Melo, Z.; Echavarria, R. Diagnostic, Prognostic, and Therapeutic Value of Non-Coding RNA Expression Profiles in Renal Transplantation. Diagnostics 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Wilflingseder, J.; Reindl-Schwaighofer, R.; Sunzenauer, J.; Kainz, A.; Heinzel, A.; Mayer, B.; Oberbauer, R. MicroRNAs in kidney transplantation. Nephrol. Dial. Transplant. 2015, 30, 910–917. [Google Scholar] [CrossRef]
- Trionfini, P.; Benigni, A.; Remuzzi, G. MicroRNAs in kidney physiology and disease. Nat. Rev. Nephrol. 2015, 11, 23–33. [Google Scholar] [CrossRef]
- Su, M.; Hu, X.; Lin, J.; Zhang, L.; Sun, W.; Zhang, J.; Tian, Y.; Qiu, W. Identification of Candidate Genes Involved in Renal Ischemia/Reperfusion Injury. DNA Cell Biol. 2019, 38, 256–262. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Dellepiane, S.; Tamagnone, M.; Medica, D.; Figliolini, F.; Messina, M.; Manzione, A.M.; Gai, M.; Tognarelli, G.; Ranghino, A.; et al. Neutrophil Gelatinase Associated Lipocalin Is an Early and Accurate Biomarker of Graft Function and Tissue Regeneration in Kidney Transplantation from Extended Criteria Donors. PLoS ONE 2015, 10, e0129279. [Google Scholar] [CrossRef]
- Bank, J.R.; Ruhaak, R.; Soonawala, D.; Mayboroda, O.; Romijn, F.P.; van Kooten, C.; Cobbaert, C.M.; de Fijter, J.W. Urinary TIMP-2 Predicts the Presence and Duration of Delayed Graft Function in Donation after Circulatory Death Kidney Transplant Recipients. Transplantation 2019, 103, 1014–1023. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Karpman, D.; Ståhl, A.-L.; Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, M.; Dellepiane, S.; Guglielmetti, G.; Merlotti, G.; Castellano, G.; Cantaluppi, V. Extracellular Vesicles as Mediators of Cellular Crosstalk between Immune System and Kidney Graft. Front. Immunol. 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Dursun, I.; Yel, S.; Unsur, E. Dynamics of circulating microparticles in chronic kidney disease and transplantation: Is it really reliable marker? World J. Transplant. 2015, 5, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Al-Massarani, G.; Vacher-Coponat, H.; Paul, P.; Widemann, A.; Arnaud, L.; Loundou, A.; Robert, S.; Berland, Y.; Dignat-George, F.; Camoin-Jau, L. Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. Am. J. Transplant. 2008, 8, 2360–2367. [Google Scholar] [CrossRef]
- Al-Massarani, G.; Vacher-Coponat, H.; Paul, P.; Arnaud, L.; Loundou, A.; Robert, S.; Moal, V.; Berland, Y.; Dignat-George, F.; Camoin-Jau, L. Kidney transplantation decreases the level and procoagulant activity of circulating microparticles. Am. J. Transplant. 2009, 9, 550–557. [Google Scholar] [CrossRef]
- Martins, S.R.; Alves, L.V.; Cardoso, C.N.; Silva, L.G.; Nunes, F.F.; de Lucas Júnior, F.M.; Silva, A.C.; Dusse, L.M.; Alpoim, P.N.; Mota, A.P. Cell-derived microparticles and von Willebrand factor in Brazilian renal transplant recipients. Nephrol. Carlton 2019, 24, 1304–1312. [Google Scholar] [CrossRef]
- Alvarez, S.; Suazo, C.; Boltansky, A.; Ursu, M.; Carvajal, D.; Innocenti, G.; Vukusich, A.; Hurtado, M.; Villanueva, S.; Carreño, J.E.; et al. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant. Proc. 2013, 45, 3719–3723. [Google Scholar] [CrossRef]
- Dimuccio, V.; Ranghino, A.; Praticò Barbato, L.; Fop, F.; Biancone, L.; Camussi, G.; Bussolati, B. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS ONE 2014, 9, e104490. [Google Scholar] [CrossRef]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A.; et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef]
- Asvapromtada, S.; Sonoda, H.; Kinouchi, M.; Oshikawa, S.; Takahashi, S.; Hoshino, Y.; Sinlapadeelerdkul, T.; Yokota-Ikeda, N.; Matsuzaki, T.; Ikeda, M. Characterization of urinary exosomal release of aquaporin-1 and -2 after renal ischemia-reperfusion in rats. Am. J. Physiol. Ren. Physiol. 2018, 314, F584–F601. [Google Scholar] [CrossRef]
- Suthanthiran, M.; Schwartz, J.E.; Ding, R.; Abecassis, M.; Dadhania, D.; Samstein, B.; Knechtle, S.J.; Friedewald, J.; Becker, Y.T.; Sharma, V.K.; et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N. Engl. J. Med. 2013, 369, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Christakoudi, S.; Runglall, M.; Mobillo, P.; Tsui, T.-L.; Duff, C.; Domingo-Vila, C.; Kamra, Y.; Delaney, F.; Montero, R.; Spiridou, A.; et al. Development of a multivariable gene-expression signature targeting T-cell-mediated rejection in peripheral blood of kidney transplant recipients validated in cross-sectional and longitudinal samples. EBioMedicine 2019, 41, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yi, Z.; Keung, K.L.; Shang, H.; Wei, C.; Cravedi, P.; Sun, Z.; Xi, C.; Woytovich, C.; Farouk, S.; et al. A Peripheral Blood Gene Expression Signature to Diagnose Subclinical Acute Rejection. J. Am. Soc. Nephrol. 2019, 30, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, E.; Gazut, S.; Yazdani, S.; Lerut, E.; de Loor, H.; Coemans, M.; Noël, L.-H.; Thorrez, L.; Van Lommel, L.; Schuit, F.; et al. Development and validation of a peripheral blood mRNA assay for the assessment of antibody-mediated kidney allograft rejection: A multicentre, prospective study. EBioMedicine 2019, 46, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, J.J.; Kurian, S.M.; Heilman, R.L.; Whisenant, T.C.; Poggio, E.D.; Marsh, C.; Baliga, P.; Odim, J.; Brown, M.M.; Ikle, D.N.; et al. Development and clinical validity of a novel blood-based molecular biomarker for subclinical acute rejection following kidney transplant. Am. J. Transplant. 2019, 19, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.; Nguyen, M.; Liberto, J.; Dobi, D.; Junger, H.; Vincenti, F.; Laszik, Z.; Sarwal, M.M. Assessment of 19 Genes and Validation of CRM Gene Panel for Quantitative Transcriptional Analysis of Molecular Rejection and Inflammation in Archival Kidney Transplant Biopsies. Front. Med. 2019, 6, 213. [Google Scholar] [CrossRef]
- Roedder, S.; Sigdel, T.; Salomonis, N.; Hsieh, S.; Dai, H.; Bestard, O.; Metes, D.; Zeevi, A.; Gritsch, A.; Cheeseman, J.; et al. The kSORT assay to detect renal transplant patients at high risk for acute rejection: Results of the multicenter AART study. PLoS Med. 2014, 11, e1001759. [Google Scholar] [CrossRef]
- Sis, B.; Jhangri, G.S.; Bunnag, S.; Allanach, K.; Kaplan, B.; Halloran, P.F. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am. J. Transplant. 2009, 9, 2312–2323. [Google Scholar] [CrossRef]
- Adam, B.A.; Smith, R.N.; Rosales, I.A.; Matsunami, M.; Afzali, B.; Oura, T.; Cosimi, A.B.; Kawai, T.; Colvin, R.B.; Mengel, M. Chronic Antibody-Mediated Rejection in Nonhuman Primate Renal Allografts: Validation of Human Histological and Molecular Phenotypes. Am. J. Transplant. 2017, 17, 2841–2850. [Google Scholar] [CrossRef]
- Stites, E.; Renner, B.; Laskowski, J.; Le Quintrec, M.; You, Z.; Freed, B.; Cooper, J.; Jalal, D.; Thurman, J.M. Complement fragments are biomarkers of antibody-mediated endothelial injury. Mol. Immunol. 2020, 118, 142–152. [Google Scholar] [CrossRef]
- Mueller, F.B.; Yang, H.; Lubetzky, M.; Verma, A.; Lee, J.R.; Dadhania, D.M.; Xiang, J.Z.; Salvatore, S.P.; Seshan, S.V.; Sharma, V.K.; et al. Landscape of innate immune system transcriptome and acute T cell-mediated rejection of human kidney allografts. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Hricik, D.E.; Nickerson, P.; Formica, R.N.; Poggio, E.D.; Rush, D.; Newell, K.A.; Goebel, J.; Gibson, I.W.; Fairchild, R.L.; Riggs, M.; et al. Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am. J. Transplant. 2013, 13, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Rabant, M.; Amrouche, L.; Morin, L.; Bonifay, R.; Lebreton, X.; Aouni, L.; Benon, A.; Sauvaget, V.; Le Vaillant, L.; Aulagnon, F.; et al. Early Low Urinary CXCL9 and CXCL10 Might Predict Immunological Quiescence in Clinically and Histologically Stable Kidney Recipients. Am. J. Transplant. 2016, 16, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Faddoul, G.; Nadkarni, G.N.; Bridges, N.D.; Goebel, J.; Hricik, D.E.; Formica, R.; Menon, M.C.; Morrison, Y.; Murphy, B.; Newell, K.; et al. Analysis of Biomarkers within the Initial 2 Years Posttransplant and 5-Year Kidney Transplant Outcomes: Results from Clinical Trials in Organ Transplantation-17. Transplantation 2018, 102, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Mühlbacher, J.; Doberer, K.; Kozakowski, N.; Regele, H.; Camovic, S.; Haindl, S.; Bond, G.; Haslacher, H.; Eskandary, F.; Reeve, J.; et al. Non-invasive Chemokine Detection: Improved Prediction of Antibody-Mediated Rejection in Donor-Specific Antibody-Positive Renal Allograft Recipients. Front. Med. 2020, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Rabant, M.; Amrouche, L.; Lebreton, X.; Aulagnon, F.; Benon, A.; Sauvaget, V.; Bonifay, R.; Morin, L.; Scemla, A.; Delville, M.; et al. Urinary C-X-C Motif Chemokine 10 Independently Improves the Noninvasive Diagnosis of Antibody-Mediated Kidney Allograft Rejection. J. Am. Soc. Nephrol. 2015, 26, 2840–2851. [Google Scholar] [CrossRef]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef]
- Huang, E.; Sethi, S.; Peng, A.; Najjar, R.; Mirocha, J.; Haas, M.; Vo, A.; Jordan, S.C. Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am. J. Transplant. 2019, 19, 1663–1670. [Google Scholar] [CrossRef]
- Sigdel, T.K.; Archila, F.A.; Constantin, T.; Prins, S.A.; Liberto, J.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.P.; et al. Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR. J. Clin. Med. 2018, 8, 19. [Google Scholar] [CrossRef]
- Oellerich, M.; Shipkova, M.; Asendorf, T.; Walson, P.D.; Schauerte, V.; Mettenmeyer, N.; Kabakchiev, M.; Hasche, G.; Gröne, H.-J.; Friede, T.; et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am. J. Transplant. 2019, 19, 3087–3099. [Google Scholar] [CrossRef]
- Jordan, S.C.; Bunnapradist, S.; Bromberg, J.S.; Langone, A.J.; Hiller, D.; Yee, J.P.; Sninsky, J.J.; Woodward, R.N.; Matas, A.J. Donor-derived Cell-free DNA Identifies Antibody-mediated Rejection in Donor Specific Antibody Positive Kidney Transplant Recipients. Transplant. Direct 2018, 4, e379. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, K.K.; Lyons, S.; Chopra, B. Letter to the Editors. Impact of kidney transplant type and previous transplant on baseline donor-derived cell free DNA. Transpl. Int. 2020. [Google Scholar] [CrossRef]
- Jordan, S.C.; Sawinsky, D.; Dholakia, S. Donor-derived cell-free DNA initiates De-Novo Donor Specific Antibody (DSA) responses. Am. J. Transplant. 2019, 19, 404–405. [Google Scholar]
- Crespo, E.; Cravedi, P.; Martorell, J.; Luque, S.; Melilli, E.; Cruzado, J.M.; Jarque, M.; Meneghini, M.; Manonelles, A.; Donadei, C.; et al. Posttransplant peripheral blood donor-specific interferon-γ enzyme-linked immune spot assay differentiates risk of subclinical rejection and de novo donor-specific alloantibodies in kidney transplant recipients. Kidney Int. 2017, 92, 201–213. [Google Scholar] [CrossRef]
- Dholakia, S.; De Vlaminck, I.; Khush, K.K. Adding Insult on Injury: Immunogenic Role for Donor-derived Cell-free DNA? Transplantation 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Moon, H.; Lee, Y.H.; Seo, J.-W.; Kim, Y.G.; Moon, J.-Y.; Kim, J.S.; Jeong, K.-H.; Lee, T.W.; Ihm, C.-G.; et al. Clinical relevance of cell-free mitochondrial DNA during the early postoperative period in kidney transplant recipients. Sci. Rep. 2019, 9, 18607. [Google Scholar] [CrossRef] [PubMed]
- Karahan, G.E.; de Vaal, Y.J.H.; Krop, J.; Wehmeier, C.; Roelen, D.L.; Claas, F.H.J.; Heidt, S. A Memory B Cell Crossmatch Assay for Quantification of Donor-Specific Memory B Cells in the Peripheral Blood of HLA-Immunized Individuals. Am. J. Transplant. 2017, 17, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Pongpirul, W.; Chancharoenthana, W.; Pongpirul, K.; Leelahavanichkul, A.; Kittikowit, W.; Jutivorakool, K.; Nonthasoot, B.; Avihingsanon, Y.; Eiam-Ong, S.; Praditpornsilpa, K.; et al. B-cell activating factor, a predictor of antibody mediated rejection in kidney transplantation recipients. Nephrol. Carlton 2018, 23, 169–174. [Google Scholar] [CrossRef]
- Hricik, D.E.; Augustine, J.; Nickerson, P.; Formica, R.N.; Poggio, E.D.; Rush, D.; Newell, K.A.; Goebel, J.; Gibson, I.W.; Fairchild, R.L.; et al. Interferon Gamma ELISPOT Testing as a Risk-Stratifying Biomarker for Kidney Transplant Injury: Results from the CTOT-01 Multicenter Study. Am. J. Transplant. 2015, 15, 3166–3173. [Google Scholar] [CrossRef]
- Gorbacheva, V.; Fan, R.; Fairchild, R.L.; Baldwin, W.M.; Valujskikh, A. Memory CD4 T Cells Induce Antibody-Mediated Rejection of Renal Allografts. J. Am. Soc. Nephrol. 2016, 27, 3299–3307. [Google Scholar] [CrossRef]
- Matz, M.; Fabritius, K.; Lorkowski, C.; Dürr, M.; Gaedeke, J.; Durek, P.; Grün, J.R.; Goestemeyer, A.; Bachmann, F.; Wu, K.; et al. Identification of T Cell-Mediated Vascular Rejection after Kidney Transplantation by the Combined Measurement of 5 Specific MicroRNAs in Blood. Transplantation 2016, 100, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Trailin, A.V.; Ostapenko, T.I.; Nykonenko, T.N.; Nesterenko, S.N.; Nykonenko, O.S. Peritransplant Soluble CD30 as a Risk Factor for Slow Kidney Allograft Function, Early Acute Rejection, Worse Long-Term Allograft Function, and Patients’ Survival. Dis. Markers 2017, 2017, 9264904. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, M.; Shahbazi, M.; Akbari, R.; Dedinská, I.; Nemati, E.; Mohammadnia-Afrouzi, M. Soluble CD30, the Immune Response, and Acute Rejection in Human Kidney Transplantation: A Systematic Review and Meta-Analysis. Front. Immunol. 2020, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, C.; Shapiro, R.; Tan, H.; Ningappa, M.; Elinoff, B.; Fedorek, S.; Sun, Q.; Higgs, B.W.; Randhawa, P.; Humar, A.; et al. Allospecific CD154+ T-cytotoxic memory cells identify recipients experiencing acute cellular rejection after renal transplantation. Transplantation 2011, 92, 433–438. [Google Scholar] [CrossRef]
- Verma, A.; Muthukumar, T.; Yang, H.; Lubetzky, M.; Cassidy, M.F.; Lee, J.R.; Dadhania, D.M.; Snopkowski, C.; Shankaranarayanan, D.; Salvatore, S.P.; et al. Urinary cell transcriptomics and acute rejection in human kidney allografts. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Ding, R.; Sharma, V.K.; Schwartz, J.E.; Suthanthiran, M. Urinary cell mRNA profiles predictive of human kidney allograft status. Immunol. Rev. 2014, 258, 218–240. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Zhang, W.; Menon, M.C.; Yi, Z.; Schröppel, B.; Gallon, L.; Luan, Y.; Rosales, I.A.; Ge, Y.; Losic, B.; et al. Biopsy transcriptome expression profiling to identify kidney transplants at risk of chronic injury: A multicentre, prospective study. Lancet Lond. Engl. 2016, 388, 983–993. [Google Scholar] [CrossRef]
- Eikmans, M.; Gielis, E.M.; Ledeganck, K.J.; Yang, J.; Abramowicz, D.; Claas, F.F.J. Non-invasive Biomarkers of Acute Rejection in Kidney Transplantation: Novel Targets and Strategies. Front. Med. 2018, 5, 358. [Google Scholar] [CrossRef]
- Jeon, H.J.; Lee, J.-G.; Kim, K.; Jang, J.Y.; Han, S.W.; Choi, J.; Ryu, J.-H.; Koo, T.Y.; Jeong, J.C.; Lee, J.W.; et al. Peripheral blood transcriptome analysis and development of classification model for diagnosing antibody-mediated rejection vs accommodation in ABO-incompatible kidney transplant. Am. J. Transplant. 2020, 20, 112–124. [Google Scholar] [CrossRef]
- Marsh, C.L.; Kurian, S.M.; Rice, J.C.; Whisenant, T.C.; David, J.; Rose, S.; Schieve, C.; Lee, D.; Case, J.; Barrick, B.; et al. Application of TruGraf v1: A Novel Molecular Biomarker for Managing Kidney Transplant Recipients with Stable Renal Function. Transplant. Proc. 2019, 51, 722–728. [Google Scholar] [CrossRef]
- Peddi, V.R.; Patel, P.S.; Schieve, C.; Rose, S.; First, M.R. Serial Peripheral Blood Gene Expression Profiling to Assess Immune Quiescence in Kidney Transplant Recipients with Stable Renal Function. Ann. Transplant. 2020, 25, e920839. [Google Scholar] [CrossRef]
- Hruba, P.; Krejcik, Z.; Stranecky, V.; Maluskova, J.; Slatinska, J.; Gueler, F.; Gwinner, W.; Bräsen, J.H.; Wohlfahrtova, M.; Parikova, A.; et al. Molecular Patterns Discriminate Accommodation and Subclinical Antibody-mediated Rejection in Kidney Transplantation. Transplantation 2019, 103, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F.; Reeve, J.; Akalin, E.; Aubert, O.; Bohmig, G.A.; Brennan, D.; Bromberg, J.; Einecke, G.; Eskandary, F.; Gosset, C.; et al. Real Time Central Assessment of Kidney Transplant Indication Biopsies by Microarrays: The INTERCOMEX Study. Am. J. Transplant. 2017, 17, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Barner, M.; DeKoning, J.; Kashi, Z.; Halloran, P. Recent Advancements in the Assessment of Renal Transplant Dysfunction with an Emphasis on Microarray Molecular Diagnostics. Clin. Lab. Med. 2018, 38, 623–635. [Google Scholar] [CrossRef]
- Wu, H.; Malone, A.F.; Donnelly, E.L.; Kirita, Y.; Uchimura, K.; Ramakrishnan, S.M.; Gaut, J.P.; Humphreys, B.D. Single-Cell Transcriptomics of a Human Kidney Allograft Biopsy Specimen Defines a Diverse Inflammatory Response. J. Am. Soc. Nephrol. 2018, 29, 2069–2080. [Google Scholar] [CrossRef]
- Stewart, B.J.; Clatworthy, M.R. Applying single-cell technologies to clinical pathology: Progress in nephropathology. J. Pathol. 2020, 250, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Stasi, A.; Fiorentino, M.; Stallone, G.; Cantaluppi, V.; Gesualdo, L.; Castellano, G. Inflammaging and Complement System: A Link between Acute Kidney Injury and Chronic Graft Damage. Front. Immunol. 2020, 11, 734. [Google Scholar] [CrossRef]
- Bobka, S.; Ebert, N.; Koertvely, E.; Jacobi, J.; Wiesener, M.; Büttner-Herold, M.; Amann, K.; Daniel, C. Is Early Complement Activation in Renal Transplantation Associated with Later Graft Outcome? Kidney Blood Press. Res. 2018, 43, 1488–1504. [Google Scholar] [CrossRef]
- Tower, C.M.; Reyes, M.; Nelson, K.; Leca, N.; Kieran, N.; Muczynski, K.; Jefferson, J.A.; Blosser, C.; Kukla, A.; Maurer, D.; et al. Plasma C4d+ Endothelial Microvesicles Increase in Acute Antibody-Mediated Rejection. Transplantation 2017, 101, 2235–2243. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Guo, M.; Han, Z.; Tao, J.; Chen, H.; Ge, Y.; Wang, K.; Tan, R.; Wei, J.-F.; et al. Impact of complement component 3/4/5 single nucleotide polymorphisms on renal transplant recipients with antibody-mediated rejection. Oncotarget 2017, 8, 94539–94553. [Google Scholar] [CrossRef]
- Lazzeri, E.; Rotondi, M.; Mazzinghi, B.; Lasagni, L.; Buonamano, A.; Rosati, A.; Pradella, F.; Fossombroni, V.; La Villa, G.; Gacci, M.; et al. High CXCL10 expression in rejected kidneys and predictive role of pretransplant serum CXCL10 for acute rejection and chronic allograft nephropathy. Transplantation 2005, 79, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Netti, G.S.; Lazzeri, E.; Stallone, G.; Bertoni, E.; Chiovato, L.; Grandaliano, G.; Gesualdo, L.; Salvadori, M.; Schena, F.P.; et al. High pretransplant serum levels of CXCL9 are associated with increased risk of acute rejection and graft failure in kidney graft recipients. Transpl. Int. 2010, 23, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Hirt-Minkowski, P.; De Serres, S.A.; Ho, J. Developing renal allograft surveillance strategies—Urinary biomarkers of cellular rejection. Can. J. Kidney Health Dis. 2015, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Wiehler, F.; Bräsen, J.H.; Gwinner, W.; Greite, R.; Kreimann, K.; Thorenz, A.; Derlin, K.; Teng, B.; Rong, S.; et al. Chemokine CXCL13 as a New Systemic Biomarker for B-Cell Involvement in Acute T Cell-Mediated Kidney Allograft Rejection. Int. J. Mol. Sci. 2019, 20, 2552. [Google Scholar] [CrossRef]
- Katou, S.; Globke, B.; Morgul, M.H.; Vogel, T.; Struecker, B.; Otto, N.M.; Reutzel-Selke, A.; Marksteiner, M.; Brockmann, J.G.; Pascher, A.; et al. Urinary Biomarkers α-GST and π-GST for Evaluation and Monitoring in Living and Deceased Donor Kidney Grafts. J. Clin. Med. 2019, 8, 1899. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, B.K.; Gwon, M.-R.; Seong, S.J.; Ohk, B.; Kang, W.Y.; Lee, H.W.; Jung, H.-Y.; Cho, J.-H.; Chung, B.H.; et al. Urinary metabolomic profiling for noninvasive diagnosis of acute T cell-mediated rejection after kidney transplantation. J. Chromatogr. B 2019, 1118, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhou, Y.; Chen, Y.; Li, X.; Lei, W.; Ge, J.; Peng, W.; Wu, J.; Liu, G.; Yang, G.; et al. Dynamics of early post-operative plasma ddcf DNA levels in kidney transplantation: A single-center pilot study. Transpl. Int. 2019, 32, 184–192. [Google Scholar] [CrossRef]
- Bloom, R.D. Using (cell-free) DNA to incriminate rejection as the cause of kidney allograft dysfunction: Do we have a verdict? Am. J. Transplant. 2019, 19, 1609–1610. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Vaitla, P.; Craici, I.M.; Leeaphorn, N.; Hansrivijit, P.; Salim, S.A.; Bathini, T.; Cabeza Rivera, F.H.; Cheungpasitporn, W. The Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Rejection and Injury Status. J. Clin. Med. 2020, 9, 1480. [Google Scholar] [CrossRef]
- Stites, E.; Kumar, D.; Olaitan, O.; Swanson, S.H.; Leca, N.; Weir, M.; Bromberg, J.; Melancon, J.; Agha, I.; Fattah, H.; et al. High levels of dd-cf DNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am. J. Transplant. 2020. [Google Scholar] [CrossRef]
- Oweira, H.; Khajeh, E.; Mohammadi, S.; Ghamarnejad, O.; Daniel, V.; Schnitzler, P.; Golriz, M.; Mieth, M.; Morath, C.; Zeier, M.; et al. Pre-transplant CD200 and CD200R1 concentrations are associated with post-transplant events in kidney transplant recipients. Med. Baltim. 2019, 98, e17006. [Google Scholar] [CrossRef] [PubMed]
- Lemerle, M.; Garnier, A.-S.; Planchais, M.; Brilland, B.; Subra, J.-F.; Blanchet, O.; Blanchard, S.; Croue, A.; Duveau, A.; Augusto, J.-F. CD45RC Expression of Circulating CD8(+) T Cells Predicts Acute Allograft Rejection: A Cohort Study of 128 Kidney Transplant Patients. J. Clin. Med. 2019, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2018, 13, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wehmeier, C.; Karahan, G.E.; Heidt, S. HLA-specific memory B-cell detection in kidney transplantation: Insights and future challenges. Int. J. Immunogenet. 2020, 47, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reinsmoen, N.L. Impact and production of Non-HLA-specific antibodies in solid organ transplantation. Int. J. Immunogenet. 2020, 47, 235–242. [Google Scholar] [CrossRef]
- Reindl-Schwaighofer, R.; Heinzel, A.; Gualdoni, G.A.; Mesnard., L.; Claas, F.H.J.; Oberbauer, R. Novel insights into non-HLA alloimmunity in kidney transplantation. Transpl. Int. 2020, 33, 5–17. [Google Scholar] [CrossRef]
- Delville, M.; Lamarthée, B. Early Acute Microvascular Kidney Transplant Rejection in the Absence of Anti-HLA Antibodies Is Associated with Preformed IgG Antibodies against Diverse Glomerular Endothelial Cell Antigens. J. Am. Soc. Nephrol. 2019, 30, 692–709. [Google Scholar] [CrossRef]
- Cardinal, H.; Dieudé, M.; Hébert, M.J. The Emerging Importance of Non-HLA Autoantibodies in Kidney Transplant Complications. J. Am. Soc. Nephrol. 2017, 28, 400–406. [Google Scholar] [CrossRef]
- Dragun, D.; Catar, R.; Aurelie, P. Non-HLA antibodies against endothelial targets bridging allo-and autoimmunity. Kidney Int. 2016, 90, 280–288. [Google Scholar] [CrossRef]
- Philogene, M.C.; Johnson, T.; Vaught, A.J.; Zakaria, S.; Fedarko., N. Antibodies against Angiotensin II Type 1 and Endothelin A Receptors: Relevance and pathogenicity. Hum. Immunol. 2019, 80, 561–567. [Google Scholar] [CrossRef]
- Sas-Strózik, A.; Krajewska, M.; Banasik, M. The significance of angiotensin II type 1 receptor (AT1 receptor) in renal transplant injury. Adv. Clin. Exp. Med. 2020, 29, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Shinaeu, Y.; Hee, J.H.; Kyo, W.L.; Park, J.B.; Kim, S.; Huh, W.; Jang, H.R.; Kwon, G.Y.; Moon, H.H.; Kang, S. Pre-Transplant Angiotensin II Type 1 Receptor Antibodies and Anti-Endothelial Cell Antibodies Predict Graft Function and Allograft Rejection in a Low-Risk Kidney Transplantation Setting. Ann. Lab. Med. 2020, 40, 398–408. [Google Scholar] [CrossRef]
- Pinelli, D.F.; Friedewald, J.J. Assessing the potential of angiotensin II type 1 receptor and donor specific anti-endothelial cell antibodies to predict long-term kidney graft outcome. Hum. Immunol. 2017, 78, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Philogene, M.C.; Zhou, S. Pre-transplant Screening for Non-HLA Antibodies: Who should be Tested? Hum. Immunol. 2018, 79, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.A.; Palmer, M.; Trofe-Clark, J.; Bloom, R.D.; Jackson, A.; Philogene, M.C.; Kamoun, M. Histopathologic changes in anti-angiotensin II type 1 receptor antibody-positive kidney transplant recipients with acute rejection and no donor specific HLA antibodies. Hum. Immunol. 2017, 78, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Divanyan, T.; Acosta, E.; Patel, D.; Constantino, D.; Lopez-Soler, R.I. Anti-vimentin antibodies in transplant and disease. Hum. Immunol. 2019, 80, 602–607. [Google Scholar] [CrossRef]
- Dieudé, M.; Cardinal, H.; Hébert, M.-J. Injury derived autoimmunity: Anti-perlecan/LG3 antibodies in transplantation. Hum. Immunol. 2019, 80, 608–613. [Google Scholar] [CrossRef]
- Riesco, L.; Irure, J.; Rodrigo, E.; Guiral, S.; Ruiz, J.C.; Gómez, J.; Hoyos, M.L.; San Segundo, D. Anti-perlecan antibodies and acute humoral rejection in hypersensitized patients without forbidden HLA specificities after kidney transplantation. Transpl. Immunol. 2019, 52, 53–56. [Google Scholar] [CrossRef]
- Padet, L.; Dieudé, M.; Karakeussian-Rimbaud, A.; Yang, B.; Turgeon, J.; Jean-Cailhier, F.; Cardinal, H.; Hébert, M.-J. New insights into immune mechanisms of antiperlecan/LG3 antibody production: Importance of T cells and innate B1 cells. Am. J. Transplant. 2019, 19, 699–712. [Google Scholar] [CrossRef]
- Yang, B.; Dieudé, M.; Hamelin, K.; Hénault-Rondeau, M.; Patey, N.; Turgeon, J.; Lan, S.; Pomerleau, L.; Quesnel, M.; Peng, J.; et al. Anti-LG3 Antibodies Aggravate Renal Ischemia-Reperfusion Injury and Long-Term Renal Allograft Dysfunction. Am. J. Transplant. 2016, 16, 3416–3429. [Google Scholar] [CrossRef]
- Jackson, A.M.; Sigdel, T.K.; Delville, M.; Hsieh, S.; Dai, H.; Bagnasco, S.; Montgomery, R.A.; Sarwal, M.M. Endothelial cell antibodies associated with novel targets and increased rejection. J. Am. Soc. Nephrol. 2015, 26, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, J.; Luo, W.; Luo, Q.; Guo, J.; Tian, F.; Ming, Y.; Zou, Y. Analysis of Sera of Recipients with Allograft Rejection Indicates that Keratin 1 Is the Target of Anti-Endothelial Antibodies. Immunol. Res. 2017, 2017, 8679841. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zapardiel, E.; Mancebo, E.; Díaz-Ordoñez, M.; de Jorge-Huerta, L.; Ruiz-Martínez, L.; Serrano, A.; Castro-Panete, M.J.; Utrero-Rico, A.; de Andrés, A.; Morales, J.M.; et al. Isolated de Novo Antiendothelial Cell Antibodies and Kidney Transplant Rejection. Am. J. Kidney Dis. 2016, 68, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Delville, M.; Lamarthée, B.; Anglicheau, D. Sensitization to endothelial cell antigens: Unraveling the cause or effect paradox. Hum. Immunol. 2019, 80, 614–620. [Google Scholar] [CrossRef]
- Angaswamy, N.; Klein, C.; Tiriveedhi, V.; Gaut, J.; Anwar, S.; Rossi, A.; Phelan, D.; Wellen, J.R.; Shenoy, S.; Chapman, W.C.; et al. Immune responses to collagen-IV and fibronectin in renal transplant recipients with transplant glomerulopathy. Am. J. Transplant. 2014, 14, 685–693. [Google Scholar] [CrossRef]
- Gunasekaran, M.; Vachharajani, N.; Gaut, J.P.; Maw, T.T.; Delos Santos, R.; Shenoy, S.; Chapman, W.C.; Wellen, J.; Mohanakumar, T. Development of immune response to tissue-restricted self-antigens in simultaneous kidney-pancreas transplant recipients with acute rejection. Clin. Transplant. 2017, 31, 8. [Google Scholar] [CrossRef]
- Soma, O.; Hatakeyama, S.; Yoneyama, T.; Saito, M.; Sasaki, H.; Tobisawa, Y.; Noro, D.; Suzuki, Y.; Tanaka, M.; Nishimura, S.-I.; et al. Serum N-glycan profiling can predict biopsy-proven graft rejection after living kidney transplantation. Clin. Exp. Nephrol. 2020, 24, 174–184. [Google Scholar] [CrossRef]
- Maehana, T.; Tanaka, T.; Kitamura, H.; Fukuzawa, N.; Ishida, H.; Harada, H.; Tanabe, K.; Masumori, N. Heat Shock Protein 90α Is a Potential Serological Biomarker of Acute Rejection after Renal Transplantation. PLoS ONE 2016, 11, e0162942. [Google Scholar] [CrossRef]
- Barbas, A.S.; Lin, L.; McRae, M.; MacDonald, A.L.; Truong, T.; Yang, Y.; Brennan, T.V. Heparan sulfate is a plasma biomarker of acute cellular allograft rejection. PLoS ONE 2018, 13, e0200877. [Google Scholar] [CrossRef]
- Kim, S.C.; Page, E.K.; Knechtle, S.J. Urine proteomics in kidney transplantation. Transplant. Rev. 2014, 28, 15–20. [Google Scholar] [CrossRef]
- Perez, J.D.; Sakata, M.M.; Colucci, J.A.; Spinelli, G.A.; Felipe, C.R.; Carvalho, V.M.; Cardozo, K.H.M.; Medina-Pestana, J.O.; Tedesco-Silva, H.J.; Schor, N.; et al. Plasma proteomics for the assessment of acute renal transplant rejection. Life Sci. 2016, 158, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, E.; Kahwaji, J.; Nast, C.C.; Li, P.; Mirocha, J.; Thomas, D.L.; Ge, S.; Vo, A.A.; Jordan, S.C.; et al. Plasma Exosomes from HLA-Sensitized Kidney Transplant Recipients Contain mRNA Transcripts Which Predict Development of Antibody-Mediated Rejection. Transplantation 2017, 101, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Qamri, Z.; Pelletier, R.; Foster, J.; Kumar, S.; Momani, H.; Ware, K.; Von Visger, J.; Satoskar, A.; Nadasdy, T.; Brodsky, S.V. Early posttransplant changes in circulating endothelial microparticles in patients with kidney transplantation. Transpl. Immunol. 2014, 31, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.K.; Ng, Y.W.; Lee, S.; Nicora, C.D.; Qian, W.-J.; Smith, R.D.; Camp, D.G., 2nd; Sarwal, M.M. Perturbations in the urinary exosome in transplant rejection. Front. Med. 2014, 1, 57. [Google Scholar] [CrossRef]
- Lim, J.-H.; Lee, C.-H.; Kim, K.Y.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Baek, M.-C.; Park, J.B.; et al. Novel urinary exosomal biomarkers of acute T cell-mediated rejection in kidney transplant recipients: A cross-sectional study. PLoS ONE 2018, 13, e0204204. [Google Scholar] [CrossRef]
- Park, J.; Lin, H.-Y.; Assaker, J.P.; Jeong, S.; Huang, C.-H.; Kurdi, A.; Lee, K.; Fraser, K.; Min, C.; Eskandari, S.; et al. Integrated Kidney Exosome Analysis for the Detection of Kidney Transplant Rejection. ACS Nano 2017, 11, 11041–11046. [Google Scholar] [CrossRef]
- Yang, C.; Qi, R.; Yang, B. Pathogenesis of Chronic Allograft Dysfunction Progress to Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 101–116. [Google Scholar] [CrossRef]
- Sellarés, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef]
- Castellano, G.; Intini, A.; Stasi, A.; Divella, C.; Gigante, M.; Pontrelli, P.; Franzin, R.; Accetturo, M.; Zito, A.; Fiorentino, M.; et al. Complement Modulation of Anti-Aging Factor Klotho in Ischemia/Reperfusion Injury and Delayed Graft Function. Am. J. Transplant. 2016, 16, 325–333. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Yiu, W.H.; Li, R.X.; Wong, D.W.L.; Wu, H.J.; Chan, K.W.; Chan, L.Y.Y.; Leung, J.C.K.; Lai, K.N.; Sacks, S.H.; Zhou, W.; et al. Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol. Dial. Transplant. 2018, 33, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lu, B.; Hatch, E.; Sacks, S.H.; Sheerin, N.S. C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J. Am. Soc. Nephrol. 2009, 20, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Xavier, S.; Sahu, R.K.; Landes, S.G.; Yu, J.; Taylor, R.P.; Ayyadevara, S.; Megyesi, J.; Stallcup, W.B.; Duffield, J.S.; Reis, E.S.; et al. Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am. J. Physiol. Ren. Physiol. 2017, 312, F516–F532. [Google Scholar] [CrossRef]
- Modena, B.D.; Kurian, S.M.; Gaber, L.W.; Waalen, J.; Su, A.I.; Gelbart, T.; Mondala, T.S.; Head, S.R.; Papp, S.; Heilman, R.; et al. Gene Expression in Biopsies of Acute Rejection and Interstitial Fibrosis/Tubular Atrophy Reveals Highly Shared Mechanisms That Correlate with Worse Long-Term Outcomes. Am. J. Transplant. 2016, 16, 1982–1998. [Google Scholar] [CrossRef] [PubMed]
- Mas, V.; Maluf, D.; Archer, K.; Yanek, K.; Mas, L.; King, A.; Gibney, E.; Massey, D.; Cotterell, A.; Fisher, R.; et al. Establishing the molecular pathways involved in chronic allograft nephropathy for testing new noninvasive diagnostic markers. Transplantation 2007, 83, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Greene, I.; Readhead, B.; Menon, M.C.; Kidd, B.A.; Uzilov, A.V.; Wei, C.; Philippe, N.; Schroppel, B.; He, J.C.; et al. Novel Therapeutics Identification for Fibrosis in Renal Allograft Using Integrative Informatics Approach. Sci. Rep. 2017, 7, 39487. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Omidi, Y.; Ardalan, M.; Samadi, N. Dysregulation of urinary miR-21 and miR-200b associated with interstitial fibrosis and tubular atrophy (IFTA) in renal transplant recipients. Clin. Biochem. 2017, 50, 32–39. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Poursadegh Zonouzi, A.; Mahmoodpoor, F.; Samadi, N.; Ardalan, M.; Omidi, Y. Circulating miR-150, miR-192, miR-200b, and miR-423-3p as Non-invasive Biomarkers of Chronic Allograft Dysfunction. Arch. Med. Res. 2017, 48, 96–104. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Poursadegh Zonouzi, A.; Ghanbarian, H.; Ghojazadeh, M.; Samadi, N.; Omidi, Y.; Ardalan, M. Differential expression of circulating miR-21, miR-142-3p and miR-155 in renal transplant recipients with impaired graft function. Int. Urol. Nephrol. 2017, 49, 1681–1689. [Google Scholar] [CrossRef]
- Matz, M.; Heinrich, F.; Lorkowski, C.; Wu, K.; Klotsche, J.; Zhang, Q.; Lachmann, N.; Durek, P.; Budde, K.; Mashreghi, M.-F. MicroRNA regulation in blood cells of renal transplanted patients with interstitial fibrosis/tubular atrophy and antibody-mediated rejection. PLoS ONE 2018, 13, e0201925. [Google Scholar] [CrossRef]
- Granata, S.; Benedetti, C.; Gambaro, G.; Zaza, G. Kidney allograft fibrosis: What we learned from latest translational research studies. J. Nephrol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.J.; Helgeson, E.S.; Gaston, R.; Cosio, F.; Mannon, R.; Kasiske, B.L.; Hunsicker, L.; Gourishankar, S.; Rush, D.; Michael Cecka, J.; et al. Inflammation in areas of fibrosis: The DeKAF prospective cohort. Am. J. Transplant. 2020. [Google Scholar] [CrossRef] [PubMed]
- Maluf, D.G.; Dumur, C.I.; Suh, J.L.; Scian, M.J.; King, A.L.; Cathro, H.; Lee, J.K.; Gehrau, R.C.; Brayman, K.L.; Gallon, L.; et al. The urine microRNA profile may help monitor post-transplant renal graft function. Kidney Int. 2014, 85, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Bontha, S.V.; Maluf, D.G.; Archer, K.J.; Dumur, C.I.; Dozmorov, M.G.; King, A.L.; Akalin, E.; Mueller, T.F.; Gallon, L.; Mas, V.R. Effects of DNA Methylation on Progression to Interstitial Fibrosis and Tubular Atrophy in Renal Allograft Biopsies: A Multi-Omics Approach. Am. J. Transplant. 2017, 17, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.M.; Hedayat, A.F.; Kanasaki, K.; Goodwin, G.E. MicroRNA Crosstalk Influences Epithelial-to-Mesenchymal, Endothelial-to-Mesenchymal, and Macrophage-to-Mesenchymal Transitions in the Kidney. Front. Pharmacol. 2019, 10, 904. [Google Scholar] [CrossRef]
- Alfieri, C.; Regalia, A.; Moroni, G.; Cresseri, D.; Zanoni, F.; Ikehata, M.; Simonini, P.; Rastaldi, M.P.; Tripepi, G.; Zoccali., C.; et al. Novel markers of graft outcome in a cohort of kidney transplanted patients: A cohort observational study. J. Nephrol. 2019, 32, 139–150. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, H.; Wang, Z.; Chen, H.; Suo, C.; Zhang, H.; Zhang, J.; Yang, Y.; Geng, L.; Gu, M.; et al. Bortezomib attenuates renal interstitial fibrosis in kidney transplantation via regulating the EMT induced by TNF-α-Smurf1-Akt-mTOR-P70S6K pathway. J. Cell. Mol. Med. 2019, 23, 5390–5402. [Google Scholar] [CrossRef]
- Hazzan, M.; Hertig, A.; Buob, D.; Copin, M.-C.; Noël, C.; Rondeau, E.; XuDubois, Y.C. Epithelial-to-mesenchymal transition predicts cyclosporine nephrotoxicity in renal transplant recipients. J. Am. Soc. Nephrol. 2011, 22, 1375–1381. [Google Scholar] [CrossRef][Green Version]
- Sosa Peña, M.D.P.; Lopez-Soler, R.; Melendez, J.A. Senescence in chronic allograft nephropathy. Am. J. Physiol. Ren. Physiol. 2018, 315, F880–F889. [Google Scholar] [CrossRef]
- Mezni, I.; Galichon, P.; Bacha, M.M.; XuDubois, Y.C.; Sfar, I.; Buob, D.; Benbouzid, S.; Goucha, R.; Gorgi, Y.; Abderrhaim, E.; et al. Urinary mRNA analysis of biomarkers to epithelial mesenchymal transition of renal allograft. Nephrol. Ther. 2018, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Galichon, P.; XuDubois, Y.C.; Buob, D.; Tinel, C.; Anglicheau, D.; Benbouzid, S.; Dahan, K.; Ouali, N.; Hertig, A.; Brocheriou, I.; et al. Urinary transcriptomics reveals patterns associated with subclinical injury of the renal allograft. Biomark. Med. 2018, 12, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Xu-Dubois, Y.-C.; Peltier, J.; Brocheriou, I.; Suberbielle-Boissel, C.; Djamali, A.; Reese, S.; Mooney, N.; Keuylian, Z.; Lion, J.; Ouali, N.; et al. Markers of Endothelial-to-Mesenchymal Transition: Evidence for Antibody-Endothelium Interaction during Antibody-Mediated Rejection in Kidney Recipients. J. Am. Soc. Nephrol. 2016, 27, 324–332. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Z.; Tao, J.; Wang, J.; Liu, X.; Zhou, W.; Xu, Z.; Zhao, C.; Wang, Z.; Tan, R.; et al. Role of endothelial-to-mesenchymal transition induced by TGF-β1 in transplant kidney interstitial fibrosis. J. Cell. Mol. Med. 2017, 21, 2359–2369. [Google Scholar] [CrossRef]
- Glover, E.K.; Jordan, N.; Sheerin, N.S.; Ali, S. Regulation of Endothelial-to-Mesenchymal Transition by MicroRNAs in Chronic Allograft Dysfunction. Transplantation 2019, 103, e64–e73. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.; Peake, P.W.; Endre, Z.H. Biomarkers of calcineurin inhibitor nephrotoxicity in transplantation. Biomark. Med. 2014, 8, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Fu, S.; Wang, Q.; Wen, Y.; Chan, S.-A.; Zhu, S.; Gao, S.; Tao, X.; Zhang, F.; Chen, W. Targeted metabolomic analysis of 33 amino acids and biogenic amines in human urine by ion-pairing HPLC-MS/MS: Biomarkers for tacrolimus nephrotoxicity after renal transplantation. Biomed. Chromatogr. BMC 2018, 32, e4198. [Google Scholar] [CrossRef] [PubMed]
- Gacka, E.; Życzkowski, M.; Bogacki, R.; Paradysz, A.; Hyla-Klekot, L. The Usefulness of Determining Neutrophil Gelatinase-Associated Lipocalin Concentration Excreted in the Urine in the Evaluation of Cyclosporine A Nephrotoxicity in Children with Nephrotic Syndrome. Dis. Markers 2016, 2016, 6872149. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Q.; Qiu, X.; Xu, L.; Jiao, Z.; Zhang, M.; Zhong, M. FKBP1A rs6041749 polymorphism is associated with allograft function in renal transplant patients. Eur. J. Clin. Pharmacol. 2019, 75, 33–40. [Google Scholar] [CrossRef]
- Carlos, C.P.; Sonehara, N.M.; Oliani, S.M.; Burdmann, E.A. Predictive usefulness of urinary biomarkers for the identification of cyclosporine. PLoS ONE 2014, 9, e103660. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, Q.; Auman, J.T.; Knight, B.; Jin, X.; Blanchard, K.T.; Chou, J.; Jayadev, S.; Paules, R.S. Genomic-derived markers for early detection of calcineurin inhibitor immunosuppressant-mediated nephrotoxicity. Toxicol. Sci. 2011, 124, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Masutani, K. Viral infections directly involved in kidney allograft function. Nephrol. Carlton 2018, 23 (Suppl. 2), 31–37. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.H.; Seo, J.-W.; Moon, H.; Kim, J.S.; Kim, Y.G.; Jeong, K.-H.; Moon, J.-Y.; Lee, T.W.; Ihm, C.-G.; et al. Urinary exosomal viral microRNA as a marker of BK virus nephropathy in kidney transplant recipients. PLoS ONE 2017, 12, e0190068. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Schaub, S.; Wiebe, C.; Gao, A.; Wehmeier, C.; Koller, M.T.; Hirsch, H.H.; Hopfer, H.; Nickerson, P.; Hirt-Minkowski, P. Urinary CXCL10 Chemokine Is Associated with Alloimmune and Virus Compartment-Specific Renal Allograft Inflammation. Transplantation 2018, 102, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Dvir, R.; Paloschi, V.; Canducci, F.; Dell’Antonio, G.; Racca, S.; Caldara, R.; Pantaleo, G.; Clementi, M.; Secchi, A. IL28B rs12979860 genotype as a predictor marker of progression to BKVirus Associated nephropathy, after kidney transplantation. Sci. Rep. 2017, 7, 6746. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lyu, Z.; Adam, B.; Zeng, G.; Wang, Z.; Huang, Y.; Abedin, Z.; Randhawa, P. Polyomavirus BK Nephropathy-Associated Transcriptomic Signatures: A Critical Reevaluation. Transplant. Direct 2018, 4, e339. [Google Scholar] [CrossRef]
- Verhoeven, J.G.H.P.; Boer, K.; Van Schaik, R.H.N.; Manintveld, O.C.; Huibers, M.M.H.; Baan, C.C.; Hesselink, D.A. Liquid Biopsies to Monitor Solid Organ Transplant Function: A Review of New Biomarkers. Ther. Drug Monit. 2018, 40, 515–525. [Google Scholar] [CrossRef]
- Peeters, L.E.J.; Andrews, L.M.; Hesselink, D.A.; de Winter, B.C.M.; van Gelder, T. Personalized immunosuppression in elderly renal transplant recipients. Pharmacol. Res. 2018, 130, 303–307. [Google Scholar] [CrossRef]
- Newell, K.A.; Adams, A.B.; Turka, L.A. Biomarkers of operational tolerance following kidney transplantation—The immune tolerance network studies of spontaneously tolerant kidney transplant recipients. Hum. Immunol. 2018, 79, 380–387. [Google Scholar] [CrossRef]
- Massart, A.; Ghisdal, L.; Abramowicz, M.; Abramowicz, D. Operational tolerance in kidney transplantation and associated biomarkers. Clin. Exp. Immunol. 2017, 189, 138–157. [Google Scholar] [CrossRef]
- Newell, K.A.; Asare, A.; Kirk, A.D.; Gisler, T.D.; Bourcier, K.; Suthanthiran, M.; Burlingham, W.J.; Marks, W.H.; Sanz, I.; Lechler, R.I.; et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J. Clin. Investig. 2010, 120, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Danger, R.; Chesneau, M.; Paul, C.; Guérif, P.; Durand, M.; Newell, K.A.; Kanaparthi, S.; Turka, L.A.; Soulillou, J.-P.; Houlgatte, R.; et al. A composite score associated with spontaneous operational tolerance in kidney transplant recipients. Kidney Int. 2017, 91, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.M.; Whisenant, T.C.; Mathew, J.M.; Miller, J.; Leventhal, J.R. Transcriptomic studies in tolerance: Lessons learned and the path forward. Hum. Immunol. 2018, 79, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Girmanova, E.; Hruba, P.; Viklicky, O. Circulating biomarkers of tolerance. Transplant. Rev. 2015, 29, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Bontha, S.V.; Fernandez-Piñeros, A.; Maluf, D.G.; Mas, V.R. Messengers of tolerance. Hum. Immunol. 2018, 79, 362–372. [Google Scholar] [CrossRef]
- Niel, O.; Bastard, P. Artificial Intelligence in Nephrology: Core Concepts, Clinical Applications, and Perspectives. Am. J. Kidney Dis. 2019, 74, 803–810. [Google Scholar] [CrossRef]
- Briganti, G.; Le Moine, O. Artificial Intelligence in Medicine: Today and Tomorrow. Front. Med. 2020, 7, 27. [Google Scholar] [CrossRef]
- Hummel, A.D.; Maciel, R.F.; Sousa, F.S.; Cohrs, F.M.; Falcão, A.E.J.; Teixeira, F.; Baptista, R.; Mancini, F.; da Costa, T.M.; Alves, D.; et al. Artificial intelligence techniques: Predicting necessity for biopsy in renal transplant recipients suspected of acute cellular rejection or nephrotoxicity. Transplant. Proc. 2011, 43, 1343–1344. [Google Scholar] [CrossRef]
- Niel, O.; Bastard, P. Artificial intelligence improves estimation of tacrolimus area under the concentration over time curve in renal transplant recipients. Transpl. Int. 2018, 31, 940–941. [Google Scholar] [CrossRef]
- Aubert, O.; Higgins, S.; Bouatou, Y.; Yoo, D.; Raynaud, M.; Viglietti, D.; Rabant, M.; Hidalgo, L.; Glotz, D.; Legendre, C.; et al. Archetype Analysis Identifies Distinct Profiles in Renal Transplant Recipients with Transplant Glomerulopathy Associated with Allograft Survival. J. Am. Soc. Nephrol. 2019, 30, 625–639. [Google Scholar] [CrossRef]
| Type of Biomarker | Meaning in Renal Transplant |
|---|---|
| Susceptibility or risk biomarker | It estimates the risk of developing a condition (e.g., AR) in a stable graft without any clinical sign of dysfunction |
| Diagnostic biomarker | It identifies patients with a disease or a subset of it (e.g., AR type) |
| Prognostic biomarker | It estimates the likelihood of a clinical event or of disease progression, staging severity of disease (e.g., severe rejection with risk of graft loss) |
| Predictive biomarker | It estimates the likelihood of achieving a favorable response from a therapy (e.g., Eculizumab for complement-fixing DSA) |
| Monitoring biomarker | It is serially measured in order to detect a change in evolution of disease or signs of drug toxicity, or to detect exposure to immunosuppressive drugs (e.g., TAC levels) |
| Pharmacodynamic/response biomarker | It verifies that a biological response has occurred after a drug exposure (e.g., DSA MFI after treatment of ABMR) |
| Safety biomarker | It estimates presence and severity of drug-related toxicity (e.g., CNI nephrotoxicity) |
| Biomarker | Source | Main Features | Author |
|---|---|---|---|
| Mitochondrial DNA | Donor plasma | It predicts DGF in DCD donors | Han F. et al. [10] |
| Complement C5a | Donor urine | It predicts DGF | Schroppel B. et al. [11] |
| miRNA | Graft preservation fluid | Several miRNAs proposed as biomarkers of DGF; miR-505-3p validated in DCD grafts | Gomez-Dos-Santos V. et al. [12] Roest H. et al. [13] |
| LDH, NGAL and MMP-2 | Perfusate of machine-perfused kidneys | Different levels according to type of donor (DCD vs. DBD vs. LD), reflecting degree of IRI | Moser M. et al. [14] |
| Exosomal mRNA for NGAL and NGAL | Perfusate of machine-perfused kidneys | They predict DGF | Cappuccilli M. et al. [15] |
| πGST | Perfusate of machine-perfused kidneys | It predicts DGF | Hall I. et al. [16] |
| Furosemide stress test | --- | Clinical test: non-responsive patients are at increased risk of DGF in the following days | Udomkarnjananun S. et al. [17] |
| miR182-5p, miR-21-3p | Recipient’s serum and urine | They predict DGF | Wilflingseder J. et al. [18] |
| miR146a-5p | Recipient’s peripheral blood and renal tissue | Increased in both DGF and AR | Milhoransa P. et al. [19] |
| miR-9, miR-10a, miR-21, miR-29a, miR-221, miR-429 | Recipient’s urine (first 5 days after KTx) | This panel predicts DGF (validated in an independent cohort) | Khalid U. et al. [20] |
| NGAL | Recipient’s serum/plasma and urine (first days after KTx) | Both bNGAL and uNGAL predict DGF and 1-year graft function, but bNGAL is more accurate. Urine NGAL predicts DGF also in KTx from LD. | Cappuccilli M. et al. [15] Maier H. et al. [21] Ramirez-Sandoval J. et al. [22] Li Y. et al. [23] Sahraei Z. et al. [24] |
| Corin | Recipient’s plasma | It is reduced in DGF | Hu X. et al. [25] |
| TLR-4 surface expression | Recipient’s circulating monocytes | It is reduced in DGF and associated with poor graft function at follow-up | Zmonarski S. et al. [26] |
| Amylase | Recipient’s serum | It increases in DGF | Comai G. et al. [27] |
| Fascin and Vimentin | Graft biopsy in recipient | Expression of these EndMT biomarkers on microvasculature correlated with long-term graft function after DGF | Xu-Dubois Y-C. et al. [28] |
| Biomarker | Type of Rejection | Main Features | Author |
|---|---|---|---|
| Three-gene signature (CTOT 04 study) | TCMR | It increases up to 20 days before histological diagnosis | Suthanthiran M et al. [51] |
| Seven-gene signature (KALIBRE study) | TCMR | It increases 7 weeks before histological diagnosis and decreased after treatment | Christakoudi S et al. [52] |
| Seventeen-gene signature (GoCAR study) | TCMR | It identifies subclinical TCMR and correlates with long-term graft survival | Zhang W et al. [53] |
| Eight-gene signature | ABMR | It correlates with histological features of acute and chronic ABMR | Van Loon E et al. [54] |
| Panel of gene signature (CTOT 08 study) | TCMR and ABMR | It correlates with clinical and histological outcomes and with de novo DSA; useful to identify immunologically quiescent patients | Friedewald J et al. [55] |
| Nineteen-gene signature | TCMR and ABMR | It includes TCMR genes. Analysis performed on RNA extracted from archival fresh frozen paraffin-embedded renal biopsy tissue. | Sigdel T et al. [56] |
| kSORT (AART study) | TCMR and ABMR | Rejection predicted 3 months before histological diagnosis in 64% of patients with stable graft function. | Roedder S et al. [57] Zhang W et al. [53] |
| ENDATs | ABMR | Analysis of endothelial transcripts predicts ABMR with excellent accuracy (AUC = 0.92). | Sis B et al. [58] Adam B et al. [59] |
| Complement fragments | ABMR | Levels correlate with ABMR | Stites E et al. [60] |
| Innate immunity genes | TCMR | Unbiased transcriptome analysis identifies increased expression of innate immune system genes | Mueller F et al. [61] |
| CXCL9 | TCMR and ABMR | High NPP (99.3%): low levels at 6 months predict low risk of rejection until 24 months. Highly accurate for ABMR diagnosis when associated with DSA. | Hricik D et al. [62] Rabant M et al. [63] Faddoul G et al. [64] Mühlbacher J et al. [65] |
| CXCL10 | ABMR and mixed | High NPP (99%). It predicts rejection at 1 month post-KTx in stable graft. | Rabant M et al. [66] |
| dd-cfDNA | ABMR and TCMR | Due to elevated negative NPP, it could help rule out especially ABMR and play a role for surveillance after a rejection episode or in sensitized patients | Bloom R et al. [67,68,69,70,71,72] |
| Allogenic circulating B- and T-cell assays | ABMR and TCMR | Useful to predict subclinical forms of rejection and DSA | Hricik D et al. [73] Crespo E et al. [74] Gorbacheva V et al. [75] |
| Peripheral blood miRNAs | TCMR | miR-15b, miR-16, miR-103a, miR-106A, miR107 predict vascular TCMR | Matz M et al. [76] |
| Peritransplant soluble CD30 (sCD30) | TCMR | Strong association between sCD30 and TCMR | Trailin A et al. [77] Mirzakhani M et al. [78] |
| CD154-positive T cytotoxic memory cells | TCMR | Association with TCMR and its histological severity in steroid-free regimen | Ashokkumar C et al. [79] |
| CD 200 and CD200R1 | TCMR and ABMR | Increased pre-transplant CD200R1/CD200 ratio identifies recipients at increased risk of AR and worse renal function | Oweira H et al. [80] |
| CD45RC | TCMR | Pre-transplant expression of CD45RC on circulating CD8+ T predicts AR | Lemerle M et al. [81] |
| N-glycan | ABMR and TCMR | N-glycan levels (integrated within a clinical score) predict rejection-free survival in KTx from LD | Soma O et al. [82] |
| HSP-90 | ABMR and TCMR | It discriminates AR from other causes of graft dysfunction | Maehana T et al. [83] |
| Heparan Sulfate | TCMR | It predicts DGF | Barbas A et al. [84] |
| Biomarker | Main Features | Author |
|---|---|---|
| Set of genes related to fibrosis (i.e., TGFβ), extracellular matrix deposition and immune response | Upregulated in IFTA | Mas V et al. [155] |
| 4-gene urinary signature (mRNA for vimentin, NKCC2, E-cadherin, and 18S rRNA) | It predicts evolution of chronic rejection towards IFTA | Lee J. et al. [86] |
| 13-gene renal tissue signature (GoCAR study) | It predicts CAD at the 12th month even with normal histology at the 3rd month | O’Connell P. et al. [87] |
| 85-gene renal tissue signature | Associated with IFTA | Li L. et al. [156] |
| Urinary mi-R21 and mi-R200b | Increased expression predicts IFTA and CAD | Zununi V. et al. [157] |
| Plasmatic miR-150, miR-192, miR-200b, and miR-423-3p | Highly accurate in identifying IFTA (AUC = 0.87; sensitivity = 78%; specificity = 91%) | Zununi V. et al. [158] |
| Plasmatic miR-21, miR-142-3p, miR-155, and mi-R 21 | Upregulated in IFTA; mi-R 21 correlates with GFR | Zununi V. et al. [159] |
| miR-145-5p expression in blood cells | Downregulated in IFTA; It can discriminate it from acute and borderline rejection | Matz M. et al. [160] |
| Biomarker | Main Features | Author |
|---|---|---|
| CD45, VIM, and POSTN | They correlate to each other and with iIFTA and graft loss | Alfieri C et al. [167] |
| Smurf 1 | It is included in a pathway involved in EMT. Its inhibition by Bortezomib may mediate its anti-fibrotic effect. | Zhou J et al. [168] |
| VIM and β-catenin | Tubular expression correlates with IFTA and long-term eGFR decline | Hazzan M et al. [169] |
| Senescence biomarkers (e.g., p16INK4a) | They mark SASP, an inflammatory phenotype connected to EMT | Sosa Pena DPM et al. [170]. |
| VIM and CD45 relative to UPK mRNA | This ratio based on urinary mRNAs correlates with VIM expression in renal tissue and may detect EMT and early graft fibrogenesis | Mezni I et al. [171] |
| Urinary transcriptomic patterns | They are associated with pEMT and subclinical graft injury | Galichon P et al. [172] |
| Biomarker | Main Features | Author |
|---|---|---|
| Urinary exosomal bkv-miR-B1-5p and bkv-miR-B1-5p/miR-16 | Excellent diagnostic accuracy for PVAN | Kim M et al. [183] |
| Urinary CXCL10 | Associated with subclinical tubule-interstitial inflammation and viremia | Ho J et al. [184] |
| IL28B SNP C/T (rs12979860) | Associated with presence of PVAN in viremic patients | Dvir R et al. [185] |
| Biomarker Features | Comment |
|---|---|
| Non-invasive and easy to measure | Urine and blood biomarkers are easily available and can be serially measured, whereas renal tissue biomarkers require renal biopsy with inherent invasiveness and limits. Urine and blood biomarkers may be used when renal biopsy is contraindicated or reduce the need for repeated surveillance biopsies. |
| Short turn-around time | Results should be available within a time frame which allows rapid, potentially pre-emptive intervention (e.g., diagnosis of subclinical AR) |
| Easy to interpret | Results should be easy to interpret, and threshold values should be established to help transplant physician in clinical practice |
| Reproducible and standardized | Results should be validated in multiple independent cohorts with different features (e.g., elderly, or highly sensitized KTx recipients, or different ethnicity) and assay standardization of analytical process performed in order to minimize inter-laboratory and inter-platform variability |
| Accuracy (sensitivity and specificity) | Biomarker levels should strictly reflect a single specific pathological process, without being influenced by other causes of kidney damage (e.g., AR vs. CNI nephrotoxicity or vs. infections) |
| Good prognostic performance (PPV and NPV) | Acceptable PPP and NPP. In general, new biomarkers should be preferably tested in subsets of patients at different immunological risk, rather than on the transplant population as a whole, in order to improve their statistical performance (e.g., higher a priori chance of AR in highly sensitized KTx recipients improves PPV compared to standard recipients). |
| Proof of cause | Reduction of a biomarker level correlates with an improvement in the underlying pathological process assessed with current gold-standard (histological examination with renal biopsy) |
| Cost-effective | Results should improve clinical management and consequently impact long-term outcomes and related economic aspects, justifying biomarker costs (e.g., a biomarker which detects subclinical AR could improve treatment, prolong graft survival and reduce costs) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaglia, M.; Merlotti, G.; Guglielmetti, G.; Castellano, G.; Cantaluppi, V. Recent Advances on Biomarkers of Early and Late Kidney Graft Dysfunction. Int. J. Mol. Sci. 2020, 21, 5404. https://doi.org/10.3390/ijms21155404
Quaglia M, Merlotti G, Guglielmetti G, Castellano G, Cantaluppi V. Recent Advances on Biomarkers of Early and Late Kidney Graft Dysfunction. International Journal of Molecular Sciences. 2020; 21(15):5404. https://doi.org/10.3390/ijms21155404
Chicago/Turabian StyleQuaglia, Marco, Guido Merlotti, Gabriele Guglielmetti, Giuseppe Castellano, and Vincenzo Cantaluppi. 2020. "Recent Advances on Biomarkers of Early and Late Kidney Graft Dysfunction" International Journal of Molecular Sciences 21, no. 15: 5404. https://doi.org/10.3390/ijms21155404
APA StyleQuaglia, M., Merlotti, G., Guglielmetti, G., Castellano, G., & Cantaluppi, V. (2020). Recent Advances on Biomarkers of Early and Late Kidney Graft Dysfunction. International Journal of Molecular Sciences, 21(15), 5404. https://doi.org/10.3390/ijms21155404






