Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Immunohistochemical Analysis of Nectin-4 and PD-L1 Expression in UTUC TMA
2.3. Association of Nectin-4 and PD-L1 Expression with Patient Prognosis
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. Immunohistochemistry
4.3. Scoring System
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UTUC | Upper tract urothelial carcinoma |
| UBC | Urinary bladder urothelial carcinoma |
| PD-L1 | Programmed Death Ligand 1 |
| PD-1 | Programmed Death 1 |
| EV | Enfortumab vedotin |
| MMAE | Monomethyl auristatin E |
| TMA | Tissue microarray |
| EDTA | Tris-ethylenediaminetetraacetic acid |
| H-score | Histochemical scoring system |
| PI3K | Phosphoinositide 3-kinase |
| AKT | Protein kinase B |
| FDA | Food and Drug Administration |
| HR | Hazard ratio |
| CI | Confidence interval |
| MI | Muscle-invasive |
| NMI | Non-muscle-invasive |
| Inf | Infinity |
References
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; Van Rhijn, B.W.G.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Chong, K.T.; Chang, S.L.; Bellmunt, J. Upper tract urothelial carcinoma: A different disease entity in terms of management. ESMO Open 2016, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4—Positive Solid Tumors, Including Metastatic Urothelial Carcinoma abstract. J. Clin. Oncol. 2020, 38, 1041. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adélaïde, J.; Geneix, J.; et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- DeRycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2015, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Wang, L.; Wu, Y.; Hao, J.; Wang, Z. A novel PI3K / AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett. 2016, 375, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Uemura, M.; Yamamoto, Y.; Tanigawa, G.; Nakata, W.; Sato, M.; Nagahara, A.; Kiuchi, H.; Nakai, Y.; Matsumiya, K.; et al. Preoperative risk stratification for cancer-specific survival of patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int. J. Clin. Oncol. 2015, 20, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Taneishi, K.; Inamoto, T.; Ishizuya, Y.; Takada, S.; Tsujihata, M.; Tanigawa, G.; Minato, N.; Nakazawa, S.; Takada, T.; et al. Adjuvant chemotherapy improves survival of patients with high-risk upper urinary tract urothelial carcinoma: A propensity score-matched analysis. BMC Urol. 2017, 17, 4–9. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arranz, J.Á.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-del-Muro, X.; De Giorgi, U.; Mencinger, M. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor 130): A multicentre, randomised, placebo-controlled trial. Lancet 2020, 1547–1557, in press. [Google Scholar] [CrossRef]
- Suzman, D.L.; Agrawal, S.; Ning, Y.; Maher, V.E.; Fernandes, L.L.; Karuri, S.; Tang, S.; Sridhara, R.; Schroeder, J.; Goldberg, K.B.; et al. FDA Approval Summary: Atezolizumab or Pembrolizumab for the Treatment of Patients with Advanced Urothelial Carcinoma Ineligible for Cisplatin-Containing Chemotherapy. Oncologist 2019, 24, 563–569. [Google Scholar] [CrossRef]
- Fujita, K.; Ujike, T.; Nagahara, A.; Uemura, M.; Tanigawa, G.; Shimazu, K.; Fushimi, H.; Yamaguchi, S.; Nonomura, N. Endoglin expression in upper urinary tract urothelial carcinoma is associated with intravesical recurrence after radical nephroureterectomy. Int. J. Urol. 2015, 22, 463–467. [Google Scholar] [CrossRef][Green Version]
- Munari, E.; Fujita, K.; Faraj, S.; Chaux, A.; Gonzalez-Roibon, N.; Hicks, J.; Meeker, A.; Nonomura, N.; Netto, G.J. Dysregulation of mammalian target of rapamycin pathway in upper tract urothelial carcinoma. Hum. Pathol. 2013, 44, 2668–2676. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Fujita, K.; Hayashi, Y.; Matsushita, M.; Nojima, S.; Jingushi, K.; Kato, T.; Kawashima, A.; Ujike, T.; Nagahara, A.; et al. STAT3 expression is a prognostic marker in upper urinary tract urothelial carcinoma. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujita, K.; Munari, E.; Uemura, M.; Miyamoto, H.; Netto, G.J. Clinical implication of the mammalian target of rapamycin pathway in upper tract urothelial carcinoma with negative GATA binding protein 3 expression. Int. J. Urol. 2019, 26, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Eich, M.L.; Tregnago, A.C.; Faraj, S.F.; Palsgrove, D.N.; Fujita, K.; Bezerra, S.M.; Munari, E.; Sharma, R.; Chaux, A.; Netto, G.J. Insulin-like growth factor-1 receptor expression in upper tract urothelial carcinoma. Virchows Arch. 2019, 474, 21–27. [Google Scholar] [CrossRef] [PubMed]

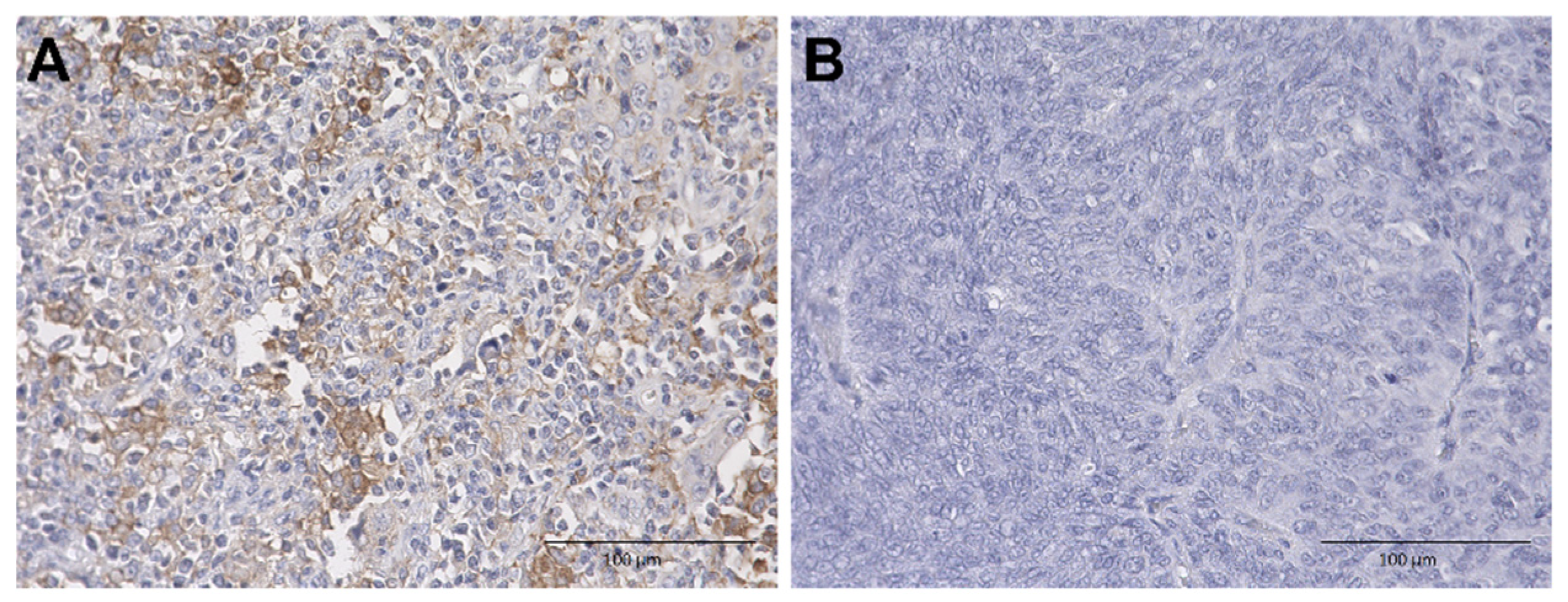
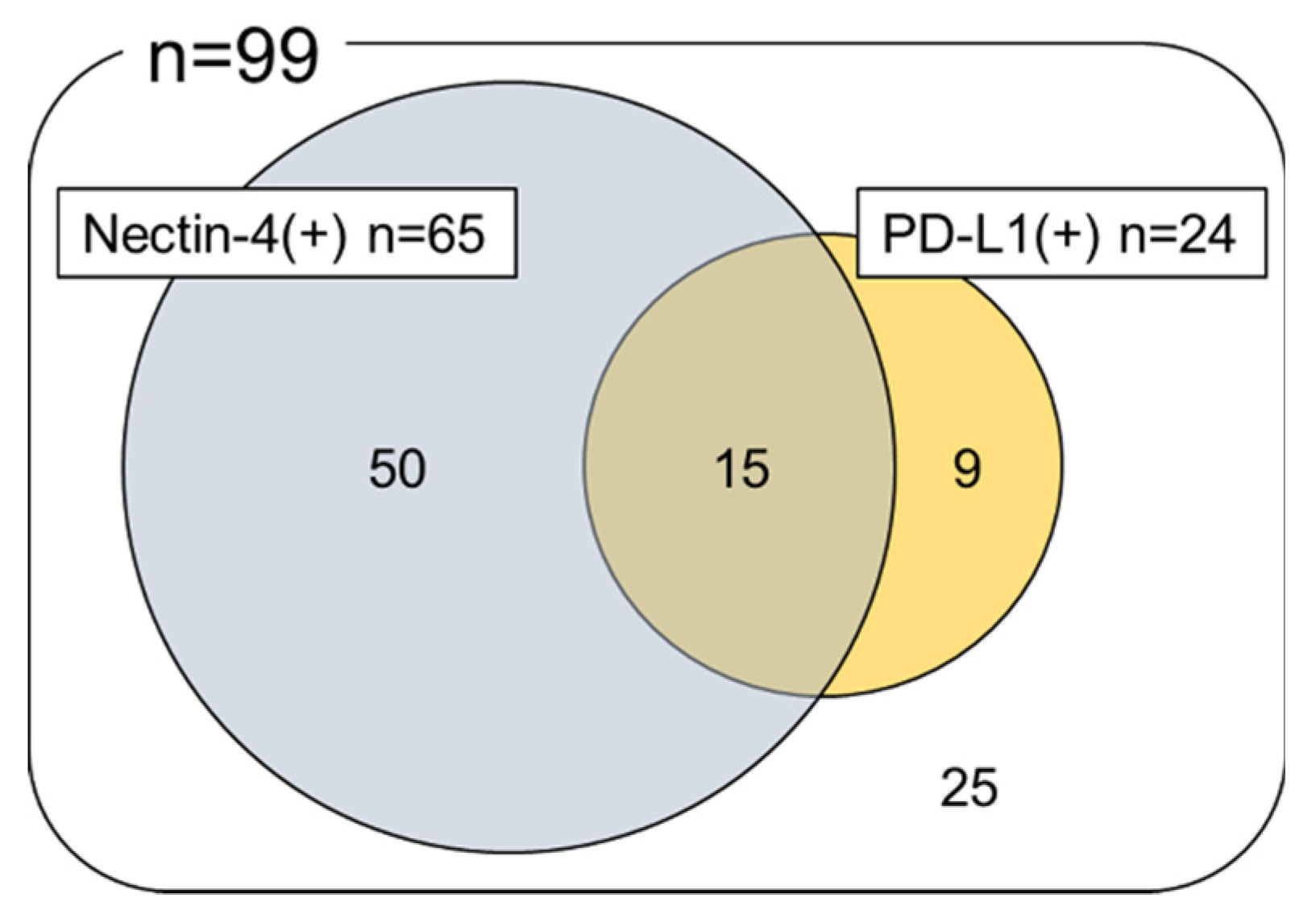
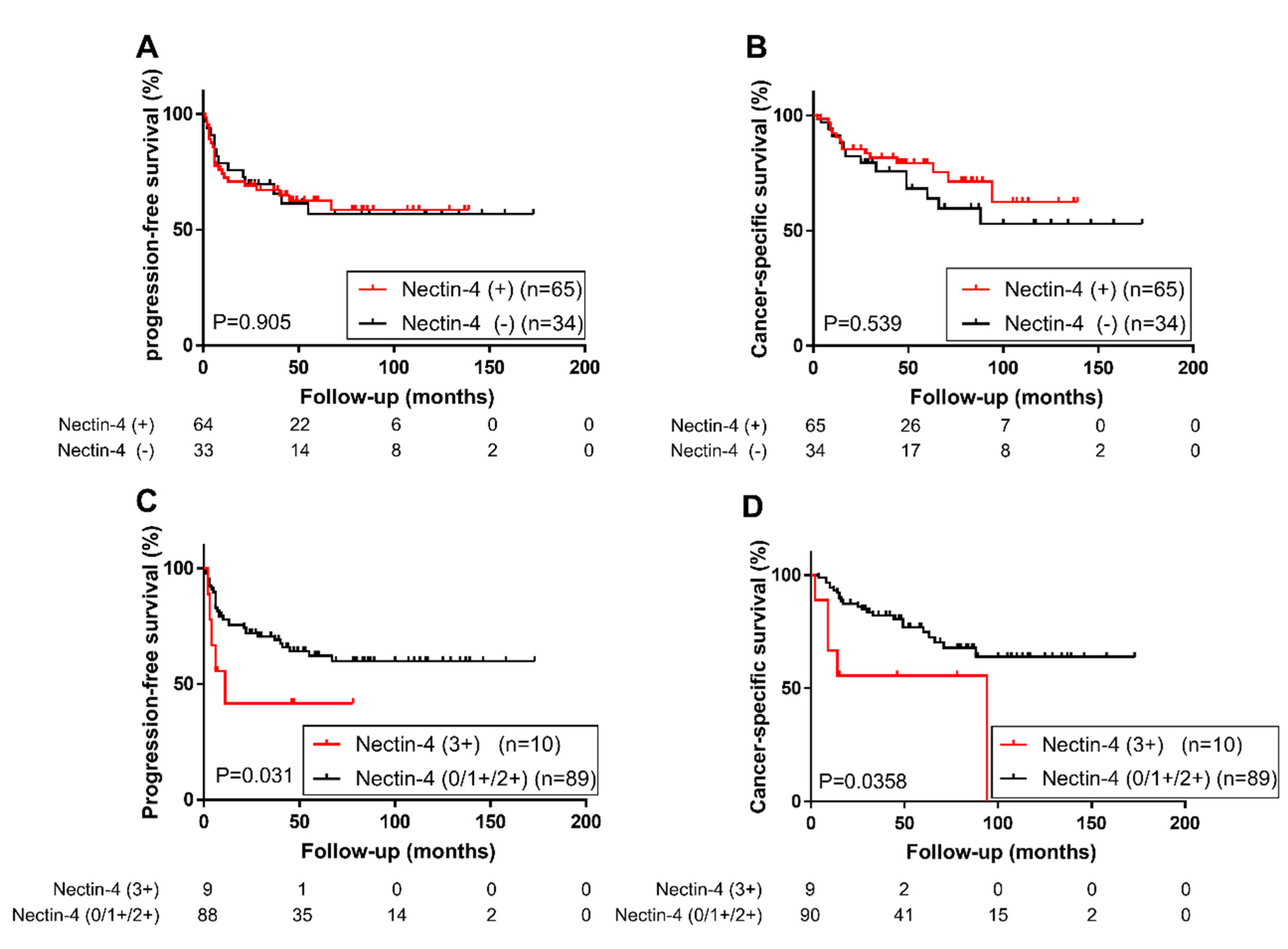
| Variable | |
|---|---|
| Age (year), median (range) | 71 (48–87) |
| Sex, n (%) | |
| Male | 60 (60.6) |
| Female | 39 (39.4) |
| Laterality, n (%) | |
| Right | 43 (43.4) |
| Left | 56 (56.6) |
| Tumor location, n (%) | |
| Renal pelvis | 45 (45.5) |
| Ureter | 50 (50.5) |
| Both | 4 (4.0) |
| Tumor grade, n (%) | |
| Low grade | 15 (15.2) |
| High grade | 84 (85.9) |
| Pathological T stage, n (%) | |
| pTa | 19 (19.2) |
| pT1 | 18 (18.2) |
| pT2 | 8 (8.1) |
| pT3 | 48 (48.5) |
| pT4 | 6 (6.1) |
| Lymphovascular invasion, n (%) | |
| No | 59 (59.6) |
| Yes | 40 (40.4) |
| Lymph node metastasis, n (%) | |
| pN0 | 84 (84.8) |
| pN+ | 12 (12.1) |
| pNx | 3 (3.0) |
| Adjuvant chemotherapy, n (%) | |
| No | 63 (63.6) |
| Yes | 26 (26.3) |
| Progression, n (%) | |
| No | 61 (61.6) |
| Yes | 38 (38.4) |
| Follow-up (month), median (range) | 37 (1–173) |
| Variable | Nectin-4 Expression, n (%) | p-Value | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 0 vs. 1+/2+/3+ | 0 vs. 3+ | |
| Sex | 0.134 | 1.000 | ||||
| Male | 17 (28.3) | 20 (33.3) | 18 (30.0) | 5 (8.3) | ||
| Female | 17 (43.6) | 11 (28.2) | 6 (15.4) | 5 (12.8) | ||
| Tumor location | 1.000 a | 0.457 | ||||
| Renal pelvis | 16 (35.6) | 13 (28.9) | 10 (22.2) | 6 (13.3) | ||
| Ureter | 18 (36.0) | 17 (34.0) | 12 (24.0) | 3 (6.0) | ||
| Both | 0 (0.0) | 1 (25.0) | 2 (50.0) | 1 (25.0) | ||
| Tumor grade | 0.376 | 0.177 | ||||
| Low grade | 7 (46.7) | 6 (40) | 2 (13.3) | 0 (0.0) | ||
| High grade | 27 (32.1) | 25 (29.8) | 22 (26.2) | 10 (11.9) | ||
| Pathological T stage | 0.829 b | 1.000 | ||||
| pTa | 7 (36.8) | 3 (15.8) | 6 (31.6) | 3 (15.8) | ||
| pT1 | 5 (27.8) | 7 (38.9) | 6 (33.3) | 0 (0.0) | ||
| pT2 | 4 (50.0) | 2 (25.0) | 1 (12.5) | 1 (12.5) | ||
| pT3 | 15 (31.3) | 17 (35.4) | 11 (22.9) | 5 (10.4) | ||
| pT4 | 3 (50.0) | 2 (33.3) | 0 (0.0) | 1 (16.7) | ||
| Lymphovascular invasion | 0.831 | 1.000 | ||||
| No | 21 (35.6) | 17 (28.8) | 15 (25.4) | 6 (10.2) | ||
| Yes | 13 (32.5) | 14 (35.0) | 9 (22.5) | 4 (10.0) | ||
| Lymph node metastasis | 0.745 c | 0.577 | ||||
| pN0 | 29 (34.5) | 26 (31.0) | 21 (25) | 8 (9.5) | ||
| pN+ | 3 (25.0) | 5 (41.7) | 2 (16.7) | 2 (16.7) | ||
| pNx | 2 (66.7) | 0 (0.0) | 1 (33.3) | 0 (0.0) | ||
| Progression-Free Survival | Cancer-Specific Survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate | Univariate | Multivariate | ||||||||
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| All cases (n = 99) | ||||||||||||
| Sex (male/female) | 1.33 | 0.69–2.69 | 0.396 | 1.33 | 0.64–2.97 | 0.457 | ||||||
| Age (70</≤70) | 1.38 | 0.73–2.70 | 0.329 | 1.65 | 0.79–3.57 | 0.182 | ||||||
| Tumor location (renal pelvis/ureter) | 0.78 | 0.40–1.54 | 0.500 | 0.84 | 0.39–1.72 | 0.608 | ||||||
| Tumor grade (high/low) | 4.44 | 1.35–27.38 | 0.010 | 4.38 | 1.28–27.58 | 0.015 | 7.77 | 1.66–138.52 | 0.005 | 5.76 | 1.21–103.12 | 0.024 |
| pT stage (MI/NMI) | 17.31 | 5.26–106.71 | <0.001 | 11.35 | 3.23–72.07 | <0.001 | 1.09 × 1010 | 13.52–Inf | <0.001 | 7.37 × 109 | 7.70–Inf | <0.001 |
| Lymphovascular invasion (yes/no) | 5.84 | 2.96–12.34 | <0.001 | 2.46 | 1.15–5.52 | 0.020 | 5.62 | 2.58–13.49 | <0.001 | 2.20 | 0.93–5.23 | 0.073 |
| Lymph node metastasis (yes/no) | 4.40 | 1.95–9.00 | <0.001 | 2.31 | 0.95–5.28 | 0.065 | 2.74 | 1.08–6.09 | 0.035 | 0.95 | 0.35–2.55 | 0.914 |
| Nectin-4 strong expression | 2.51 | 0.94–5.61 | 0.064 | 2.73 | 0.97–6.65 | 0.056 | 2.69 | 0.90–6.50 | 0.072 | 2.10 | 0.64–5.81 | 0.179 |
| Overall Survival | ||||||
|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate | ||||
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| All cases (n = 99) | ||||||
| Sex (male/female) | 1.21 | 0.59–2.62 | 0.608 | |||
| Age (70</≤70) | 1.74 | 0.84–3.75 | 0.134 | |||
| Tumor location (renal pelvis/ureter) | 0.89 | 0.43–1.84 | 0.757 | |||
| Tumor grade (high/low) | 8.05 | 1.72–143.58 | 0.004 | 5.99 | 1.26–107.27 | 0.019 |
| pT stage (MI/NMI) | 1.09 × 1010 | 14.00–Inf | <0.001 | 7.70 × 1010 | 8.53–Inf | <0.001 |
| Lymphovascular invasion (yes/no) | 5.02 | 2.38–11.51 | <0.001 | 1.97 | 0.87–4.70 | 0.105 |
| Lymph node metastasis (yes/no) | 2.63 | 1.04–5.81 | 0.041 | 0.95 | 0.35–2.55 | 0.914 |
| Nectin-4 strong expression | 2.60 | 0.88–6.25 | 0.081 | 2.09 | 0.71–6.14 | 0.181 |
| Progression-Free Survival | Cancer-Specific Survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate | Univariate | Multivariate | ||||||||
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| High-risk group * (n = 50) | ||||||||||||
| Sex (male/female) | 1.35 | 0.68–2.81 | 0.394 | 1.23 | 0.58–2.75 | 0.601 | ||||||
| Age (70</≤70) | 1.11 | 0.57–2.23 | 0.771 | 1.57 | 0.75–3.48 | 0.238 | ||||||
| Tumor location (renal pelvis/ureter) | 0.95 | 0.47–1.90 | 0.894 | 0.97 | 0.45–2.04 | 0.932 | ||||||
| Tumor grade (high/low) | 6.96 | 1.49–123.94 | 0.008 | 6.30 | 1.34–112.50 | 0.014 | 6.41 | 1.36–114.68 | 0.014 | 5.73 | 1.20–102.73 | 0.025 |
| Nectin-4 strong expression | 3.79 | 1.37–9.11 | 0.013 | 3.32 | 1.20–7.98 | 0.027 | 2.78 | 0.92–6.87 | 0.067 | 2.31 | 0.76–5.77 | 0.128 |
| Overall survival | ||||||
|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate | ||||
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| High-risk group * (n = 50) | ||||||
| Sex (male/female) | 1.12 | 0.54–2.44 | 0.768 | |||
| Age (70</≤70) | 1.69 | 0.80–3.67 | 0.176 | |||
| Tumor location (renal pelvis/ureter) | 1.04 | 0.50–2.17 | 0.909 | |||
| Tumor grade (high/low) | 6.68 | 1.42–119.40 | 0.011 | 6.01 | 1.26–107.63 | 0.020 |
| Nectin-4 strong expression | 2.73 | 0.91–6.71 | 0.072 | 2.26 | 0.75–5.61 | 0.136 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomiyama, E.; Fujita, K.; Rodriguez Pena, M.D.C.; Taheri, D.; Banno, E.; Kato, T.; Hatano, K.; Kawashima, A.; Ujike, T.; Uemura, M.; et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2020, 21, 5390. https://doi.org/10.3390/ijms21155390
Tomiyama E, Fujita K, Rodriguez Pena MDC, Taheri D, Banno E, Kato T, Hatano K, Kawashima A, Ujike T, Uemura M, et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. International Journal of Molecular Sciences. 2020; 21(15):5390. https://doi.org/10.3390/ijms21155390
Chicago/Turabian StyleTomiyama, Eisuke, Kazutoshi Fujita, Maria Del Carmen Rodriguez Pena, Diana Taheri, Eri Banno, Taigo Kato, Koji Hatano, Atsunari Kawashima, Takeshi Ujike, Motohide Uemura, and et al. 2020. "Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma" International Journal of Molecular Sciences 21, no. 15: 5390. https://doi.org/10.3390/ijms21155390
APA StyleTomiyama, E., Fujita, K., Rodriguez Pena, M. D. C., Taheri, D., Banno, E., Kato, T., Hatano, K., Kawashima, A., Ujike, T., Uemura, M., Takao, T., Yamaguchi, S., Fushimi, H., Yoshimura, K., Uemura, H., Netto, G. J., & Nonomura, N. (2020). Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. International Journal of Molecular Sciences, 21(15), 5390. https://doi.org/10.3390/ijms21155390






