Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome
Abstract
1. Introduction
2. Results
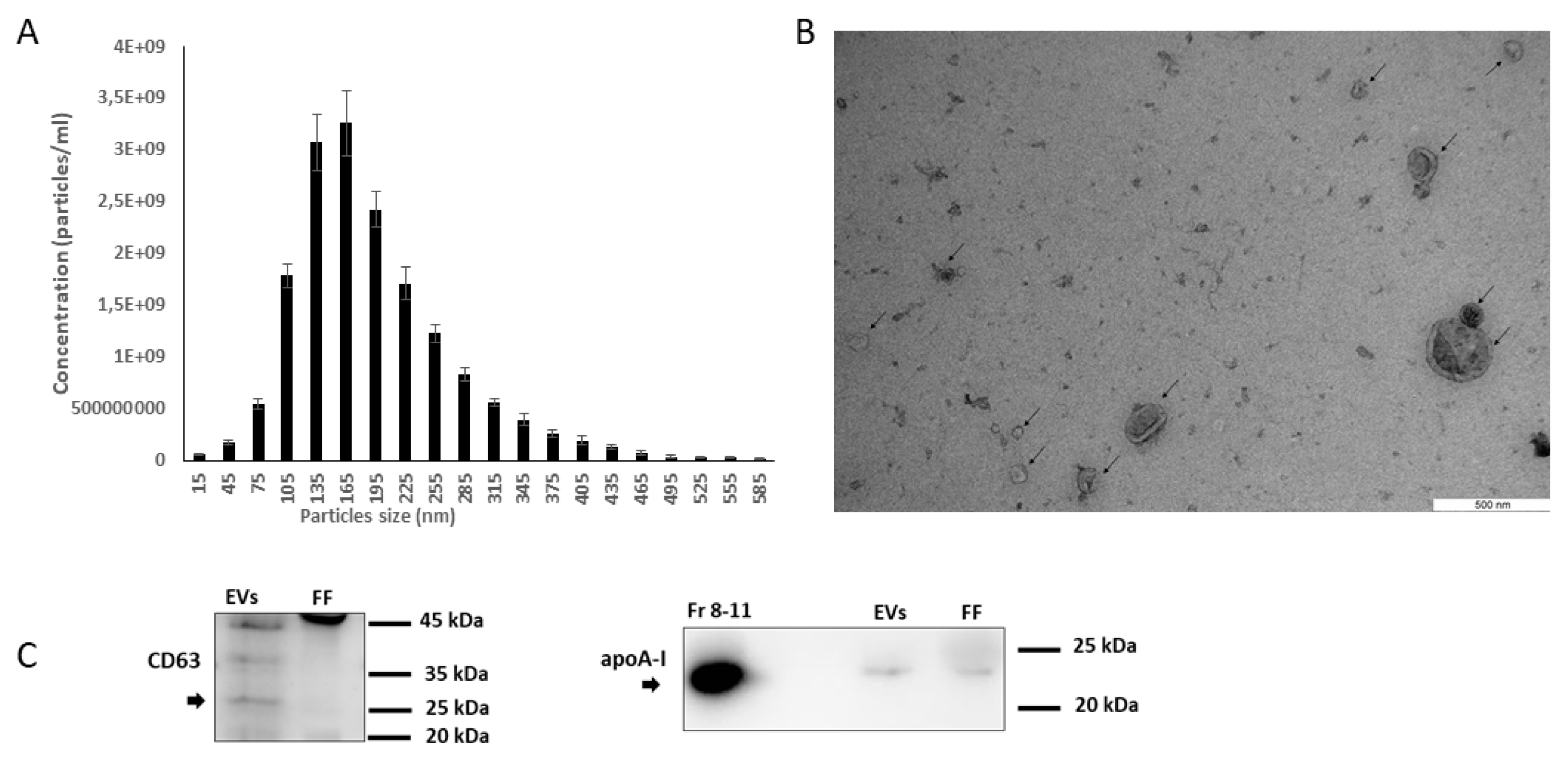
2.1. Size-Exclusion Chromatography Column-Based Isolation of Extracellular Vesicles (EVs) from Bovine Follicular Fluid (FF)
2.2. Characterization of EVs
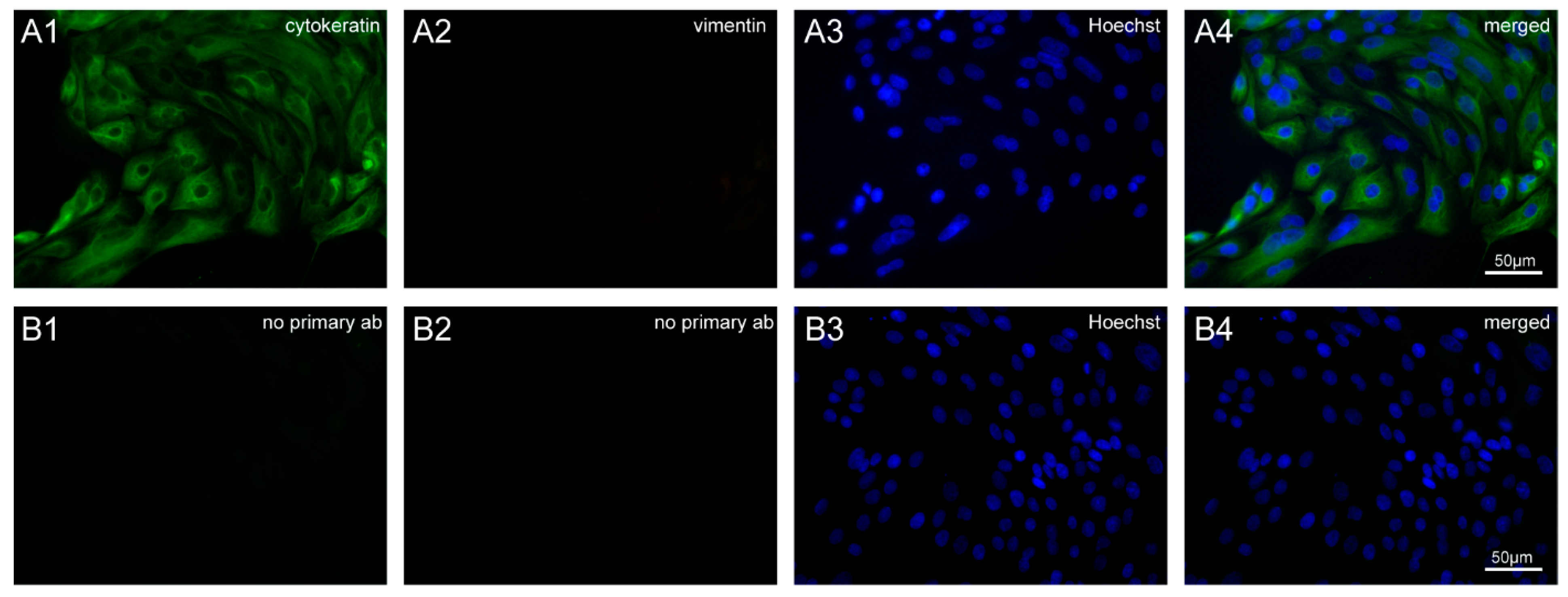
2.3. Characterization of BOECs by Immunofluorescence Staining
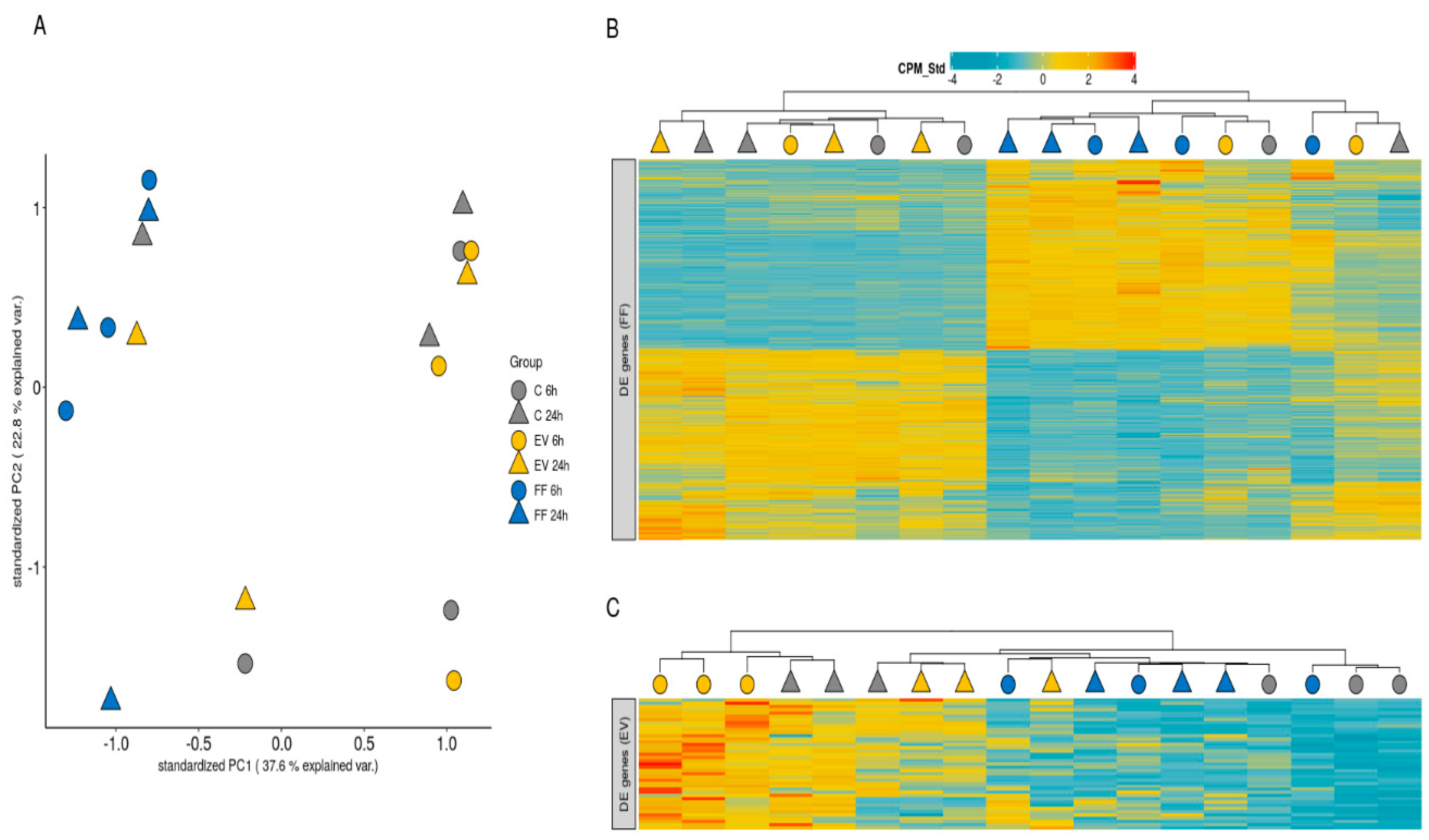
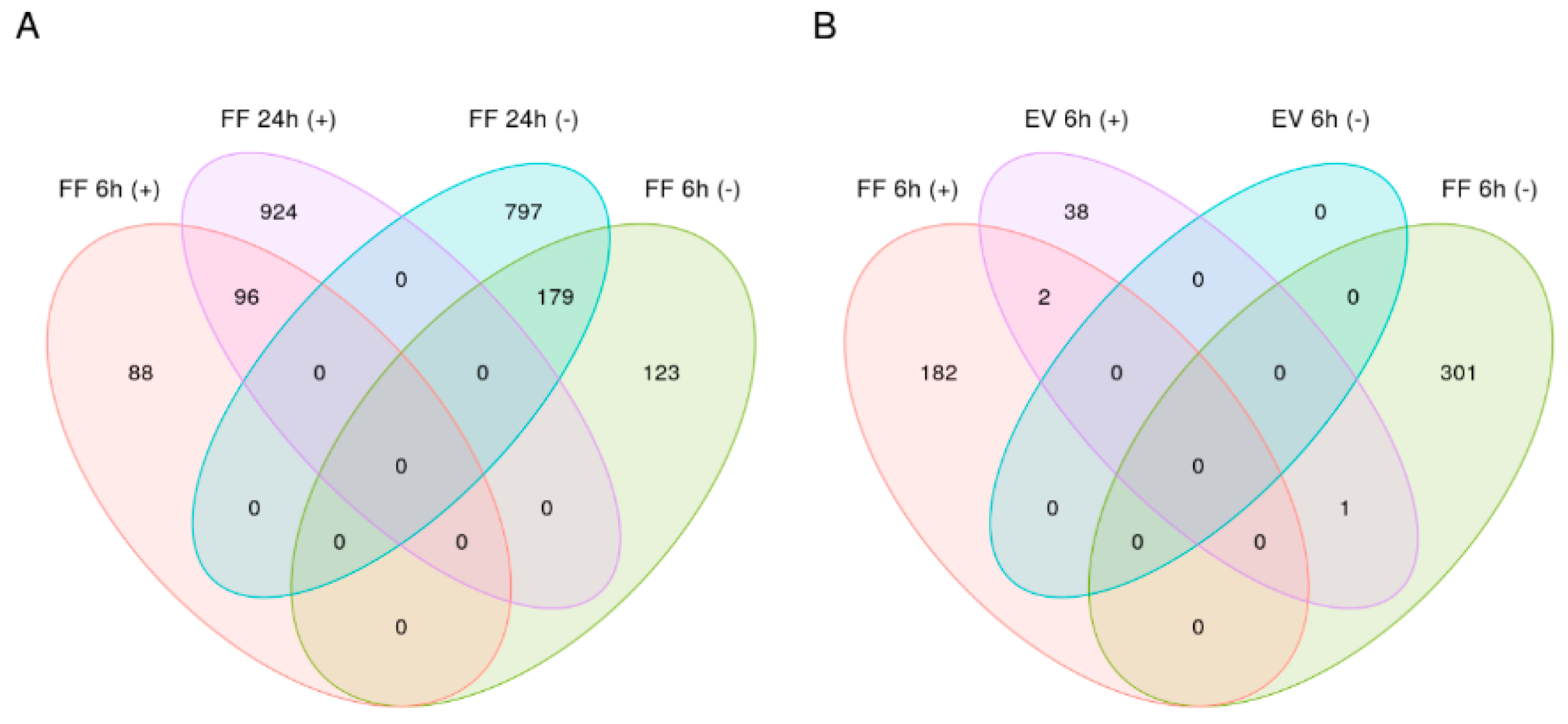
2.4. Differential Gene Expression Analysis
2.5. Pathway Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Bovine Oviductal Epithelial Cells
4.2. Collection of Follicular Fluid
4.3. Isolation of EVs from Bovine FF
4.4. Nanoparticle Tracking Analysis
4.5. Western Blot Analysis
4.6. Transmission Electron Microscopy
4.7. Immunofluorescence Staining
4.8. Supplementation and Bovine Oviductal Epithelial Cells Culture
4.9. RNA Isolation, Library Preparation, and Sequencing
4.9.1. RNA Isolation
4.9.2. RNA-Seq Library Preparation
4.9.3. Processing, Alignment, and Quantification of RNA-Seq Reads
4.10. Differential Gene Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FF | Follicular fluid |
| EV | Extracellular vesicle |
| BOECs | Bovine oviductal epithelial cells |
| DEGs | Differentially expressed genes |
| GSEA | Gene Set Enrichment Analysis |
| SEC | size-exclusion chromatography |
| NTA | Nanoparticle tracking analysis |
| TEM | Transmission Electron microscopy |
| WB | Western blot analysis |
| RNA seq | RNA sequencing |
References
- Nandi, S.; Kumar, V.G.; Manjunatha, B.M.; Gupta, P.S.P. Biochemical composition of ovine follicular fluid in relation to follicle size. Dev. Growth Differ. 2007, 49, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.S.E.; Hafez, B. Folliculogenesis, Egg Maturation, and Ovulation. In Reproduction in Farm. Animals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 68–81. [Google Scholar]
- Baka, S.; Malamitsi-Puchner, A. Novel follicular fluid factors influencing oocyte developmental potential in IVF: A review. Reprod. Biomed. Online 2006, 12, 500–506. [Google Scholar] [CrossRef]
- Carpintero, N.L.; Suárez, O.A.; Mangas, C.C.; Varea, C.G.; Rioja, R.G. Follicular steroid hormones as markers of oocyte quality and oocyte development potential. J. Hum. Reprod. Sci. 2014, 7, 187. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stahl, P.D. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9. [Google Scholar] [CrossRef]
- Da Silveira, J.C.; Veeramachaneni, D.N.R.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-Secreted Vesicles in Equine Ovarian Follicular Fluid Contain miRNAs and Proteins: A Possible New Form of Cell Communication Within the Ovarian Follicle1. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of MicroRNAs in Human Follicular Fluid: Characterization of MicroRNAs That Govern Steroidogenesis in Vitro and Are Associated With Polycystic Ovary Syndrome in Vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef]
- Sohel, M.M.H.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and Non-Exosomal Transport of Extra-Cellular microRNAs in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef]
- Brücher, B.L.D.M.; Jamall, I.S. Cell-Cell Communication in the Tumor Microenvironment, Carcinogenesis, and Anticancer Treatment. Cell. Physiol. Biochem. 2014, 34, 213–243. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.M. Roles of Cell-to-Cell Communication in Development. Biol. Reprod. 1985, 32, 27–42. [Google Scholar] [CrossRef][Green Version]
- Pyun, B.Y. Extracellular Vesicle: An Unknown Environmental Factor for Causing Airway Disease. Allergy Asthma Immunol. Res. 2016, 8, 179–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rybak, K.; Robatzek, S. Functions of Extracellular Vesicles in Immunity and Virulence1[OPEN]. Plant. Physiol. 2019, 179, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Coy, P.; García-Vázquez, F.A.; Visconti, P.E.; Avilés, M. Roles of the oviduct in mammalian fertilization. Reprod. Camb. Engl. 2012, 144, 649–660. [Google Scholar] [CrossRef]
- Fazeli, A.; Affara, N.A.; Hubank, M.; Holt, W.V. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol. Reprod. 2004, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Geisert, R.D.; Schmitt, R.A.M. Early embryonic survival in the pig: Can it be improved? J. Anim. Sci. 2002, 80, E54–E65. [Google Scholar] [CrossRef]
- Holt, W.V.; Fazeli, A. Sperm Storage in the Female Reproductive Tract. Annu. Rev. Anim. Biosci. 2016, 4, 291–310. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Kim, G.A.; Choi, Y.B.; Jo, Y.K.; Setyawan, E.M.N.; Lee, B.C. Oocyte maturation-related gene expression in the canine oviduct, cumulus cells, and oocytes and effect of co-culture with oviduct cells on in vitro maturation of oocytes. J. Assist. Reprod. Genet. 2017, 34, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Srikandakumar, A.; Downey, B.R. Presence of follicular fluid in the porcine oviduct and its contribution to the acrosome reaction. Mol. Reprod. Dev. 1991, 30, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic Cph. Den. 2002, 3, 321–330. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Fazeli, A.; Pewsey, E. Maternal communication with gametes and embryos: A complex interactome. Brief. Funct. Genomic. Proteomic. 2008, 7, 111–118. [Google Scholar] [CrossRef]
- Guerin, P.; el Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef]
- Miki, K.; Clapham, D.E. Rheotaxis Guides Mammalian Sperm. Curr. Biol. 2013, 23, 443–452. [Google Scholar] [CrossRef]
- Croxatto, H.B. Physiology of gamete and embryo transport through the Fallopian tube*. Reprod. Biomed. Online 2002, 4, 160–169. [Google Scholar] [CrossRef]
- Nichol, R.; Hunter, R.H.F.; Gardner, D.K.; Leese, H.J.; Cooke, G.M. Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri-ovulatory period. Reproduction 1992, 96, 699–707. [Google Scholar] [CrossRef]
- Nieder, G.L.; Corder, C.N. Pyruvate and Lactate Levels in Oviducts of Cycling, Pregnant, and Pseudopregnant Mice. Biol. Reprod. 1983, 28, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Nieder, G.L.; Corder, C.N. Quantitative histochemical measurement of pyruvate and lactate in mouse oviduct during the estrous cycle. J. Histochem. Cytochem. 1982, 30, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Lane, M.; Calderon, I.; Leeton, J. Environment of the preimplantation human embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil. Steril. 1996, 65, 349–353. [Google Scholar] [CrossRef]
- Ballard, S.T.; Hunter, J.H.; Taylor, A.E. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu. Rev. Nutr. 1995, 15, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.J.; Fleming, T.P. Tight junction biogenesis during early development. Biochim. Biophys. Acta 2008, 1778, 717–728. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Samuelsson, B. Arachidonic acid metabolism: Role in inflammation. Z. Rheumatol. 1991, 50, 3–6. [Google Scholar]
- Hansen, P.J. The immunology of early pregnancy in farm animals. Reprod. Domest. Anim. Zucht. 2011, 46, 18–30. [Google Scholar] [CrossRef]
- Hansen, P.J. Regulation of immune cells in the uterus during pregnancy in ruminants. J. Anim. Sci. 2007, 85, E30–E31. [Google Scholar] [CrossRef][Green Version]
- Binelli, M.; Gonella-Diaza, A.M.; Mesquita, F.S.; Membrive, C.M.B. Sex Steroid-Mediated Control of Oviductal Function in Cattle. Biology 2018, 7, 15. [Google Scholar] [CrossRef]
- Wood, G.A.; Fata, J.E.; Watson, K.L.M.; Khokha, R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 2007, 133, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Palma-Vera, S.E.; Schoen, J.; Chen, S. Periovulatory follicular fluid levels of estradiol trigger inflammatory and DNA damage responses in oviduct epithelial cells. PLoS ONE 2017, 12, e0172192. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.J.; Coons, L.W.; Radicke, D.A.; Hodgson, B.J.; Valenzuela, G. Role of prostaglandins in contractile activity of the ampulla of the rabbit oviduct. Am. J. Physiol. 1980, 238, E157–E166. [Google Scholar] [CrossRef]
- Kodithuwakku, S.P.; Miyamoto, A.; Wijayagunawardane, M.P.B. Spermatozoa stimulate prostaglandin synthesis and secretion in bovine oviductal epithelial cells. Reproduction 2007, 133, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Freeman, D.A.; Vanderwall, D.K.; Woods, G.L. Prostaglandin E2 Hastens Oviductal Transport of Equine Embryos. Biol. Reprod. 1991, 45, 544–546. [Google Scholar] [CrossRef]
- Hiradate, Y.; Inoue, H.; Kobayashi, N.; Shirakata, Y.; Suzuki, Y.; Gotoh, A.; Roh, S.; Uchida, T.; Katoh, K.; Yoshida, M.; et al. Neurotensin Enhances Sperm Capacitation and Acrosome Reaction in Mice. Biol. Reprod. 2014, 91. [Google Scholar] [CrossRef]
- Umezu, K.; Hiradate, Y.; Oikawa, T.; Ishiguro, H.; Numabe, T.; Hara, K.; Tanemura, A.K. Exogenous neurotensin modulates sperm function in Japanese Black cattle. J. Reprod. Dev. 2016, 62, 409–414. [Google Scholar] [CrossRef]
- Ireland, J.J.; Murphee, R.L.; Coulson, P.B. Accuracy of Predicting Stages of Bovine Estrous Cycle by Gross Appearance of the Corpus Luteum1. J. Dairy Sci. 1980, 63, 155–160. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Lehrich, B.M.; Liang, Y.; Khosravi, P.; Federoff, H.J.; Fiandaca, M.S. Fetal Bovine Serum-Derived Extracellular Vesicles Persist within Vesicle-Depleted Culture Media. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Picelli, S.; Faridani, O.R.; Björklund, A.K.; Winberg, G.; Sagasser, S.; Sandberg, R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014, 9, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef]
- Aken, B.L.; Ayling, S.; Barrell, D.; Clarke, L.; Curwen, V.; Fairley, S.; Banet, J.F.; Billis, K.; Girón, C.G.; Hourlier, T.; et al. The Ensembl gene annotation system. Database J. Biol. Databases Curation 2016. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]

| Pathway ID | Pathway Name | DE Genes | FDR |
|---|---|---|---|
| bta00590 | Arachidonic acid metabolism | 5 | 0.012 |
| bta00140 | Steroid hormone biosynthesis | 4 | 0.026 |
| bta04913 | Ovarian steroidogenesis | 4 | 0.026 |
| bta04137 | Mitophagy—animal | 6 | 0.026 |
| bta04145 | Phagosome | 8 | 0.026 |
| bta04140 | Autophagy—animal | 8 | 0.033 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Viil, J.; Lättekivi, F.; Ord, J.; Reshi, Q.U.A.; Jääger, K.; Velthut-Meikas, A.; Andronowska, A.; Jaakma, Ü.; Salumets, A.; et al. Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome. Int. J. Mol. Sci. 2020, 21, 5365. https://doi.org/10.3390/ijms21155365
Hasan MM, Viil J, Lättekivi F, Ord J, Reshi QUA, Jääger K, Velthut-Meikas A, Andronowska A, Jaakma Ü, Salumets A, et al. Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome. International Journal of Molecular Sciences. 2020; 21(15):5365. https://doi.org/10.3390/ijms21155365
Chicago/Turabian StyleHasan, Mohammad Mehedi, Janeli Viil, Freddy Lättekivi, James Ord, Qurat Ul Ain Reshi, Kersti Jääger, Agne Velthut-Meikas, Aneta Andronowska, Ülle Jaakma, Andres Salumets, and et al. 2020. "Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome" International Journal of Molecular Sciences 21, no. 15: 5365. https://doi.org/10.3390/ijms21155365
APA StyleHasan, M. M., Viil, J., Lättekivi, F., Ord, J., Reshi, Q. U. A., Jääger, K., Velthut-Meikas, A., Andronowska, A., Jaakma, Ü., Salumets, A., & Fazeli, A. (2020). Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome. International Journal of Molecular Sciences, 21(15), 5365. https://doi.org/10.3390/ijms21155365







