Pseudomonas Aeruginosa Induced Cell Death in Acute Lung Injury and Acute Respiratory Distress Syndrome
Abstract
1. Introduction
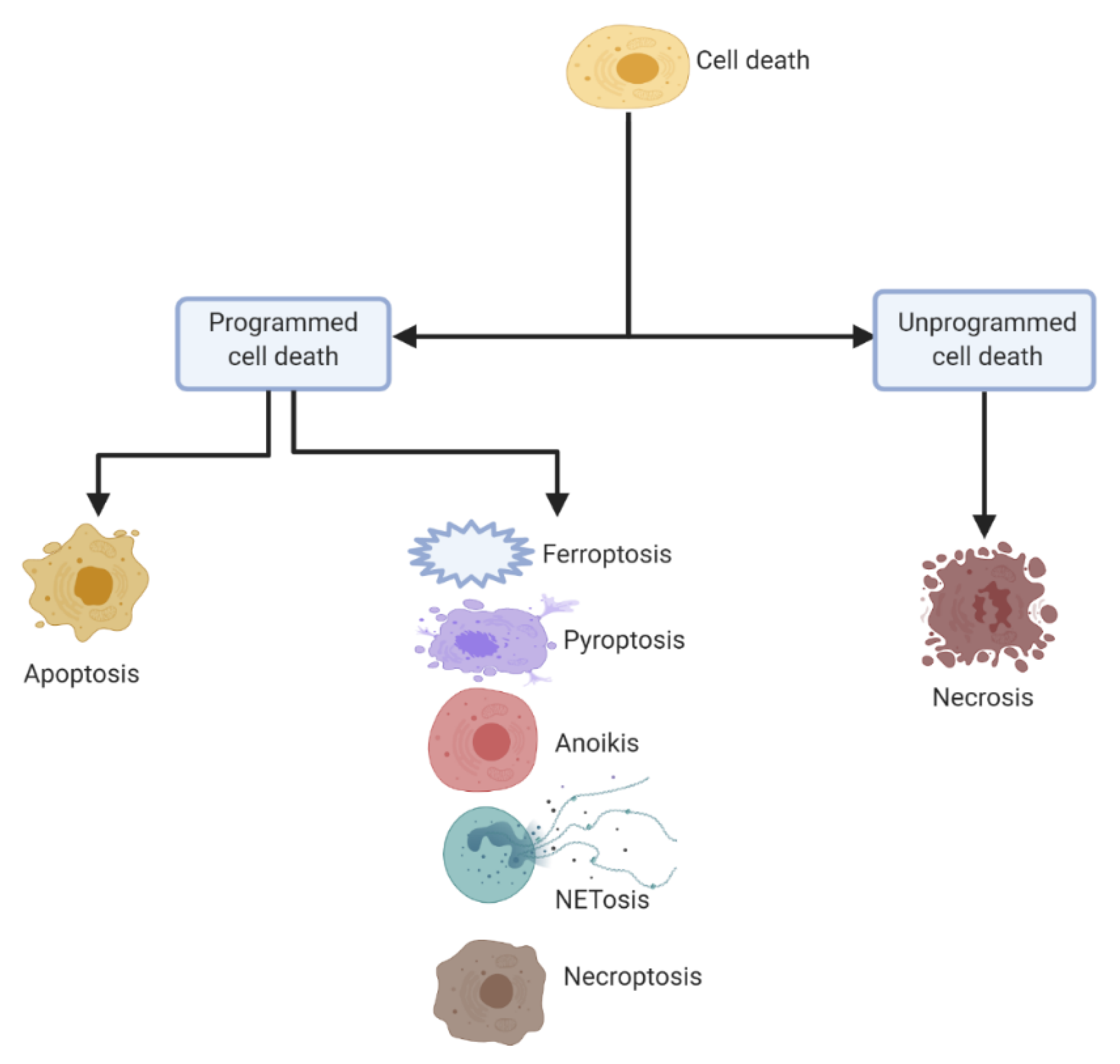
2. Distinct Cell Death Mechanisms
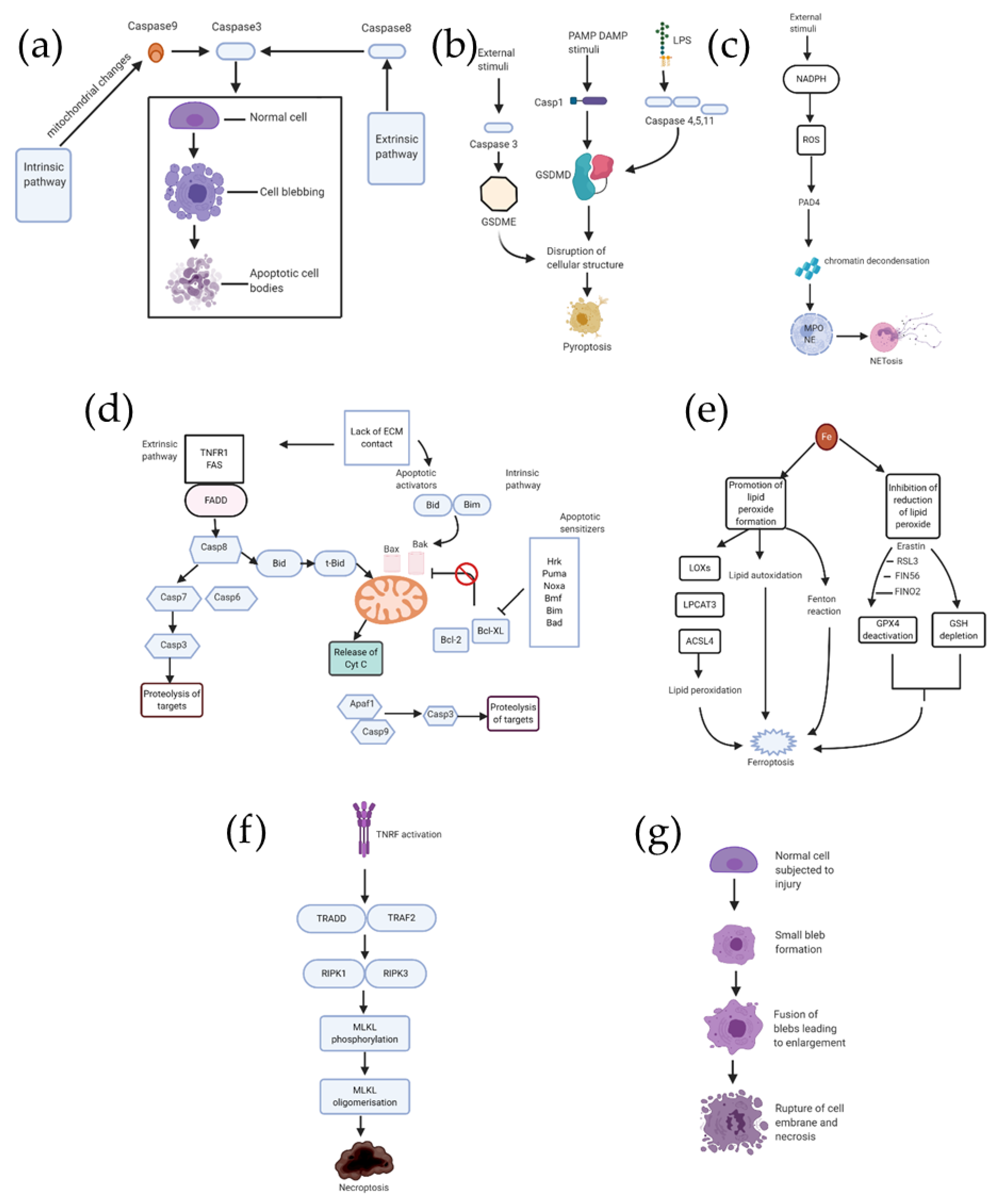
2.1. Apoptosis (Figure 2a)
2.2. Pyroptosis (Figure 2b)
2.3. NETosis (Figure 2c)
2.4. Anoikis (Figure 2d)
2.5. Ferroptosis (Figure 2e)
2.6. Necroptosis (Figure 2f)
2.7. Necrosis (Figure 2g)
3. P. aeruginosa Induced Lung Epithelial Cell Death
3.1. P. aeruginosa Triggers Apoptosis in Lung Epithelial Cells (Figure 3a)
3.2. Epithelial Anoikis Caused by P. aeruginosa Infection (Figure 3b)
3.3. P. aeruginosa Induced Pyroptosis (Figure 3c)
3.4. P. aeruginosa Associated NETosis (Figure 3d)
3.5. Epithelial Ferroptosis in Context of P. aeruginosa (Figure 3e)
4. Cell Death Mediation and Therapeutic Strategies
4.1. Role of IL-15 in Apoptosis Prevention
4.2. Platelets Inhibit Epithelial Cell Apoptosis
4.3. Mediation of Epithelial Cell Death in P. aeruginosa Pneumonia by Morf4l1
4.4. Inhibition of Cellular Apoptosis and Autophagy Due to Tremella Polysaccharides
5. Conclusions and Future Directions
Funding
Conflicts of Interest
Abbreviations
| AA-PE | Arachidonic acid-phosphatidylethanolamines |
| ACSL4 | Acyl-CoA synthetase long-chain family 4 |
| ADPRT | (ADP-ribosyl) transferase |
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| BAD | BCL2 associated agonist of cell death |
| BAX | Bcl-2-associated X protein |
| BIK | BCL2 Interacting Killer |
| BIM | Bcl-2-like protein 11 |
| BMF | Bcl2 Modifying Factor |
| C. elegans | Caenorhabditis elegans |
| CD8 | Cluster of differentiation 8 |
| CD95 | Cluster of differentiation 95 |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| COPD | Chronic obstructive pulmonary disease |
| COVID–19 | Coronavirus disease of 2019 |
| CX43 | Connexin 43 |
| DAMPs | Danger-associated molecular patterns |
| DC | Dendritic cells |
| DISC | Death-inducing signaling complex |
| DOPC | 1-2-dioleoyl-sn-glycero–3-phosphocholine |
| DPPG | 1,2-Dipalmitoyl-sn-glycero–3-phosphoglycerol |
| ExoT | Exoenzyme T |
| FADD | Fas-associated death domain protein |
| FBXL18 | F-Box and Leucine Rich Repeat Protein 18 |
| GAP | GTPase-accelerating protein |
| GPX4 | Glutathione peroxidase 4 |
| GSDMD | Gasdermin-D |
| GSDME | Gasdermin E |
| GSH | Glutathione |
| HBE | Human bronchial epithelial |
| HRK | Harakiri |
| IFNs | Interferons |
| IL–15 | Interleukin-15 |
| JNK | c-Jun N-terminal kinases |
| LecA | Lectins PA-IL |
| LecB | Lectins PA-IIL |
| LOXs | Lipoxygenases |
| LPCAT3 | Lysophosphatidylcholine acyltransferase 3 |
| LPS | Lipopolysaccharide |
| MLKL | Mixed lineage kinase domain-like protein |
| Morf4l1 | Mortality factor 4 like 1 |
| MPO | Myeloperoxidase |
| NE | Neutrophil elastase |
| NK | Natural killer cells |
| NLRC4 | NLR Family CARD Domain Containing 4 |
| NOXA | Phorbol-12-myristate-13-acetate-induced protein 1 |
| P. aeruginosa | Pseudomonas aeruginosa |
| PAMPs | Pathogen-associated molecular patterns |
| PAMPs | Pathogen-associated molecular patterns |
| PGC-1α | Proliferator-activated receptor-γ coactivator-1α |
| PRR | Pattern recognition receptors |
| PUMA | p53 upregulated modulator of apoptosis |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting protein kinase 3 |
| ROS | Reactive oxidation species |
| SARS-CoV2 | Severe acute respiratory syndrome coronavirus 2 |
| SIRT1 | Sirtuin 1 |
| T3SS | Type III secretion system |
| TFP | Type IV pili |
| THAM | Tris-hydroxymethyl aminomethane |
| TLR5 | Toll-like receptor 5 |
| TNF | Tumor necrosis factor |
| TNFR | Tumor necrosis factor receptor |
| TRADD | TNFR-associated death protein |
| TRAF2 | TNFR-associated factor 2 |
| 3-oc | (3-oxo-dodecanoyl) homoserine lactone |
References
- COVID-19 Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 July 2020).
- Vincent, J. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Frederiksen, B. Microbiology of cystic fibrosis. In Cystic Fibrosis; Arnold Publishers-International Book and Journal Publishers: London, UK, 2000; pp. 83–107. [Google Scholar]
- Murphy, T.F. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2009, 15, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2012, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, A.A.; Oglesby-Sherrouse, A.G. Regulation of Pseudomonas aeruginosa Virulence by Distinct Iron Sources. Genes 2016, 7, 126. [Google Scholar] [CrossRef]
- Bain, W.; Olonisakin, T.; Yu, M.; Qu, Y.; Hulver, M.; Xiong, Z.; Li, H.; Pilewski, J.; Mallampalli, R.K.; Nouraie, M.; et al. Platelets inhibit apoptotic lung epithelial cell death and protect mice against infection-induced lung injury. Blood Adv. 2019, 3, 432–445. [Google Scholar] [CrossRef]
- Rashid, M.H.; Kornberg, A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 4885–4890. [Google Scholar] [CrossRef]
- Bucior, I.; Pielage, J.F.; Engel, J. Pseudomonas aeruginosa Pili and Flagella Mediate Distinct Binding and Signaling Events at the Apical and Basolateral Surface of Airway Epithelium. PLoS Pathog. 2012, 8, e1002616. [Google Scholar] [CrossRef]
- Burrows, L.L. Pseudomonas aeruginosa Twitching Motility: Type IV Pili in Action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef]
- Irvin, R.T.; Doig, P.; Lee, K.K.; Sastry, P.A.; Paranchych, W.; Todd, T.; Hodges, R.S. Characterization of the Pseudomonas aeruginosa pilus adhesin: Confirmation that the pilin structural protein subunit contains a human epithelial cell-binding domain. Infect. Immun. 1989, 57, 3720–3726. [Google Scholar] [CrossRef]
- Gupta, S.K.; Berk, R.S.; Masinick, S.; Hazlett, L.D. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect. Immun. 1994, 62, 4572–4579. [Google Scholar] [CrossRef]
- Vallet, I.; Olson, J.W.; Lory, S.; Lazdunski, A.; Filloux, A. The chaperone/usher pathways of Pseudomonas aeruginosa: Identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA 2001, 98, 6911–6916. [Google Scholar] [CrossRef] [PubMed]
- Chemani, C.; Imberty, A.; De Bentzmann, S.; Pierre, M.; Wimmerová, M.; Guery, B.; Faure, K. Role of LecA and LecB Lectins in Pseudomonas aeruginosa-Induced Lung Injury and Effect of Carbohydrate Ligands. Infect. Immun. 2009, 77, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Nishizawa, H.; Kitao, S.; Deguchi, S.; Nakamura, T.; Fujimoto, A.; Shikata, M.; Gotoh, N. Pseudomonas aeruginosainjects type III effector ExoS into epithelial cells through the function of type IV pili. FEBS Lett. 2015, 589, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2011, 19, 107–120. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Genet. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Gong, W.; Shi, Y.; Ren, J. Research progresses of molecular mechanism of pyroptosis and its related diseases. Immunobiology 2020, 225, 151884. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.-C.; Tyurin, V.A.; Krieger, J.; Croix, C.M.S.; Watkins, S.; Bayir, E.; et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Investig. 2018, 128, 4639–4653. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Methods 2016, 13, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; Croix, C.M.S.; Dar, H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, O.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of Ferroptosis and Relations with Regulated Cell Death: A Review. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Dixon, S.J.; Winter, G.E.; Musavi, L.S.; Lee, E.D.; Snijder, B.; Rebsamen, M.; Superti-Furga, G.; Stockwell, B.R. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015, 10, 1604–1609. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Methods 2016, 13, 91–98. [Google Scholar] [CrossRef]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Ito, F.; Yamashita, K.; Okazaki, Y.; Akatsuka, S. Iron and thiol redox signaling in cancer: An exquisite balance to escape ferroptosis. Free Radic. Biol. Med. 2017, 108, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Central Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2019, 36, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Jendrossek, V.; Gulbins, E. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 2001, 6, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H. CD95/CD95 Ligand Interactions on Epithelial Cells in Host Defense to Pseudomonas aeruginosa. Science 2000, 290, 527–530. [Google Scholar] [CrossRef]
- Valente, E.; Assis, M.C.; Alvim, I.M.; Pereira, G.M.; Plotkowski, M.C. Pseudomonas aeruginosa induces apoptosis in human endothelial cells. Microb. Pathog. 2000, 29, 345–356. [Google Scholar] [CrossRef]
- Aballay, A.; Ausubel, F.M. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc. Natl. Acad. Sci. USA 2001, 98, 2735–2739. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Yuan, Z.; Goldberg, J.B.; Koval, M.; Hart, C.M.; Sadikot, R.T. Pseudomonas aeruginosa Induced Host Epithelial Cell Mitochondrial Dysfunction. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Song, D.; Meng, J.; Cheng, J.; Fan, Z.; Chen, P.; Ruan, H.; Tu, Z.; Kang, N.; Li, N.; Xu, Y.; et al. Pseudomonas aeruginosa quorum-sensing metabolite induces host immune cell death through cell surface lipid domain dissolution. Nat. Microbiol. 2018, 4, 97–111. [Google Scholar] [CrossRef]
- Losa, D.; Köhler, T.; Bellec, J.; Dudez, T.; Crespin, S.; Bacchetta, M.; Boulanger, P.; Hong, S.S.; Morel, S.; Nguyen, T.H.; et al. Pseudomonas aeruginosa–Induced Apoptosis in Airway Epithelial Cells Is Mediated by Gap Junctional Communication in a JNK-Dependent Manner. J. Immunol. 2014, 192, 4804–4812. [Google Scholar] [CrossRef]
- Ryu, J.-C.; Kim, M.-J.; Kwon, Y.; Oh, J.-H.; Yoon, S.S.; Shin, S.J.; Yoon, J.-H.; Ryu, J.-H. Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol. 2016, 10, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Pylaeva, E.; Lang, S.; Jablonska, J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Cohen, T.S.; Alhede, M.; Harfenist, B.S.; Martin, F.J.; Prince, A. Induction of Type I Interferon Signaling by Pseudomonas aeruginosa Is Diminished in Cystic Fibrosis Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2012, 46, 6–13. [Google Scholar] [CrossRef]
- Pylaeva, E.; Bordbari, S.; Spyra, I.; Decker, A.S.; Häussler, S.; Vybornov, V.; Lang, S.; Jablonska, J. Detrimental Effect of Type I IFNs During Acute Lung Infection With Pseudomonas aeruginosa Is Mediated Through the Stimulation of Neutrophil NETosis. Front. Immunol. 2019, 10, 2190. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Philip, L.M.; Cheung, G.; Vadakepeedika, S.; Grasemann, H.; Sweezey, N.; Palaniyar, N. Regulating NETosis: Increasing pH Promotes NADPH Oxidase-Dependent NETosis. Front. Med. 2018, 5. [Google Scholar] [CrossRef]
- Shafikhani, S.H.; Morales, C.; Engel, J. The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell. Microbiol. 2008, 10, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Goldufsky, J.; Shafikhani, S.H. Pseudomonas aeruginosa ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin. PLoS Pathog. 2015, 11, e1004934. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Bello, D.; Masood, S.; Shafikhani, S.H. Pseudomonas aeruginosaExoT Induces Mitochondrial Apoptosis in Target Host Cells in a Manner That Depends on Its GTPase-activating Protein (GAP) Domain Activity. J. Biol. Chem. 2015, 290, 29063–29073. [Google Scholar] [CrossRef]
- Scannapieco, F.; Wang, B.; Shiau, H.J. Oral Bacteria and Respiratory Infection: Effects on Respiratory Pathogen Adhesion and Epithelial Cell Proinflammatory Cytokine Production. Ann. Periodontol. 2001, 6, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Teng, D.; Burke, A.C.; Haase, E.; Scannapieco, F. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb. Pathog. 2009, 46, 73–79. [Google Scholar] [CrossRef]
- Torres, I.M.; Demirdjian, S.; Vargas, J.; Goodale, B.C.; Berwin, B. Acidosis increases the susceptibility of respiratory epithelial cells to Pseudomonas aeruginosa-induced cytotoxicity. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L126–L137. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Boone, D.L.; Lodolce, J.P. The Pleiotropic Functions of Interleukin 15. J. Exp. Med. 2000, 191, 753–756. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and relevance to human disease. Blood 2001, 97, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Demirci, G.; Ferrari-Lacraz, S.; Groves, C.; Coyle, A.; Malek, T.R.; Strom, T.B. IL-15 and IL-2: A matter of life and death for T cells in vivo. Nat. Med. 2001, 7, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Schachterle, W.; Oberle, K.; Aichele, P.; Diefenbach, A. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity 2007, 26, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Unsinger, J.; Davis, C.G.; Muenzer, J.T.; Ferguson, T.A.; Chang, K.; Osborne, D.F.; Clark, A.T.; Coopersmith, C.M.; McDunn, J.E.; et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J. Immunol. 2009, 184, 1401–1409. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.M.; Willis, C.R.; et al. Reversible Defects in Natural Killer and Memory Cd8 T Cell Lineages in Interleukin 15–Deficient Mice. J. Exp. Med. 2000, 191, 771–780. [Google Scholar] [CrossRef]
- Ma, A.; Koka, R.; Burkett, P. DIVERSE FUNCTIONS OF IL-2, IL-15, AND IL-7 IN LYMPHOID HOMEOSTASIS. Annu. Rev. Immunol. 2006, 24, 657–679. [Google Scholar] [CrossRef]
- Maeurer, M.; Trinder, P.; Hommel, G.; Walter, W.; Freitag, K.; Atkins, D.; Storkel, S. Interleukin-7 or Interleukin-15 Enhances Survival of Mycobacterium tuberculosis-Infected Mice. Infect. Immun. 2000, 68, 2962–2970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motegi, A.; Kinoshita, M.; Inatsu, A.; Habu, Y.; Saitoh, D.; Seki, S. IL-15-induced CD8+CD122+T cells increase antibacterial and anti-tumor immune responses: Implications for immune function in aged mice. J. Leukoc. Biol. 2008, 84, 1047–1056. [Google Scholar] [CrossRef]
- Hiromatsu, T.; Yajima, T.; Matsuguchi, T.; Nishimura, H.; Wajjwalku, W.; Arai, T.; Nimura, Y.; Yoshikai, Y. Overexpression of Interleukin-15 Protects againstEscherichia coli–Induced Shock Accompanied by Inhibition of Tumor Necrosis Factor-α-Induced Apoptosis. J. Infect. Dis. 2003, 187, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Li, J.; Xiong, S.; Chen, Y.; Wu, Q.; Li, X.; Weathington, N.M.; Han, S.H.; Snavely, C.; Chen, B.B.; et al. Mortality factor 4 like 1 protein mediates epithelial cell death in a mouse model of pneumonia. Sci. Transl. Med. 2015, 7, 311ra171. [Google Scholar] [CrossRef]
- Shi, X.; Wei, W.; Wang, N. Tremella polysaccharides inhibit cellular apoptosis and autophagy induced by Pseudomonas aeruginosa lipopolysaccharide in A549 cells through sirtuin 1 activation. Oncol. Lett. 2018, 15, 9609–9616. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]

| Type of Cell Death | Description | Mechanism | Reference Numbers |
|---|---|---|---|
| Apoptosis | CD95/CD95-ligand system for P. aeruginosa triggered apoptosis | P. aeruginosa causes an up-regulation of CD95/CD95ligand on the cell surface, responsible for the triggering of apoptosis | [37,38,39,40] |
| Apoptosis | Disruption of mitochondrial morphology using 3-oxo-C12-HSL | Quorum sensing molecule 3-oxo-C12-HSL activates the apoptosis by disrupting the mitochondrial structure, attenuating cellular respiration and inducing ROS generation | [41] |
| Apoptosis | Caspase 3-caspase 8-mediated apoptosis | Necrosis factor receptor 1 expelled into the disordered lipid phase triggers cell death | [42] |
| Apoptosis | Apoptosis due to Cx43-mediated cell-to-cell communication | Cx43-mediated gap junctional communication enhances apoptosis in PAO1-infected airway epithelial cells, while on the other hand JNK signaling inhibits Cx43 function | [43] |
| Pyroptosis | PAO1 flagellin induced CASP1-dependent neutrophil pyroptosis | PAO1-induced pyroptosis depends on NLRC4 and Toll-like receptor 5 (TLR5) in neutrophils | [44] |
| NETosis | Type I interferon associated NETosis | Excessive activation of neutrophils by type I IFNs causes aboost in NETosis which triggers biofilm formation by P. aeruginosa, thereby supporting its persistence in the infected lung. | [45,46,47,48] |
| NETosis | NADPH Oxidase-Dependent NETosis | Increase in pH in neutrophils stimulates Nox activity and ROS production requiredl for NETosis | [49] |
| Anoikis | Pseudomonas aeruginosa ExoT induced atypical anoikis | GAP domain of ExoT is responsible for triggering the mitochondrial intrinsic pathway of anoikis apoptosis | [50,51,52] |
| Ferroptosis | P. aeruginosa produced biofilm induces ferroptosis | Caused by enhancing expression of pLoxA as well as oxidising host cell AA-PE to 15-HOOAA-PE | [24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshpande, R.; Zou, C. Pseudomonas Aeruginosa Induced Cell Death in Acute Lung Injury and Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020, 21, 5356. https://doi.org/10.3390/ijms21155356
Deshpande R, Zou C. Pseudomonas Aeruginosa Induced Cell Death in Acute Lung Injury and Acute Respiratory Distress Syndrome. International Journal of Molecular Sciences. 2020; 21(15):5356. https://doi.org/10.3390/ijms21155356
Chicago/Turabian StyleDeshpande, Rushikesh, and Chunbin Zou. 2020. "Pseudomonas Aeruginosa Induced Cell Death in Acute Lung Injury and Acute Respiratory Distress Syndrome" International Journal of Molecular Sciences 21, no. 15: 5356. https://doi.org/10.3390/ijms21155356
APA StyleDeshpande, R., & Zou, C. (2020). Pseudomonas Aeruginosa Induced Cell Death in Acute Lung Injury and Acute Respiratory Distress Syndrome. International Journal of Molecular Sciences, 21(15), 5356. https://doi.org/10.3390/ijms21155356