UP256 Inhibits Hyperpigmentation by Tyrosinase Expression/Dendrite Formation via Rho-Dependent Signaling and by Primary Cilium Formation in Melanocytes
Abstract
1. Introduction
2. Results
2.1. Inhibitory Effects of UP256 on Melanin Synthesis in Melanocytes
2.2. Effects of UP256 on the Expression of Melanogenic Enzymes in Melanocytes
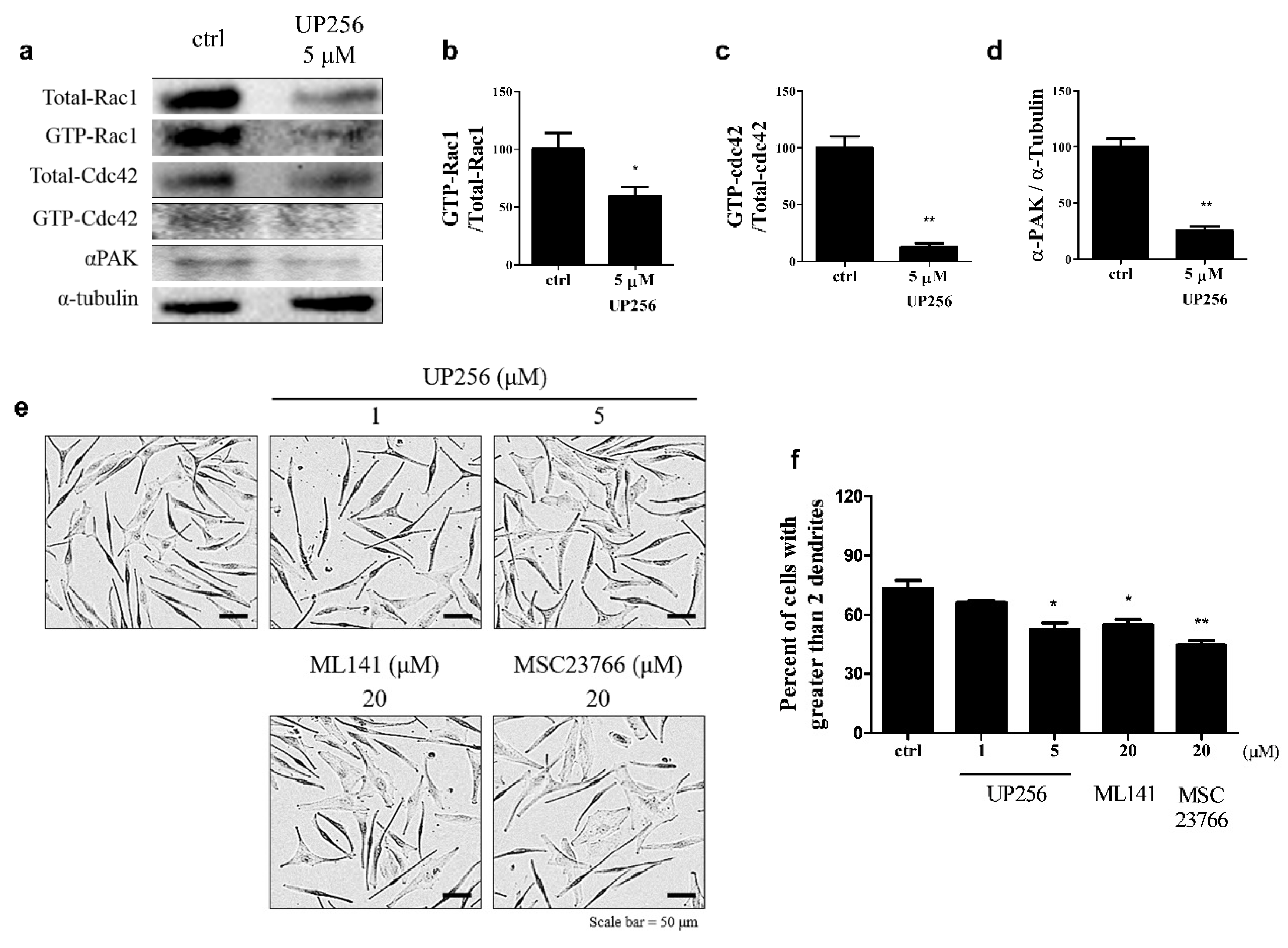
2.3. Effects of UP256 on Rac1, Cdc42, and α-PAK Signaling Proteins in Melanocytes
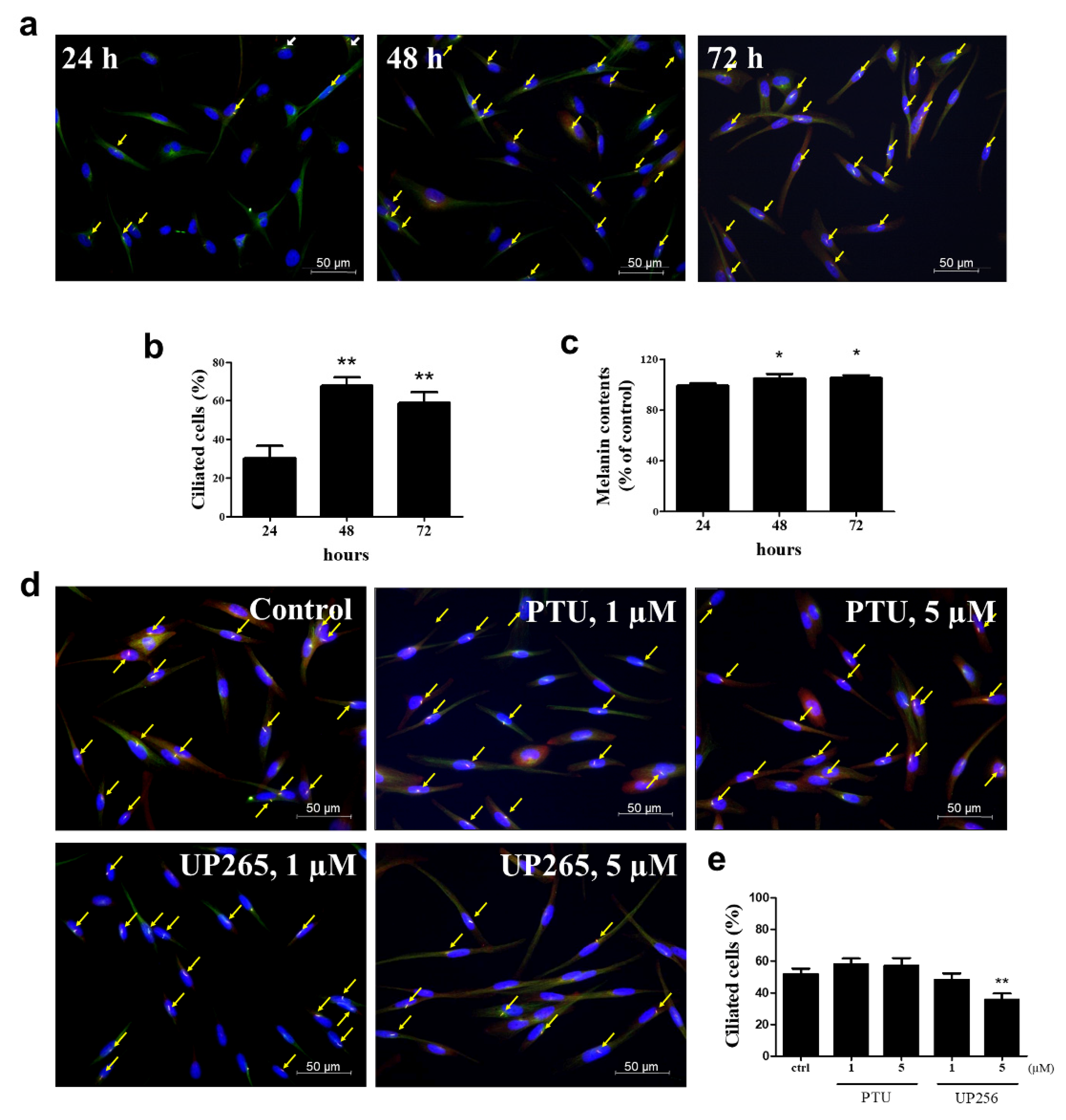
2.4. Inhibitory Effects of UP256 on Cilia Formation in Melanocytes
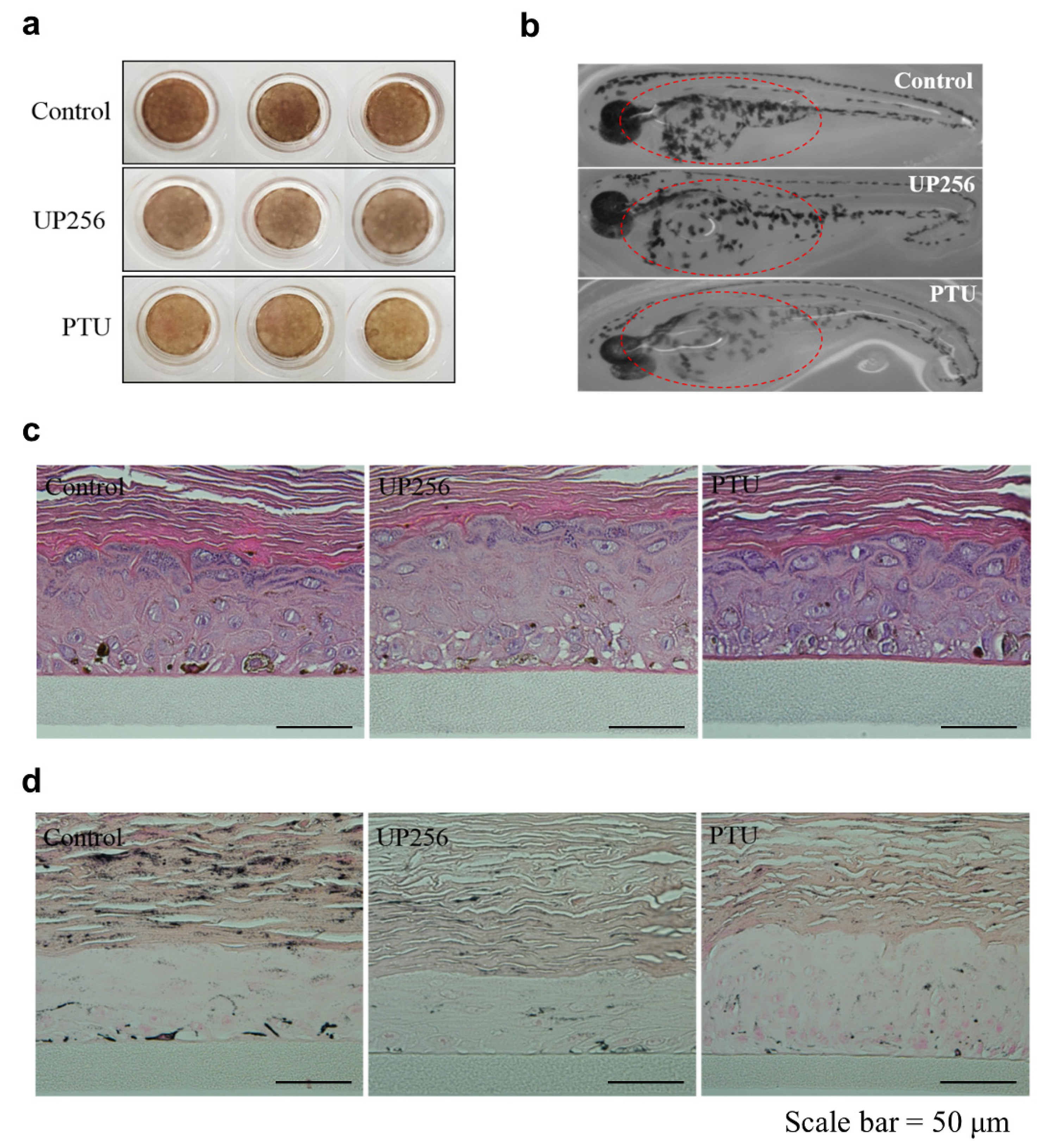
2.5. Effects of UP256 on Melanogenesis In Vivo and Ex Vivo
3. Discussion
4. Materials and Methods
4.1. Preparation of UP256
4.2. Cell Culture and Treatments
4.3. Cell Viability and In Situ Tyrosinase Activity
4.4. Western Blot Analysis
4.5. Zebrafish Model
4.6. 3D Tissue Model
4.7. Detection of Primary Cilia
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Juhasz, M.L.W.; Levin, M.K. The role of systemic treatments for skin lightening. J. Cosmet. Dermatol. 2018, 17, 1144–1157. [Google Scholar] [CrossRef]
- Woolery-Lloyd, H.; Kammer, J.N. Treatment of hyperpigmentation. Semin. Cutan. Med. Surg. 2011, 30, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Takahashi, K.; Zmudzka, B.Z.; Kornhauser, A.; Miller, S.A.; Tadokoro, T.; Berens, W.; Beer, J.Z.; Hearing, V.J. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006, 20, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Murase, D.; Hachiya, A.; Amano, Y.; Ohuchi, A.; Kitahara, T.; Takema, Y. The essential role of p53 in hyperpigmentation of the skin via regulation of paracrine melanogenic cytokine receptor signaling. J. Biol. Chem. 2009, 284, 4343–4353. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Lee, T.H.; Lee, W.J.; Shim, W.S.; Yeo, E.J.; Kim, S.; Kim, S.Y. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigment Cell Melanoma Res. 2016, 29, 81–91. [Google Scholar] [CrossRef]
- Chalupa, A.; Koshoffer, A.; Galan, E.; Yu, L.; Liu, F.T.; Boissy, R.E. Melanocytic galectin-3 is associated with tyrosinase-related protein-1 and pigment biosynthesis. J. Investig. Dermatol. 2015, 135, 202–211. [Google Scholar] [CrossRef]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Park, S.; Yoo, G.; Jang, C.; Kim, M.H.; Kim, S.H.; Kim, S.Y. Demethyleugenol beta-Glucopyranoside Isolated from Agastache rugosa Decreases Melanin Synthesis via Down-regulation of MITF and SOX9. J. Agric. Food Chem. 2016, 64, 7733–7742. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Valencia, J.; Bertolotto, C.; Hoashi, T.; Pape, E.; Takahashi, K.; Ballotti, R.; Hearing, V. Sox9 is a key player in ultraviolet B-induced melanocyte differentiation and pigmentation. Proc. Natl. Acad. Sci. USA 2007, 104, 13984–13989. [Google Scholar] [CrossRef]
- Delevoye, C. Melanin transfer: The keratinocytes are more than gluttons. J. Investig. Dermatol. 2014, 134, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kannamaru, A.; Tada, A. Centaureidin Promotes Dendrite Retraction of Melanocytes by Activating Rho. Biochim. Biophys. Acta 2006, 1760, 487–494. [Google Scholar] [CrossRef]
- Jung, E.; Hwang, W.; Kim, S.; Kim, Y.; Kim, Y.; Lee, J.; Park, D. Depigmenting Action of Platycodin D Depends on the cAMP/Rho-dependent Signalling Pathway. Exp. Dermatol. 2011, 20, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for Hyperpigmentation: What is Available? J. Cutan. Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Skin hyperpigmentation treatment using herbs: A review of clinical evidences. J. Cosmet. Laser Ther. Dermatol. 2017, 20, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Park, K. Current clinical use of depigmenting agents. Dermatol. Sin. 2014, 32, 205–210. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J. Soc. Cosmet. Chem. 1991, 42, 361–368. [Google Scholar]
- Hollinger, J.C.; Angra, K.; Halder, R.M. Are Natural Ingredients Effective in the Management of Hyperpigmentation? A Systematic Review. J. Clin. Aesthet. Dermatol. 2018, 11, 28–37. [Google Scholar]
- Polakova, K.; Fauger, A.; Sayag, M.; Jourdan, E. A dermocosmetic containing bakuchiol, Ginkgo biloba extract and mannitol improves the efficacy of adapalene in patients with acne vulgaris: Result from a controlled randomized trial. Clin. Cosmet. Investig. Dermatol. 2015, 8, 187–191. [Google Scholar]
- Shoji, M.; Arakaki, Y.; Esumi, T.; Kohnomi, S.; Yamamoto, C.; Suzuki, Y.; Takahashi, E.; Konishi, S.; Kido, H.; Kuzuhara, T. Bakuchiol Is a Phenolic Isoprenoid with Novel Enantiomer-selective Anti-influenza A Virus Activity Involving Nrf2 Activation. J. Biol. Chem. 2015, 290, 28001–28017. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Liu, C.C.; Lee, L.S.; Man, C.; Cheng, S.H. Phytoestrogen Bakuchiol Exhibits In Vitro and In Vivo Anti-breast Cancer Effects by Inducing S Phase Arrest and Apoptosis. Front. Pharmacol. 2016, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Shalita, A.R.; Geen, S.C.; Lee, W.L.; Yaping, E. A clinical study evaluating the dermatologic benefits of topical bakuchiol (up256) cream on facial acne. J. Am. Acad. Dermatol. 2011, 64, AB19. [Google Scholar]
- Poma, A.; Bianchini, S.; Miranda, M. Inhibition of L-tyrosine-induced micronuclei production by phenylthiourea in human melanoma cells. Mutat. Res. 1999, 446, 143–148. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- del Marmol, V.; Ito, S.; Jackson, I.; Vachtenheim, J.; Berr, P.; Ghanem, G.; Morandini, R.; Wakamatsu, K.; Huez, G. TRP-1 expression correlates with eumelanogenesis in human pigment cells in culture. FEBS Lett. 1993, 327, 307–310. [Google Scholar] [CrossRef]
- Harris, M.L.; Baxter, L.L.; Loftus, S.K.; Pavan, W.J. Sox proteins in melanocyte development and melanoma. Pigment Cell Melanoma Res. 2010, 23, 496–513. [Google Scholar] [CrossRef]
- Newton, R.A.; Cook, A.L.; Roberts, D.W.; Leonard, J.H.; Sturm, R.A. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J. Investig. Dermatol. 2007, 127, 2216–2227. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef]
- Impey, S.; Davare, M.; Lesiak, A.; Fortin, D.; Ando, H.; Varlamova, O.; Obrietan, K.; Soderling, T.R.; Goodman, R.H.; Wayman, G.A. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol. Cell. Neurosci. 2010, 43, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Chavali, P.L.; Gergely, F. Cilia born out of shock and stress. EMBO J. 2013, 32, 3011–3013. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Airik, R.; Ghosh, A.K.; Giles, R.H.; Chen, R.; Slaats, G.G.; Wang, H.; Hurd, T.W.; Zhou, W.; Cluckey, A.; et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 2012, 150, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Shin, J.H.; Kim, E.S.; Park, S.J.; Bae, I.H.; Jo, Y.K.; Jeong, I.Y.; Kim, H.J.; Lee, Y.; Park, H.C.; et al. Primary Cilia Negatively Regulate Melanogenesis in Melanocytes and Pigmentation in a Human Skin Model. PLoS ONE 2016, 11, e0168025. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.C.; Lee, J.-W.; Lee, T.H.; Subedi, L.; Wahedi, H.M.; Do, S.-G.; Shin, E.; Moon, E.-Y.; Kim, S.Y. UP256 Inhibits Hyperpigmentation by Tyrosinase Expression/Dendrite Formation via Rho-Dependent Signaling and by Primary Cilium Formation in Melanocytes . Int. J. Mol. Sci. 2020, 21, 5341. https://doi.org/10.3390/ijms21155341
Kang MC, Lee J-W, Lee TH, Subedi L, Wahedi HM, Do S-G, Shin E, Moon E-Y, Kim SY. UP256 Inhibits Hyperpigmentation by Tyrosinase Expression/Dendrite Formation via Rho-Dependent Signaling and by Primary Cilium Formation in Melanocytes . International Journal of Molecular Sciences. 2020; 21(15):5341. https://doi.org/10.3390/ijms21155341
Chicago/Turabian StyleKang, Min Cheol, Jae-Wook Lee, Taek Hwan Lee, Lalita Subedi, Hussain M. Wahedi, Seon-Gil Do, Eunju Shin, Eun-Yi Moon, and Sun Yeou Kim. 2020. "UP256 Inhibits Hyperpigmentation by Tyrosinase Expression/Dendrite Formation via Rho-Dependent Signaling and by Primary Cilium Formation in Melanocytes " International Journal of Molecular Sciences 21, no. 15: 5341. https://doi.org/10.3390/ijms21155341
APA StyleKang, M. C., Lee, J.-W., Lee, T. H., Subedi, L., Wahedi, H. M., Do, S.-G., Shin, E., Moon, E.-Y., & Kim, S. Y. (2020). UP256 Inhibits Hyperpigmentation by Tyrosinase Expression/Dendrite Formation via Rho-Dependent Signaling and by Primary Cilium Formation in Melanocytes . International Journal of Molecular Sciences, 21(15), 5341. https://doi.org/10.3390/ijms21155341




