Abstract
p62 is a versatile protein involved in the delicate balance between cell death and survival, which is fundamental for cell fate decision in the context of both cancer and neurodegenerative diseases. As an autophagy adaptor, p62 recognizes polyubiquitin chains and interacts with LC3, thereby targeting the selected cargo to the autophagosome with consequent autophagic degradation. Beside this function, p62 behaves as an interactive hub in multiple signalling including those mediated by Nrf2, NF-κB, caspase-8, and mTORC1. The protein is thus crucial for the control of oxidative stress, inflammation and cell survival, apoptosis, and metabolic reprogramming, respectively. As a multifunctional protein, p62 falls into the category of those factors that can exert opposite roles in the cells. Chronic p62 accumulation was found in many types of tumors as well as in stress granules present in different forms of neurodegenerative diseases. However, the protein seems to have a Janus behaviour since it may also serve protective functions against tumorigenesis or neurodegeneration. This review describes the diversified roles of p62 through its multiple domains and interactors and specifically focuses on its oncoJanus and neuroJanus roles.
1. Introduction
p62 is a multifunctional protein that was originally identified as a component of the sequestosome, a cytoplasmic structure which serves as a storage place for ubiquitinated proteins. [1]. The p62 encoding gene was then isolated and termed sequestosome 1 (SQSTM1) [2]. The protein was the first adaptor involved in selective autophagy identified in mammals [3]. Autophagy is a widely described physiological process of recycling cell components through autophagosome formation and lysosomal cargo digestion. The autophagic process requires a number of gene products named Atg proteins [4] and satisfies two major cellular requirements: response to metabolic stress due to nutrient deprivation and detoxification through waste removal. It is thus not surprising that basal autophagy occurs in the cells under normal conditions and that the process highly increases under particular cellular stresses [5,6]. While in “non-selective” autophagy, the autophagosome randomly sequesters cytoplasmic material, in selective autophagy the autophagosome targets specific cargoes and requires selective adaptors [7]. Among selective cargoes, poly-ubiquitin tagged proteins are recognized by specific adaptors or alternatively transmembrane receptor proteins directly bind to cargoes without requiring ubiquitin [8]. Studies on selective protein degradation reveal a crucial role of p62 in autophagic digestion of poly-ubiquitinated protein cargo [9]. During the early steps of autophagosome formation, a dimeric form of p62 is phosphorylated in Ser407 by Atg1/ULK1 kinase, one of the upstream ATG gene products that trigger the autophagic flux. This phosphorylation destabilizes the p62 dimer and renders the protein prone to undergo subsequent phosphorylation by other kinases (casein kinase2 or TANK-binding kinase1) to increase the binding affinity of p62 for ubiquitin chains [10]. It is well known that p62 can preferentially bind to certain ubiquitin chain types and this might represent a subtle level of regulation during selective autophagy. Generally, while Lys48-linked ubiquitin chains are recognized by the 26S proteasome, Lys63-linked ubiquitin chains are also associated with selective autophagy and are preferentially recognized by p62 [11].
Following binding to ubiquitinated proteins, p62 undergoes oligomerization and interacts with the autophagosomal membrane protein LC3, thus delivering the cargo aggregates to the autophagosome [12]. LC3 exerts multiple roles in autophagy including membrane fusion, cargo selection and autophagosome mobilization. LC3/p62 interaction is indispensable for autophagic degradation of polyubiquitinated cargo. Moreover, the same p62 becomes degraded by the autophagosome, which makes the protein a marker to monitor the autophagic flux. Indeed, a precocious increase in p62 cellular levels is usually observed during autophagy execution, and a subsequent decrease, due to autophagic degradation of the protein, occurs later when autophagy is completed [13,14,15,16].
As a multifunctional protein, p62 not only takes part in selective autophagy, but also interacts with many factors that play important roles in determining cell fate. p62 behaves indeed as a signalling hub and can be considered a main regulator of key pathways including the nuclear factor (erythroid-derived 2)-like 2/Kelch-like ECH-associated protein 1 (Nrf2/Keap1) oxidative defense system, NF-kB mediated-inflammation and pro-survival response, mTORC1 related nutrient sensing, and caspase-8- mediated apoptosis [17,18,19,20]. Due to its versatile behaviour and capability of recruiting diverse binding partners to influence many cellular processes, p62 is clearly involved in important diseases including cancer and neurodegenerative disorders [21,22,23]. A common denominator for such a wide range of issues is represented by the balance between cell death and survival, which involves strategic crosstalk between apoptosis and autophagy, metabolic programming, redox balancing, and fine regulation of functional networks determining cell fate. p62 seems to take part in all of these processes and its contribution to their regulation is commonly considered noteworthy, as revealed by a number of studies correlating the factor to both cancer development and neurodegeneration. Intriguingly, p62 behaviour in tumor environment as well as in neuronal circuits is not always univocal. This review aims to highlight the double face of p62, critically discussing the most recent findings in the literature and evidencing the interconnections between pathways involved in cancer development and in neurodegenerative diseases.
2. p62 Structure, Post-translational Modifications, and Conformational Changes
Human p62 protein is encoded by SQSTM1 gene, which is located on chromosome 5 and contains 8 exons distributed along 16 kb. Transcriptional activation of SQSTM1 gene can be promoted by various cellular stresses. The SQSTM1 gene promoter indeed contains specific response elements including kB, CLEAR, and ARE, which respond to the respective master gene regulators NF-kB, Mit/TFE, and Nrf2. Specifically, NF-kB can activate p62 under inflammatory or pro-survival responses, Mit/TFE and Nrf2 stimulate p62 expression under metabolic stress and oxidative stress, respectively [24,25,26].
Concerning the structure, although human p62 protein is homologous to mouse and rat p62, very different alternative splicing mechanisms have been identified in these models [27,28]. Human p62 exists in two protein isoforms that originate by alternative splicing of three mRNA variants [29]. p62 mRNA variant 1 encodes the full length 440-aa protein. The other two mRNA variants (2 and 3) share the same coding sequence but differ in their 5′UTR regions. Both these variants encode p62 isoform 2, which is 84 amino acids shorter than p62 isoform 1 at the N terminus. While the precise function of this isoform remains unknown, the full length p62 is widely expressed and represents the predominant one. The full length p62 protein structure, including domains and multiple interactors, is reported in Figure 1.
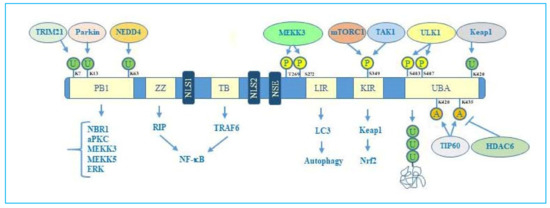
Figure 1.
p62 structure, functional domains, post-translational modifications, and interactors. In particular, the figure shows Phox/Bem1p (PB1) domain, which is important for p62 oligomerization, with indicated ubiquitination sites, relative ubiquitinating enzymes (Trim21, parkin, NEDD4) and interactors (NBR1, atypical protein kinase C (aPKC), MEKK3, MEKK5, ERK); Zinc finger ZZ and TRAF bindingTB domains necessary for interaction with receptor interacting protein (RIP) and TRAF6, respectively, to activate NF-κB signalling; nuclear localization sequences (NLS1 and NLS2), and nuclear export sequence (NES) which account for nuclear-cytoplasmic shuttling of p62; LYR and Keap1-interacting region (KIR) domains that interact with LC3 and Keap1 respectively to promote selective autophagy and nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-mediated signalling. Ubiquitin associated (UBA) domain is fundamental for recognition of poly-ubiquitinated cargo during selective autophagy. Post-translational modification sites including phosphorylation, acetylation, and ubiquitination together with the respective regulative enzymes (MEKK3, mTORC1, TAK1, Keap1, Tip60, HDAC6) are also indicated.
2.1. PB1 Domain and p62 Oligomerization
In the N-terminal region Phox/Bem1p (PB1) domain is present, which is responsible for homo-dimerization or formation of p62 oligomeric filaments [30]. While the dimeric form of p62 is substantially inactive with respect to autophagy, polymerization of p62 in a filamentous form, which renders the protein functional, is promoted by the interaction of the protein with the autophagic receptor NBR1 (the neighbor of BRCA1 gene). This factor specifically binds to PB1 domain and cooperates with p62 in targeting poly-ubiquitinated proteins to the autophagosome during selective autophagy [31]. Jacobi at al. have recently elucidated the structural basis of p62 helical filaments formation through the PB1 domain and their role in autophagic cellular cargo uptake [32]. Phase condensation of p62 in non-membrane liquid compartment is crucial for its role of cargo receptor. In this regard, evidence has been provided that p62 “puncta formation”, the process of liquid–liquid phase separation that is needed for p62 oligomerization is enhanced by the cytoplasmic histone H3F3/H3.3 chaperone DAXX [33].
PB1 domain can also interact with other PB1 containing proteins involved in signal transduction including atypical protein kinase C (aPKC), cAMP-degrading phosphodiesterase-4 (PDE4) and MAP kinase pathway members such as MEKK3, MEK5, and ERK [34]. Another p62 binding partner in this region is NEDD4 (neuronal precursor cell expressed developmentally down regulated protein 4). This factor acts as a ubiquitin ligase and is involved in p62 ubiquitination in the PB1 domain facilitating autophagy [35].
2.2. The Zinc Finger ZZ Domain, Nuclear Localization, and Export Sequences
The zinc finger domain (ZZ) is located near the PB1 domain and is specifically recognized by receptor interacting protein (RIP). This interaction leads to the activation NF-κB through aPKC- mediated pathway [21]. ZZ is a typical DNA binding domain, however, although two nuclear localization sequences (NLS1 and NLS2) together with a nuclear export sequence (NES) are present, there are not direct evidences that p62 can bind to the DNA.
Regarding the nuclear localization of p62, data present in the literature indicate that p62 knockdown increases chromatin poly-ubiquitination. In this regard, Feng et al. have proposed that p62 is involved in autophagy-regulated DNA repair through inhibition of the E3 ligase RNF168-dependent ubiquitination of histones [36]. Notably, Salmina et al. found nuclear clustering of p62 foci together with the telomere capping protein TRF2 and the DNA damage molecular marker βH2AX in doxorubicin treated breast cancer cells. These authors also found that damaged DNA in the form of telomere fragments was sorted into the cytoplasm in association with p62 [37]. Another interesting study of p62 nuclear localization regards the involvement of the protein in a nuclear form of cell death known as parthanatos, which is dependent on poly (ADPribose) polymerase-1 (PARP-1) [38]. In this study, p62 was shown to be required for nuclear accumulation of aggresome-like structures, resulting in the induction of parthanatos. p62 was also found in association with transcription factors such as TFIIE [39] and TFIIH [40], suggesting a possible role in transcriptional regulation.
2.3. The TRAF binding TB Domain
The TRAF binding domain (TB) is located between the NLS1 and NLS2 regions and is specifically recognized by RING finger domain proteins such as tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6). This factor is a ubiquitin E3 ligase, whose activity is critical for activation of NF-κB signalling in response to IL-1 [41]. Therefore, through this domain, p62 also behaves as a significant intermediate of IL-1 signalling pathway to stimulate NF-κB activation during inflammation or pro-survival response. The connection of p62 with NF-κB will be discussed in the next paragraph.
2.4. The LC3-Interacting Region (LIR) Domain and Autophagy
LC3-interacting region (LIR) is required for autophagy since it represents the binding site for LC3 to promote the autophagosome formation, thus linking the core autophagy machinery to the target cargo identified by p62. LIR motif, indeed, guides the ubiquitinated cargo captured by p62, through the ubiquitin associated domain (UBA), to Atg8/LC3, which is anchored to the surface of the autophagosomal membrane. Beyond LC3 binding, LIR domain can also interact with other autophagic proteins including beclin and BNIP3 [42,43], representing a fundamental domain for selective autophagy execution.
2.5. The Keap1-Interacting Region (KIR) Domain and Nrf2 Signalling Activation
Notably, in addition to degradation of ubiquitinated cargo during selective autophagy, p62 is also involved in the degradation of other substrates such as Kelch-like ECH-associated protein 1 (Keap1), the well-known inhibitor of the oxidative response transcription factor Nuclear factor erythroid-2 related factor 2 (Nrf2). Keap1 binds to a specific motif in p62, called Keap1-interacting region (KIR) which is located next to the LIR domain. KIR is required for p62 to activate Nrf2 through suppression of Keap1 in response to oxidative stress [44]. Phosphorylation of Ser349 in this domain has been shown to be crucial for Keap1 recognition and sequestration [45].
Kageyama et al. have recently identified a p62 splicing variant in mice lacking the KIR domain, this variant produced opposite effects increasing the amount of Keap1, ubiquitination of Nrf2, and consequent suppression of its transcriptional targets [28].
2.6. The Ubiquitin Associated (UBA) Domain, Phosphorylation, and Acetylation of p62
The ubiquitin associated domain (UBA) is located next to KIR domain, at the carboxy-terminal region of p62. This domain is a core regulator of the interaction between p62 and the poly-ubiquitinated cargo and is thus necessary for selective autophagy-mediated protein degradation. In an inactive form, homodimerization of UBA domain prevails with consequent impediment of ubiquitinated cargo recognition. Upon autophagic stimuli, phosphorylation by ULK1 kinase in Ser407 present in the UBA domain induces dissociation of the p62 dimer to form a monomer that is subsequently phosphorylated by ULK1 or other kinases such as casein kinase 2 or TANK binding kinase. This modification enhances p62 binding to poly-ubiquitinated proteins. Intriguingly, it has been shown that upon ubiquitin binding p62 acquires liquid like properties since it forms droplets that serve as interactive nodes in the context of selective autophagy. The droplet formation is favoured by the interaction through the PB1 domain of p62 with NBR1, as previously mentioned [31]. Beside p62 regulative phosphorylation within the UBA domain, another important post translational modification is represented by acetylation. In this regard, You et al. have shown that Lys420 and Lys435 in the UBA domain are the main acetylation sites, which respond to TIP60 acetyltransferase and HDAC6 deacetylase [46]. The same authors showed that p62 acetylation occurs under nutrient stress and is required to promote p62 binding to poly-ubiquitinated cargo during selective autophagy.
Overall, the flexible structure of p62 and the fine regulation due to post translational changes makes this protein a versatile interactor and a key factor regulating diverse cellular processes.
3. p62 Functional Interplay with Key Factors Regulating Cell Death and Survival
Understanding the molecular basis of highly complex pathologies like cancer and neurodegenerative diseases cannot prescind from an accurate analysis of the delicate balance between cell death and survival within the cells. It is currently recognized that autophagy plays a double role in cancer development as well as in neurodegenerative diseases [47,48]. The process can indeed be promoted as a pro-survival response to particular cellular stresses or it can evolve into a cell death mechanism, namely autophagic cell death, also known as type II programmed cell death [49]. Intriguingly, crosstalk between autophagy and other forms of programmed cell death, including classic apoptosis and/or necroptosis, represents a key point to dissect those networks that establish the cell fate towards either tumorigenesis or neurodegeneration.
p62 has been shown to exert a fundamental role in both autophagy and apoptosis regulation due to its ability to interact with key factors regulating these processes.
Beyond the important role of p62 in binding poly-ubiquitinated cargoes and LC3 recognition during the autophagosome formation, the protein function in autophagy regulation is also correlated with interactions with other specific partners.
It is well known that autophagy is promoted by diverse cellular stresses including oxidative stress, metabolic stress under nutrient deprivation, endoplasmic reticulum (ER) stress, and inflammation.
3.1. Interplay p62-Nrf2/Keap1
Concerning oxidative stress, p62 is known to take part in the Nrf2/Keap1 mediated signalling, an important cytoprotective and antioxidant response mediated by the activation of the transcription factor Nrf2. The levels of Nrf2 are kept low in the cells under normal conditions since the factor is degraded by the ubiquitin proteasome system. This event is promoted by the Nrf2 inhibitor Keap1, which binds Nrf2 and promotes its ubiquitination and proteasome-mediated degradation. Upon oxidative stress, Keap1 undergoes a conformational change that decreases affinity to Nrf2 [50]. Consequently, Nrf2 is released and translocates to the nucleus, where it promotes the expression of antioxidant and pro-survival genes. p62 has been shown to interact, through its KIR domain, with Keap1 at the same binding site for Nrf2 and thus competitively inhibits the Keap1/Nrf2 interaction resulting in Nrf2 activation (Figure 2a). As previously mentioned, binding to Keap1 is favoured by phosphorylation of Ser349 present in p62 KIR domain [45]. Different kinases including mechanistic target of rapamycin complex 1 (mTORC1) and TGF-β activated kinase (TAK1) can phosphorylate p62 in this residue thereby promoting Nrf2 activation. Notably, p62 and Nrf2 have a mutual relationship since Nrf2, which can be activated by p62, can in its turn stimulate SQSTM1 gene transcription [51]. Moreover, p62 binding to Keap1 seems to target Keap1 for inclusion in the autophagosome and consequent degradation by selective autophagy [52]. On the other hand, evidence has been provided that Keap1 can favour p62 ubiquitination in the UBA domain, an event that promotes its degradation when autophagy is completed. These evidence suggest that Nrf2/Keap1 pathway and p62 mediated-autophagy are coupled with each other. This connection is particularly important in cancer development and will be discussed in the next section.
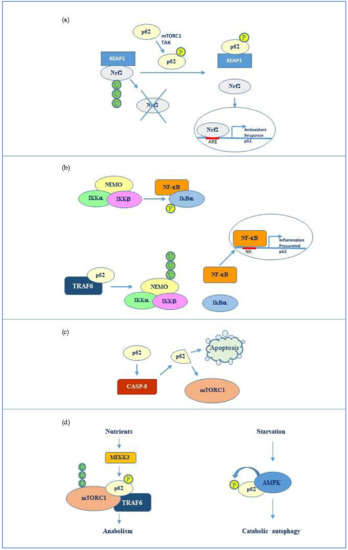
Figure 2.
p62 molecular interactors and relative signalling. (a) Phosphorylated p62 (Ser349) activates nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-mediated oxidative response and cell survival by sequestering Kelch-like ECH-associated protein 1 (Keap1). p62 expression is then activated by Nrf2 in an amplification loop. (b) p62 promotes NF-kappa-B essential modulator (NEMO) ubiquitination via TRAF6 favouring NF-κB activation and consequent transcription of genes involved in inflammation and pro-survival response. NF-κB also induces the same p62 expression. (c) p62 stimulates caspase-8 which in turns cleaves p62 thus promoting apoptosis or mTORC1-mediated nutrient sensing. (d) mTORC1 modulation by p62 via TRAF6 and stimulation of anabolism in the presence of nutrients and AMP kinase mediated p62 phosphorylation during starvation and consequent catabolic autophagy.
3.2. p62 Relationship with NF-κB and Caspase 8
Nuclear factor-κB (NF-κB) represents a family of transcription factors that are fundamental in inflammatory responses and are considered among the most important molecules linking chronic inflammation to both cancer and neurodegenerative diseases [53,54]. Recent evidences support an interesting interplay between p62 and NF-κB mediated signalling, which can be decisive to balance autophagy with classic apoptosis.
P62 can activate NF-κB via its tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6) binding motif (TB), thereby enhancing inflammatory responses mediated by IL-1 or TNF [41] or anti-apoptotic and pro-survival signals [55]. NF-κB, in its turn, can stimulate p62 gene transcription, constituting a feed-forward loop that amplifies its signalling effects [24]. Concerning TRAF6, p62 seems to promote TRAF6-mediated Lys63-linked ubiquitination of NEMO, a regulatory factor of the NF-κB inhibitory complex (IKK), thus promoting NF-κB release and activation [56] (Figure 2b). Oppositely, p62 may also inhibit TRAF6 signalling [57] and promote caspase-3 activation and consequent apoptosis execution [58]. In addition, Young et al. have shown that poly-ubiquitination of p62 favours Fas/CD95 aggregation to induce caspase-dependent apoptosis [59]. In this scenario, the role of p62 in apoptosis appears to be dual since the protein can either block apoptosis favouring autophagy in an initial phase or promote apoptosis switch following autophagy triggering. For instance, p62 has been shown to activate caspase-8 under oroxylin treatment in hepatocellular carcinoma and to be further degraded by the same active caspase. This generated a cleaved p62 form that lost the ability to activate Nrf2, thus promoting oxidative stress and cell death [60]. In another experimental model, Garrido et al. have shown that a cleaved p62 form obtained from proteolytic cleavage by caspase 8, instead of functioning in autophagy or in apoptosis, was selectively involved in nutrient-sensing and cell homeostasis through mTORC1 [61] (Figure 2c).
3.3. p62 Interaction with mTORC1
Among p62 interactors, mTORC1 represents another key factor in cell fate regulation. Specifically, mTORC1 is a complex composed of mTOR (the effective kinase subunit) associated with Raptor, a component important for mTORC1 localization and substrate recognition, and mLST8, which promotes mTOR kinase activity. The activity of the whole complex is negatively modulated by PRAS40 and DEPTOR, two inhibitory subunits that are also present in the complex [23]. mTORC1 is a major complex involved in nutrient sensing and represents a crossroad between catabolic and anabolic pathways in the cells [62]. In the presence of nutrients, mTORC1 promotes anabolic processes including protein, lipid, and nucleotide synthesis to favour cell growth, concomitantly inhibiting catabolic pathways such as autophagy. The inhibitory effect of mTORC1 on autophagy is related to phosphorylation of ULK1, an autophagy-initiating kinase, promoted by the complex [63]. Surprisingly, p62, which is clearly involved in autophagy, has been shown to activate mTORC1 [64]. In particular, evidence has been provided that p62 is phosphorylated at Thr269 and Ser272 by MEKK3 in response to amino acid abundance [65]. In this phosphorylated form, p62 recruits the ubiquitin ligase TRAF6 and mTORC1 resulting in Lys63-linked ubiquitination and consequent activation of mTOR (Figure 2d). This most likely represents an inhibitory feedback that p62 promotes with respect to catabolic autophagy under nutrient abundance, coupling this condition to anabolism and cell growth.
Conversely, energy reduction due to nutrient deprivation activates the metabolic sensor AMP kinase which inhibits mTORC1 directly by phosphorylation and indirectly by stimulation of its inhibitor mTORC negative regulator (TSC) [66]. AMP kinase has been shown to phosphorylate p62 in Ser294 following insulin withdrawal in hippocampal neural stem cells, thus promoting autophagy in this model [67] (Figure 2d). Implication of p62 in cell metabolism regulation together with its role in balancing cell death and survival open a new perspective to understand the molecular basis of both cancer and neurodegenerative diseases.
4. OncoJanus Role of p62
The versatile properties of p62 and diversified roles deriving from the broad spectrum of interactors make the protein a double-edged sword in cancer. A number of papers support an oncogenic role of p62 in a wide variety of human cancers [21]. However, recent findings also suggest that the protein may behave oppositely counteracting tumor development and exerting a pro-apoptotic function [20]. This paragraph aims to critically revise the most recent literature in this regard and discuss the oncoJanus role of p62.
4.1. Pro-Tumor Role of p62
Accumulation of p62 has been found in several forms of tumors including hepatocarcinoma, breast, lung, gastric, colon, and ovarian cancers [68,69,70,71,72]. Increasing evidence demonstrates that abnormal expression of p62 is associated with malignancy in most cases. For instance, an amplified p62 copy number on chromosome 5q has been found in renal cancer and overexpression of the SQSTM1 gene was identified in many other tumors [23,73]. Notably, p62 knockout or knockdown have been shown to abrogate tumor growth in different cancer models [74,75]. In such a way, p62 may be considered as an oncogene.
From a molecular point of view, given that p62 abundance may favour pro-survival tumor-associated autophagy, increased activity of p62 was also correlated with increased phosphorylation. In particular, the phosphorylated form of p62 in Ser349 leads to persistent activation of Nrf2 signalling promoting cell survival and consequent tumor growth. Interestingly, Ichimura et al. have identified a specific inhibitor for the Keap1/phospho-p62 (Ser349) interaction, the acetonyl naphthalene derivative K67 [45]. These authors showed that treatment of hepatocarcinoma cells with K67 suppressed proliferation and reduced tumor resistance to cisplatin or sorafenib [76].
p62/Nrf2 signalling can be also negatively regulated by the tumor suppressor PCDC4 (programmed cell death 4). In this regard, evidence has been provided that induction of PDCD4 overexpression in lung cancer increased Keap1 levels and reduced the activity of the p62/Nrf2 pathway, thereby inhibiting tumorigenesis [77]. In addition, other authors have shown that p62 knockdown in hepatoma cells upregulated PCDC4 levels and demonstrated that p62, PCDC4 and LC3 co-localized in particles to promote PCDC4 autophagic degradation [78].
Other molecular events that may explain p62 oncogenic potential are correlated with NF-κB or mTORC1 activation. Recently, Sanchez Lopez et al. have found NF-κB-driven p62 expression in chronic lymphocytic leukemia (CLL) correlates with both Nrf2 and mTORC1 activation [79].
Of relevance is the finding of these authors that CLL cells expressing on the surface high ROR1, an onco-embryonic orphan receptor, display increased p62/Nrf2-mediated antioxidant response and consequent resistance to pro-oxidative drugs such as venetoclax. Notably, they propose a possible targeted therapy for this neoplasia consisting in administration of a drug that is selectively activated by antioxidant enzymes induced by p62/Nrf2 signalling.
As previously discussed, mTORC1 is strictly associated to anabolism and cell growth, therefore cancer cells are highly benefited by increased activity of this factor. Activation of mTORC1 related to p62 upregulation was found, for instance, in hepatocellular carcinoma [68]. Moreover, p62 has been recently shown to play a key role in cellular redox homeostasis by keeping mitochondrial integrity and glutathione levels necessary to support mTORC1 hyperactivation [73]. Notably, Tamura et al. have shown that combination of a dual PI3K/mTOR inhibitor with p62 knockdown targeted human bladder cancer either in vitro or in vivo [80]. More recently, Xu et al. provided evidence that p62 was enriched in small cell lung cancer spheroids with cancer stem-like properties and that p62 knockdown sensitized these cells to cisplatin [81].
4.2. Antitumor Role of p62
The other side of the coin is that p62 can exert a protective function against tumorigenesis. For instance, p62 levels were found to be reduced in the stroma of several tumors and loss of p62 in the tumor microenvironment resulted in increased tumorigenesis [82]. In particular, the same authors showed that inactivation of mTORC1 in p62-deficient stromal fibroblasts caused a metabolic reprogramming that increased stromal IL-6 production, which promoted inflammation and tumor growth. Therefore, in this context, p62 seemed to behave as an anti-inflammatory and tumor suppressor.
Metabolic reprogramming is a hallmark of tumor cells. A fascinating study of how p62 is implicated in the communication between metabolic tissues and cancer cells is provided Yuang et al. Specifically, these authors propose p62 as a potential metabolic tumor suppressor and describe the symbiotic collaboration between adipose tissue and tumor cells that profoundly affects cancer metabolic fitness [83]. In addition, p62-deficient tumor stroma has been shown to produce asparagine that is released and sustains tumor metabolism in conditions of glutamine deprivation [84].
In the scenario of tumor microenvironment and communication of tumor cells with other cell types, a relevant function of p62 has been identified in tumor surrounding macrophages.
Induction of p62 through NF-κB in macrophages serves to limit the release of cytokines, such as IL-1β and consequent inflammation, which is likely to promote tumor survival and growth [24]. Moreover, p62 exerts a protective function in macrophages since p62-dependent autophagy promotes the clearance of mitochondria that have been altered following inflammasome activation in these cells [85]. It is well known that inflammasome activation in tumor associated macrophages promotes tumorigenesis. These evidences indicate that p62 preserves macrophage homeostasis and reduces their oncogenic potential. Overall, p62 displays a key role in the tumor microenvironment through a fine regulation of the crosstalk between different cell types and cancer cells in the tumor niche.
Intriguingly, p62 has also been shown to exert an anti-tumor function within cancer cells. For instance, at the nuclear level p62 can control DNA damage through inhibition of Histone H2A ubiquitination, thus affecting DNA repair and increasing the sensitivity of tumor cells to radiation [86]. In such a perspective, high levels of p62 can even represent an advantage for cancer therapy.
In accordance, Yan et al. have shown that overexpressed wild-type p62 in ovarian cancer cells significantly increased activation of caspase 8, with consequent apoptosis, and ameliorates the response to cisplatin [20]. Moreover, evidence has been provided that p62 ectopic expression in vivo reverts tumor grade, changes tumor stroma and enhances anticancer immunity [87]. Even more recently, Choi et al. showed increased levels of nuclear p62 upon ascorbic acid treatment in breast cancer cells correlated with endoplasmic reticulum stress and cell death [88]. Although the precise role of nuclear p62 remains to be clarified, it is relevant that this localization seems to be related with an anti-tumor function. In this regard, Liu et al. showed that low nuclear level of p62 was associated with aggressive clinic pathologic features and unfavourable prognosis of oral squamous cell carcinoma [89].
Intriguingly, a p62 encoding plasmid named Elenagen was used in association with standard chemotherapy to treat a chemoresistant triple-negative breast cancer patient with significant improvement in the response and increase in progression-free survival [90].
Taken together, all these findings strongly support the dual role of p62 in cancer. If on the one hand we consider p62 as an oncogene because of its overexpression in many tumors, on the other hand this condition may be strategically used against cancer to improve the therapeutic efficacy. Of relevance is the role of p62 in cells of the tumor microenvironment, which sustains its tumor suppressor properties. Elucidating the interaction mechanisms of tumor cells with other cell types within the tumor niche will definitely shed light on the specific p62 function in tumor development and progression. From a general perspective, we have to consider that the behaviour of the factor can be cell specific and may depend on a number of variables and interaction networks. Pro-tumor and anti-tumor activities of p62 are summarized in Table 1.

Table 1.
Highlights of research findings of p62double role in cancer.
5. NeuroJanus Role of p62
Given the role of p62 in selective autophagy and implications in regulating the balance between cell death and survival, it is not surprising that this multifunctional protein is also involved in the pathogenesis of neurodegenerative diseases. Due to its functional plasticity, p62 serves indeed as a signalling hub for multiple pathways implicated in neurodegeneration. p62 may be neuroprotective since it promotes pro-survival autophagy against various types of neuronal stress or it may be neurotoxic when overexpressed or deregulated. Moreover, many evidences indicate that aberrant p62 is found in association with specific aggregates that are typical of different neurodegenerative diseases [22,99]. This paragraph summarizes and discussed the most recent findings about the opposite roles of p62 in neurodegeneration, focusing on three major neurodegenerative disorders: Alzheimer disease (AD), Parkinson disease (PD), and Amyotrophic lateral sclerosis (ALS).
5.1. p62 in Alzheimer Disease
AD is one of the most widespread neurodegenerative diseases and the most common cause of dementia. It is a progressive disorder that causes severe cognitive dysfunctions and decline of memory. The molecular basis of AD is related with β-amyloid (Aβ) plaques accumulation in the brain and hyperphosphorylated tau-containing neurofibrillary tangles within the neurons [100].
Low expression of p62 has been found in many forms of AD and p62 loss of function has been shown to result in Aβ accumulation, tau hyperphosphorylation, and consequent neurodegeneration [101,102].
Interestingly, overexpression of p62 was able to rescue cognitive deficit in transgenic AD mice models, increasing neuronal pro-survival autophagy and affecting Aβ level and amyloid plaque formation. The protective role of p62 is most likely correlated with its capability of promoting the degradation of misfolded protein through the autophagy pathway. Moreover, p62 has been shown to inhibit Aβ-induced neuronal death through TRAF6-mediated ubiquitination of p75 neurotrophin receptor, which is known to bind Aβ and transduce death signals [103].
Deregulation of p62 in AD may also depend on increased p62 phosphorylation. In particular, as previously discussed, increased phosphorylation of Ser349 in KIR domain of p62 is related with Nrf2 activation. Tanji et al. revealed that the ratio of phosphorylated (Ser349) p62 to total p62 was considerably increased in the brains of AD patients compared to controls [104]. The role of Nrf2 in Alzheimer disease has been well described in a comprehensive review by Fão et al. [105].
The discussion above sustains that functional p62 level is decreased in AD patients, causing autophagy failure. However, increased autophagy can occur during Alzheimer development and its deregulation may cause neuronal damage [106]. For instance, in some cases, mitophagy, the selective autophagic degradation of mitochondria involving p62, was found excessively intensified in neurons leading to synaptic deterioration and axon degeneration [99]. In this regard, Wang et al. have recently shown that Aβ promotes the accumulation of mitochondrial p62, which was associated with impaired mitophagy correlated with neuronal cell death. In such a way, p62 seems to appear neurotoxic. Restoration of Aβ-induced mitochondrial dysfunction and impaired mitophagy was obtained by overexpressing parkin [107], an ubiquitin ligase that will be described in the next subsection. Moreover, in this connection, autophagy inhibition has been proposed as a tool to attenuate Aβ toxicity and neurodegeneration in Alzheimer transgenic mice models [108].
5.2. p62 in Parkinson Disease
PD represents the second most common neurodegenerative disease associated with age, following Alzheimer [109]. Additionally, in this case, it is a progressive disorder in the central nervous system, but it is particularly connected with movement affection since it predominantly compromises dopaminergic neurons involved in motor control. Tremors are indeed the most common signs of early stage PD, then muscle rigidity, slowed movements, and walking difficulty progressively occur. Mental and cognitive problems appear in the advanced stages of PD. The pathogenesis of PD is connected with both genetic alterations, including mutations in PARK genes, and environmental and epigenetic factors. One of the principle pathological factors of PD is the intracellular accumulation of Lewy bodies, consisting of aggregated proteins including α-synuclein, parkin, and ubiquitinated proteins [110].
p62, together with parkin, has an important role in the prevention of dopaminergic neurodegeneration. For instance, loss of p62-dependent autophagy in neurons was associated with Lewy pathology and motor dysfunction in aged mice [111]. In addition, p62-knockdown experiments demonstrated that p62 is required for autophagic clearance of α-synuclein inclusions [112]. Notably, evidence has been provided that autophagy failure can lead to p62 association with α-synuclein accumulation into Lewy bodies. Moreover, it has been shown that aberrant expression of p62 could enhance the amount of α-synuclein in the pathological inclusions [113]. Therefore, a double role of p62 also appears in neurodegeneration. In this context, an important function is exerted by parkin, the product of PARK2 gene, which serves a protective function in dopaminergic neurons.
Being an E3 ubiquitin ligase, parkin has been shown to ubiquitinate p62, thus promoting its proteasomal degradation [114]. The same authors found p62 accumulation and aggregation in dopamingeric neuronal cells of parkin deficient mice. Through the control of p62 levels, parkin, together with the mitochondrial factor pink1, is also associated with p62-dependent mitophagy. In normal cells, dysfunctional mitochondria accumulate pink1 in the outer mitochondrial membrane, with consequent recruitment from cytosol to damaged mitochondria and phosphorylation of parkin. In this form, parkin ubiquitinates mitochondrial substrates that are then recognized by p62 to undergo mitophagy.
Deregulation of pink1/parkin/p62 pathway has been associated to increased vulnerability of neuronal cells toward PD development [114]. In particular, parkin loss of function results in p62 accumulation and association to Lewy bodies.
Overall, if on one hand mitophagy failure leads to p62 accumulation and association with α-synuclein and PD pathogenesis, on the other hand, p62 is fundamental together with parkin and pink1 to maintain neuronal homeostasis and survival.
5.3. p62 in Amyotrophic Lateral Sclerosis
Much evidence indicates that p62 is also involved in ALS, a progressive and fatal neurodegenerative disease, which is caused by the gradual depletion of motor neurons in either the cerebral cortex, the brain stem, or the spinal cord. The consequence is an impairment of voluntary muscle movements that leads to paralysis, respiratory arrest, and death. ALS can be familial or sporadic, although the distinction between the two forms can be difficult to evaluate [115]. The pathogenesis is highly correlated with genetic factors, but environmental exposure can also contribute. One common aspect of different ALS forms is the aggregation of insoluble proteins within cells. In particular, intrinsically disordered proteins such as DNA-binding protein 43 (TDP-43) and fused in sarcoma (FUS) are frequently associated to the pathogenic aggregates within motor neurons of ALS patients [116]. A minor percentage of patients (about 20%) have accumulations of superoxide dismutase 1 (SOD1), a key antioxidant enzyme involved in toxic superoxide radicals disposal within the cell [117]. Accumulation of such proteins in amorphous aggregates is due to genetic mutations in their encoding genes or aberrant expression of their inactive forms. Most of these inclusions have been shown to be ubiquitin-immunoreactive and in many cases they contain p62.
It is interesting to note that p62 mutations have been found in a large cohort of both familial and sporadic ALS patients [118,119,120]. These result in accumulation of p62 in the protein aggregates within the motor neurons. Gal et al. showed that accumulation of p62 can enhance the formation of polyubiquitinated proteins and mutant SOD1 aggregates in the spinal cord in a mouse model of ALS [121]. They also provided evidence that p62 can directly bind to a mutated form of SOD1, suggesting that p62 can target mutant SOD1 to selective autophagy in an ubiquitin-dependent mechanism. Autophagy impairment or p62 loss of function may therefore contribute in ALS pathogenesis [122]. Moreover, functional p62, through stimulation of Nrf2- mediated antioxidant response, also serves a protective function against oxidative stress related with SOD1 mutations [123].
More recent findings indicate that p62 can also interact with both TDP43 and FUS, the main components of stress granules in ALS affected motor neurons. In this regard, evidence has been provided that p62 contributes to elimination of stress granules by autophagy [124], thus serving a neuroprotective function.
However, Lee et al. found that functional modulation of p62 can switch toward neurodegeneration. In particular, they showed that p62 phosphorylation at Ser403 increased upon TDP43 overexpression in neuronal cells and this was correlated with accumulation of insoluble poly-ubiquitinated proteins and neurotoxicity [125]. This evidence, together with the observation that p62 can be mutated or functionally altered in ALS patients, confirms that p62 can either suppress or promote neurodegeneration depending on particular circumstances, cell context, and interactive networks. Neurotoxic and neuroprotective roles of p62 are summarized in Table 2.

Table 2.
Highlights of research findings of p62 double role in neurodegeneration.
6. Conclusions
Biochemical complexity is a hallmark of multi-factorial diseases, including cancer and neurodegenerative disorders. In a wide and intricate molecular scenario, it is not easy to strictly classify a factor as a positive or negative regulator in the pathogenesis and disease development.
P62 perfectly fits the feature of a double-faced protein depending on cell context, interacting networks, expression levels, and regulative post-translational modifications.
If we can consider p62 a “benefactor” when it contributes to protect the cells from tumorigenesis or neurodegeneration, we have to consider it a “maleficent” when it is de-regulated or overexpressed thus causing the opposite effects. Discriminating such behaviour becomes important to understand the molecular basis of the above-mentioned diseases and to perform appropriate targeted therapies. For instance, future research could be focused on pharmacological tools to target p62 accumulation in particular tumor types or in those neuron-specific aggregates contributing to neurodegeneration. On the other hand, stimulation of p62-mediated autophagy or p62/Nrf2-mediated antioxidant response may result as useful to counteract neurodegeneration or to selectively target tumor cells by autophagic cell death and redox homeostasis control.
Author Contributions
S.E. conceived the review, wrote, reviewed, and edited the article. M.L., A.D., D.C., A.D.B. and D.D.L. reviewed the article. M.G. contributed to the general design of the article and to the development of figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Acknowledgments
We apologize to authors whose references were not included because of space limitations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shin, J. P62 and the sequestosome, a novel mechanism for protein metabolism. Arch. Pharm. Res. 1998, 21, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.G.R. Identification and confirmation of a module of coexpressed genes. Genome Res. 2002, 12, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological functions of autophagy genes: A disease perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of autophagy in oxidative stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef]
- Sánchez-Martín, P.; Komatsu, M. Physiological stress response by selective autophagy. J. Mol. Biol. 2020, 432, 53–62. [Google Scholar] [CrossRef]
- Zaffagnini, G.; Martens, S. Mechanisms of selective autophagy. J. Mol. Biol. 2016, 428, 1714–1724. [Google Scholar] [CrossRef]
- Rogov, V.; Dötsch, V.; Johansen, T.; Kirkin, V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell 2014, 53, 167–178. [Google Scholar] [CrossRef]
- Danieli, A.; Martens, S. p62-mediated phase separation at the intersection of the ubiquitin-proteasome system and autophagy. J. Cell Sci. 2018, 131, jcs214304. [Google Scholar] [CrossRef]
- Lim, J.; Lachenmayer, M.L.; Wu, S.; Liu, W.; Kundu, M.; Wang, R.; Komatsu, M.; Oh, Y.J.; Zhao, Y.; Yue, Z. Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 2015, 11, e1004987. [Google Scholar] [CrossRef]
- Sun, D.; Wu, R.; Zheng, J.; Li, P.; Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 2018, 28, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Ciuffa, R.; Lamark, T.; Tarafder, A.K.; Guesdon, A.; Rybina, S.; Hagen, W.J.H.; Johansen, T.; Sachse, C. The selective autophagy receptor p62 forms a flexible filamentous helical scaffold. Cell Rep. 2015, 11, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Ting, Y.; Mingtao, G.; Xiaofei, T.; Dong, Y.; Chenguang, Z.; Wei, D. Monitoring autophagic flux using p62/SQSTM1 based luciferase reporters in glioma cells. Exp. Cell Res. 2018, 363, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, C.; Emanuele, S.; Ferrante, F.; Celesia, A.; Giuliano, M.; Fiore, T. Tributyltin(IV) ferulate, a novel synthetic ferulic acid derivative, induces autophagic cell death in colon cancer cells: From chemical synthesis to biochemical effects. J. Inorg. Biochem. 2020, 205, 110999. [Google Scholar] [CrossRef]
- Cernigliaro, C.; D’Anneo, A.; Carlisi, D.; Giuliano, M.; Marino Gammazza, A.; Barone, R.; Longhitano, L.; Cappello, F.; Emanuele, S.; Distefano, A.; et al. Ethanol-mediated stress promotes autophagic survival and aggressiveness of colon cancer cells via activation of Nrf2/HO-1 pathway. Cancers 2019, 11, 505. [Google Scholar] [CrossRef]
- Emanuele, S.; Notaro, A.; Palumbo Piccionello, A.; Maggio, A.; Lauricella, M.; D’Anneo, A.; Cernigliaro, C.; Calvaruso, G.; Giuliano, M. Sicilian litchi fruit extracts induce autophagy versus apoptosis switch in human colon cancer cells. Nutrients 2018, 10, 1490. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.-K.; Kwon, S.W.; Han, D.H.; Lee, Y.-H.; Bae, S.H. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 1–25. [Google Scholar] [CrossRef]
- Schwob, A.; Teruel, E.; Dubuisson, L.; Lormières, F.; Verlhac, P.; Abudu, Y.P.; Gauthier, J.; Naoumenko, M.; Cloarec-Ung, F.-M.; Faure, M.; et al. SQSTM-1/p62 potentiates HTLV-1 Tax-mediated NF-κB activation through its ubiquitin binding function. Sci. Rep. 2019, 9, 16014. [Google Scholar] [CrossRef]
- Linares, J.F.; Duran, A.; Yajima, T.; Pasparakis, M.; Moscat, J.; Diaz-Meco, M.T. K63 Polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol. Cell 2013, 51, 283–296. [Google Scholar] [CrossRef]
- Yan, X.; Zhong, X.; Yu, S.; Zhang, L.; Liu, Y.; Zhang, Y.; Sun, L.; Su, J. p62 aggregates mediated Caspase 8 activation is responsible for progression of ovarian cancer. J. Cell. Mol. Med. 2019, 23, 4030–4042. [Google Scholar] [CrossRef]
- Islam, M.; Sooro, M.; Zhang, P. Autophagic regulation of p62 is critical for cancer therapy. Int. J. Mol. Sci. 2018, 19, 1405. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Attarwala, I.Y.; Xie, X.-Q. SQSTM1/p62: A potential target for neurodegenerative disease. ACS Chem. Neurosci. 2019, 10, 2094–2114. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, P.; Saito, T.; Komatsu, M. p62/SQSTM1: “Jack of all trades” in health and cancer. FEBS J. 2019, 286, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, E.; Tang, L.; Lei, Y.; Sun, X.; Hu, J.; Dong, H.; Yang, S.-M.; Gao, M.; Tang, B. Emerging roles and regulation of MiT/TFE transcriptional factors. Cell. Commun. Signal. 2018, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Hanawa, N.; Katsumata, S.-I.; Katsumata-Tsuboi, R.; Takahashi, N.; Uehara, M. Iron deficiency induces autophagy and activates Nrf2 signal through modulating p62/SQSTM. Biomed. Res. 2017, 38, 343–350. [Google Scholar] [CrossRef]
- Gong, J. Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science 1999, 285, 1565–1569. [Google Scholar] [CrossRef]
- Kageyama, S.; Saito, T.; Obata, M.; Koide, R.; Ichimura, Y.; Komatsu, M. Negative regulation of the Keap1-Nrf2 pathway by a p62/Sqstm1 splicing variant. Mol. Cell. Biol. 2018, 38, e00642-17. [Google Scholar] [CrossRef]
- Wang, L.; Cano, M.; Handa, J.T. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochem. Biophys. Acta BBA Mol. Cell. Res. 2014, 1843, 1248–1258. [Google Scholar] [CrossRef]
- Kwon, D.H.; Park, O.H.; Kim, L.; Jung, Y.O.; Park, Y.; Jeong, H.; Hyun, J.; Kim, Y.K.; Song, H.K. Insights into degradation mechanism of N-end rule substrates by p62/SQSTM1 autophagy adapter. Nat. Commun. 2018, 9, 3291. [Google Scholar] [CrossRef]
- Sánchez-Martín, P.; Sou, Y.-S.; Kageyama, S.; Koike, M.; Waguri, S.; Komatsu, M. NBR1-mediated p62-liquid droplets enhance the Keap1-Nrf2 system. EMBO Rep. 2020, 21, e48902. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, A.J.; Huber, S.T.; Mortensen, S.A.; Schultz, S.W.; Palara, A.; Kuhm, T.; Shrestha, B.K.; Lamark, T.; Hagen, W.J.H.; Wilmanns, M.; et al. Structural basis of p62/SQSTM1 helical filaments and their role in cellular cargo uptake. Nat. Commun. 2020, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Willis, T.L.; Button, R.W.; Strang, C.J.; Fu, Y.; Wen, X.; Grayson, P.R.C.; Evans, T.; Sipthorpe, R.J.; Roberts, S.L.; et al. Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2-mediated stress response. Nat. Commun. 2019, 10, 3759. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T.; Albert, A.; Campuzano, S. Cell signaling and function organized by PB1 domain interactions. Mol. Cell 2006, 23, 631–640. [Google Scholar] [CrossRef]
- Lin, Q.; Dai, Q.; Meng, H.; Sun, A.; Wei, J.; Peng, K.; Childress, C.; Chen, M.; Shao, G.; Yang, W. The HECT E3 ubiquitin ligase NEDD4 interacts with and ubiquitylates SQSTM1 for inclusion body autophagy. J. Cell. Sci. 2017, 130, 3839–3850. [Google Scholar] [CrossRef]
- Feng, Y.; Klionsky, D.J. Autophagy regulates DNA repair through SQSTM1/p62. Autophagy 2017, 13, 995–996. [Google Scholar] [CrossRef]
- Salmina, K.; Bojko, A.; Inashkina, I.; Staniak, K.; Dudkowska, M.; Podlesniy, P.; Rumnieks, F.; Vainshelbaum, N.M.; Pjanova, D.; Sikora, E.; et al. “Mitotic slippage” and extranuclear DNA in cancer chemoresistance: A focus on telomeres. Int. J. Mol.Sci. 2020, 21, 2779. [Google Scholar] [CrossRef]
- Noguchi, T.; Suzuki, M.; Mutoh, N.; Hirata, Y.; Tsuchida, M.; Miyagawa, S.; Hwang, G.-W.; Aoki, J.; Matsuzawa, A. Nuclear-accumulated SQSTM1/p62-based ALIS act as microdomains sensing cellular stresses and triggering oxidative stress-induced parthanatos. Cell Death Dis. 2018, 9, 1193. [Google Scholar] [CrossRef]
- Yan, C.; Dodd, T.; He, Y.; Tainer, J.A.; Tsutakawa, S.E.; Ivanov, I. Transcription preinitiation complex structure and dynamics provide insight into genetic diseases. Nat. Struct. Mol. Biol. 2019, 26, 397–406. [Google Scholar] [CrossRef]
- Greber, B.J.; Toso, D.B.; Fang, J.; Nogales, E. The complete structure of the human TFIIH core complex. eLife 2019, 8, e44771. [Google Scholar] [CrossRef]
- Schimmack, G.; Schorpp, K.; Kutzner, K.; Gehring, T.; Brenke, J.K.; Hadian, K.; Krappmann, D. YOD1/TRAF6 association balances p62-dependent IL-1 signaling to NF-κB. eLife 2017, 6, e22416. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Lee, D.-H.; Dilly, A.-K.; Lee, Y.-S.; Choudry, H.A.; Kwon, Y.T.; Bartlett, D.L.; Lee, Y.J. Crosstalk between apoptosis and autophagy is regulated by the arginylated BiP/Beclin-1/p62 complex. Mol Cancer Res. 2018, 16, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Godar, R.J.; Liu, H.; Diwan, A. Enhancing lysosome biogenesis attenuates BNIP3-induced cardiomyocyte death. Autophagy 2012, 8, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Rad. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Komatsu, M. Activation of p62/SQSTM1–Keap1–nuclear factor erythroid 2-related factor 2 pathway in cancer. Front. Oncol. 2018, 8, 210. [Google Scholar] [CrossRef]
- You, Z.; Jiang, W.-X.; Qin, L.-Y.; Gong, Z.; Wan, W.; Li, J.; Wang, Y.; Zhang, H.; Peng, C.; Zhou, T.; et al. Requirement for p62 acetylation in the aggregation of ubiquitylated proteins under nutrient stress. Nat. Commun. 2019, 10, 5792. [Google Scholar] [CrossRef]
- Yun, C.; Lee, S. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy: Autophagy in neurodegenerative diseases. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Fujikawa, N.; Komatsu, M.; Ishii, T.; Unno, M.; Akaike, T.; Motohashi, H.; Yamamoto, M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 13561–13566. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Sibon, O.C.M.; Dijkers, P.F. Inhibition of NF-κB in astrocytes is sufficient to delay neurodegeneration induced by proteotoxicity in neurons. J. Neuroinflam. 2018, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Moreno-Villanueva, M.; Krieger, S.; Ramesh, G.; Neelam, S.; Wu, H. Transcriptomics, NF-κB pathway, and their potential spaceflight-related health consequences. Int. J. Mol. Sci. 2017, 18, 1166. [Google Scholar] [CrossRef] [PubMed]
- Zotti, T.; Scudiero, I.; Settembre, P.; Ferravante, A.; Mazzone, P.; D’Andrea, L.; Reale, C.; Vito, P.; Stilo, R. TRAF6-mediated ubiquitination of NEMO requires p62/sequestosome-1. Mol. Immun. 2014, 58, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ozato, K. The sequestosome 1/p62 attenuates cytokine gene expression in activated macrophages by inhibiting IFN regulatory factor 8 and TNF receptor-associated factor 6/NF-κB activity. J. Immunol. 2009, 182, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kageyama, S.; Ichimura, Y. p62/SQSTM1/A170: Physiology and pathology. Pharmacol. Res. 2012, 66, 457–462. [Google Scholar] [CrossRef]
- Young, M.M.; Takahashi, Y.; Khan, O.; Park, S.; Hori, T.; Yun, J.; Sharma, A.K.; Amin, S.; Hu, C.-D.; Zhang, J.; et al. Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated Caspase-8 activation and apoptosis. J. Biol. Chem. 2012, 287, 12455–12468. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Q.; Bu, X.; Zhou, Y.; Bai, D.; Guo, Q.; Gao, Y.; Lu, N. Triggering apoptosis by oroxylin A through caspase-8 activation and p62/SQSTM1 proteolysis. Redox Biol. 2020, 29, 101392. [Google Scholar] [CrossRef]
- Sanchez-Garrido, J.; Sancho-Shimizu, V.; Shenoy, A.R. Regulated proteolysis of p62/SQSTM1 enables differential control of autophagy and nutrient sensing. Sci. Signal. 2018, 11, eaat6903. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Korolchuk, V. mTORC1 and nutrient homeostasis: The central role of the lysosome. Int. J. Mol. Sci. 2018, 19, 818. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Amanchy, R.; Linares, J.F.; Joshi, J.; Abu-Baker, S.; Porollo, A.; Hansen, M.; Moscat, J.; Diaz-Meco, M.T. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell 2011, 44, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.F.; Duran, A.; Reina-Campos, M.; Aza-Blanc, P.; Campos, A.; Moscat, J.; Diaz-Meco, M.T. Amino acid activation of mTORC1 by a PB1-domain-driven kinase complex cascade. Cell Rep. 2015, 12, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef]
- Ha, S.; Jeong, S.-H.; Yi, K.; Chung, K.M.; Hong, C.J.; Kim, S.W.; Kim, E.-K.; Yu, S.-W. Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells. J. Biol. Chem. 2017, 292, 13795–13808. [Google Scholar] [CrossRef]
- Umemura, A.; He, F.; Taniguchi, K.; Nakagawa, H.; Yamachika, S.; Font-Burgada, J.; Zhong, Z.; Subramaniam, S.; Raghunandan, S.; Duran, A.; et al. p62, Upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell 2016, 29, 935–948. [Google Scholar] [CrossRef]
- Ryoo, I.; Choi, B.; Ku, S.-K.; Kwak, M.-K. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: Implications for cancer stem cell resistance. Redox Biol. 2018, 17, 246–258. [Google Scholar] [CrossRef]
- Schläfli, A.M.; Adams, O.; Galván, J.A.; Gugger, M.; Savic, S.; Bubendorf, L.; Schmid, R.A.; Becker, K.-F.; Tschan, M.P.; Langer, R.; et al. Prognostic value of the autophagy markers LC3 and p62/SQSTM1 in early-stage non-small cell lung cancer. Oncotarget 2016, 7, 39544–39555. [Google Scholar] [CrossRef]
- Masuda, G.O.; Yashiro, M.; Kitayama, K.; Miki, Y.; Kasashima, H.; Kinoshita, H.; Morisaki, T.; Fukuoka, T.; Hasegawa, T.; Sakurai, K.; et al. Clinicopathological correlations of autophagy-related proteins LC3, Beclin 1 and p62 in gastric cancer. Anticancer Res. 2016, 36, 129–136. [Google Scholar]
- Lei, C.; Zhao, B.; Liu, L.; Zeng, X.; Yu, Z.; Wang, X. Expression and clinical significance of p62 protein in colon cancer. Medicine 2020, 99, e18791. [Google Scholar] [CrossRef]
- Lam, H.C.; Baglini, C.V.; Lope, A.L.; Parkhitko, A.A.; Liu, H.-J.; Alesi, N.; Malinowska, I.A.; Ebrahimi-Fakhari, D.; Saffari, A.; Yu, J.J.; et al. p62/SQSTM1 cooperates with hyperactive mTORC1 to regulate glutathione production, maintain mitochondrial integrity, and promote tumorigenesis. Cancer Res. 2017, 77, 3255–3267. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, C.; Croce, C.M.; Guan, J.-L. p62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes Dev. 2014, 28, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, D.; Wu, K. p62 promotes bladder cancer cell growth by activating KEAP1/NRF2-dependent antioxidative response. Cancer Sci. 2020, 111, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, D.; Ohe, T.; Takahashi, K.; Imamura, R.; Kojima, H.; Okabe, T.; Ichimura, Y.; Komatsu, M.; Yamamoto, M.; Nagano, T.; et al. Inhibitors of the protein–protein interaction between phosphorylated p62 and Keap1 attenuate chemoresistance in a human hepatocellular carcinoma cell line. Free Rad. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Jeong, Y.-J.; Chang, Y.-C. PDCD4 inhibits lung tumorigenesis by the suppressing p62-Nrf2 signaling pathway and upregulating Keap1 expression. Am. J. Cancer Res. 2020, 10, 424–439. [Google Scholar]
- Manirujjaman, M.; Ozaki, I.; Murata, Y.; Guo, J.; Xia, J.; Nishioka, K.; Perveen, R.; Takahashi, H.; Anzai, K.; Matsuhashi, S. Degradation of the tumor suppressor PDCD4 is impaired by the suppression of p62/SQSTM1 and autophagy. Cells 2020, 9, 218. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Ghia, E.M.; Antonucci, L.; Sharma, N.; Rassenti, L.Z.; Xu, J.; Sun, B.; Kipps, T.J.; Karin, M. NF-κB-p62-NRF2 survival signaling is associated with high ROR1 expression in chronic lymphocytic leukemia. Cell Death Differ. 2020. [Google Scholar] [CrossRef]
- Tamura, K.; Watanabe, K.; Matsushita, Y.; Watanabe, H.; Motoyama, D.; Ito, T.; Sugiyama, T.; Otsuka, A.; Miyake, H. Enhanced sensitivity to NVP-BEZ235 by inhibition of p62/SQSTM1 in human bladder cancer KoTCC-1 cells both in vitro and in vivo. In Vivo 2020, 34, 1001–1008. [Google Scholar] [CrossRef]
- Xu, L.; Xu, F.; Kong, Q.; Yang, T.; Tan, D.; Zhang, X.; Li, N.; Zhao, S.; Zhao, J.; Li, M. Inhibition of p62/SQSTM1 sensitizes small-cell lung cancer cells to cisplatin-induced cytotoxicity by targeting NEDD9 expression. Mol. Carcinog. 2020, mc.23215. [Google Scholar] [CrossRef]
- Valencia, T.; Kim, J.Y.; Abu-Baker, S.; Moscat-Pardos, J.; Ahn, C.S.; Reina-Campos, M.; Duran, A.; Castilla, E.A.; Metallo, C.M.; Diaz-Meco, M.T.; et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell 2014, 26, 121–135. [Google Scholar] [CrossRef]
- Huang, J.; Diaz-Meco, M.T.; Moscat, J. The macroenviromental control of cancer metabolism by p62. Cell Cycle 2018, 17, 2110–2121. [Google Scholar] [CrossRef]
- Linares, J.F.; Cordes, T.; Duran, A.; Reina-Campos, M.; Valencia, T.; Ahn, C.S.; Castilla, E.A.; Moscat, J.; Metallo, C.M.; Diaz-Meco, M.T. ATF4-induced metabolic reprograming is a synthetic vulnerability of the p62-deficient tumor stroma. Cell Metabolism 2017, 26, 817–829.e6. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.-G.; Zhao, Y. Autophagy substrate SQSTM1/p62 regulates chromatin ubiquitination during the DNA damage response. Autophagy 2017, 13, 212–213. [Google Scholar] [CrossRef]
- Venanzi, F.M.; Gabai, V.; Mariotti, F.; Magi, G.E.; Vullo, C.; Sufianov, A.A.; Kolesnikov, S.I.; Shneider, A. p62-DNA-encoding plasmid reverts tumor grade, changes tumor stroma, and enhances anticancer immunity. Aging 2019, 11, 10711–10722. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kang, J.-I.; Han, S.; Kim, Y.R.; Jo, J.; Kang, Y.W.; Choo, D.R.; Hyun, J.W.; Koh, Y.S.; Yoo, E.-S.; et al. L-ascorbic acid inhibits breast cancer growth by inducing IRE/JNK/CHOP-related endoplasmic reticulum stress-mediated p62/SQSTM1 accumulation in the nucleus. Nutrients 2020, 12, 1351. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, F.-F.; Lung, J.; Lo, C.-H.; Lee, F.-H.; Lu, Y.-C.; Hung, C.-H. Prognostic significance of p62/SQSTM1 subcellular localization and LC3B in oral squamous cell carcinoma. Br. J. Cancer 2014, 111, 944–954. [Google Scholar] [CrossRef]
- Ponomarenko, D.M.; Gabai, V.L.; Sufianov, A.A.; Kolesnikov, S.I.; Shneider, A.M. Response of a chemo-resistant triple-negative breast cancer patient to a combination of p62-encoding plasmid, Elenagen, and CMF chemotherapy. Oncotarget 2020, 11, 294–299. [Google Scholar] [CrossRef]
- Lou, J.-S.; Bi, W.-C.; Chan, G.K.L.; Jin, Y.; Wong, C.-W.; Zhou, Z.-Y.; Wang, H.-Y.; Yao, P.; Dong, T.T.X.; Tsim, K.W.K. Ginkgetin induces autophagic cell death through p62/SQSTM1-mediated autolysosome formation and redox setting in non-small cell lung cancer. Oncotarget 2017, 8, 93131–93148. [Google Scholar] [CrossRef]
- Li, S.-S.; Xu, L.-Z.; Zhou, W.; Yao, S.; Wang, C.-L.; Xia, J.-L.; Wang, H.-F.; Kamran, M.; Xue, X.-Y.; Dong, L.; et al. p62/SQSTM1 interacts with vimentin to enhance breast cancer metastasis. Carcinogenesis 2017, 38, 1092–1103. [Google Scholar] [CrossRef]
- Xu, L.-Z.; Li, S.-S.; Zhou, W.; Kang, Z.-J.; Zhang, Q.-X.; Kamran, M.; Xu, J.; Liang, D.-P.; Wang, C.-L.; Hou, Z.-J.; et al. p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene 2017, 36, 304–317. [Google Scholar] [CrossRef]
- Kim, J.S.; Bae, G.E.; Kim, K.-H.; Lee, S.-I.; Chung, C.; Lee, D.; Lee, T.H.; Kwon, I.S.; Yeo, M.-K. Prognostic significance of LC3B and p62/SQSTM1 expression in gastric adenocarcinoma. Anticancer Res. 2019, 39, 6711–6722. [Google Scholar] [CrossRef]
- Yan, X.-Y.; Zhang, Y.; Zhang, J.-J.; Zhang, L.-C.; Liu, Y.-N.; Wu, Y.; Xue, Y.-N.; Lu, S.-Y.; Su, J.; Sun, L.-K. p62/SQSTM1 as an oncotarget mediates cisplatin resistance through activating RIP1-NF-κB pathway in human ovarian cancer cells. Cancer Sci. 2017, 108, 1405–1413. [Google Scholar] [CrossRef]
- Wu, Q.; Xiang, M.; Wang, K.; Chen, Z.; Long, L.; Tao, Y.; Liang, Y.; Yan, Y.; Xiao, Z.; Qiu, S.; et al. Overexpression of p62 induces autophagy and promotes proliferation, migration and invasion of nasopharyngeal carcinoma cells through promoting ERK signaling pathway. Curr. Cancer Drug Targets 2020, 20. [Google Scholar] [CrossRef]
- Saito, T.; Ichimura, Y.; Taguchi, K.; Suzuki, T.; Mizushima, T.; Takagi, K.; Hirose, Y.; Nagahashi, M.; Iso, T.; Fukutomi, T.; et al. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming. Nat. Commun. 2016, 7, 12030. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, I. p62 manipulation affects chlorin e6-mediated photodynamic therapy efficacy in colorectal cancer cell lines. Oncol. Lett. 2020. [Google Scholar] [CrossRef]
- Liu, H.; Dai, C.; Fan, Y.; Guo, B.; Ren, K.; Sun, T.; Wang, W. From autophagy to mitophagy: The roles of P62 in neurodegenerative diseases. J. Bioenerg. Biomembr. 2017, 49, 413–422. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18. [Google Scholar] [CrossRef]
- Nilsson, P.; Sekiguchi, M.; Akagi, T.; Izumi, S.; Komori, T.; Hui, K.; Sörgjerd, K.; Tanaka, M.; Saito, T.; Iwata, N.; et al. Autophagy-related protein 7 deficiency in amyloid β (Aβ) precursor protein transgenic mice decreases Aβ in the multivesicular bodies and induces Aβ accumulation in the Golgi. Am. J. Pathol. 2015, 185, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, W.; Liu, R.; Huang, H.; Zhang, R.; Sun, L. Effect of p62 on tau hyperphosphorylation in a rat model of Alzheimer’s disease. Neural Regen. Res. 2012, 7, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Zheng, C.; McGregor, W.C.; Douglas White, B.; Diaz-Meco, M.T.; Moscat, J.; Babu, J.R. TRAF6 and p62 inhibit amyloid β-induced neuronal death through p75 neurotrophin receptor. Neurochem. Intern. 2012, 61, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Tanji, K.; Miki, Y.; Ozaki, T.; Maruyama, A.; Yoshida, H.; Mimura, J.; Matsumiya, T.; Mori, F.; Imaizumi, T.; Itoh, K.; et al. Phosphorylation of serine 349 of p62 in Alzheimer’s disease brain. Acta Neuropathol. Commun. 2014, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Fão, L.; Mota, S.I.; Rego, A.C. Shaping the Nrf2-ARE-related pathways in Alzheimer’s and Parkinson’s diseases. Ageing Res. Rev. 2019, 54, 100942. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Siu, W.; Li, L.; Jin, Y.; Liang, S.; Cao, M.; Ma, M.; Wu, Z. Autophagy in Alzheimer’s disease and promising modulatory effects of herbal medicine. Exp. Geront. 2019, 119, 100–110. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Ge, X.; Chen, J.; Zhao, Y.; Fu, J. Parkin overexpression attenuates Aβ-induced mitochondrial dysfunction in HEK293 cells by restoring impaired mitophagy. Life Sci. 2020, 244, 117322. [Google Scholar] [CrossRef]
- Deng, M.; Huang, L.; Zhong, X. β-asarone modulates Beclin-1, LC3 and p62 expression to attenuate Aβ40 and Aβ42 levels in APP/PS1 transgenic mice with Alzheimer’s disease. Mol. Med. Rep. 2020. [Google Scholar] [CrossRef]
- Cacabelos, R. Parkinson’s disease: From pathogenesis to pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R.; Shakeel, N.U.A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Sato, S.; Uchihara, T.; Fukuda, T.; Noda, S.; Kondo, H.; Saiki, S.; Komatsu, M.; Uchiyama, Y.; Tanaka, K.; Hattori, N. Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep. 2018, 8, 2813. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatebe, H.; Taguchi, K.; Endo, Y.; Tokuda, T.; Mizuno, T.; Nakagawa, M.; Tanaka, M. p62/SQSTM1-dependent autophagy of Lewy body-like α-synuclein inclusions. PLoS ONE 2012, 7, e52868. [Google Scholar] [CrossRef]
- Wu, F.; Xu, H.-D.; Guan, J.-J.; Hou, Y.-S.; Gu, J.-H.; Zhen, X.-C.; Qin, Z.-H. Rotenone impairs autophagic flux and lysosomal functions in Parkinson’s disease. Neuroscience 2015, 284, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, S.; Wu, H.; Gao, R.; Rao, G.; Wang, D.; Chen, Z.; Ma, B.; Wang, H.; Sui, N.; et al. Parkin promotes proteasomal degradation of p62: Implication of selective vulnerability of neuronal cells in the pathogenesis of Parkinson’s disease. Protein Cell 2016, 7, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Ajroud-Driss, S.; Siddique, T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochem. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 679–684. [Google Scholar] [CrossRef]
- Nowicka, N.; Juranek, J.; Juranek, J.K.; Wojtkiewicz, J. Risk factors and emerging therapies in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2019, 20, 2616. [Google Scholar] [CrossRef]
- Pansarasa, O.; Bordoni, M.; Diamanti, L.; Sproviero, D.; Gagliardi, S.; Cereda, C. SOD1 in amyotrophic lateral sclerosis: “Ambivalent” behavior connected to the disease. Int. J. Mol. Sci. 2018, 19, 1345. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.T.; Morris, A.; de Belleroche, J.S. Sequestosome-1 (SQSTM1) sequence variants in ALS cases in the UK: Prevalence and coexistence of SQSTM1 mutations in ALS kindred with PDB. Eur. J. Hum. Genet. 2014, 22, 492–496. [Google Scholar] [CrossRef]
- Teyssou, E.; Takeda, T.; Lebon, V.; Boillée, S.; Doukouré, B.; Bataillon, G.; Sazdovitch, V.; Cazeneuve, C.; Meininger, V.; LeGuern, E.; et al. Mutations in SQSTM1 encoding p62 in amyotrophic lateral sclerosis: Genetics and neuropathology. Acta Neuropathol. 2013, 125, 511–522. [Google Scholar] [CrossRef]
- Yilmaz, R.; Müller, K.; Brenner, D.; Volk, A.E.; Borck, G.; Hermann, A.; Meitinger, T.; Strom, T.M.; Danzer, K.M.; Ludolph, A.C.; et al. SQSTM1/p62 variants in 486 patients with familial ALS from Germany and Sweden. Neurobiol. Aging 2020, 87, 139.e9–139.e15. [Google Scholar] [CrossRef]
- Gal, J.; Ström, A.-L.; Kwinter, D.M.; Kilty, R.; Zhang, J.; Shi, P.; Fu, W.; Wooten, M.W.; Zhu, H. Sequestosome 1/p62 links familial ALS mutant SOD1 to LC3 via an ubiquitin-independent mechanism. J. Neurochem. 2009, 111, 1062–1073. [Google Scholar] [CrossRef]
- Vicencio, E.; Beltrán, S.; Labrador, L.; Manque, P.; Nassif, M.; Woehlbier, U. Implications of selective autophagy dysfunction for ALS pathology. Cells 2020, 9, 381. [Google Scholar] [CrossRef]
- Deng, Z.; Lim, J.; Wang, Q.; Purtell, K.; Wu, S.; Palomo, G.M.; Tan, H.; Manfredi, G.; Zhao, Y.; Peng, J.; et al. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy 2020, 16, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.H.; Thombre, R.; Wang, J. Autophagy as a common pathway in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 697, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jeon, Y.-M.; Cha, S.J.; Kim, S.; Kwon, Y.; Jo, M.; Jang, Y.-N.; Lee, S.; Kim, J.; Kim, S.R.; et al. PTK2/FAK regulates UPS impairment via SQSTM1/p62 phosphorylation in TARDBP/TDP-43 proteinopathies. Autophagy 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).