P2 Purinergic Signaling in the Distal Lung in Health and Disease
Abstract
1. Introduction
2. Functional Relevance of P2 Receptor Signaling in the Distal Lung
2.1. Expression of P2 Receptors in the Distal Lung
2.2. ATP in the Alveolus
2.3. Surfactant Secretion
2.4. Epithelial Fluid Transport
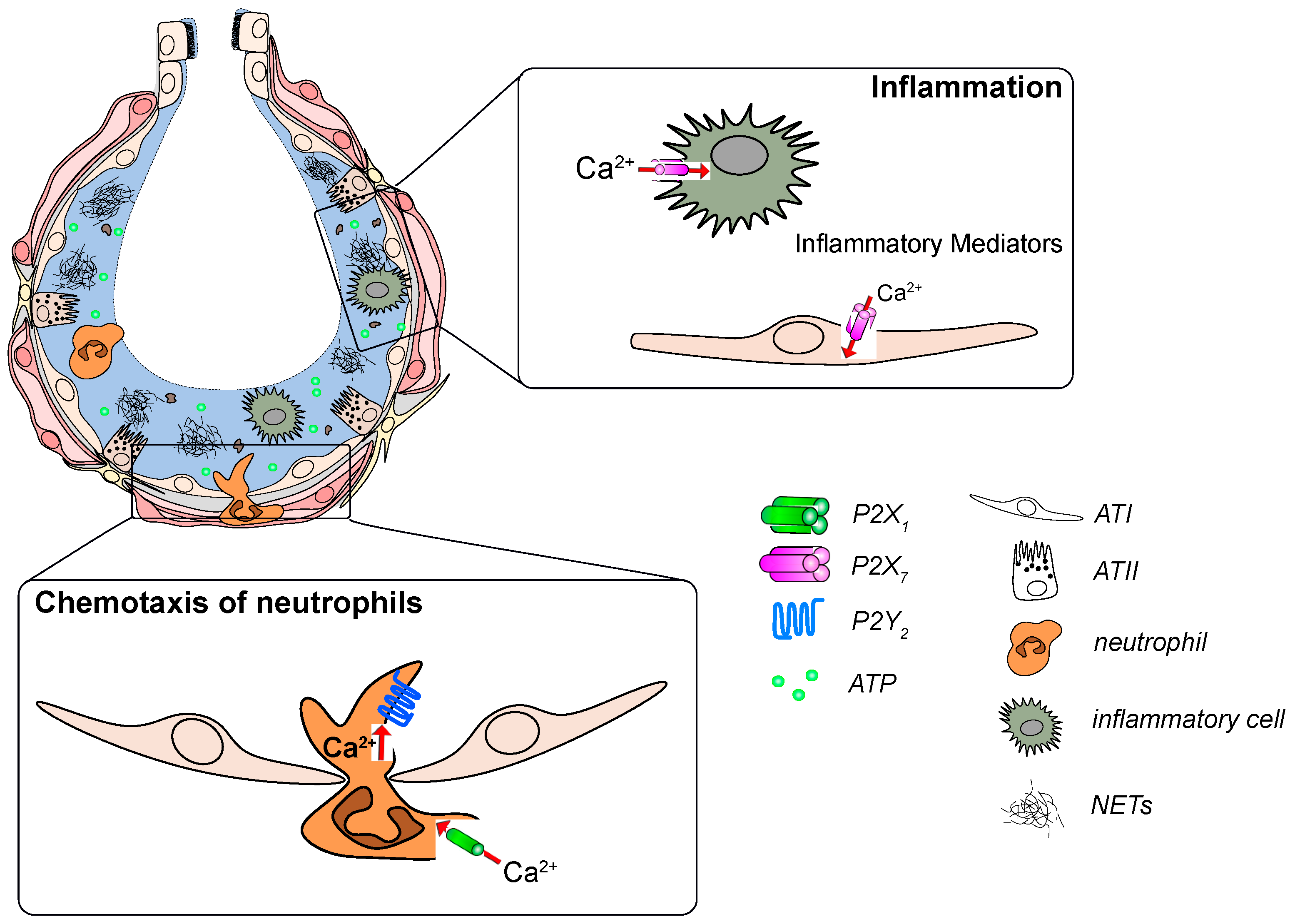
2.5. Host Defense
3. The Role of P2 Receptors in Lung Disease
3.1. Infectious Pneumonia
3.2. ALI/ARDS
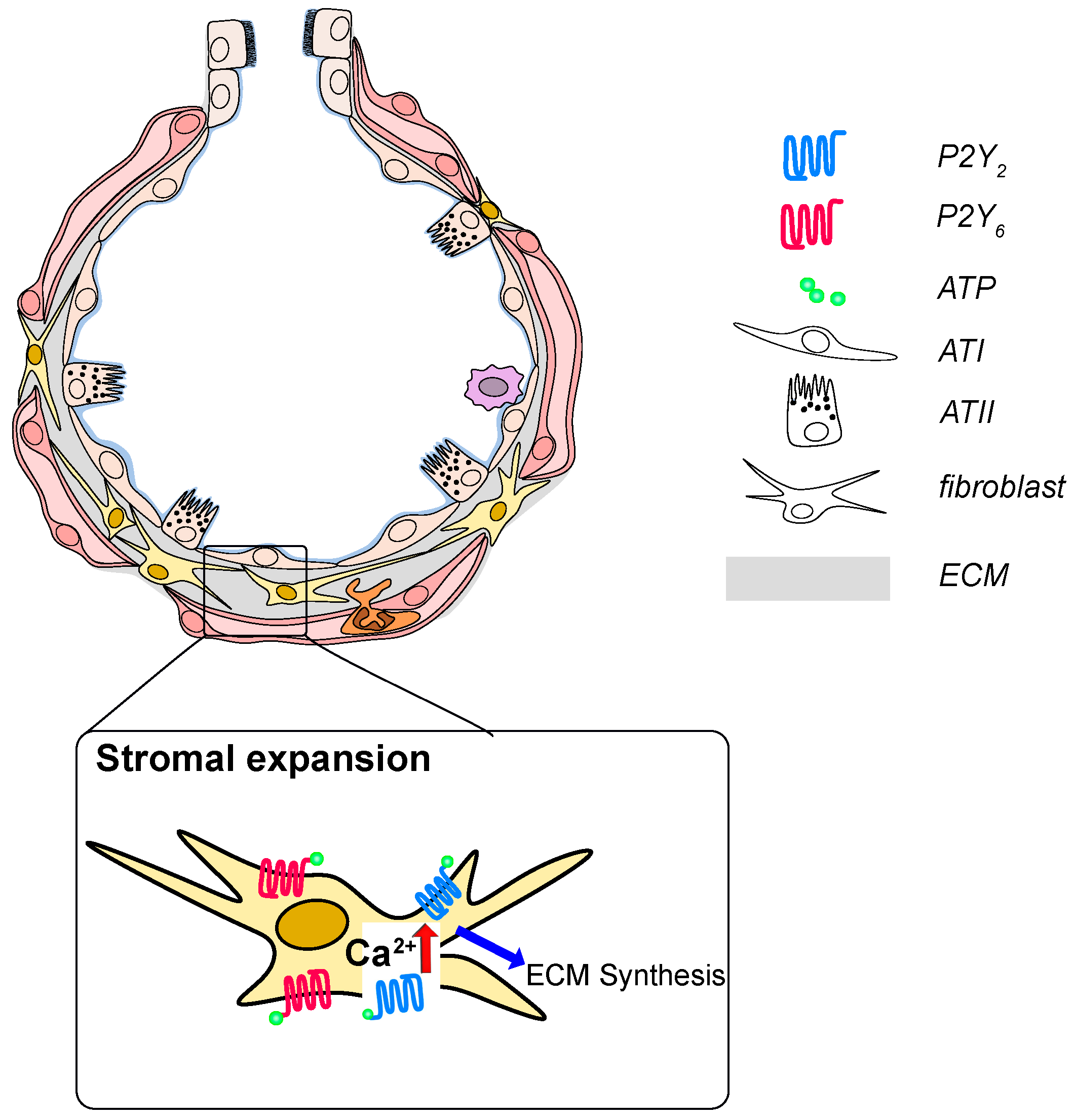
3.3. Idiopathic Pulmonary Fibrosis
3.4. Pulmonary Arterial Hypertension (PAH)
4. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALI/ARDS | Acute Lung Injury/Acute Respiratory Distress Syndrome |
| AM | Alveolar macrophage |
| ASL | Alveolar surface liquid (thin fluid layer, also termed hypophase) |
| ATI | Alveolar type I epithelial cell |
| ATII | Alveolar type II epithelial cell |
| BAL | Bronchoalveolar lavage |
| CaCC | Ca2+-activated Cl- channels |
| CD39 | Ectonucleotidase, converts ATP into AMP |
| CD73 | Ectonucleotidase, dephosphorylates AMP into adenosine |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| ENaC | Epithelial Na+ channel |
| FACE | Fusion activated Ca2+-entry |
| Hypophase | Thin fluid layer covering the luminal alveolar surface |
| Inflammasome | Cytosolic multiprotein oligomers, involved in inflammatory responses |
| LB | Lamellar Body, surfactant storing organelle in alveolar type II epithelial cells |
| NK | Natural killer cell |
| NLRP3 | Nucleotide-binding domain leucine-rich repeat (NLR) and pyrin domain containing receptor 3, Pattern recognition receptor |
| Treg | Regulatory T cell |
References
- Ochs, M.; Nyengaard, J.R.; Jung, A.; Knudsen, L.; Voigt, M.; Wahlers, T.; Richter, J.; Gundersen, H.J.G. The Number of Alveoli in the Human Lung. Am. J. Respir. Crit. Care Med. 2004. [Google Scholar] [CrossRef]
- Weibel, E.R. Lung morphometry: The link between structure and function. Cell Tissue Res. 2017, 367, 413–426. [Google Scholar] [CrossRef]
- Hsia, C.C.W.; Hyde, D.M.; Weibel, E.R. Lung structure and the intrinsic challenges of gas exchange. Compr. Physiol. 2016. [Google Scholar] [CrossRef]
- Isakson, B.E.; Seedorf, G.J.; Lubman, R.L.; Evans, W.H.; Boitano, S. Cell-Cell Communication in Heterocellular Cultures of Alveolar Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2003. [Google Scholar] [CrossRef]
- Mishra, A.; Chintagari, N.R.; Guo, Y.; Weng, T.; Su, L.; Liu, L. Purinergic P2X7 receptor regulates lung surfactant secretion in a paracrine manner. J. Cell. Sci. 2011. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Reigada, D.; Mitchell, C.H.; Bates, S.R.; Margulies, S.S.; Koval, M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L489–L496. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C. Alveolar Type I Cells: Molecular Phenotype and Development. Annu. Rev. Physiol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Crapo, J.D.; Barry, B.E.; Gehr, P.; Bachofen, M.; Weibel, E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982, 126, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R. On the tricks alveolar epithelial cells play to make a good lung. Am. J. Respir. Crit. Care Med. 2015. [Google Scholar] [CrossRef]
- Dietl, P.; Haller, T.; Frick, M. Spatio-temporal aspects, pathways and actions of Ca2+ in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium 2012, 52, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dietl, P.; Haller, T.; Mair, N.; Frick, M. Mechanisms of surfactant exocytosis in alveolar type II cells in vitro and in vivo. News Physiol. Sci. 2001. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.; Eschertzhuber, S.; Haller, T.; Mair, N.; Dietl, P. Secretion in alveolar type II cells at the interface of constitutive and regulated exocytosis. Am. J. Respir. Cell Mol. Biol. 2001. [Google Scholar] [CrossRef]
- Parra, E.; Pérez-Gil, J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem. Phys. Lipids 2015. [Google Scholar] [CrossRef] [PubMed]
- Goerke, J. Pulmonary surfactant: Functions and molecular composition. Biochim. Biophys. Acta Mol. Basis Dis. 1998. [Google Scholar] [CrossRef]
- Rao Tata, P.; Rajagopal, J. Plasticity in the lung: Making and breaking cell identity. Development 2017, 144, 755–766. [Google Scholar] [CrossRef]
- Olajuyin, A.M.; Zhang, X.; Ji, H.L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discov. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.; Ochs, M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Marsland, B.J. Lung Homeostasis: Influence of Age, Microbes, and the Immune System. Immunity 2017, 46, 549–561. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell. Immunol. 2018. [Google Scholar] [CrossRef]
- Garbi, N.; Lambrecht, B.N. Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflugers Arch. Eur. J. Physiol. 2017, 469, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Evren, E.; Ringqvist, E.; Willinger, T. Origin and ontogeny of lung macrophages: From mice to humans. Immunology 2019. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, G.K. Neutrophils in the lung: “The first responders”. Cell Tissue Res. 2018, 371, 577–588. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, N.; Narasaraju, T.; Chen, J.; McFarland, L.R.; Scott, M.; Liu, L. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem. Biophys. Res. Commun. 2004, 319, 774–780. [Google Scholar] [CrossRef]
- Belete, H.A.; Hubmayr, R.D.; Wang, S.; Singh, R.D. The role of purinergic signaling on deformation induced injury and repair responses of alveolar epithelial cells. PLoS ONE 2011. [Google Scholar] [CrossRef][Green Version]
- Miklavc, P.; Mair, N.; Wittekindt, O.H.; Haller, T.; Dietl, P.; Felder, E.; Timmler, M.; Frick, M. Fusion-activated Ca 2+ entry via vesicular P2X 4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 14503–14508. [Google Scholar] [CrossRef]
- Olotu, C.; Kiefmann, M.; Ronneburg, C.; Lehmensiek, F.; Cuvenhaus, A.; Meidl, V.; Goetz, A.E.; Kiefmann, R. Analysis of purine receptor expression and functionality in alveolar epithelial cells. Purinergic Signal. 2020. [Google Scholar] [CrossRef]
- Rice, W.R.; Burton, F.M.; Fiedeldey, D.T. Cloning and expression of the alveolar type II cell P2u-purinergic receptor. Am. J. Respir. Cell Mol. Biol. 1995. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Lüneburg, N.; Stage, A.; Schmitz, M.; Körbelin, J.; Harbaum, L.; Matuszcak, C.; Mienert, J.; Bokemeyer, C.; Böger, R.H.; et al. The P2-receptor-mediated Ca2+ signalosome of the human pulmonary endothelium—Implications for pulmonary arterial hypertension. Purinergic Signal. 2019, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Qi, Z.; Sokabe, M.; Ando, J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Schwiebert, L.M.; Rice, W.C.; Kudlow, B.A.; Taylor, A.L.; Schwiebert, E.M. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2002, 282, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sokabe, T.; Ohura, N.; Nakatsuka, H.; Kamiya, A.; Ando, J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 793–803. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Zemskov, E.A.; Gonzales, J.; Gorshkov, B.A.; Sridhar, S.; Chakraborty, T.; Lucas, R.; Verin, A.D. Extracellular β-nicotinamide adenine dinucleotide (β-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. J. Cell. Physiol. 2010, 223, 215–223. [Google Scholar] [CrossRef]
- Zemskov, E.; Lucas, R.; Verin, A.D.; Umapathy, N.S. P2Y receptors as regulators of lung endothelial barrier integrity. J. Cardiovasc. Dis. Res. 2011, 2, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Helenius, M.H.; Vattulainen, S.; Orcholski, M.; Aho, J.; Komulainen, A.; Taimen, P.; Wang, L.; De Jesus Perez, V.A.; Koskenvuo, J.W.; Alastalo, T.P. Suppression of endothelial CD39/ENTPD1 is associated with pulmonary vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1046–L1057. [Google Scholar] [CrossRef]
- Janssen, L.J.; Farkas, L.; Rahman, T.; Kolb, M.R.J. ATP stimulates Ca2+-waves and gene expression in cultured human pulmonary fibroblasts. Int. J. Biochem. Cell Biol. 2009, 41, 2477–2484. [Google Scholar] [CrossRef]
- Myrtek, D.; Müller, T.; Geyer, V.; Derr, N.; Ferrari, D.; Zissel, G.; Dürk, T.; Sorichter, S.; Luttmann, W.; Kuepper, M.; et al. Activation of Human Alveolar Macrophages via P2 Receptors: Coupling to Intracellular Ca 2+ Increases and Cytokine Secretion. J. Immunol. 2008, 181, 2181–2188. [Google Scholar] [CrossRef]
- Kessler, S.; Clauss, W.G.; Günther, A.; Kummer, W.; Fronius, M. Expression and functional characterization of P2X receptors in mouse alveolar macrophages. Pflugers Arch. Eur. J. Physiol. 2011, 462, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Bowler, J.W.; Bailey, R.J.; North, R.A.; Surprenant, A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br. J. Pharmacol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Stokes, L.; Surprenant, A. Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur. J. Immunol. 2009, 39, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Stokes, L.; Surprenant, A. Purinergic P2Y 2 Receptors Induce Increased MCP-1/CCL2 Synthesis and Release from Rat Alveolar and Peritoneal Macrophages. J. Immunol. 2007, 179, 6016–6023. [Google Scholar] [CrossRef]
- Vaughan, K.R.; Stokes, L.; Prince, L.R.; Marriott, H.M.; Meis, S.; Kassack, M.U.; Bingle, C.D.; Sabroe, I.; Surprenant, A.; Whyte, M.K.B. Inhibition of Neutrophil Apoptosis by ATP Is Mediated by the P2Y 11 Receptor. J. Immunol. 2007, 179, 8544–8553. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Raible, D.G.; McDermott, L.J.; Pelleg, A.; Schulman, E.S. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: Activation of purinergic P2Y receptors. J. Allergy Clin. Immunol. 2001, 107, 849–855. [Google Scholar] [CrossRef]
- Lecut, C.; Frederix, K.; Johnson, D.M.; Deroanne, C.; Thiry, M.; Faccinetto, C.; Marée, R.; Evans, R.J.; Volders, P.G.A.; Bours, V.; et al. P2X 1 Ion Channels Promote Neutrophil Chemotaxis through Rho Kinase Activation. J. Immunol. 2009, 183, 2801–2809. [Google Scholar] [CrossRef]
- Alkayed, F.; Kashimata, M.; Koyama, N.; Hayashi, T.; Tamura, Y.; Azuma, Y. P2Y11 purinoceptor mediates the ATP-enhanced chemotactic response of rat neutrophils. J. Pharmacol. Sci. 2012, 120, 288–295. [Google Scholar] [CrossRef]
- Suh, B.-C.; Kim, J.-S.; Namgung, U.; Ha, H.; Kim, K.-T. P2X 7 Nucleotide Receptor Mediation of Membrane Pore Formation and Superoxide Generation in Human Promyelocytes and Neutrophils. J. Immunol. 2001, 166, 6754–6763. [Google Scholar] [CrossRef]
- Martel-Gallegos, G.; Rosales-Saavedra, M.T.; Reyes, J.P.; Casas-Pruneda, G.; Toro-Castillo, C.; Pérez-Cornejo, P.; Arreola, J. Human neutrophils do not express purinergic P2X7 receptors. Purinergic Signal. 2010, 6, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shukla, A.; Namiki, S.; Insel, P.A.; Junger, W.G. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J. Leukoc. Biol. 2004, 76, 245–253. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef]
- Scrivens, M.; Dickenson, J.M. Functional expression of the P2Y14 receptor in human neutrophils. Eur. J. Pharmacol. 2006, 543, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G. Purinoceptors: Are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 1994, 64, 445–475. [Google Scholar] [CrossRef]
- Burnstock, G.; Kennedy, C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen. Pharmacol. 1985, 16, 433–440. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M., VI. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar] [PubMed]
- North, R.A. P2X receptors. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002. [Google Scholar] [CrossRef] [PubMed]
- Surprenant, A.; North, R.A. Signaling at Purinergic P2X Receptors. Annu. Rev. Physiol. 2009. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Evans, R.J. ATP-Gated P2X Receptor Channels: Molecular Insights into Functional Roles. Annu. Rev. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Petersen, O.H.; Michalak, M.; Verkhratsky, A. Calcium signalling: Past, present and future. Cell Calcium 2005. [Google Scholar] [CrossRef]
- Kaczmarek-Hájek, K.; Lörinczi, É.; Hausmann, R.; Nicke, A. Molecular and functional properties of P2X receptors-recent progress and persisting challenges. Purinergic Signal. 2012. [Google Scholar] [CrossRef] [PubMed]
- Coddou, C.; Yan, Z.; Obsil, T.; Huidobro-Toro, J.P.; Stojilkovic, S.S. Activation and Regulation of Purinergic P2X Receptor Channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef]
- Habermacher, C.; Dunning, K.; Chataigneau, T.; Grutter, T. Molecular structure and function of P2X receptors. Neuropharmacology 2016. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Müller, C.E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006. [Google Scholar] [CrossRef]
- Von Kügelgen, I.; Hoffmann, K. Pharmacology and structure of P2Y receptors. Neuropharmacology 2016. [Google Scholar] [CrossRef]
- Kennedy, C.; Qi, A.D.; Herold, C.L.; Harden, T.K.; Nicholas, R.A. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4. Mol. Pharmacol. 2000, 57, 926–931. [Google Scholar] [PubMed]
- Jacobson, K.A.; Paoletta, S.; Katritch, V.; Wu, B.; Gao, Z.G.; Zhao, Q.; Stevens, R.C.; Kiselev, E. Nucleotides acting at P2Y receptors: Connecting structure and function. Mol. Pharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Von Kugelgen, I.; Harden, T.K. Molecular Pharmacology, Physiology, and Structure of the P2Y Receptors. No Adv. Pharmacol. 2011. [Google Scholar] [CrossRef]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Von Kügelgen, I. Pharmacology of P2Y receptors. Brain Res. Bull. 2019. [Google Scholar] [CrossRef] [PubMed]
- Homolya, L.; Watt, W.C.; Lazarowski, E.R.; Koller, B.H.; Boucher, R.C. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y2 receptor (-/-) mice. J. Biol. Chem. 1999. [Google Scholar] [CrossRef][Green Version]
- Savio, L.E.B.; Mello, P. de A.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 receptor in inflammatory diseases: Angel or demon? Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Dagvadorj, J.; Shimada, K.; Chen, S.; Jones, H.D.; Tumurkhuu, G.; Zhang, W.; Wawrowsky, K.A.; Crother, T.R.; Arditi, M. Lipopolysaccharide Induces Alveolar Macrophage Necrosis via CD14 and the P2X7 Receptor Leading to Interleukin-1α Release. Immunity 2015, 42, 640–653. [Google Scholar] [CrossRef]
- Moore, D.J.; Murdock, P.R.; Watson, J.M.; Faull, R.L.M.; Waldvogel, H.J.; Szekeres, P.G.; Wilson, S.; Freeman, K.B.; Emson, P.C. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: Possible role in neuroimmune function. Mol. Brain Res. 2003, 118, 10–23. [Google Scholar] [CrossRef]
- Darbousset, R.; Delierneux, C.; Mezouar, S.; Hego, A.; Lecut, C.; Guillaumat, I.; Riederer, M.A.; Evans, R.J.; Dignat-George, F.; Panicot-Dubois, L.; et al. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood 2014, 124, 2575–2585. [Google Scholar] [CrossRef]
- Lucattelli, M.; Cicko, S.; Müller, T.; Lommatzsch, M.; De Cunto, G.; Cardini, S.; Sundas, W.; Grimm, M.; Zeiser, R.; Dürk, T.; et al. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 2011, 44, 423–429. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Le Duc, D.; Schulz, A.; Lede, V.; Schulze, A.; Thor, D.; Brüser, A.; Schöneberg, T. P2Y Receptors in Immune Response and Inflammation. Adv. Immunol. 2017, 136, 85–121. [Google Scholar]
- Gorini, S.; Callegari, G.; Romagnoli, G.; Mammi, C.; Mavilio, D.; Rosano, G.; Fini, M.; Di Virgilio, F.; Gulinelli, S.; Falzoni, S.; et al. ATP secreted by endothelial cells blocks CX 3CL1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y 11 receptor activation. Blood 2010, 116, 4492–4500. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A. New insights of P2X7 receptor signaling pathway in alveolar functions. J. Biomed. Sci. 2013, 20, 26. [Google Scholar] [CrossRef]
- Saéz, P.J.; Vargas, P.; Shoji, K.F.; Harcha, P.A.; Lennon-Duméni, A.M.; Saéz, J.C. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptors. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Aswad, F.; Dennert, G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell. Immunol. 2006, 243, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, W.; Xu, X.; Xiong, Y.; Zhang, Y.; Zhang, H.; Sun, B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc. Natl. Acad. Sci. USA 2017, 114, 4483–4488. [Google Scholar] [CrossRef]
- El Mdawar, M.B.; Maître, B.; Magnenat, S.; Gachet, C.; Hechler, B.; de la Salle, H. The ATP-gated P2X 1 ion channel contributes to the severity of antibody-mediated Transfusion-Related Acute Lung Injury in mice. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Visovatti, S.H.; Hyman, M.C.; Goonewardena, S.N.; Anyanwu, A.C.; Kanthi, Y.; Robichaud, P.; Wang, J.; Petrovic-Djergovic, D.; Rattan, R.; Burant, C.F.; et al. Purinergic dysregulation in pulmonary hypertension. Am. J. Physiol. Hear. Circ. Physiol. 2016, 311, H286–H298. [Google Scholar] [CrossRef] [PubMed]
- Murrell-Lagnado, R.D.; Frick, M. P2X4 and lysosome fusion. Curr. Opin. Pharmacol. 2019, 47, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Neuland, K.; Sharma, N.; Frick, M. Synaptotagmin-7 links fusion-activated Ca2+ entry and fusion pore dilation. J. Cell Sci. 2014, 127, 5218–5227. [Google Scholar] [CrossRef]
- Thompson, K.E.; Korbmacher, J.P.; Hecht, E.; Hobi, N.; Wittekindt, O.H.; Dietl, P.; Kranz, C.; Frick, M. Fusion-activated cation entry (FACE) via P2X4 couples surfactant secretion and alveolar fluid transport. FASEB J. 2013, 27, 1772–1783. [Google Scholar] [CrossRef]
- Ledderose, C.; Liu, K.; Kondo, Y.; Slubowski, C.J.; Dertnig, T.; Denicoló, S.; Arbab, M.; Hubner, J.; Konrad, K.; Fakhari, M.; et al. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J. Clin. Invest. 2018, 128, 3583–3594. [Google Scholar] [CrossRef]
- Hasan, D.; Satalin, J.; van der Zee, P.; Kollisch-Singule, M.; Blankman, P.; Shono, A.; Somhorst, P.; den Uil, C.; Meeder, H.; Kotani, T.; et al. Excessive extracellular ATP desensitizes P2Y2 and P2X4 ATP receptors provoking surfactant impairment ending in ventilation-induced lung injury. Int. J. Mol. Sci. 2018, 19, 1185. [Google Scholar] [CrossRef] [PubMed]
- Mawatwal, S.; Behura, A.; Ghosh, A.; Kidwai, S.; Mishra, A.; Deep, A.; Agarwal, S.; Saha, S.; Singh, R.; Dhiman, R. Calcimycin mediates mycobacterial killing by inducing intracellular calcium-regulated autophagy in a P2RX7 dependent manner. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Mawatwal, S.; Behura, A.; Mishra, A.; Singh, R.; Dhiman, R. Calcimycin induced IL-12 production inhibits intracellular mycobacterial growth by enhancing autophagy. Cytokine 2018, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Guo, Y.; Zhang, L.; More, S.; Weng, T.; Chintagari, N.R.; Huang, C.; Liang, Y.; Pushparaj, S.; Gou, D.; et al. A Critical Role for P2X7 Receptor–Induced VCAM-1 Shedding and Neutrophil Infiltration during Acute Lung Injury. J. Immunol. 2016, 197, 2828–2837. [Google Scholar] [CrossRef]
- Lee, B.H.; Hwang, D.M.; Palaniyar, N.; Grinstein, S.; Philpott, D.J.; Hu, J. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Leyva-Grado, V.H.; Ermler, M.E.; Schotsaert, M.; Gonzalez, M.G.; Gillespie, V.; Lim, J.K.; García-Sastrea, A. Contribution of the purinergic receptor P2X7 to development of lung immunopathology during influenza virus infection. MBio 2017. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Coutinho-Silva, R. Purinergic signalling, DAMPs and inflammation. Am. J. Physiol. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H.; Wang, L.; Zhang, N.; Huang, H.; Xiong, Q.; Yan, Y.; Wu, N.; Ren, H.; Han, H.; et al. Virus-Triggered ATP Release Limits Viral Replication through Facilitating IFN-β Production in a P2X7-Dependent Manner. J. Immunol. 2017, 199, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, C.H. Control of Tissue-Resident Invariant NKT Cells by Vitamin A Metabolites and P2X7-Mediated Cell Death. J. Immunol. 2019, 203, 1189–1197. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, J.; Bian, H.; Cai, J.; Zhu, G. Suppression of P2X7/NF-κB pathways by Schisandrin B contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Arch. Pharm. Res. 2016, 39, 499–507. [Google Scholar] [CrossRef]
- Cicko, S.; Köhler, T.C.; Korcan Ayata, C.; Müller, T.; Ehrat, N.; Meyer, A.; Hossfeld, M.; Zech, A.; Di Virgilio, F.; Idzko, M. Extracellular ATP is a danger signal activating P2X7 receptor in a LPS mediated inflammation (ARDS/ALI). Oncotarget 2018, 9, 30635–30648. [Google Scholar] [CrossRef] [PubMed]
- Cicko, S.; Meyer, A.; Ehrat, N.; Ayata, C.K.; Zech, A.; Hossfeld, M.; Idzko, M. Extracellular ATP is a danger signal activating P2X7 Receptor in a LPS mediated inflammation (ARDS). ERS 2015, 46. [Google Scholar] [CrossRef]
- Galam, L.; Rajan, A.; Failla, A.; Soundararajan, R.; Lockey, R.F.; Kolliputi, N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, 572–581. [Google Scholar] [CrossRef]
- Ibrahim, M.; Wang, X.; Puyo, C.A.; Montecalvo, A.; Huang, H.J.; Hachem, R.R.; Andreetti, C.; Menna, C.; Chen, R.; Krupnick, A.S.; et al. Human recombinant apyrase therapy protects against canine pulmonary ischemia-reperfusion injury. J. Hear. Lung Transplant. 2015, 34, 247–253. [Google Scholar] [CrossRef]
- Li, Q.C.; Liang, Y.; Su, Z.B. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L1107–L1117. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Chen, X.; Shi, Q.; Su, W.; Zhao, H. SOCS-1 Suppresses Inflammation Through Inhibition of NALP3 Inflammasome Formation in Smoke Inhalation-Induced Acute Lung Injury. Inflammation 2018, 41, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.A. Regulation of surfactant secretion. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2001. [Google Scholar] [CrossRef]
- Leipziger, J. Control of epithelial transport via luminal P2 receptors. Am. J. Physiol. Ren. Physiol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Novak, I. Purinergic signalling in epithelial ion transport: Regulation of secretion and absorption. Acta Physiol. 2011. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G. Purinergic regulation of neutrophil chemotaxis. Cell. Mol. Life Sci. 2008, 65, 2528–2540. [Google Scholar] [CrossRef]
- Müller, T.; Fay, S.; Vieira, R.P.; Harry, K.Q.; Cicko, S.; Ayata, K.; Zissel, G.; Goldmann, T.; Lungarella, G.; Ferrari, D.; et al. The purinergic receptor subtype P2Y2 mediates chemotaxis of neutrophils and fibroblasts in fibrotic lung disease. Oncotarget 2017, 8, 35962–35972. [Google Scholar] [CrossRef]
- Bove, P.F.; Grubb, B.R.; Okada, S.F.; Ribeiro, C.M.P.; Rogers, T.D.; Randell, S.H.; O’Neal, W.K.; Boucher, R.C. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J. Biol. Chem. 2010. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Watt, W.C.; Stutts, M.J.; Boucher, R.C.; Harden, T.K. Pharmacological selectivity of the cloned human P2U-purinoceptor: Potent activation by diadenosine tetraphosphate. Br. J. Pharmacol. 1995. [Google Scholar] [CrossRef]
- Brunschweiger, A.; Muller, C. P2 Receptors Activated by Uracil Nucleotides—An Update. Curr. Med. Chem. 2006. [Google Scholar] [CrossRef]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Sesma, J.I.; Seminario-Vidal, L.; Kreda, S.M. Molecular Mechanisms of Purine and Pyrimidine Nucleotide Release. Adv. Pharmacol. 2011. [Google Scholar] [CrossRef]
- Fois, G.; Winkelmann, V.E.; Bareis, L.; Staudenmaier, L.; Hecht, E.; Ziller, C.; Ehinger, K.; Schymeinsky, J.; Kranz, C.; Frick, M. ATP is stored in lamellar bodies to activate vesicular P2X4 in an autocrine fashion upon exocytosis. J. Gen. Physiol. 2018, 150, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Boudreault, F.; Adam, D.; Brochiero, E.; Grygorczyk, R. Type 2 secretory cells are primary source of ATP release in mechanically stretched lung alveolar cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Grygorczyk, R.; Boudreault, F.; Tan, J.J.; Ponomarchuk, O.; Sokabe, M.; Furuya, K. Mechanosensitive ATP release in the lungs: New insights from real-time luminescence imaging studies. Curr. Top. Membr. 2019, 83, 45–76. [Google Scholar] [CrossRef]
- Ramsingh, R.; Grygorczyk, A.; Solecki, A.; Cherkaoui, L.S.; Berthiaume, Y.; Grygorczyk, R. Cell deformation at the air-liquid interface induces Ca2+-dependent ATP release from lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011. [Google Scholar] [CrossRef][Green Version]
- Furuya, K.; Tan, J.J.; Boudreault, F.; Sokabe, M.; Berthiaume, Y.; Grygorczyk, R. Real-time imaging of inflation-induced ATP release in the ex vivo rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L956–L969. [Google Scholar] [CrossRef] [PubMed]
- Dietl, P.; Frick, M.; Mair, N.; Bertocchi, C.; Haller, T. Pulmonary consequences of a deep breath revisited. Biol. Neonate 2004. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.; Bertocchi, C.; Jennings, P.; Haller, T.; Mair, N.; Singer, W.; Pfaller, W.; Ritsch-Marte, M.; Dietl, P. Ca2+ entry is essential for cell strain-induced lamellar body fusion in isolated rat type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, H.R.; Dobbs, L.G. The effects of mechanical forces on lung functions. Respir. Physiol. 2000. [Google Scholar] [CrossRef]
- Edwards, Y.S. Stretch stimulation: Its effects on alveolar type II cell function in the lung. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2001. [Google Scholar] [CrossRef]
- Kuehn, A.; Kletting, S.; De Souza Carvalho-Wodarz, C.; Repnik, U.; Griffiths, G.; Fischer, U.; Meese, E.; Huwer, H.; Wirth, D.; May, T.; et al. Human alveolar epithelial cells expressing tight junctions to model the air-blood barrier. ALTEX 2016, 33, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M. Purines in tissue inflammation and fibrosis. Purinergic Signal. 2014. [Google Scholar] [CrossRef][Green Version]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.J.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.B.; Douillet, C.D.; Mahler, S.A.; Husain, S.A.; Boucher, R.C. Adenosine triphosphate is released during injurious mechanical ventilation and contributes to lung edema. J. Trauma 2003. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Blankman, P.; Nieman, G.F. Purinergic signalling links mechanical breath profile and alveolar mechanics with the pro-inflammatory innate immune response causing ventilation-induced lung injury. Purinergic Signal. 2017, 13, 363–386. [Google Scholar] [CrossRef]
- Tatur, S.; Groulx, N.; Orlov, S.N.; Grygorczyk, R. Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides. J. Physiol. 2007. [Google Scholar] [CrossRef]
- Dietl, P.; Liss, B.; Felder, E.; Miklavc, P.; Wirtz, H. Lamellar body exocytosis by cell stretch or purinergic stimulation: Possible physiological roles, messengers and mechanisms. Cell. Physiol. Biochem. 2010, 25, 1–12. [Google Scholar] [CrossRef]
- Andreeva, A.V.; Kutuzov, M.A.; Voyno-Yasenetskaya, T.A. Regulation of surfactant secretion in alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293. [Google Scholar] [CrossRef]
- Olmeda, B.; Martínez-Calle, M.; Pérez-Gil, J. Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat. 2017, 209, 78–92. [Google Scholar] [CrossRef]
- Perez-Gil, J.; Weaver, T.E. Pulmonary surfactant pathophysiology: Current models and open questions. Physiology 2010, 25, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chander, A.; Fisher, A.B. Regulation of lung surfactant secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 1990. [Google Scholar] [CrossRef]
- Rice, W.R. Effects of Extracellular ATP on Surfactant Secretion. Ann. N. Y. Acad. Sci. 1990. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Rooney, S.A. Purinoceptor agonists stimulate phosphatidylcholine secretion in primary cultures of adult rat type II pneumocytes. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1987. [Google Scholar] [CrossRef]
- Griese, M.; Gobran, L.I.; Rooney, S.A. ATP-stimulated inositol phospholipid metabolism and surfactant secretion in rat type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 1991. [Google Scholar] [CrossRef]
- Haller, T.; Auktor, K.; Frick, M.; Mair, N.; Dietl, A.P. Threshold calcium levels for lamellar body exocytosis in type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999, 277, L893–L900. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Frick, M.; Haller, T.; Bernet, S.; Ritsch-Marte, M.; Dietl, P. Mechanical forces impeding exocytotic surfactant release revealed by optical tweezers. Biophys. J. 2003, 84, 1344–1351. [Google Scholar] [CrossRef]
- Haller, T.; Dietl, P.; Pfaller, K.; Frick, M.; Mair, N.; Paulmichl, M.; Hess, M.W.; Fürst, J.; Maly, K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 2001, 155, 279–289. [Google Scholar] [CrossRef]
- Miklavc, P.; Ehinger, K.; Sultan, A.; Felder, T.; Paul, P.; Gottschalk, K.-E.E.; Frick, M. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J. Cell Sci. 2015, 128, 1193–1203. [Google Scholar] [CrossRef]
- Miklavc, P.; Frick, M.; Wittekindt, O.H.; Haller, T.; Dietl, P. Fusion-Activated Ca2+ entry: An “active zone” of elevated Ca2+ during the postfusion stage of lamellar body exocytosis in rat Type II Pneumocytes. PLoS ONE 2010. [Google Scholar] [CrossRef]
- Fois, G.; Föhr, K.J.; Kling, C.; Fauler, M.; Wittekindt, O.H.; Dietl, P.; Frick, M. P2X4 receptor re-sensitization depends on a protonation/deprotonation cycle mediated by receptor internalization and recycling. J. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewski, R.; Matalon, S.; Collawn, J.F. Ion channels of the lung and their role in disease pathogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L859–L872. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, O.H.; Dietl, P. Aquaporins in the lung. Pflugers Arch. Eur. J. Physiol. 2019, 471, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, M.I.; Richter, K.; Fronius, M. Ion transport by pulmonary epithelia. J. Biomed. Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Bastacky, J.; Lee, C.Y.C.; Goerke, J.; Koushafar, H.; Yager, D.; Kenaga, L.; Speed, T.P.; Chen, Y.; Clements, J.A. Alveolar lining layer is thin and continuous: Low-temperature scanning electron microscopy of rat lung. J. Appl. Physiol. 1995, 79, 1615–1628. [Google Scholar] [CrossRef]
- Berthiaume, Y.; Matthay, M.A. Alveolar edema fluid clearance and acute lung injury. Respir. Physiol. Neurobiol. 2007. [Google Scholar] [CrossRef]
- Kemp, P.J.; Kim, K.J. Spectrum of ion channels in alveolar epithelial cells: Implications for alveolar fluid balance. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, L.G.; Johnson, M.D. Alveolar epithelial transport in the adult lung. Respir. Physiol. Neurobiol. 2007. [Google Scholar] [CrossRef]
- Peteranderl, C.; Sznajder, J.I.; Herold, S.; Lecuona, E. Inflammatory responses regulating alveolar ion transport during pulmonary infections. Front. Immunol. 2017. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Makhlina, M.; Martsen, E.; Thomas, E.J.; Lethem, M.I.; Boucher, R.C. Permeabilization via the P2X7 purinoreceptor reveals the presence of a Ca2+ -activated Cl- conductance in the apical membrane of murine tracheal epithelial cells. J. Biol. Chem. 2000. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Schreiber, R.; Cook, D. Mechanisms for the inhibition of amiloride-sensitive Na+ absorption by extracellular nucleotides in mouse trachea. Pflugers Arch. Eur. J. Physiol. 2002. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Kunzelmann, K. Purinergic P2Y 6 receptors induce Ca 2+ and CFTR dependent Cl—Secretion in mouse trachea. Cell. Physiol. Biochem. 2005. [Google Scholar] [CrossRef] [PubMed]
- Blaug, S.; Rymer, J.; Jalickee, S.; Miller, S.S. P2 purinoceptors regulate calcium-activated chloride and fluid transport in 31EG4 mammary epithelia. Am. J. Physiol. Cell Physiol. 2003. [Google Scholar] [CrossRef][Green Version]
- Pochynyuk, O.; Bugaj, V.; Vandewalle, A.; Stockand, J.D. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am. J. Physiol. Ren. Physiol. 2008. [Google Scholar] [CrossRef]
- Faria, D.; Schreiber, R.; Kunzelmann, K. CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflugers Arch. Eur. J. Physiol. 2009. [Google Scholar] [CrossRef]
- Jin, W.; Hopfer, U. Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: Elevated cytosolic Ca2+ is not required. Am. J. Physiol. Cell Physiol. 1997. [Google Scholar] [CrossRef]
- Miklavc, P.; Thompson, K.E.; Frick, M. A new role for P2X4 receptors as modulators of lung surfactant secretion. Front. Cell. Neurosci. 2013, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Hartl, D.; Tirouvanziam, R.; Laval, J.; Greene, C.M.; Habiel, D.; Sharma, L.; Yildirim, A.Ö.; Dela Cruz, C.S.; Hogaboam, C.M. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J. Innate Immun. 2018. [Google Scholar] [CrossRef]
- Le, T.T.T.; Berg, N.K.; Harting, M.T.; Li, X.; Eltzschig, H.K.; Yuan, X. Purinergic signaling in pulmonary inflammation. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Vuerich, M. Purinergic signaling in the immune system. Auton. Neurosci. Basic Clin. 2015. [Google Scholar] [CrossRef]
- Morandini, A.; Savio, L.; Coutinho-Silva, R. The role of p2x7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed. J. 2014. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Falzoni, S.; Di Virgilio, F. The P2X7 receptor-interleukin-1 liaison. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Krautloher, A.; Ramírez-Fernández, A.; Nicke, A. P2X7 Interactions and Signaling—Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- Barberà-Cremades, M.; Gómez, A.I.; Baroja-Mazo, A.; Martínez-Alarcón, L.; Martínez, C.M.; de Torre-Minguela, C.; Pelegrín, P. P2X7 receptor induces tumor necrosis factor-α converting enzyme activation and release to boost TNF-α production. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Dubyak, G.R. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell. Microbiol. 2012, 14, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 2015. [Google Scholar] [CrossRef]
- Scott, I.C.; Majithiya, J.B.; Sanden, C.; Thornton, P.; Sanders, P.N.; Moore, T.; Guscott, M.; Corkill, D.J.; Erjefält, J.S.; Cohen, E.S. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J. Immunol. 2011. [Google Scholar] [CrossRef]
- Soni, S.; O’Dea, K.P.; Tan, Y.Y.; Cho, K.; Abe, E.; Romano, R.; Cui, J.; Ma, D.; Sarathchandra, P.; Wilson, M.R.; et al. ATP redirects cytokine trafficking and promotes novel membrane TNF signaling via microvesicles. FASEB J. 2019, 33, 6442–6455. [Google Scholar] [CrossRef]
- Soni, S.; Wilson, M.R.; O’Dea, K.P.; Yoshida, M.; Katbeh, U.; Woods, S.J.; Takata, M. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax 2016, 71, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Pizzirani, C.; Ferrari, D.; Chiozzi, P.; Adinolfi, E.; Sandonà, D.; Savaglio, E.; Di Virgilio, F. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood 2007, 109, 3856–3864. [Google Scholar] [CrossRef] [PubMed]
- Kronlage, M.; Song, J.; Sorokin, L.; Isfort, K.; Schwerdtle, T.; Leipziger, J.; Robaye, B.; Conley, P.B.; Kim, H.C.; Sargin, S.; et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal. 2010, 3. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, Y.; Ledderose, C.; Li, L.; Junger, W.G. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J. Biol. Chem. 2013, 288, 22650–22657. [Google Scholar] [CrossRef]
- Bao, Y.; Ledderose, C.; Graf, A.F.; Brix, B.; Birsak, T.; Lee, A.; Zhang, J.; Junger, W.G. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J. Cell Biol. 2015, 210, 1153–1164. [Google Scholar] [CrossRef]
- Li, P.; Cao, J.; Chen, Y.; Wang, W.; Yang, J. Apyrase protects against allergic airway inflammation by decreasing the chemotactic migration of dendritic cells in mice. Int. J. Mol. Med. 2014, 34, 269–275. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Burnstock, G.; Boeynaems, J.M. Purinergic signalling and immune cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar]
- Shin, A.; Toy, T.; Rothenfusser, S.; Robson, N.; Vorac, J.; Dauer, M.; Stuplich, M.; Endres, S.; Cebon, J.; Maraskovsky, E.; et al. P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood 2008, 111, 3062–3069. [Google Scholar] [CrossRef]
- Ledderose, C.; Bao, Y.; Lidicky, M.; Zipperle, J.; Li, L.; Strasser, K.; Shapiro, N.I.; Junger, W.G. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J. Biol. Chem. 2014, 289, 25936–25945. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, C.; Bao, Y.; Zhang, J.; Junger, W.G. Novel method for real-time monitoring of ATP release reveals multiple phases of autocrine purinergic signalling during immune cell activation. Acta Physiol. 2015, 213, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Ploia, C.; Anselmi, F.; Sarukhan, A.; Viola, A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 2014, 33, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Brouns, I.; Adriaensen, D.; Timmermans, J.-P. Purinergic signaling in the airways. Pharmacol. Rev. 2012. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The purinergic system as a pharmacological target for the treatment of immune-mediated inflammatory diseases. Pharmacol. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling: Therapeutic developments. Front. Pharmacol. 2017, 8, 1–55. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Tate, M.D.; Ong, J.D.H.; Dowling, J.K.; McAuley, J.L.; Robertson, A.B.; Latz, E.; Drummond, G.R.; Cooper, M.A.; Hertzog, P.J.; Mansell, A. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Rosli, S.; Kirby, F.J.; Lawlor, K.E.; Rainczuk, K.; Drummond, G.R.; Mansell, A.; Tate, M.D. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br. J. Pharmacol. 2019, 176, 3834–3844. [Google Scholar] [CrossRef]
- Santos, A.A.; Rodrigues-Junior, V.; Zanin, R.F.; Borges, T.J.; Bonorino, C.; Coutinho-Silva, R.; Takyia, C.M.; Santos, D.S.; Campos, M.M.; Morrone, F.B. Implication of purinergic P2X7 receptor in M. tuberculosis infection and host interaction mechanisms: A mouse model study. Immunobiology 2013, 218, 1104–1112. [Google Scholar] [CrossRef]
- Amaral, E.P.; Ribeiro, S.C.M.; Lanes, V.R.; Almeida, F.M.; de Andrade, M.R.M.; Bomfim, C.C.B.; Salles, É.M.; Bortoluci, K.R.; Coutinho-Silva, R.; Hirata, M.H.; et al. Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis. PLoS Pathog. 2014. [Google Scholar] [CrossRef] [PubMed]
- Olotu, C.; Lehmensiek, F.; Koch, B.; Kiefmann, M.; Riegel, A.K.; Hammerschmidt, S.; Kiefmann, R. Streptococcus pneumoniae inhibits purinergic signaling and promotes purinergic receptor P2Y2 internalization in alveolar epithelial cells. J. Biol. Chem. 2019, 294, 12795–12806. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Amaya, F.; Hashimoto, S.; Ueno, H.; Beppu, S.; Mizuta, M.; Shime, N.; Ishizaka, A.; Hashimoto, S. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: An experimental study. Respir. Res. 2008, 9, 1–13. [Google Scholar] [CrossRef]
- Reutershan, J.; Vollmer, I.; Stark, S.; Wagner, R.; Ngamsri, K.; Eltzschig, H.K. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009, 23, 473–482. [Google Scholar] [CrossRef]
- Mansour, A.; Bachelot-Loza, C.; Nesseler, N.; Gaussem, P.; Gouin-Thibault, I. P2Y12 inhibition beyond thrombosis: Effects on inflammation. Int. J. Mol. Sci. 2020, 21, 1391. [Google Scholar] [CrossRef]
- Liverani, E.; Rico, M.C.; Yaratha, L.; Tsygankov, A.Y.; Kilpatrick, L.E.; Kunapuli, S.P. LPS-induced systemic inflammation is more severe in P2Y 12 null mice. J. Leukoc. Biol. 2014, 95, 313–323. [Google Scholar] [CrossRef]
- Amison, R.T.; Arnold, S.; O’Shaughnessy, B.G.; Cleary, S.J.; Ofoedu, J.; Idzko, M.; Page, C.P.; Pitchford, S.C. Lipopolysaccharide (LPS) induced pulmonary neutrophil recruitment and platelet activation is mediated via the P2Y1 and P2Y14 receptors in mice. Pulm. Pharmacol. Ther. 2017, 45, 62–68. [Google Scholar] [CrossRef]
- Liverani, E. Lung injury during LPS-induced inflammation occurs independently of the receptor P2Y1. Purinergic Signal. 2017, 13, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.; Wagner, K.; Weber, S.; Gröger, M.; Wepler, M.; McCook, O.; Scheuerle, A.; Stahl, B.; Huber-Lang, M.; Jung, B.; et al. Role of the purinergic receptor P2XR4 after blunt chest trauma in cigarette smoke-exposed mice. Shock 2017, 47, 193–199. [Google Scholar] [CrossRef]
- Dixit, A.; Cheema, H.; George, J.; Iyer, S.; Dudeja, V.; Dawra, R.; Saluja, A.K. Extracellular release of ATP promotes systemic inflammation during acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G463–G475. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, I.A.; Mirzapoiazova, T.; Moreno-Vinasco, L.; Sammani, S.; Garcia, J.G.N.; Verin, A.D. Protective effect of purinergic agonist ATPγS against acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294. [Google Scholar] [CrossRef]
- Li, X.; Kondo, Y.; Bao, Y.; Staudenmaier, L.; Lee, A.; Zhang, J.; Ledderose, C.; Junger, W.G. Systemic Adenosine Triphosphate Impairs Neutrophil Chemotaxis and Host Defense in Sepsis. Crit. Care Med. 2017, 45, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Hoegl, S.; Burns, N.; Angulo, M.; Francis, D.; Osborne, C.M.; Mills, T.W.; Blackburn, M.R.; Eltzschig, H.K.; Vohwinkel, C.U. Capturing the multifactorial nature of ARDS—“Two-hit” approach to model murine acute lung injury. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, Y.; Islam, D.; Wen, X.Y.; Wu, S.; Streutker, C.; Luo, A.; Li, M.; Khang, J.; Han, B.; et al. Dual effects of human neutrophil peptides in a mouse model of pneumonia and ventilator-induced lung injury. Respir. Res. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Bagnato, G.; Harari, S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur. Respir. Rev. 2015, 24, 102–114. [Google Scholar] [CrossRef]
- Wuyts, W.A.; Agostini, C.; Antoniou, K.M.; Bouros, D.; Chambers, R.C.; Cottin, V.; Egan, J.J.; Lambrecht, B.N.; Lories, R.; Parfrey, H.; et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur. Respir. J. 2013. [Google Scholar] [CrossRef]
- Müller, T.; Fay, S.; Vieira, R.P.; Karmouty-Quintana, H.; Cicko, S.; Ayata, C.K.; Zissel, G.; Goldmann, T.; Lungarella, G.; Ferrari, D.; et al. P2Y6 receptor activation promotes inflammation and tissue remodeling in pulmonary fibrosis. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Moore, B.B.; Lawson, W.E.; Oury, T.D.; Sisson, T.H.; Raghavendran, K.; Hogaboam, C.M. Animal models of fibrotic lung disease. Am. J. Respir. Cell Mol. Biol. 2013, 49, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Günther, S.; Dorfmüller, P.; Perros, F.; Girerd, B.; Garcia, G.; Jaïs, X.; Savale, L.; Artaud-Macari, E.; Price, L.C.; et al. Pulmonary arterial hypertension. Orphanet J. Rare Dis. 2013, 8, 97. [Google Scholar] [CrossRef]
- Visovatti, S.H.; Hyman, M.C.; Bouis, D.; Neubig, R.; McLaughlin, V.V.; Pinsky, D.J. Increased CD39 nucleotidase activity on microparticles from patients with idiopathic pulmonary arterial hypertension. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ohashi, Y.; Watanabe, H.; Tsubota, K. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: A Japanese phase 2 clinical trial. Ophthalmology 2012. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.E.; Baqi, Y.; Namasivayam, V. Agonists and antagonists for purinergic receptors. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020. [Google Scholar]
- Baqi, Y.; Müller, C.E. Antithrombotic P2Y 12 receptor antagonists: Recent developments in drug discovery. Drug Discov. Today 2019. [Google Scholar] [CrossRef] [PubMed]
- Abdulqawi, R.; Dockry, R.; Holt, K.; Layton, G.; Mccarthy, B.G.; Ford, A.P.; Smith, J.A. P2X3 receptor antagonist (AF-219) in refractory chronic cough: A randomised, double-blind, placebo-controlled phase 2 study. Lancet 2015. [Google Scholar] [CrossRef]
- Martin, T.R.; Matute-Bello, G. Experimental Models and Emerging Hypotheses for Acute Lung Injury. Crit. Care Clin. 2011. [Google Scholar] [CrossRef]
- Miller, A.J.; Spence, J.R. In vitro models to study human lung development, disease and homeostasis. Physiology 2017. [Google Scholar] [CrossRef]
- Potkay, J.A.; Magnetta, M.; Vinson, A.; Cmolik, B. Bio-inspired, efficient, artificial lung employing air as the ventilating gas. Lab. Chip 2011. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010. [Google Scholar] [CrossRef] [PubMed]
- Huh, D. A human breathing lung-on-a-chip. Ann. Am. Thorac. Soc. 2015. [Google Scholar] [CrossRef] [PubMed]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab. Chip 2015. [Google Scholar] [CrossRef] [PubMed]
- Konar, D.; Devarasetty, M.; Yildiz, D.V.; Atala, A.; Murphy, S.V. Lung-On-A-Chip Technologies for Disease Modeling and Drug Development. Biomed. Eng. Comput. Biol. 2016. [Google Scholar] [CrossRef]

| Cell Type | P2 Receptor | Expression |
|---|---|---|
| ATI | P2X7 | mRNA (r [27]), protein (r [27]) |
| P2Y2 | protein (r [28]) | |
| ATI like | P2Y2 | protein (h [28], r [28]) |
| ATII | P2X4 | mRNA (r [29,30]), protein (r [29,30]) |
| (P2X7) | mRNA (r [30]), protein (r [30]) | |
| P2Y2 | mRNA (r [30,31]), protein (r [30]) | |
| P2Y6 | mRNA (r [30]), protein (r [30]) | |
| EC | P2X1 | mRNA (h [32]), protein (h [32]) |
| P2X4 | mRNA (h [32,33,34,35]), protein (h [32]) | |
| P2X5 | mRNA (h [32,34,35]), protein (h [32]) | |
| P2X6 | mRNA (h [35]) | |
| P2X7 | mRNA (h [35]) | |
| P2Y1 | mRNA (h [32,35,36,37]), protein (h [32,36]) | |
| P2Y2 | mRNA (h [35,37]) | |
| P2Y6 | mRNA (h [32]), protein (h [32]) | |
| P2Y11 | mRNA (h [32,36,37,38]), protein (h [32,36]) | |
| P2Y12 | mRNA (h [37]) | |
| P2Y14 | mRNA (h [37]) | |
| Fibroblasts | P2Y2 | mRNA (h [39]) |
| P2Y4 | mRNA (h [39]) | |
| P2Y6 | mRNA (h [39]) | |
| P2Y11 | mRNA (h [39]) | |
| Alveolar macrophages | P2X1 | mRNA (h [40], m [41], r [42]) |
| P2X3 | mRNA (m [41] h [40]) | |
| P2X4 | mRNA (h [40], m [41], r [42]), protein (h [43], r [43,44]), | |
| P2X5 | mRNA (h [40]) | |
| P2X7 | mRNA (h [40], r [42]) | |
| P2Y1 | mRNA (h [40], r [42]) | |
| P2Y2 | mRNA (h [40], r [42]) | |
| P2Y4 | mRNA (h [40], r [42]) | |
| P2Y6 | mRNA (h [40]) | |
| P2Y11 | mRNA (h [40]) | |
| P2Y12 | mRNA (r [42]) | |
| P2Y13 | mRNA (h [40]) | |
| P2Y14 | mRNA (h [40]) | |
| Neutrophils | P2X1 | mRNA (h [45,46,47], r [48]), protein (h [47]) |
| P2X4 | mRNA (h [46,47], r [48]) | |
| P2X5 | mRNA (h [45,46,47], r [48]) | |
| P2X6 | mRNA (h [47]) | |
| P2X7 | ambiguous data ([45,46,48,49,50,51,52]) | |
| P2Y1 | mRNA (h [46]) | |
| P2Y2 | mRNA (h [46,51,53]), protein (h [51], r [48]) | |
| P2Y4 | mRNA (h [51]) | |
| P2Y6 | mRNA (h [51]) | |
| P2Y11 | mRNA (h [51]), protein (h [45], r [48]) | |
| P2Y14 | mRNA (h [54]) |
| P2 Receptor | Physiological Function | Implication in Lung Diseases |
|---|---|---|
| P2X1 | Host defense | Transfusion-related acute lung injury [89] Contribution to development of IPAH [90] |
| P2X4 | Surfactant secretion/ release [29,91,92] Fluid resorption [93] Host defense
| Development of VILI [95] |
| P2X7 | Host defense | Pulmonary tuberculosis [77] Pathogenesis and progression of ARDS [99,105,106,107,108,109,110,111] |
| P2Y2 | Surfactant secretion [12,112] Fluid transport [113,114] Host defense
| Development of VILI [96] Fibrotic remodelling [117] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirsching, E.; Fauler, M.; Fois, G.; Frick, M. P2 Purinergic Signaling in the Distal Lung in Health and Disease. Int. J. Mol. Sci. 2020, 21, 4973. https://doi.org/10.3390/ijms21144973
Wirsching E, Fauler M, Fois G, Frick M. P2 Purinergic Signaling in the Distal Lung in Health and Disease. International Journal of Molecular Sciences. 2020; 21(14):4973. https://doi.org/10.3390/ijms21144973
Chicago/Turabian StyleWirsching, Eva, Michael Fauler, Giorgio Fois, and Manfred Frick. 2020. "P2 Purinergic Signaling in the Distal Lung in Health and Disease" International Journal of Molecular Sciences 21, no. 14: 4973. https://doi.org/10.3390/ijms21144973
APA StyleWirsching, E., Fauler, M., Fois, G., & Frick, M. (2020). P2 Purinergic Signaling in the Distal Lung in Health and Disease. International Journal of Molecular Sciences, 21(14), 4973. https://doi.org/10.3390/ijms21144973





