P2X7 Receptor at the Crossroads of T Cell Fate
Abstract
1. Introduction
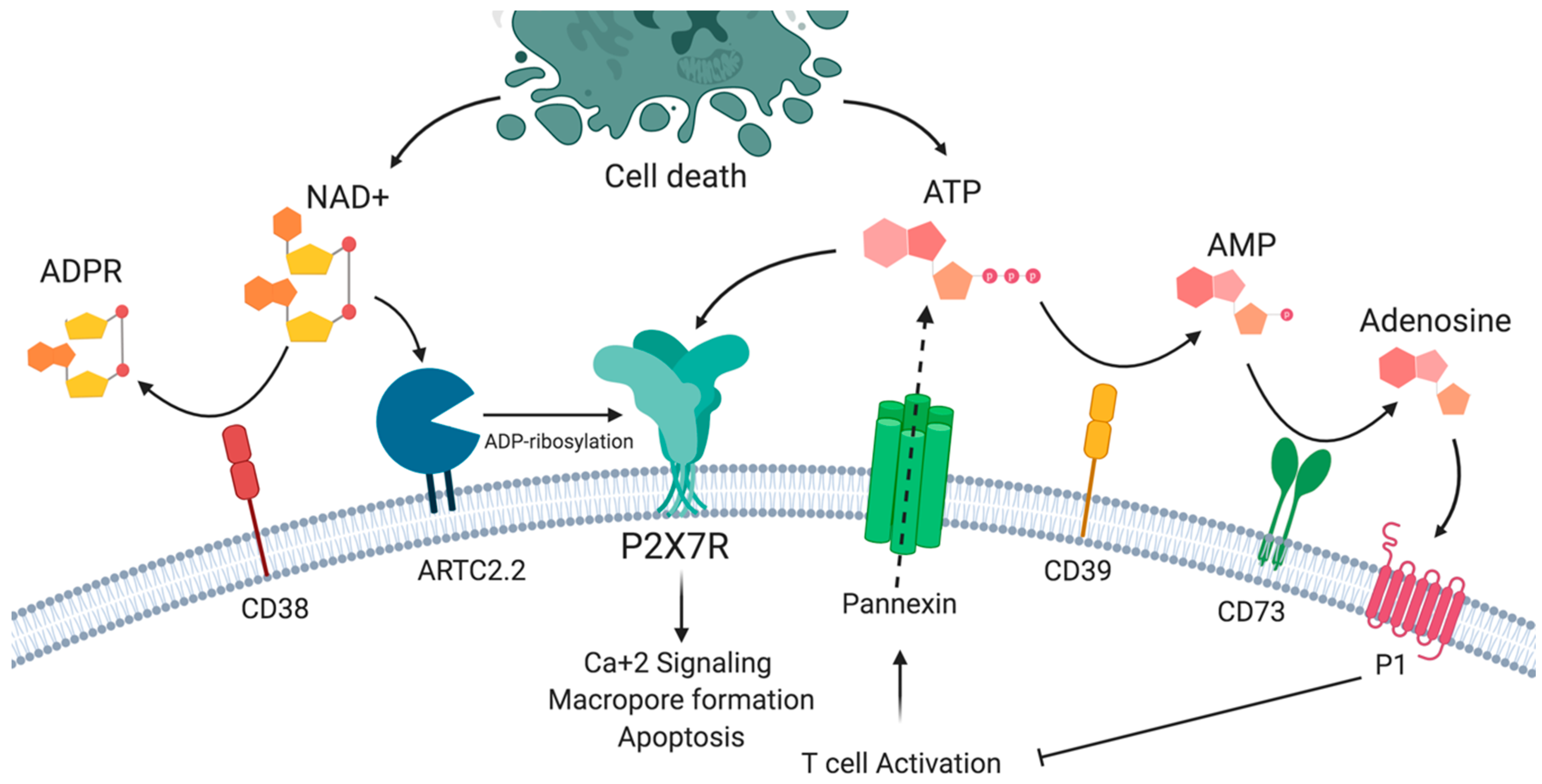
2. P2X7 Receptor Structure and Functions
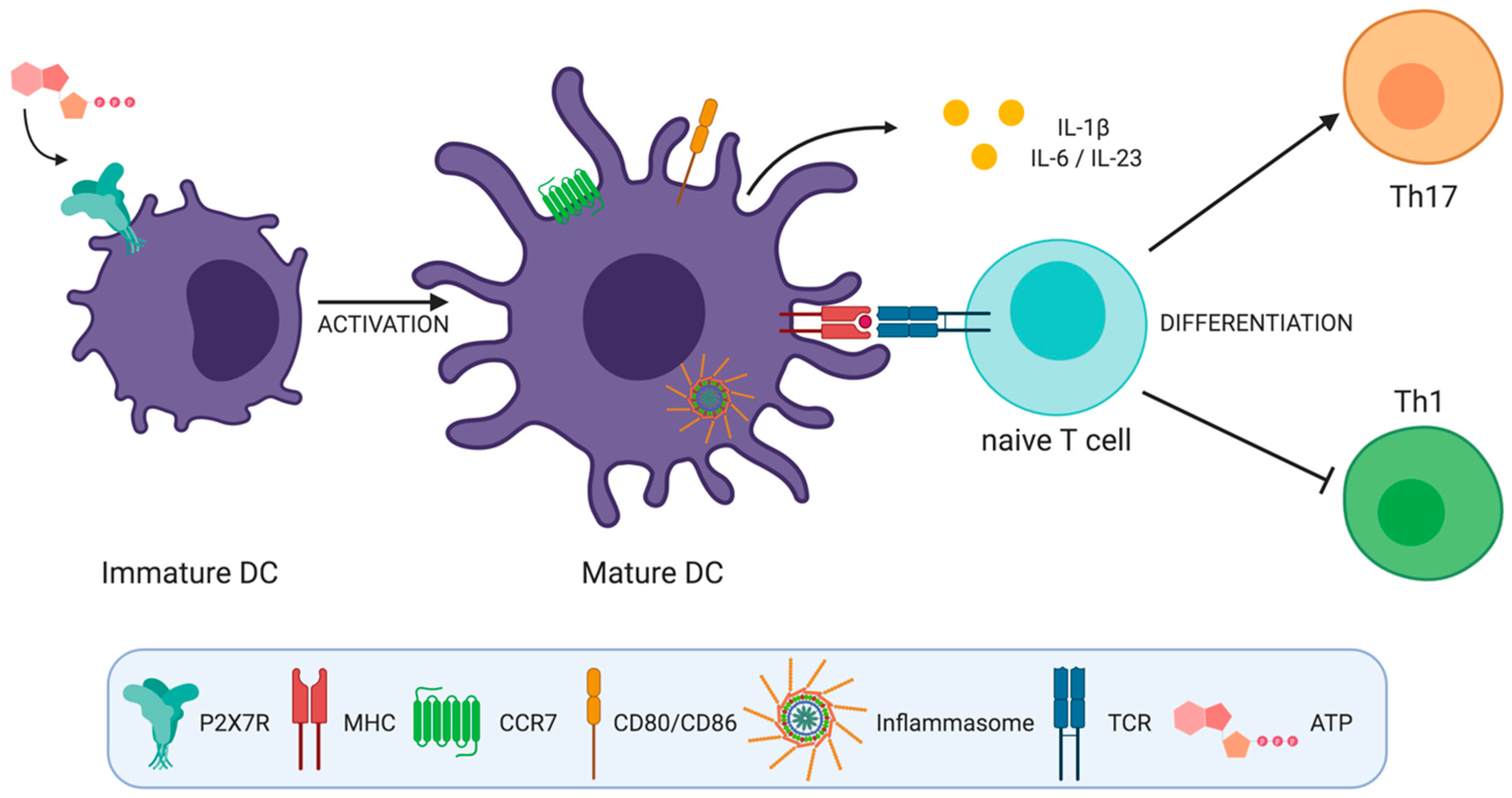
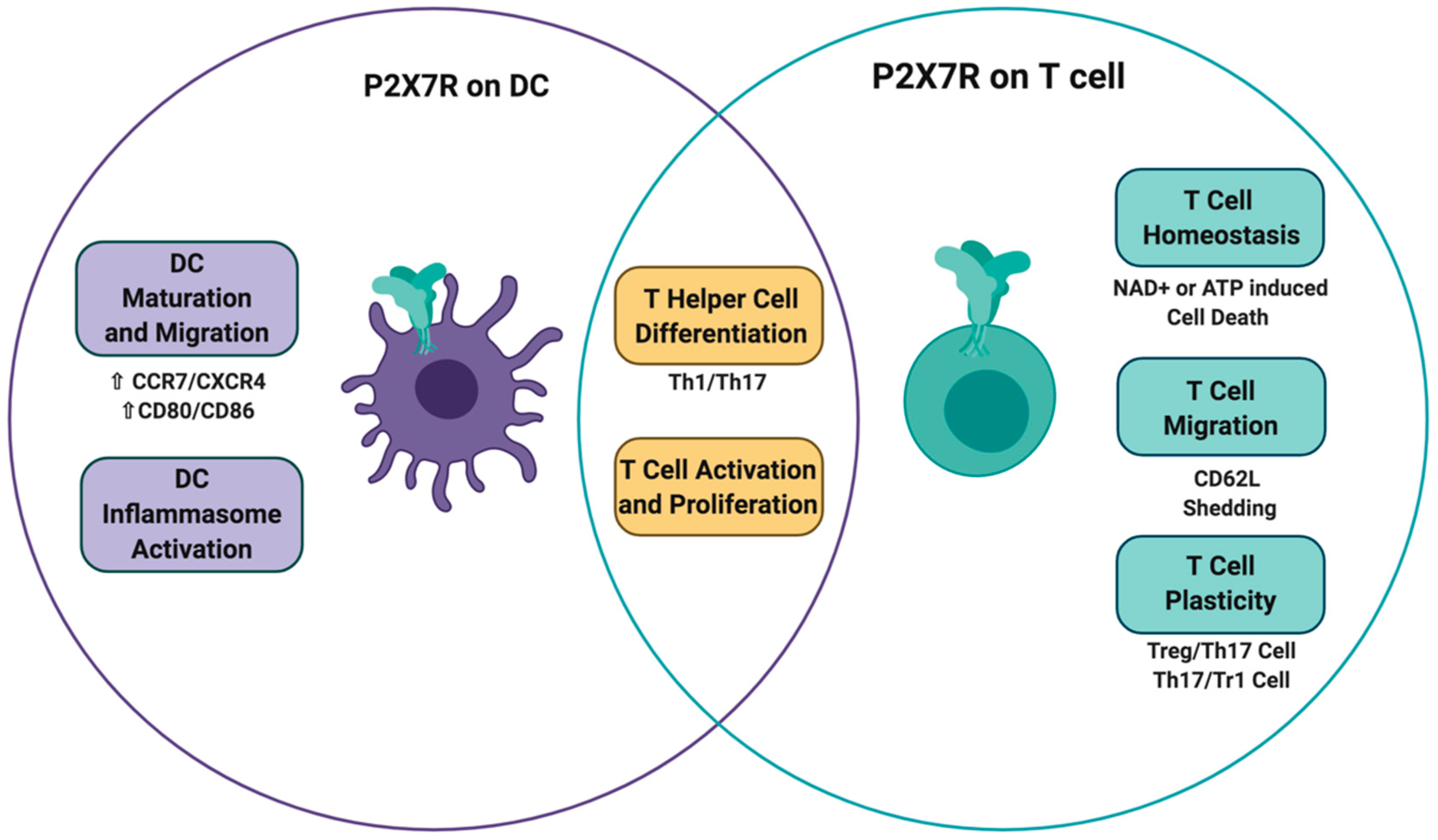
3. P2X7 Receptor on Dendritic Cells
3.1. Inflammasome Activation
3.2. DC Maturation and Migration
3.3. Cytokine Secretion and Polarization of T Helper Cells
3.3.1. Th1 Cell Differentiation
3.3.2. Th17 Cell Differentiation
4. P2X7 Receptor on T Cells
4.1. T Cell Activation
4.2. T Cell Migration and Motility
4.3. Differential Sensitivity to ATP and NAD+ Induced Cell Death
4.3.1. Expansion of Primed T Cells in Lymph Nodes
4.3.2. Regulation of P2X7R Expression in the Gut by Retinoic Acid
4.3.3. Survival of T Cells in Inflamed Tissues
4.4. T Cell Differentiation and Function
4.4.1. Control of CD8+ Memory T Cell Fitness
4.4.2. Control of Regulatory T Cell Differentiation and Plasticity
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bours, M.J.; Swennen, E.; Di Virgilio, F.; Cronstein, B.; Dagnelie, P. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006, 112, 358–404. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Sitkovsky, M.V.; Robson, S.C. Purinergic signaling during inflammation. N. Engl. J. Med. 2012, 367, 2322–2333. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Vuerich, M. Purinergic signaling in the immune system. Auton. Neurosci. 2015, 191, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic Control of T Cell Activation by ATP Released Through Pannexin-1 Hemichannels. Sci. Signal. 2008, 1, ra6. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. et Biophys. Acta (BBA) Bioenerg. 2008, 1783, 673–694. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Sylvester Vizi, E.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Robson, S.C.; Sévigny, J.; Zimmermann, H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006, 2, 409–430. [Google Scholar] [CrossRef]
- Regateiro, F.S.; Cobbold, S.; Waldmann, H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin. Exp. Immunol. 2012, 171, 1–7. [Google Scholar] [CrossRef]
- Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.R.; Fernandez, D.; Flores-Santibáñez, F.; Rosemblatt, M.; Sauma, D.; Flores-Santibañez, F. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015, 589, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Niemi, K.; Teirilä, L.; Lappalainen, J.; Rajamäki, K.; Baumann, M.H.; Öörni, K.; Wolff, H.; Kovanen, P.T.; Matikainen, S.; Eklund, K.K. Serum Amyloid A Activates the NLRP3 Inflammasome via P2X7 Receptor and a Cathepsin B-Sensitive Pathway. J. Immunol. 2011, 186, 6119–6128. [Google Scholar] [CrossRef]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Forchap, S.; Sitta, B.; Turchet, L.; Falzoni, S.; Minelli, M.; Baricordi, R.; Di Virgilio, F. The antibiotic polymyxin B modulates P2X7 receptor function. J. Immunol. 2004, 173, 4652–4660. [Google Scholar] [CrossRef]
- Mutini, C.; Falzoni, S.; Ferrari, D.; Chiozzi, P.; Morelli, A.; Baricordi, O.R.; Collo, G.; Ricciardi-Castagnoli, P.; Di Virgilio, F. Mouse dendritic cells express the P2X7 purinergic receptor: Characterization and possible participation in antigen presentation. J. Immunol. 1999, 163, 1958–1965. [Google Scholar]
- Burnstock, G.; Knight, G.E. Cellular Distribution and Functions of P2 Receptor Subtypes in Different Systems. Nat. Eng. Resist. Plant Viruses Part II 2004, 240, 31–304. [Google Scholar] [CrossRef]
- Scheuplein, F.; Rissiek, B.; Driver, J.P.; Chen, Y.-G.; Koch-Nolte, F.; Serreze, D.V. A recombinant heavy chain antibody approach blocks ART2 mediated deletion of an iNKT cell population that upon activation inhibits autoimmune diabetes. J. Autoimmun. 2010, 34, 145–154. [Google Scholar] [CrossRef][Green Version]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Adriouch, S.; Bannas, P.; Schwarz, N.; Fliegert, R.; Guse, A.H.; Seman, M.; Haag, F.; Koch-Noke, F.; Koch-Nolte, F. ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J. 2007, 22, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Seman, M.; Adriouch, S.; Scheuplein, F.; Krebs, C.; Freese, D.; Glowacki, G.; Deterre, P.; Haag, F.; Koch-Nolte, F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 2003, 19, 571–582. [Google Scholar] [CrossRef]
- Lee, H.C. Structure and Enzymatic Functions of Human CD38. Mol. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Rissiek, B.; Haag, F.; Boyer, O.; Koch-Nolte, F.; Adriouch, S. P2X7 on Mouse T Cells: One Channel, Many Functions. Front. Immunol. 2015, 6, 204. [Google Scholar] [CrossRef]
- Haag, F.; Koch-Nolte, F.; Kühl, M.; Lorenzen, S.; Thiele, H.-G. Premature Stop Codons Inactivate the RT6 Genes of the Human and Chimpanzee Species. J. Mol. Boil. 1994, 243, 537–546. [Google Scholar] [CrossRef]
- Haag, F.; Adriouch, S.; Braß, A.; Jung, C.; Möller, S.; Scheuplein, F.; Bannas, P.; Seman, M.; Koch-Nolte, F. Extracellular NAD and ATP: Partners in immune cell modulation. Purinergic Signal. 2007, 3, 71–81. [Google Scholar] [CrossRef]
- Krebs, C.F.; Adriouch, S.; Braasch, F.; Koestner, W.; Leiter, E.H.; Seman, M.; Lund, F.E.; Oppenheimer, N.; Haag, F.; Koch-Nolte, F. CD38 controls ADP-ribosyltransferase-2-catalyzed ADP-ribosylation of T cell surface proteins. J. Immunol. 2005, 174, 3298–3305. [Google Scholar] [CrossRef]
- Mizumoto, N.; Kumamoto, T.; Robson, S.C.; Sévigny, J.; Matsue, H.; Enjyoji, K.; Takashima, A. CD39 is the dominant Langerhans cell–associated ecto-NTPDase: Modulatory roles in inflammation and immune responsiveness. Nat. Med. 2002, 8, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schoneberg, T.; Schaefer, M.; Krügel, U.; et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef]
- Gudipaty, L.; Munetz, L.; Verhoef, P.A.; Dubyak, G.R. Essential role for Ca2+ in regulation of IL-1beta secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am. J. Physiol. Cell. Physiol. 2003, 285, C286–C299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-H. Inhibition of P2X7 receptors by divalent cations: Old action and new insight. Eur. Biophys. J. 2008, 38, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.D.; Chessell, I.; Humphrey, P.P.A. Ionic effects on human recombinant P2X7 receptor function. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999, 359, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Virginio, C.; Church, D.; North, R.; Surprenant, A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 1997, 36, 1285–1294. [Google Scholar] [CrossRef]
- Bradley, H.J.; E Browne, L.; Yang, W.; Jiang, L.-H. Pharmacological properties of the rhesus macaque monkey P2X7 receptor. Br. J. Pharmacol. 2011, 164, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Chessell, I.; Simon, J.; Hibell, A.D.; Michel, A.D.; A Barnard, E.; Humphrey, P. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998, 439, 26–30. [Google Scholar] [CrossRef]
- Rassendren, F.; Buell, G.N.; Virginio, C.; Collo, G.; North, R.A.; Surprenant, A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 1997, 272, 5482–5486. [Google Scholar] [CrossRef]
- Roman, S.; Cusdin, F.; Fonfria, E.; Goodwin, J.; Reeves, J.; Lappin, S.; Chambers, L.; Walter, D.; Clay, W.; Michel, A. Cloning and pharmacological characterization of the dog P2X7 receptor. Br. J. Pharmacol. 2009, 158, 1513–1526. [Google Scholar] [CrossRef]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G.; Muragaki, Y.; Mundlos, S.; Upton, J.; Olsen, B.R. The Cytolytic P2Z Receptor for Extracellular ATP Identified as a P2X Receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef]
- Karasawa, A.; Kawate, T. Structural basis for subtype-specific inhibition of the P2X7 receptor. eLife 2016, 5, 73. [Google Scholar] [CrossRef]
- Volonté, C.; Apolloni, S.; Skaper, S.D.; Burnstock, G. P2X7 receptors: Channels, pores and more. CNS Neurol. Disord. Drug Targets 2012, 11, 705–721. [Google Scholar] [CrossRef]
- Garcia-Marcos, M.; Pérez-Andrés, E.; Tandel, S.; Fontanils, U.; Kumps, A.; Kabré, E.; Gomez-Muñoz, A.; Marino, A.; Dehaye, J.-P.; Pochet, S. Coupling of two pools of P2X7receptors to distinct intracellular signaling pathways in rat submandibular gland. J. Lipid Res. 2006, 47, 705–714. [Google Scholar] [CrossRef]
- Morelli, A.; Chiozzi, P.; Chiesa, A.; Ferrari, D.; Sanz, J.M.; Falzoni, S.; Pinton, P.; Rizzuto, R.; Olson, M.F.; Di Virgilio, F. Extracellular ATP Causes ROCK I-dependent Bleb Formation in P2X7-transfected HEK293 Cells. Mol. Boil. Cell 2003, 14, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Chiozzi, P.; Falzoni, S.; Ferrari, D.; Sanz, J.M.; Venketaraman, V.; Baricordi, O.R. Cytolytic P2X purinoceptors. Cell Death Differ. 1998, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.X.; Defarias, F.P.; Alves, L.A. Are second messengers crucial for opening the pore associated with P2X7receptor? Am. J. Physiol. Physiol. 2005, 288, C260–C271. [Google Scholar] [CrossRef] [PubMed]
- Beyer, E.C.; Steinberg, T.H. Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. J. Boil. Chem. 1991, 266, 7971–7974. [Google Scholar]
- Locovei, S.; Scemes, E.; Qiu, F.; Spray, D.C.; Dahl, G. Pannexin1 is part of the pore forming unit of the P2X7receptor death complex. FEBS Lett. 2007, 581, 483–488. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo. J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef]
- Alberto, A.V.P.; Faria, R.X.; Couto, C.G.C.; Ferreira, L.G.B.; Souza, C.A.M.; Teixeira, P.C.N.; Froes, M.; Alves, L.A. Is pannexin the pore associated with the P2X7 receptor? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 775–787. [Google Scholar] [CrossRef]
- Karasawa, A.; Michalski, K.; Mikhelzon, P.; Kawate, T. The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. eLife 2017, 6. [Google Scholar] [CrossRef]
- Smart, M.L.; Gu, B.; Panchal, R.G.; Wiley, J.; Cromer, B.; Williams, D.A.; Petrou, S. P2X7 Receptor Cell Surface Expression and Cytolytic Pore Formation Are Regulated by a Distal C-terminal Region. J. Boil. Chem. 2002, 278, 8853–8860. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandonà, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered Cytokine Production in Mice Lacking P2X7Receptors. J. Boil. Chem. 2000, 276, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, J.; Novak, I. P2X7 receptor activates extracellular signal-regulated kinases ERK1 and ERK2 independently of Ca2+ influx. Biochem. J. 2003, 374, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Shemon, A.N.; Sluyter, R.; Wiley, J.S. Rottlerin inhibits P2X 7 receptor-stimulated phospholipase D activity in chronic lymphocytic leukaemia B-lymphocytes. Immunol. Cell Boil. 2006, 85, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Panenka, W.J.; Jijon, H.; Herx, L.M.; Armstrong, J.N.; Feighan, D.; Wei, T.; Yong, V.W.; Ransohoff, R.M.; MacVicar, B.A. P2X7-Like Receptor Activation in Astrocytes Increases Chemokine Monocyte Chemoattractant Protein-1 Expression via Mitogen-Activated Protein Kinase. J. Neurosci. 2001, 21, 7135–7142. [Google Scholar] [CrossRef]
- Gavala, M.L.; Pfeiffer, Z.A.; Bertics, P.J. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J. Leukoc. Boil. 2008, 84, 1159–1171. [Google Scholar] [CrossRef]
- Le Gall, S.M.; Bobé, P.; Reiss, K.; Horiuchi, K.; Niu, X.-D.; Lundell, D.; Gibb, D.R.; Conrad, D.; Saftig, P.; Blobel, C.P. ADAMs 10 and 17 Represent Differentially Regulated Components of a General Shedding Machinery for Membrane Proteins Such as Transforming Growth Factor α, L-Selectin, and Tumor Necrosis Factor α. Mol. Boil. Cell 2009, 20, 1785–1794. [Google Scholar] [CrossRef]
- Nicke, A.; Kuan, Y.-H.; Masin, M.; Rettinger, J.; Marquez-Klaka, B.; Bender, O.; Górecki, D.C.; Murrell-Lagnado, R.D.; Soto, F. A Functional P2X7 Splice Variant with an Alternative Transmembrane Domain 1 Escapes Gene Inactivation in P2X7 Knock-out Mice*. J. Boil. Chem. 2009, 284, 25813–25822. [Google Scholar] [CrossRef]
- Schwarz, N.; Drouot, L.; Nicke, A.; Fliegert, R.; Boyer, O.; Guse, A.H.; Haag, F.; Adriouch, S.; Koch-Nolte, F. Alternative Splicing of the N-Terminal Cytosolic and Transmembrane Domains of P2X7 Controls Gating of the Ion Channel by ADP-Ribosylation. PLoS ONE 2012, 7, e41269. [Google Scholar] [CrossRef]
- Benzaquen, J.; Heeke, S.; Hreich, S.J.D.; Douguet, L.; Marquette, C.H.; Hofman, P.; Vouret-Craviari, V. Alternative splicing of P2RX7 pre-messenger RNA in health and diseases: Myth or reality? Biomed. J. 2019, 42, 141–154. [Google Scholar] [CrossRef]
- Xu, X.J.; Boumechache, M.; Robinson, L.E.; Marschall, V.; Górecki, D.C.; Masin, M.; Murrell-Lagnado, R.D. Splice variants of the P2X7 receptor reveal differential agonist dependence and functional coupling with pannexin-1. J. Cell Sci. 2012, 125, 3776–3789. [Google Scholar] [CrossRef]
- Adriouch, S.; Dox, C.; Welge, V.; Seman, M.; Koch-Nolte, F.; Haag, F. Cutting edge: A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J. Immunol. 2002, 169, 4108–4112. [Google Scholar] [CrossRef] [PubMed]
- Aswad, F.; Dennert, G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell. Immunol. 2006, 243, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Aswad, F.; Kawamura, H.; Dennert, G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: A role for P2X7 receptors. J. Immunol. 2005, 175, 3075–3083. [Google Scholar] [CrossRef]
- Kawamura, H.; Aswad, F.; Minagawa, M.; Malone, K.; Kaslow, H.; Koch-Nolte, F.; Schott, W.H.; Leiter, E.H.; Dennert, G.; Koch-Nolte, F. P2X7 Receptor-Dependent and -Independent T Cell Death Is Induced by Nicotinamide Adenine Dinucleotide. J. Immunol. 2005, 174, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Cheewatrakoolpong, B.; Gilchrest, H.; Anthes, J.C.; Greenfeder, S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem. Biophys. Res. Commun. 2005, 332, 17–27. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Ben, D.D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Liang, X.; Samways, D.S.K.; Wolf, K.J.; Bowles, E.A.; Richards, J.P.; Bruno, J.; Dutertre, S.; DiPaolo, R.J.; Egan, T.M. Quantifying Ca2+Current and Permeability in ATP-gated P2X7 Receptors. J. Boil. Chem. 2015, 290, 7930–7942. [Google Scholar] [CrossRef]
- Killeen, M.E.; Ferris, L.; Kupetsky, E.; Falo, L.; Mathers, A.R. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: Implications in the pathogenesis of psoriasis. J. Immunol. 2013, 190, 4324–4336. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Colognesi, D.; Ricco, T.; Roncato, C.; Capece, M.; Amoroso, F.S.; Wang, Q.G.; De Marchi, E.; Gartland, A.; Di Virgilio, F.; et al. Trophic Activity of Human P2X7 Receptor Isoforms A and B in Osteosarcoma. PLoS ONE 2014, 9, e107224. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; Fernando, S.L.; Sluyter, R.; Britton, W.J.; Wiley, J.S. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J. Immunol. 2003, 171, 5442–5446. [Google Scholar] [CrossRef]
- Wiley, J.S.; Dao-Ung, L.P.; Gu, B.J.; Sluyter, R.; Shemon, A.N.; Li, C.; Taper, J.; Gallo, J.; Manoharan, A. A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: A molecular study. Lancet 2002, 359, 1114–1119. [Google Scholar] [CrossRef]
- Sluyter, R.; Shemon, A.N.; Wiley, J.S. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J. Immunol. 2004, 172, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Wesselius, A.; Bours, M.J.; Arts, I.C.W.; Theunisz, E.H.; Geusens, P.; Dagnelie, P.C. The P2X7 loss-of-function Glu496Ala polymorphism affects ex vivo cytokine release and protects against the cytotoxic effects of high ATP-levels. BMC Immunol. 2012, 13, 64. [Google Scholar] [CrossRef]
- Howard, C.J.; Charleston, B.; Stephens, S.A.; Sopp, P.; Hope, J. The role of dendritic cells in shaping the immune response. Anim. Heal. Res. Rev. 2004, 5, 191–195. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.C.; Smits, H.H.; Kapsenberg, M.L. Dendritic cell-mediated T cell polarization. Springer Semin. Immunopathol. 2004, 26, 289–307. [Google Scholar] [CrossRef]
- Zhong, Y.; Kinio, A.; Saleh, M. Functions of NOD-Like Receptors in Human Diseases. Front. Immunol. 2013, 4, 333. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Doyle, S.L.; Ozaki, E.; Campbell, M. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Santarlasci, V.; Cosmi, L.; Maggi, L.; Liotta, F.; Annunziato, F. IL-1 and T Helper Immune Responses. Front. Immunol. 2013, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; La Sala, A.; Chiozzi, P.; Morelli, A.; Falzoni, S.; Girolomoni, G.; Idzko, M.; Dichmann, S.; Norgauer, J.; Di Virgilio, F. The P2 purinergic receptors of human dendritic cells: Identification and coupling to cytokine release. FASEB J. 2000, 14, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Capece, M.; Franceschini, A.; Falzoni, S.; Giuliani, A.L.; Rotondo, A.; Sarti, A.C.; Bonora, M.; Syberg, S.; Corigliano, D.; et al. Accelerated Tumor Progression in Mice Lacking the ATP Receptor P2X7. Cancer Res. 2014, 75, 635–644. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, R.; Zhu, F.; Xu, W.; Zha, G.; He, G.; Cao, H.; Wang, Y.; Yang, J. ATP/P2X7-NLRP3 axis of dendritic cells participates in the regulation of airway inflammation and hyper-responsiveness in asthma by mediating HMGB1 expression and secretion. Exp. Cell Res. 2018, 366, 1–15. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Ricart, B.G.; John, B.; Lee, O.; Hunter, C.A.; Hammer, D.A. Dendritic Cells Distinguish Individual Chemokine Signals through CCR7 and CXCR4. J. Immunol. 2010, 186, 53–61. [Google Scholar] [CrossRef]
- Myrtek, D.; Idzko, M. Chemotactic activity of extracellular nucleotideson human immune cells. Purinergic Signal. 2007, 3, 5–11. [Google Scholar] [CrossRef]
- Sáez, P.J.; Vargas, P.; Shoji, K.F.; Harcha, P.A.; Lennon-Duménil, A.-M.; Sáez, J. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7receptors. Sci. Signal. 2017, 10, eaah7107. [Google Scholar] [CrossRef]
- Bretou, M.; Sáez, P.J.; Sanséau, D.; Maurin, M.; Lankar, D.; Chabaud, M.; Spampanato, C.; Malbec, O.; Barbier, L.; Muallem, S.; et al. Lysosome signaling controls the migration of dendritic cells. Sci. Immunol. 2017, 2, eaak9573. [Google Scholar] [CrossRef]
- Hoffman, L.; Farley, M.M.; Waxham, M.N. Calcium-Calmodulin-Dependent Protein Kinase II Isoforms Differentially Impact the Dynamics and Structure of the Actin Cytoskeleton. Biochemistry 2013, 52, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.; Ganesan, J.; Müller, T.; Dürr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Jüttner, E.; et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, S.; Wei, S.; Zhong, Y.; Yi, G.; Chen, H.; Liang, L.; Chen, H.; Lu, X. Extracellular ATP activates P2X7R-NF-κB (p65) pathway to promote the maturation of bone marrow-derived dendritic cells of mice. Cytokine 2019, 119, 175–181. [Google Scholar] [CrossRef] [PubMed]
- La Sala, A.; Ferrari, D.; Corinti, S.; Cavani, A.; Di Virgilio, F.; Girolomoni, G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 2001, 166, 1611–1617. [Google Scholar] [CrossRef]
- Wilkin, F.; Stordeur, P.; Goldman, M.; Boeynaems, J.-M.; Robaye, B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: Dual effect on IL-12 and stimulation of IL-10. Eur. J. Immunol. 2002, 32, 2409–2417. [Google Scholar] [CrossRef]
- Schnurr, M.; Toy, T.; Shin, A.; Wagner, M.; Cebon, J.; Maraskovsky, E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: A novel role for the cAMP pathway. Blood 2005, 105, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- La Sala, A.; Sebastiani, S.; Ferrari, D.; Di Virgilio, F.; Idzko, M.; Norgauer, J.; Girolomoni, G. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood 2002, 99, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.J.; E Polak, P.; Simonini, V.; Lin, S.X.; Richardson, J.C.; Bongarzone, E.R.; Feinstein, D.L. P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J. Neuroinflammation 2008, 5, 33. [Google Scholar] [CrossRef]
- Vergani, A.; Fotino, C.; D’Addio, F.; Tezza, S.; Podetta, M.; Gatti, F.; Chin, M.; Bassi, R.; Molano, R.; Corradi, D.; et al. Effect of the Purinergic Inhibitor Oxidized ATP in a Model of Islet Allograft Rejection. Diabetes 2013, 62, 1665–1675. [Google Scholar] [CrossRef]
- Vergani, A.; Tezza, S.; D’Addio, F.; Fotino, C.; Liu, K.; Niewczas, M.; Bassi, R.; Molano, R.; Kleffel, S.; Petrelli, A.; et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 2012, 127, 463–475. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Dai, C.; Wang, H.; Zhang, H.; Huang, Y.; Wang, S.; Gaskin, F.; Yang, N.; Man Fu, S. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013, 65, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Benjamim, C.; Martinez, C.; Kurtenbach, E.; Takiya, C.M.; Coutinho-Silva, R. The P2X7 Receptor Contributes to the Development of the Exacerbated Inflammatory Response Associated with Sepsis. J. Innate Immun. 2015, 7, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, J.B.; Ferraro, A.A.; Sananez, I.; Gancedo, M.C.; Baz, P.; Billordo, L.A.; Fainboim, L.; Arruvito, L. ATP-Induced Inflammation Drives Tissue-Resident Th17 Cells in Metabolically Unhealthy Obesity. J. Immunol. 2016, 196, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Li, H.; Jia, X.; Zhang, X.; Xia, Y.; Wang, Y.; Fu, L.; Xiao, C.; Geng, D. Increased expression of P2X7 receptor in peripheral blood mononuclear cells correlates with clinical severity and serum levels of Th17-related cytokines in patients with myasthenia gravis. Clin. Neurol. Neurosurg. 2017, 157, 88–94. [Google Scholar] [CrossRef]
- Atarashi, K.; Nishimura, J.; Shima, T.; Umesaki, Y.; Yamamoto, M.; Onoue, M.; Yagita, H.; Ishii, N.; Evans, R.; Honda, K. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008, 455, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-D.; Zhang, Y.-Y.; Guo, Y.-H.; Huang, N.; Ma, H.-H.; Huang, H.; Yu, H.-G. Involvement of P2X7 receptor signaling on regulating the differentiation of Th17 cells and type II collagen-induced arthritis in mice. Sci. Rep. 2016, 6, 35804. [Google Scholar] [CrossRef]
- Woehrle, T.; Yip, L.; Elkhal, A.; Sumi, Y.; Chen, Y.; Yao, Y.; Insel, P.A.; Junger, W.G. Pannexin-1 hemichannel–mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 2010, 116, 3475–3484. [Google Scholar] [CrossRef]
- Yip, L.; Woehrle, T.; Corriden, R.; Hirsh, M.; Chen, Y.; Inoue, Y.; Ferrari, V.; Insel, P.A.; Junger, W.G. Autocrine regulation of T-cell activation by ATP release and P2X 7 receptors. FASEB J. 2009, 23, 1685–1693. [Google Scholar] [CrossRef]
- Labasi, J.M.; Petrushova, N.; Donovan, C.; McCurdy, S.; Lira, P.; Payette, M.M.; Brissette, W.; Wicks, J.R.; Audoly, L.; Gabel, C.A. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J. Immunol. 2002, 168, 6436–6445. [Google Scholar] [CrossRef]
- Arbonés, M.L.; Ord, D.C.; Ley, K.; Ratech, H.; Maynard-Curry, C.; Otten, G.; Capon, D.J.; Teddert, T.F. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1994, 1, 247–260. [Google Scholar] [CrossRef]
- Masopust, D.; Schenkel, J.M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013, 13, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Carter, E.; Kilty, I.; MacKenzie, A.B.; Ward, S.G. Mitochondrial superoxide generation enhances P2X7R-mediated loss of cell surface CD62L on naive human CD4+ T lymphocytes. J. Immunol. 2013, 190, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Safya, H.; Mellouk, A.; Legrand, J.; Le Gall, S.M.; Benbijja, M.; Kanellopoulos-Langevin, C.; Kanellopoulos, J.M.; Bobé, P. Variations in Cellular Responses of Mouse T Cells to Adenosine-5′-Triphosphate Stimulation Do Not Depend on P2X7 Receptor Expression Levels but on Their Activation and Differentiation Stage. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Ploia, C.; Anselmi, F.; Sarukhan, A.; Viola, A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 2014, 33, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Pizzirani, C.; Idzko, M.; Panther, E.; Norgauer, J.; Di Virgilio, F.; Ferrari, D. P2X7 receptor: Death or life? Purinergic Signal. 2005, 1, 219–227. [Google Scholar] [CrossRef] [PubMed]
- La Sala, A.; Ferrari, D.; Di Virgilio, F.; Idzko, M.; Norgauer, J.; Girolomoni, G. Alerting and tuning the immune response by extracellular nucleotides. J. Leukoc. Boil. 2003, 73, 339–343. [Google Scholar] [CrossRef]
- Tsukimoto, M.; Maehata, M.; Harada, H.; Ikari, A.; Takagi, K.; Degawa, M. P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J. Immunol. 2006, 177, 2842–2850. [Google Scholar] [CrossRef]
- Taylor, S.; Gonzalez-Begne, M.; Dewhurst, S.; Chimini, G.; Higgins, C.F.; Melvin, J.E.; Elliott, J.I. Sequential shrinkage and swelling underlie P2X7-stimulated lymphocyte phosphatidylserine exposure and death. J. Immunol. 2008, 180, 300–308. [Google Scholar] [CrossRef]
- Tsukimoto, M.; Harada, H.; Ikari, A.; Takagi, K. Involvement of Chloride in Apoptotic Cell Death Induced by Activation of ATP-sensitive P2X7Purinoceptor. J. Boil. Chem. 2004, 280, 2653–2658. [Google Scholar] [CrossRef]
- Le Stunff, H.; Auger, R.; Kanellopoulos, J.; Raymond, M.-N. The Pro-451 to Leu Polymorphism within the C-terminal Tail of P2X7 Receptor Impairs Cell Death but Not Phospholipase D Activation in Murine Thymocytes. J. Boil. Chem. 2004, 279, 16918–16926. [Google Scholar] [CrossRef] [PubMed]
- Scheuplein, F.; Schwarz, N.; Adriouch, S.; Krebs, C.F.; Bannas, P.; Rissiek, B.; Seman, M.; Haag, F.; Koch-Nolte, F. NAD+and ATP Released from Injured Cells Induce P2X7-Dependent Shedding of CD62L and Externalization of Phosphatidylserine by Murine T Cells. J. Immunol. 2009, 182, 2898–2908. [Google Scholar] [CrossRef]
- Auger, R.; Motta, I.; Benihoud, K.; Ojcius, D.M.; Kanellopoulos, J.M. A role for mitogen-activated protein kinase(Erk1/2) activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J. Biol. Chem. 2005, 280, 28142–28151. [Google Scholar] [CrossRef] [PubMed]
- Adriouch, S.; Hubert, S.; Pechberty, S.; Koch-Nolte, F.; Haag, F.; Seman, M. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J. Immunol. 2007, 179, 186–194. [Google Scholar] [CrossRef]
- Schenk, U.; Frascoli, M.; Proietti, M.; Geffers, R.; Traggiai, E.; Buer, J.; Ricordi, C.; Westendorf, A.M.; Grassi, F. ATP Inhibits the Generation and Function of Regulatory T Cells Through the Activation of Purinergic P2X Receptors. Sci. Signal. 2011, 4, ra12. [Google Scholar] [CrossRef]
- Hubert, S.; Rissiek, B.; Klages, K.; Huehn, J.; Sparwasser, T.; Haag, F.; Koch-Nolte, F.; Boyer, O.; Seman, M.; Adriouch, S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2–P2X7 pathway. J. Exp. Med. 2010, 207, 2561–2568. [Google Scholar] [CrossRef]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef]
- Adriouch, S.; Haag, F.; Boyer, O.; Seman, M.; Koch-Nolte, F. Extracellular NAD+: A danger signal hindering regulatory T cells. Microbes Infect. 2012, 14, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Robson, S.C.; Wu, Y.; Sun, X.; Knosalla, C.; Dwyer, K.M.; Enjyoji, K. Ectonucleotidases of CD39 Family Modulate Vascular Inflammation and Thrombosis in Transplantation. Semin. Thromb. Hemost. 2005, 31, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Latner, N.R.; Zilliox, M.J.; McCausland, M.; Akondy, R.; Penaloza-MacMaster, P.; Hale, J.S.; Ye, L.; Mohammed, A.-U.-R.; Yamaguchi, T.; et al. Identification of novel markers for mouse CD4(+) T follicular helper cells. Eur. J. Immunol. 2013, 43, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Cornacchione, V.; Jost, T.R.; Romagnani, A.; Faliti, C.E.; Perruzza, L.; Rigoni, R.; Radaelli, E.; Caprioli, F.; Preziuso, S.; et al. ATP-Gated Ionotropic P2X7 Receptor Controls Follicular T Helper Cell Numbers in Peyer’s Patches to Promote Host-Microbiota Mutualism. Immunity 2014, 41, 789–801. [Google Scholar] [CrossRef] [PubMed]
- De Salles, É.M.; De Menezes, M.N.; Siqueira, R.; Da Silva, H.B.; Amaral, E.P.; Castillo, S.; Cunha, I.; Cassado, A.D.A.; Vieira, F.S.; Olivieri, D.N.; et al. P2X7 receptor drives Th1 cell differentiation and controls the follicular helper T cell population to protect against Plasmodium chabaudi malaria. PLoS Pathog. 2017, 13, e1006595. [Google Scholar] [CrossRef]
- Faliti, C.E.; Gualtierotti, R.; Rottoli, E.; Gerosa, M.; Perruzza, L.; Romagnani, A.; Pellegrini, G.; Conti, B.D.P.; Rossi, R.L.; Idzko, M.; et al. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J. Exp. Med. 2019, 216, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Künzli, M.; Schreiner, D.; Pereboom, T.C.; Swarnalekha, N.; Litzler, L.C.; Lötscher, J.; Ertuna, Y.I.; Roux, J.; Geier, F.; Jakob, R.P.; et al. Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci. Immunol. 2020, 5, eaay5552. [Google Scholar] [CrossRef] [PubMed]
- Heiss, K.; Jänner, N.; Mähnß, B.; Schumacher, V.; Koch-Nolte, F.; Haag, F.; Mittrücker, H.-W.; Mähnss, B. High Sensitivity of Intestinal CD8+ T Cells to Nucleotides Indicates P2X7 as a Regulator for Intestinal T Cell Responses. J. Immunol. 2008, 181, 3861–3869. [Google Scholar] [CrossRef]
- Hashimoto-Hill, S.; Friesen, L.; Kim, M.; Kim, J. Contraction of intestinal effector T cells by retinoic acid-induced purinergic receptor P2X7. Mucosal Immunol. 2016, 10, 912–923. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Höpner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L.; et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 2007, 110, 1225–1232. [Google Scholar] [CrossRef]
- Read, S.; Mauze, S.; Asseman, C.; Bean, A.; Coffman, R.; Powrie, F.M. CD38+ CD45RBlow CD4+ T cells: A population of T cells with immune regulatory activities in vitro. Eur. J. Immunol. 1998, 28, 3435–3447. [Google Scholar] [CrossRef]
- Cortés, J.R.; Sánchez-Díaz, R.; Bovolenta, E.R.; Barreiro, O.; Lasarte, S.; Matesanz-Marín, A.; Toribio, M.L.; Sanchez-Madrid, F.; Martín, P. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 2014, 55, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.T.; Wilson, M.D.; Rowan, W.C.; Soond, D.R.; Okkenhaug, K. The PI3K p110δ Regulates Expression of CD38 on Regulatory T Cells. PLoS ONE 2011, 6, e17359. [Google Scholar] [CrossRef]
- Masopust, D.; Soerens, A.G. Tissue-Resident T Cells and Other Resident Leukocytes. Annu. Rev. Immunol. 2019, 37, 521–546. [Google Scholar] [CrossRef]
- Stark, R.; Wesselink, T.H.; Behr, F.M.; Kragten, N.A.M.; Arens, R.; Koch-Nolte, F.; van Gisbergen, K.P.J.M.; van Lier, R.A.W. T RM maintenance is regulated by tissue damage via P2RX7. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef]
- Da Silva, H.B.; Beura, L.K.; Wang, H.; Hanse, E.A.; Gore, R.; Scott, M.C.; Walsh, D.A.; Block, K.E.; Fonseca, R.; Yan, Y.; et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature 2018, 559, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Boil. 2017, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Mellouk, A.; Bobé, P. CD8 +, but not CD4 + effector/memory T cells, express the CD44 high CD45RB high phenotype with aging, which displays reduced expression levels of P2X 7 receptor and ATP-induced cellular responses. FASEB J. 2018, 33, 3225–3236. [Google Scholar] [CrossRef]
- Krummey, S.M.; Morris, A.B.; Jacobs, J.R.; McGuire, D.J.; Ando, S.; Tong, K.P.; Zhang, W.; Robertson, J.; Guasch, S.A.; Araki, K.; et al. CD45RB Status of CD8+ T Cell Memory Defines T Cell Receptor Affinity and Persistence. Cell Rep. 2020, 30, 1282–1291.e5. [Google Scholar] [CrossRef]
- Wanhainen, K.M.; Jameson, S.C.; Da Silva, H.B. Self-Regulation of Memory CD8 T Cell Metabolism through Extracellular ATP Signaling. Immunometabolism 2019, 1. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Trabanelli, S.; Ocadlikova, D.; Gulinelli, S.; Curti, A.; Salvestrini, V.; Vieira, R.D.P.; Idzko, M.; Di Virgilio, F.; Ferrari, D.; Lemoli, R.M. Extracellular ATP Exerts Opposite Effects on Activated and Regulatory CD4+ T Cells via Purinergic P2 Receptor Activation. J. Immunol. 2012, 189, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef]
- Mascanfroni, I.D.; Takenaka, M.C.; Yeste, A.; Patel, B.; Wu, Y.; Kenison, J.E.; Siddiqui, S.; Basso, A.; Otterbein, L.E.; Pardoll, E.M.; et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 2015, 21, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Pot, C.; Apetoh, L.; Awasthi, A.; Kuchroo, V.K. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin. Immunol. 2011, 23, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.; Flores-Santibáñez, F.; Neira, J.; Osorio-Barrios, F.; Tejón, G.; Nunez, S.; Hidalgo, Y.; Fuenzalida, M.J.; Meza, D.; Ureta, G.; et al. Purinergic Signaling as a Regulator of Th17 Cell Plasticity. PLoS ONE 2016, 11, e0157889. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Yáñez, E.; Barrera-Avalos, C.; Parra-Tello, B.; Briceño, P.; Rosemblatt, M.V.; Saavedra-Almarza, J.; Rosemblatt, M.; Acuña-Castillo, C.; Bono, M.R.; Sauma, D. P2X7 Receptor at the Crossroads of T Cell Fate. Int. J. Mol. Sci. 2020, 21, 4937. https://doi.org/10.3390/ijms21144937
Rivas-Yáñez E, Barrera-Avalos C, Parra-Tello B, Briceño P, Rosemblatt MV, Saavedra-Almarza J, Rosemblatt M, Acuña-Castillo C, Bono MR, Sauma D. P2X7 Receptor at the Crossroads of T Cell Fate. International Journal of Molecular Sciences. 2020; 21(14):4937. https://doi.org/10.3390/ijms21144937
Chicago/Turabian StyleRivas-Yáñez, Elizabeth, Carlos Barrera-Avalos, Brian Parra-Tello, Pedro Briceño, Mariana V. Rosemblatt, Juan Saavedra-Almarza, Mario Rosemblatt, Claudio Acuña-Castillo, María Rosa Bono, and Daniela Sauma. 2020. "P2X7 Receptor at the Crossroads of T Cell Fate" International Journal of Molecular Sciences 21, no. 14: 4937. https://doi.org/10.3390/ijms21144937
APA StyleRivas-Yáñez, E., Barrera-Avalos, C., Parra-Tello, B., Briceño, P., Rosemblatt, M. V., Saavedra-Almarza, J., Rosemblatt, M., Acuña-Castillo, C., Bono, M. R., & Sauma, D. (2020). P2X7 Receptor at the Crossroads of T Cell Fate. International Journal of Molecular Sciences, 21(14), 4937. https://doi.org/10.3390/ijms21144937





