Angiotensin II Infusion Leads to Aortic Dissection in LRP8 Deficient Mice
Abstract
1. Introduction
2. Results
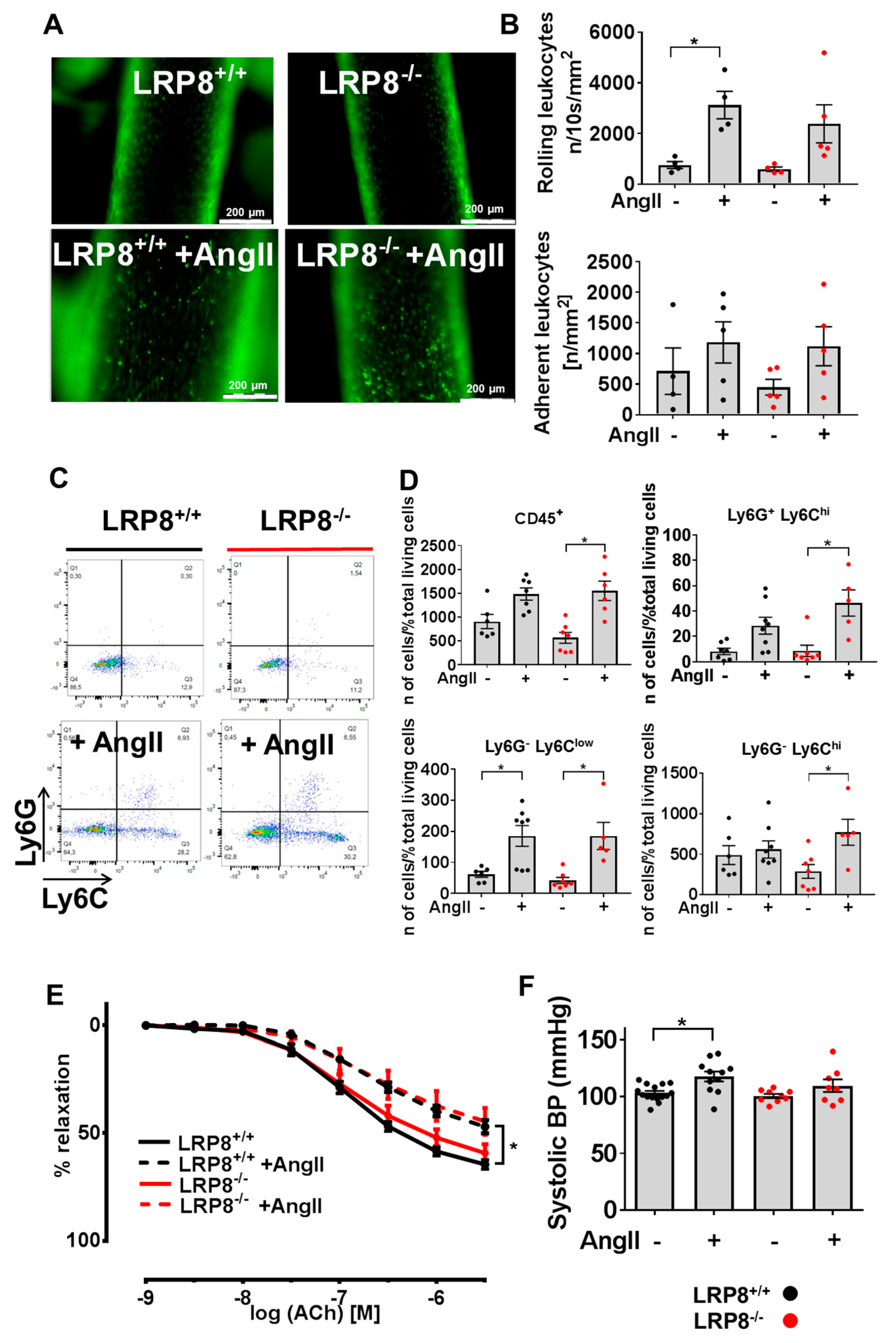
2.1. AngII Induces Vascular Dysfunction and Immune Cell Infiltration in LRP8+/+ and LRP8−/− Mice
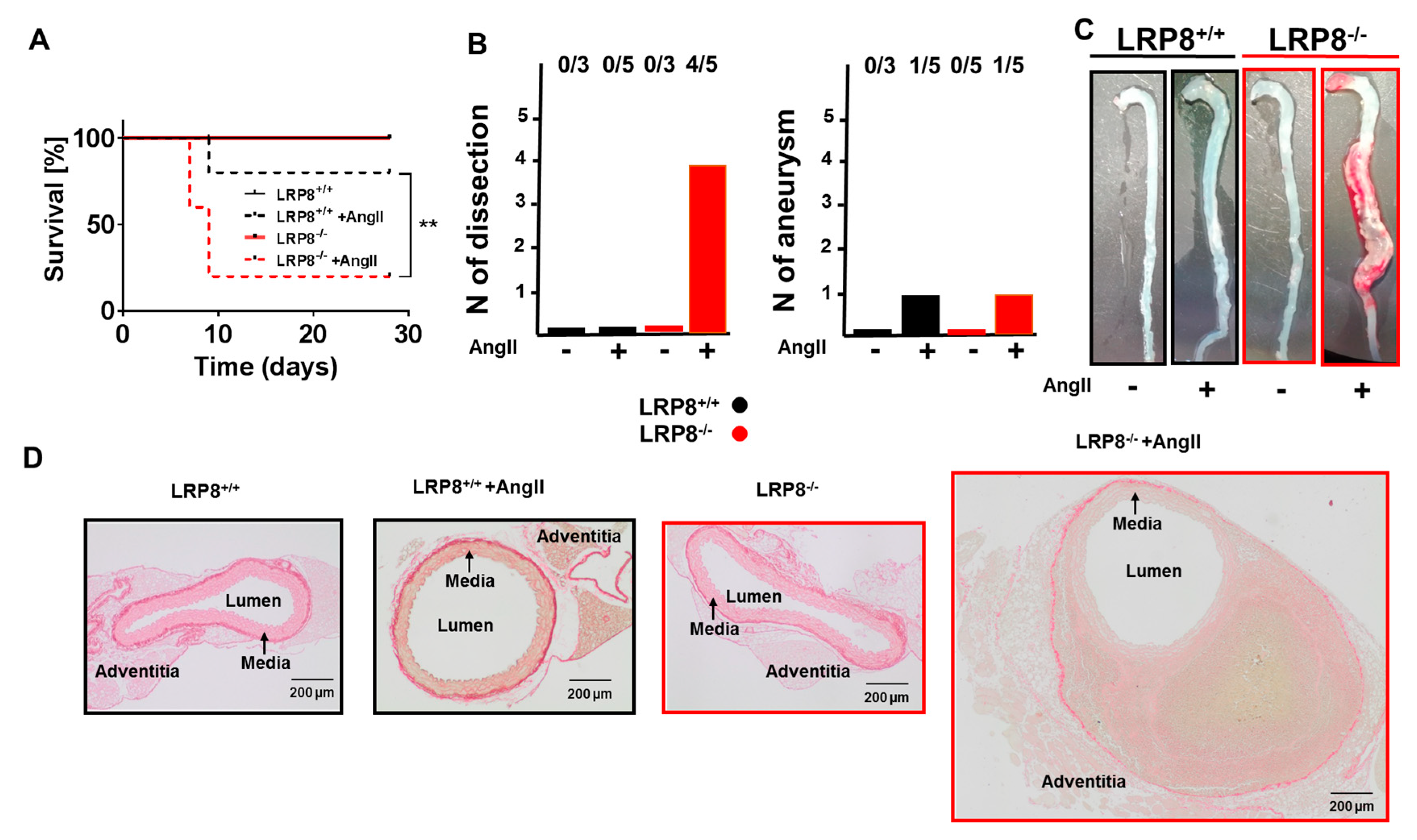
2.2. LRP8 Deficient Mice Infused with AngII Develop Aortic Dissections
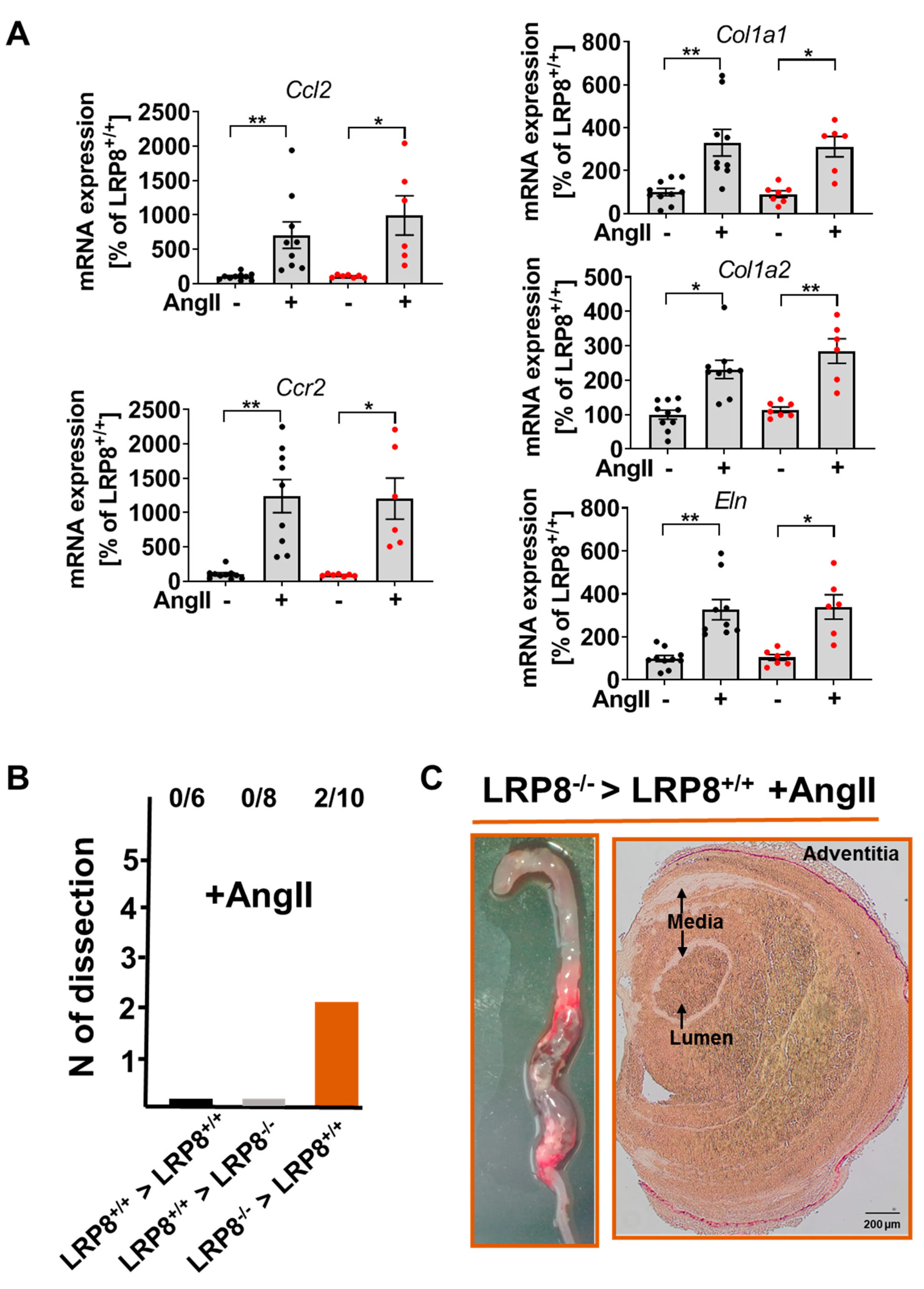
2.3. AngII-Induced Aortic Dissections are Driven by LRP8 Deficient Bone Marrow Derived Cells
3. Discussion
4. Materials and Methods
4.1. Animals, In Vivo Treatment and Blood Pressure Recording
4.2. Vascular Reactivity Studies
4.3. Intravital Fluorescence Microscopy
4.4. Flow Cytometric Analysis of Aortic Lysates
4.5. Picro-Sirius Red Staining
4.6. mRNA Expression Analysis
4.7. Bone Marrow Transplantation
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AngII | Angiotensin II |
| ApoER2 | Apolipoprotein E receptor 2 |
| FXI | Factor XI |
| GPIbα | Glycoprotein Ib alpha |
| LRP8 | Low-density lipoprotein receptor-related protein 8 |
References
- Shen, G.-Q.; Li, L.; Girelli, D.; Seidelmann, S.B.; Rao, S.; Fan, C.; Park, J.E.; Xi, Q.; Li, J.; Hu, Y.; et al. An LRP8 variant is associated with familial and premature coronary artery disease and myocardial infarction. Am. J. Hum. Genet. 2007, 81, 780–791. [Google Scholar] [CrossRef]
- Shen, G.-Q.; Girelli, D.; Li, L.; Rao, S.; Archacki, S.; Olivieri, O.; Martinelli, N.; Park, J.E.; Chen, Q.; Topol, E.J.; et al. A novel molecular diagnostic marker for familial and early-onset coronary artery disease and myocardial infarction in the LRP8 gene. Circ. Cardiovasc. Genet. 2014, 7, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Norlander, A.E.; Madhur, M.S.; Harrison, D.G. The immunology of hypertension. J. Exp. Med. 2018, 215, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Waltmann, M.D.; Basford, J.E.; Konaniah, E.S.; Weintraub, N.L.; Hui, D.Y. Apolipoprotein E receptor-2 deficiency enhances macrophage susceptibility to lipid accumulation and cell death to augment atherosclerotic plaque progression and necrosis. Biochim. Biophys. Acta 2014, 1842, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.G.; Derksen, R.H.; Urbanus, R.T. The role of LRP8 (ApoER2′) in the pathophysiology of the antiphospholipid syndrome. Lupus 2010, 19, 389–393. [Google Scholar] [CrossRef]
- Damås, J.K.; Otterdal, K.; Yndestad, A.; Aass, H.; Solum, N.O.; Frøland, S.S.; Simonsen, S.; Aukrust, P.; Andreassen, A.K. Soluble CD40 ligand in pulmonary arterial hypertension: Possible pathogenic role of the interaction between platelets and endothelial cells. Circulation 2004, 110, 999–1005. [Google Scholar] [CrossRef]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef]
- Kossmann, S.; Lagrange, J.; Jäckel, S.; Jurk, K.; Ehlken, M.; Schönfelder, T.; Weihert, Y.; Knorr, M.; Brandt, M.; Xia, N.; et al. Platelet-localized FXI promotes a vascular coagulation-inflammatory circuit in arterial hypertension. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- White-Adams, T.C.; Berny, M.A.; Tucker, E.I.; Gertz, J.M.; Gailani, D.; Urbanus, R.T.; de Groot, P.G.; Gruber, A.; McCarty, O.J.T. Identification of coagulation factor XI as a ligand for platelet apolipoprotein E receptor 2 (ApoER2). Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1602–1607. [Google Scholar] [CrossRef]
- Sinha, R.K.; Yang, X.V.; Fernández, J.A.; Xu, X.; Mosnier, L.O.; Griffin, J.H. Apolipoprotein E receptor 2 mediates activated protein C-induced endothelial akt activation and endothelial barrier stabilization. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 518–524. [Google Scholar] [CrossRef]
- Robertson, J.O.; Li, W.; Silverstein, R.L.; Topol, E.J.; Smith, J.D. Deficiency of LRP8 in mice is associated with altered platelet function and prolonged time for in vivo thrombosis. Thromb. Res. 2009, 123, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Riddell, D.R.; Vinogradov, D.V.; Stannard, A.K.; Chadwick, N.; Owen, J.S. Identification and characterization of LRP8 (apoER2) in human blood platelets. J. Lipid Res. 1999, 40, 1925–1930. [Google Scholar] [PubMed]
- White, T.C.; Berny, M.A.; Tucker, E.I.; Urbanus, R.T.; de Groot, P.G.; Fernández, J.A.; Griffin, J.H.; Gruber, A.; McCarty, O.J.T. Protein C supports platelet binding and activation under flow: Role of glycoprotein Ib and apolipoprotein E receptor 2. J. Thromb. Haemost. 2008, 6, 995–1002. [Google Scholar] [CrossRef]
- Chen, X.; Tang, L.; Jiang, J.; Jiang, J.; Hu, X.; Yu, W.; Wang, J. Increased levels of lipoprotein(a) in non-smoking aortic dissection patients. Clin. Exp. Med. 2008, 8, 123–127. [Google Scholar] [CrossRef]
- Komaravolu, R.K.; Waltmann, M.D.; Konaniah, E.; Jaeschke, A.; Hui, D.Y. ApoER2 (Apolipoprotein E receptor-2) deficiency accelerates smooth muscle cell senescence via cytokinesis impairment and promotes fibrotic neointima after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2132–2144. [Google Scholar] [CrossRef]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Faugeroux, J.; Nematalla, H.; Li, W.; Clement, M.; Robidel, E.; Frank, M.; Curis, E.; Ait-Oufella, H.; Caligiuri, G.; Nicoletti, A.; et al. Angiotensin II promotes thoracic aortic dissections and ruptures in Col3a1 haploinsufficient mice. Hypertension 2013, 62, 203–208. [Google Scholar] [CrossRef]
- Patel, J.; Douglas, G.; Kerr, A.G.; Hale, A.B.; Channon, K.M. Effect of irradiation and bone marrow transplantation on angiotensin II-induced aortic inflammation in ApoE knockout mice. Atherosclerosis 2018, 276, 74–82. [Google Scholar] [CrossRef]
- Hackam, D.G.; Thiruchelvam, D.; Redelmeier, D.A. Angiotensin-converting enzyme inhibitors and aortic rupture: A population-based case-control study. Lancet Lond. Engl. 2006, 368, 659–665. [Google Scholar] [CrossRef]
- Yoshida, S.; Yamamoto, M.; Aoki, H.; Fukuda, H.; Akasu, K.; Takagi, K.; Shojima, T.; Fukumoto, Y.; Akashi, H.; Tanaka, H. STAT3 activation correlates with adventitial neutrophil infiltration in human aortic dissection. Ann. Vasc. Dis. 2019, 12, 187–193. [Google Scholar] [CrossRef]
- Anzai, A.; Shimoda, M.; Endo, J.; Kohno, T.; Katsumata, Y.; Matsuhashi, T.; Yamamoto, T.; Ito, K.; Yan, X.; Shirakawa, K.; et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ. Res. 2015, 116, 612–623. [Google Scholar] [CrossRef]
- Hatipoglu, O.F.; Miyoshi, T.; Yonezawa, T.; Kondo, M.; Amioka, N.; Yoshida, M.; Akagi, S.; Nakamura, K.; Hirohata, S.; Ito, H. Deficiency of CD44 prevents thoracic aortic dissection in a murine model. Sci. Rep. 2020, 10, 6869. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Ramirez, F. Therapies for thoracic aortic aneurysms and acute aortic dissections. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Habashi, J.P.; Judge, D.P.; Patel, N.; Loeys, B.; Dietz, H.C. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N. Engl. J. Med. 2008, 358, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Trachet, B.; Aslanidou, L.; Piersigilli, A.; Fraga-Silva, R.A.; Sordet-Dessimoz, J.; Villanueva-Perez, P.; Stampanoni, M.F.M.; Stergiopulos, N.; Segers, P. Angiotensin II infusion into ApoE-/- mice: A model for aortic dissection rather than abdominal aortic aneurysm? Cardiovasc. Res. 2017, 113, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Izawa-Ishizawa, Y.; Imanishi, M.; Zamami, Y.; Toya, H.; Nagao, T.; Morishita, M.; Tsuneyama, K.; Horinouchi, Y.; Kihira, Y.; Takechi, K.; et al. Development of a novel aortic dissection mouse model and evaluation of drug efficacy using in-vivo assays and database analyses. J. Hypertens. 2019, 37, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Gawinecka, J.; Schönrath, F.; von Eckardstein, A. Acute aortic dissection: Pathogenesis, risk factors and diagnosis. Swiss Med. Wkly. 2017, 147, w14489. [Google Scholar] [CrossRef]
- Kossmann, S.; Schwenk, M.; Hausding, M.; Karbach, S.H.; Schmidgen, M.I.; Brandt, M.; Knorr, M.; Hu, H.; Kröller-Schön, S.; Schönfelder, T.; et al. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1313–1319. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
| LRP8+/+ | LRP8+/+ + AngII | LRP8−/− | LRP8−/− + AngII | |
|---|---|---|---|---|
| n | 7 | 6 | 6 | 6 |
| WBC (103/µL) | 4.0 ± 0.4 | 4.5 ± 0.6 | 4.7 ± 0.6 | 4.6 ± 0.5 |
| RBC (106/µL) | 8.0 ± 0.1 | 8.8 ± 0.3 * | 8.3 ± 0.1 | 9.1 ± 0.4 |
| HGB (g/dL) | 12.0 ± 0.1 | 12.8 ± 0.4 | 12.0 ± 0.2 | 13.1 ± 0.5 |
| HCT (%) | 42 ± 0.4 | 46 ± 1.4 * | 43 ± 0.6 | 47 ± 2.1 |
| Platelets (103/µL) | 967 ± 23 | 1035 ± 90 | 1001 ± 79 | 929 ± 74 |
| MPV (fl) | 5.7 ± 0.1 | 6.0 ± 0.1 * | 5.7 ± 0.1 | 5.9 ± 0.1 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagrange, J.; Finger, S.; Kossmann, S.; Garlapati, V.; Ruf, W.; Wenzel, P. Angiotensin II Infusion Leads to Aortic Dissection in LRP8 Deficient Mice. Int. J. Mol. Sci. 2020, 21, 4916. https://doi.org/10.3390/ijms21144916
Lagrange J, Finger S, Kossmann S, Garlapati V, Ruf W, Wenzel P. Angiotensin II Infusion Leads to Aortic Dissection in LRP8 Deficient Mice. International Journal of Molecular Sciences. 2020; 21(14):4916. https://doi.org/10.3390/ijms21144916
Chicago/Turabian StyleLagrange, Jeremy, Stefanie Finger, Sabine Kossmann, Venkata Garlapati, Wolfram Ruf, and Philip Wenzel. 2020. "Angiotensin II Infusion Leads to Aortic Dissection in LRP8 Deficient Mice" International Journal of Molecular Sciences 21, no. 14: 4916. https://doi.org/10.3390/ijms21144916
APA StyleLagrange, J., Finger, S., Kossmann, S., Garlapati, V., Ruf, W., & Wenzel, P. (2020). Angiotensin II Infusion Leads to Aortic Dissection in LRP8 Deficient Mice. International Journal of Molecular Sciences, 21(14), 4916. https://doi.org/10.3390/ijms21144916






