Allosterism of Nicotinic Acetylcholine Receptors: Therapeutic Potential for Neuroinflammation Underlying Brain Trauma and Degenerative Disorders
Abstract
1. Introduction
2. Nicotinic Acetylcholine Receptors and Allosterism
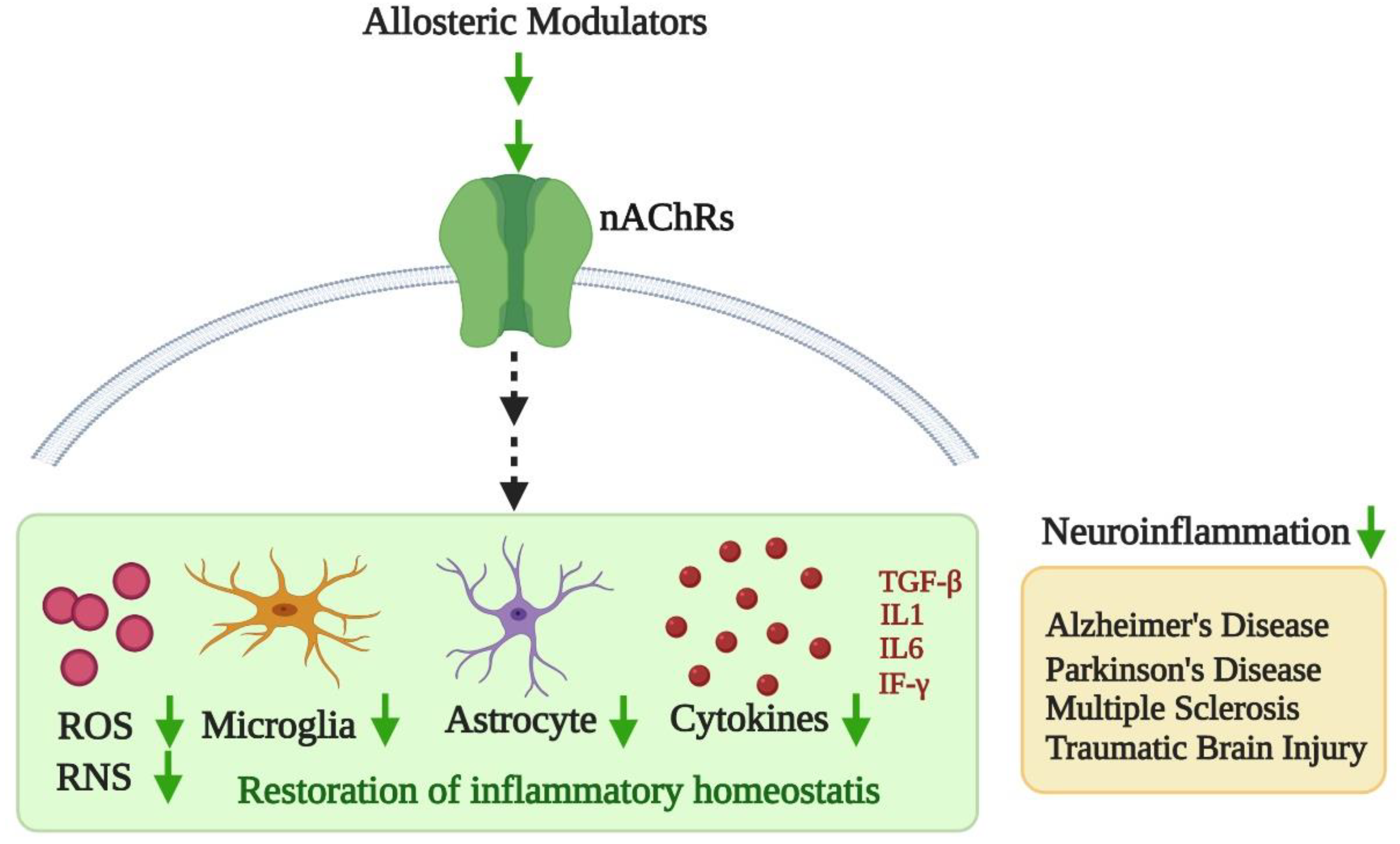
3. Neuroinflammation and Potential of Cholinergic Allosterism
3.1. Alzheimer’s Disease (AD)
3.2. Parkinson’s Disease (PD)
3.3. Multiple Sclerosis (MS)
3.4. Traumatic Brain Injury (TBI)
4. Potential Allosteric Modulators as Therapeutics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lennertz, R.C.; Kossyreva, E.A.; Smith, A.K.; Stucky, C.L. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS ONE 2012, 7, e43597. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, M. Neuronal nicotinic receptors as novel targets for inflammation and neuroprotection: Mechanistic considerations and clinical relevance. Acta Pharmacol. Sin. 2009, 30, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, M.; Lovette, M.E.; Fowler, K.W.; Arrington, S.; Reeves, L.; Caldwell, W.S.; Lippiello, P.M. RJR-2403: A nicotinic agonist with CNS selectivity I. In vitro characterization. J. Pharmacol. Exp. Ther. 1996, 279, 1413–1421. [Google Scholar] [PubMed]
- Weiner, H.L.; Selkoe, D.J. Inflammation and therapeutic vaccination in CNS diseases. Nature 2002, 420, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Vigano, P.; Rabellotti, E.; Pagliardini, L.; Somigliana, E.; Candiani, M.; Vercellini, P. Progesterone Resistance, Aromatase, and Inflammation: The Important Relationships Between Hormones and Inflammation. Curr. Obstet. Gynecol. Rep. 2012, 1, 146–152. [Google Scholar] [CrossRef][Green Version]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, Costs, and Natural Variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Roach, J.C.; Glusman, G.; Rowen, L.; Kaur, A.; Purcell, M.K.; Smith, K.D.; Hood, L.E.; Aderem, A. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 9577–9582. [Google Scholar] [CrossRef]
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Bell, J.K.; Mullen, G.E.D.; Leifer, C.A.; Mazzoni, A.; Davies, D.R.; Segal, D.M. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003, 24, 528–533. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.A.; Klop, B.; Janssen, H.W.; Njo, T.L.; Westerman, E.M.; Castro Cabezas, M. Postprandial inflammation: Targeting glucose and lipids. Adv. Exp. Med. Biol. 2014, 824, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Herieka, M.; Erridge, C. High-fat meal induced postprandial inflammation. Mol. Nutr. Food Res. 2014, 58, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Arsenis, N.C.; Disanzo, B.L.; Lamonte, M.J. Effects of exercise training on chronic inflammation in obesity: Current evidence and potential mechanisms. Sports Med. 2013, 43, 243–256. [Google Scholar] [CrossRef]
- Gjevestad, G.O.; Holven, K.B.; Ulven, S.M. Effects of Exercise on Gene Expression of Inflammatory Markers in Human Peripheral Blood Cells: A Systematic Review. Curr. Cardiovasc. Risk Rep. 2015, 9, 34. [Google Scholar] [CrossRef]
- Davidson, A.; Diamond, B. Autoimmune diseases. N. Engl. J. Med. 2001, 345, 340–350. [Google Scholar] [CrossRef]
- Franklin, R.J.; Ffrench-Constant, C.; Edgar, J.M.; Smith, K.J. Neuroprotection and repair in multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 624–634. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, V.G.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef]
- Liu, Q.; Whiteaker, P.; Morley, B.J.; Shi, F.-D.; Lukas, R.J. Distinctive Roles for α7*- and α9*-Nicotinic Acetylcholine Receptors in Inflammatory and Autoimmune Responses in the Murine Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.B.; Deisseroth, A.; Morganroth, J.; Carpenter, C.B.; Merrill, J.P. Alteration of the cytotoxic action of sensitized lymphocytes by cholinergic agents and activators of adenylate cyclase. Proc. Natl. Acad. Sci. USA 1972, 69, 2995–2999. [Google Scholar] [CrossRef] [PubMed]
- Middlebrook, A.J.; Martina, C.; Chang, Y.; Lukas, R.J.; DeLuca, D. Effects of nicotine exposure on T cell development in fetal thymus organ culture: Arrest of T cell maturation. J. Immunol. 2002, 169, 2915–2924. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Guinet, E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology 2003, 109, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Shoop, R.D.; Martone, M.E.; Yamada, N.; Ellisman, M.H.; Berg, D.K. Neuronal acetylcholine receptors with alpha7 subunits are concentrated on somatic spines for synaptic signaling in embryonic chick ciliary ganglia. J. Neurosci. 1999, 19, 692–704. [Google Scholar] [CrossRef]
- Voitenko, L.P.; Voitenko, S.V.; Skok, M.V.; Purnyn, H.E.; Skok, V.I. Nicotinic acetylcholine receptor subtypes in rat superior cervical ganglion neurons as studied by sequential application of two alpha-subunit-specific antibodies. Neurosci. Lett. 2001, 303, 37–40. [Google Scholar] [CrossRef]
- Rau, K.K.; Johnson, R.D.; Cooper, B.Y. Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. J. Neurophysiol. 2005, 93, 1358–1371. [Google Scholar] [CrossRef]
- Genzen, J.R.; Van Cleve, W.; McGehee, D.S. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J. Neurophysiol. 2001, 86, 1773–1782. [Google Scholar] [CrossRef]
- Patel, H.; McIntire, J.; Ryan, S.; Dunah, A.; Loring, R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J. Neuroinflammation 2017, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Marrero, M.B.; Bencherif, M. Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: Central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res. 2009, 1256, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F. Allosteric modulators: An emerging concept in drug discovery. ACS Med. Chem. Lett. 2015, 6, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Clementi, F. Neuronal nicotinic receptors: From structure to pathology. Prog. Neurobiol. 2004, 74, 363–396. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.C.; Raggenbass, M.; Bertrand, D. Nicotinic acetylcholine receptors: From structure to brain function. Rev. Physiol. Biochem. Pharmacol. 2003, 147, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Corringer, P.J.; Le Novère, N.; Changeux, J.P. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 431–458. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Weltzin, M.M.; Huang, Y.; Schulte, M.K. Allosteric modulation of alpha4beta2 nicotinic acetylcholine receptors by HEPES. Eur. J. Pharmacol. 2014, 732, 159–168. [Google Scholar] [CrossRef]
- Zwart, R.; Vijverberg, H.P. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol. Pharmacol. 1998, 54, 1124–1131. [Google Scholar] [CrossRef]
- McGranahan, T.M.; Patzlaff, N.E.; Grady, S.R.; Heinemann, S.F.; Booker, T.K. α4β2 Nicotinic Acetylcholine Receptors on Dopaminergic Neurons Mediate Nicotine Reward and Anxiety Relief. J. Neurosci. 2011, 31, 10891–10902. [Google Scholar] [CrossRef]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018, 592, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; López-Hernández, B.; Ceña, V. Nicotinic receptors in neurodegeneration. Curr. Neuropharmacol. 2013, 11, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.-P. The nicotinic acetylcholine receptor: A typical ‘allosteric machine’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170174. [Google Scholar] [CrossRef] [PubMed]
- Lentz, T.L. Rabies virus binding to an acetylcholine receptor alpha-subunit peptide. J. Mol. Recognit. 1990, 3, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hueffer, K.; Khatri, S.; Rideout, S.; Harris, M.B.; Papke, R.L.; Stokes, C.; Schulte, M.K. Rabies virus modifies host behaviour through a snake-toxin like region of its glycoprotein that inhibits neurotransmitter receptors in the CNS. Sci. Rep. 2017, 7, 12818. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.K.; Wang, J.; Papke, R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol. 2011, 82, 915–930. [Google Scholar] [CrossRef]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.S.; Jayakar, S.S.; Hamouda, A.K. Orthosteric and Allosteric Ligands of Nicotinic Acetylcholine Receptors for Smoking Cessation. Front. Mol. Neurosci. 2015, 8, 71. [Google Scholar] [CrossRef]
- Luttmann, E.; Ludwig, J.; Höffle-Maas, A.; Samochocki, M.; Maelicke, A.; Fels, G. Structural model for the binding sites of allosterically potentiating ligands on nicotinic acetylcholine receptors. ChemMedChem 2009, 4, 1874–1882. [Google Scholar] [CrossRef]
- Taly, A.; Corringer, P.-J.; Guedin, D.; Lestage, P.; Changeux, J.-P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Mulet, J.; Reddy, K.P.; Bernal, J.A.; Wikman, P.; Valor, L.M.; Peters, L.; König, G.M.; Criado, M.; Sala, S. Potentiation of human alpha4beta2 neuronal nicotinic receptors by a Flustra foliacea metabolite. Neurosci. Lett. 2005, 373, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.K.; Woodin, K.S.; Berry, V.; Boezio, A.A.; Cao, L.; Clarkin, K.; Harmange, J.C.; Hierl, M.; Knop, J.; Malmberg, A.B.; et al. Synthesis and activity of substituted carbamates as potentiators of the alpha4beta2 nicotinic acetylcholine receptor. Bioorg. Med. Chem. Lett. 2008, 18, 5643–5647. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, B.K.; Berry, V.; Boezio, A.A.; Cao, L.; Clarkin, K.; Guo, W.; Harmange, J.C.; Hierl, M.; Huang, L.; Janosky, B.; et al. Discovery and optimization of substituted piperidines as potent, selective, CNS-penetrant alpha4beta2 nicotinic acetylcholine receptor potentiators. Bioorg. Med. Chem. Lett. 2008, 18, 5209–5212. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, D.B.; Sandager-Nielsen, K.; Dyhring, T.; Smith, M.; Jacobsen, A.M.; Nielsen, E.; Grunnet, M.; Christensen, J.K.; Peters, D.; Kohlhaas, K.; et al. Augmentation of cognitive function by NS9283, a stoichiometry-dependent positive allosteric modulator of α2- and α4-containing nicotinic acetylcholine receptors. Br. J. Pharmacol. 2012, 167, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.A.; Ahring, P.K.; Kastrup, J.S.; Gajhede, M.; Balle, T. Structural and functional studies of the modulator NS9283 reveal agonist-like mechanism of action at α4β2 nicotinic acetylcholine receptors. J. Biol. Chem. 2014, 289, 24911–24921. [Google Scholar] [CrossRef]
- Uteshev, V.V. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur. J. Pharmacol. 2014, 727, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Grønlien, J.H.; Håkerud, M.; Ween, H.; Thorin-Hagene, K.; Briggs, C.A.; Gopalakrishnan, M.; Malysz, J. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol. Pharmacol. 2007, 72, 715–724. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Mikkelsen, J.D. Type I and II positive allosteric modulators differentially modulate agonist-induced up-regulation of α7 nicotinic acetylcholine receptors. J. Neurochem. 2012, 123, 73–83. [Google Scholar] [CrossRef]
- Foster, D.J.; Conn, P.J. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 2017, 94, 431–446. [Google Scholar] [CrossRef]
- Chatzidaki, A.; Millar, N.S. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015, 97, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Huang, Y.; Whiteaker, P. Mechanism of Allosteric Modulation of the Cys-loop Receptors. Pharmaceuticals 2010, 3, 2592–2609. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P.; Christopoulos, A. Allosteric Modulation as a Unifying Mechanism for Receptor Function and Regulation. Cell 2016, 166, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 2002, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Aguilera, G.; Colín-González, A.L.; Rangel-López, E.; Chavarría, A.; Santamaría, A. Redox Signaling, Neuroinflammation, and Neurodegeneration. Antioxid. Redox Signal. 2017, 28, 1626–1651. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Hensley, K.; Maidt, M.L.; Yu, Z.; Sang, H.; Markesbery, W.R.; Floyd, R.A. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci. 1998, 18, 8126–8132. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Boche, D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimers Res. Ther. 2015, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Mrak, R.E.; Griffin, W.S. Potential inflammatory biomarkers in Alzheimer’s disease. J. Alzheimers Dis. 2005, 8, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Mrak, R.E.; Sheng, J.G.; Griffin, W.S. Glial cytokines in Alzheimer’s disease: Review and pathogenic implications. Hum. Pathol. 1995, 26, 816–823. [Google Scholar] [CrossRef]
- Griffin, W.S.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., 3rd; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Luber-Narod, J.; Styren, S.D.; Civin, W.H. Expression of immune system-associated antigens by cells of the human central nervous system: Relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging 1988, 9, 339–349. [Google Scholar] [CrossRef]
- McGeer, P.L.; Schulzer, M.; McGeer, E.G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology 1996, 47, 425–432. [Google Scholar] [CrossRef]
- Luterman, J.D.; Haroutunian, V.; Yemul, S.; Ho, L.; Purohit, D.; Aisen, P.S.; Mohs, R.; Pasinetti, G.M. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch. Neurol. 2000, 57, 1153–1160. [Google Scholar] [CrossRef]
- Van der Wal, E.A.; Gómez-Pinilla, F.; Cotman, C.W. Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport 1993, 4, 69–72. [Google Scholar] [CrossRef]
- Rogers, J.; Cooper, N.R.; Webster, S.; Schultz, J.; McGeer, P.L.; Styren, S.D.; Civin, W.H.; Brachova, L.; Bradt, B.; Ward, P.; et al. Complement activation by beta-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10016–10020. [Google Scholar] [CrossRef]
- Wood, J.A.; Wood, P.L.; Ryan, R.; Graff-Radford, N.R.; Pilapil, C.; Robitaille, Y.; Quirion, R. Cytokine indices in Alzheimer’s temporal cortex: No changes in mature IL-1β or IL-1RA but increases in the associated acute phase proteins IL-6, α2-macroglobulin and C-reactive protein. Brain Res. 1993, 629, 245–252. [Google Scholar] [CrossRef]
- Du Yan, S.; Fu, J.; Soto, C.; Chen, X.; Zhu, H.; Al-Mohanna, F.; Collison, K.; Zhu, A.; Stern, E.; Saido, T.; et al. An intracellular protein that binds amyloid-β peptide and mediates neurotoxicity in Alzheimer’s disease. Nature 1997, 389, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Reich, E.E.; Markesbery, W.R.; Roberts, L.J., 2nd; Swift, L.L.; Morrow, J.D.; Montine, T.J. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am. J. Pathol. 2001, 158, 293–297. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Aksenova, M.V.; Butterfield, D.A.; Geddes, J.W.; Markesbery, W.R. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience 2001, 103, 373–383. [Google Scholar] [CrossRef]
- Williamson, K.S.; Gabbita, S.P.; Mou, S.; West, M.; Pye, Q.N.; Markesbery, W.R.; Cooney, R.V.; Grammas, P.; Reimann-Philipp, U.; Floyd, R.A.; et al. The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide 2002, 6, 221–227. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Su, F.; Bai, F.; Zhang, Z. Inflammatory Cytokines and Alzheimer’s Disease: A Review from the Perspective of Genetic Polymorphisms. Neurosci. Bull. 2016, 32, 469–480. [Google Scholar] [CrossRef]
- Auld, D.S.; Kornecook, T.J.; Bastianetto, S.; Quirion, R. Alzheimer’s disease and the basal forebrain cholinergic system: Relations to beta-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 2002, 68, 209–245. [Google Scholar] [CrossRef]
- Mufson, E.J.; Ginsberg, S.D.; Ikonomovic, M.D.; DeKosky, S.T. Human cholinergic basal forebrain: Chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 2003, 26, 233–242. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Skok, M.; Lykhmus, O. The Role of α7 Nicotinic Acetylcholine Receptors and α7-Specific Antibodies in Neuroinflammation Related to Alzheimer Disease. Curr. Pharm. Des. 2016, 22, 2035–2049. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Calissano, P. Nerve growth factor and Alzheimer’s disease: New facts for an old hypothesis. Mol. Neurobiol. 2012, 46, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Triaca, V.; Calissano, P. Impairment of the nerve growth factor pathway driving amyloid accumulation in cholinergic neurons: The incipit of the Alzheimer’s disease story? Neural Regen. Res. 2016, 11, 1553–1556. [Google Scholar] [CrossRef]
- Turnbull, M.T.; Boskovic, Z.; Coulson, E.J. Acute Down-regulation of BDNF Signaling Does Not Replicate Exacerbated Amyloid-β Levels and Cognitive Impairment Induced by Cholinergic Basal Forebrain Lesion. Front. Mol. Neurosci. 2018, 11, 51. [Google Scholar] [CrossRef]
- Cuello, A.C.; Bruno, M.A.; Bell, K.F. NGF-cholinergic dependency in brain aging, MCI and Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 351–358. [Google Scholar] [CrossRef]
- Iulita, M.F.; Cuello, A.C. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol. Sci. 2014, 35, 338–348. [Google Scholar] [CrossRef]
- Iulita, M.F.; Bistué Millón, M.B.; Pentz, R.; Aguilar, L.F.; Do Carmo, S.; Allard, S.; Michalski, B.; Wilson, E.N.; Ducatenzeiler, A.; Bruno, M.A.; et al. Differential deregulation of NGF and BDNF neurotrophins in a transgenic rat model of Alzheimer’s disease. Neurobiol. Dis. 2017, 108, 307–323. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef] [PubMed]
- Warpman, U.; Nordberg, A. Epibatidine and ABT 418 reveal selective losses of α4β2 nicotinic receptors in Alzheimer brains. Neuroreport 1995, 6, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Marutle, A.; Warpman, U.; Bogdanovic, N.; Lannfelt, L.; Nordberg, A. Neuronal nicotinic receptor deficits in Alzheimer patients with the Swedish amyloid precursor protein 670/671 mutation. J. Neurochem. 1999, 72, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-Z.; Zhang, X.; Ravid, R.; Nordberg, A. Decreased Protein Levels of Nicotinic Receptor Subunits in the Hippocampus and Temporal Cortex of Patients with Alzheimer’s Disease. J. Neurochem. 2000, 74, 237–243. [Google Scholar] [CrossRef]
- Burghaus, L.; Schütz, U.; Krempel, U.; de Vos, R.A.; Jansen Steur, E.N.; Wevers, A.; Lindstrom, J.; Schröder, H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res. Mol. Brain Res. 2000, 76, 385–388. [Google Scholar] [CrossRef]
- Wevers, A.; Burghaus, L.; Moser, N.; Witter, B.; Steinlein, O.K.; Schütz, U.; Achnitz, B.; Krempel, U.; Nowacki, S.; Pilz, K.; et al. Expression of nicotinic acetylcholine receptors in Alzheimer’s disease: Postmortem investigations and experimental approaches. Behav. Brain Res. 2000, 113, 207–215. [Google Scholar] [CrossRef]
- Shaw, S.; Bencherif, M.; Marrero, M.B. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1-42) amyloid. J. Biol. Chem. 2002, 277, 44920–44924. [Google Scholar] [CrossRef]
- Kihara, T.; Shimohama, S.; Sawada, H.; Kimura, J.; Kume, T.; Kochiyama, H.; Maeda, T.; Akaike, A. Nicotinic receptor stimulation protects neurons against β-amyloid toxicity. Ann. Neurol. 1997, 42, 159–163. [Google Scholar] [CrossRef]
- Medeiros, R.; Castello, N.A.; Cheng, D.; Kitazawa, M.; Baglietto-Vargas, D.; Green, K.N.; Esbenshade, T.A.; Bitner, R.S.; Decker, M.W.; LaFerla, F.M. α7 Nicotinic receptor agonist enhances cognition in aged 3xTg-AD mice with robust plaques and tangles. Am. J. Pathol. 2014, 184, 520–529. [Google Scholar] [CrossRef]
- Nordberg, A.; Hellström-Lindahl, E.; Lee, M.; Johnson, M.; Mousavi, M.; Hall, R.; Perry, E.; Bednar, I.; Court, J. Chronic nicotine treatment reduces β-amyloidosis in the brain of a mouse model of Alzheimer’s disease (APPsw). J. Neurochem. 2002, 81, 655–658. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Jones, A.K.; Brown, L.A.; Sattelle, D.B. Nicotinic acetylcholine receptor signalling: Roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol. Rev. 2009, 61, 39–61. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, W.J.; van der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Lilja, A.M.; Porras, O.; Storelli, E.; Nordberg, A.; Marutle, A. Functional interactions of fibrillar and oligomeric amyloid-β with alpha7 nicotinic receptors in Alzheimer’s disease. J. Alzheimers Dis. 2011, 23, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Yang, K.H.; Petroianu, G. On the interaction of β-amyloid peptides and α7-nicotinic acetylcholine receptors in Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 618–630. [Google Scholar] [CrossRef]
- Lasala, M.; Fabiani, C.; Corradi, J.; Antollini, S.; Bouzat, C. Molecular Modulation of Human α7 Nicotinic Receptor by Amyloid-β Peptides. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. (Sch. Ed.) 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Michel, P.P.; Toulorge, D.; Guerreiro, S.; Hirsch, E.C. Specific needs of dopamine neurons for stimulation in order to survive: Implication for Parkinson disease. FASEB J. 2013, 27, 3414–3423. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Dubois, B.; Pillon, B. Cognitive deficits in Parkinson’s disease. J. Neurol. 1996, 244, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Gatto, N.M.; Rhodes, S.L.; Manthripragada, A.D.; Bronstein, J.; Cockburn, M.; Farrer, M.; Ritz, B. α-Synuclein Gene May Interact with Environmental Factors in Increasing Risk of Parkinson’s Disease. Neuroepidemiology 2010, 35, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Warner, T.T.; Schapira, A.H. Genetic and environmental factors in the cause of Parkinson’s disease. Ann. Neurol. 2003, 53, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.M.; Quinlan, P.J.; Ross, G.W.; Marras, C.; Meng, C.; Bhudhikanok, G.S.; Comyns, K.; Korell, M.; Chade, A.R.; Kasten, M.; et al. Solvent exposures and parkinson disease risk in twins. Ann. Neurol. 2012, 71, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M.; Mitra, S.; Bult-Ito, A.; Taylor, B.E.; Vayndorf, E.M. Behavioral Phenotyping and Pathological Indicators of Parkinson’s Disease in C. elegans Models. Front. Genet. 2017, 8, 77. [Google Scholar] [CrossRef]
- Quik, M.; Bordia, T.; Huang, L.; Perez, X. Targeting nicotinic receptors for Parkinson’s disease therapy. CNS Neurol. Disord. Drug Targets 2011, 10, 651–658. [Google Scholar] [CrossRef]
- Quik, M.; O’Neill, M.; Perez, X. Nicotine neuroprotection against nigrostriatal damage: Importance of the animal model. Trends Pharmacol. Sci. 2007, 28, 229–235. [Google Scholar] [CrossRef]
- Quik, M.; O’Leary, K.; Tanner, C.M. Nicotine and Parkinson’s disease: Implications for therapy. Mov. Disord. 2008, 23, 1641–1652. [Google Scholar] [CrossRef]
- Lu, J.Y.D.; Su, P.; Barber, J.E.M.; Nash, J.E.; Le, A.D.; Liu, F.; Wong, A.H.C. The neuroprotective effect of nicotine in Parkinson’s disease models is associated with inhibiting PARP-1 and caspase-3 cleavage. PeerJ 2017, 5, 3933. [Google Scholar] [CrossRef]
- Costa, G.; Abin-Carriquiry, J.A.; Dajas, F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain Res. 2001, 888, 336–342. [Google Scholar] [CrossRef]
- Parain, K.; Hapdey, C.; Rousselet, E.; Marchand, V.; Dumery, B.; Hirsch, E.C. Cigarette smoke and nicotine protect dopaminergic neurons against the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Parkinsonian toxin. Brain Res. 2003, 984, 224–232. [Google Scholar] [CrossRef]
- Bordia, T.; McGregor, M.; Papke, R.L.; Decker, M.W.; McIntosh, J.M.; Quik, M. The α7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions. Exp. Neurol. 2015, 263, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Campos, C.; Bordia, T.; Strachan, J.-P.; Zhang, J.; McIntosh, J.M.; Letchworth, S.; Jordan, K. α4β2 Nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology 2013, 71, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Chen, L.; Parameswaran, N.; Xie, X.; Langston, J.W.; McCallum, S.E. Chronic Oral Nicotine Normalizes Dopaminergic Function and Synaptic Plasticity in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Lesioned Primates. J. Neurosci. 2006, 26, 4681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Kulak, J.M.; McIntosh, J.M.; Quik, M. Loss of nicotinic receptors in monkey striatum after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment is due to a decline in alpha-conotoxin MII sites. Mol. Pharmacol. 2002, 61, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Sum, J.D.; Whiteaker, P.; McCallum, S.E.; Marks, M.J.; Musachio, J.; McIntosh, J.M.; Collins, A.C.; Grady, S.R. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol. Pharmacol. 2003, 63, 1169–1179. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1β, interleukin-6, epidermal growth factor and transforming growth factor-α are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Nagatsu, T. Brain β2-microglobulin levels are elevated in the striatum in Parkinson’s diseaselevels are elevated in the striatum in Parkinson’s disease. J. Neural Transm. Park. Dis. Dement. Sect. 1995, 9, 87–92. [Google Scholar] [CrossRef]
- Blum-Degena, D.; Müller, T.; Kuhn, W.; Gerlach, M.; Przuntek, H.; Riederer, P. Interleukin-1β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995, 202, 17–20. [Google Scholar] [CrossRef]
- Mount, M.P.; Lira, A.; Grimes, D.; Smith, P.D.; Faucher, S.; Slack, R.; Anisman, H.; Hayley, S.; Park, D.S. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J. Neurosci. 2007, 27, 3328–3337. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Wong, S.C.; Tan, E.K. Evidence of inflammatory system involvement in Parkinson’s disease. Biomed. Res. Int. 2014, 2014, 308654. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; O’Neill, M.J.; Duty, S. Nicotine, but neither the α4β2 ligand RJR2403 nor an α7 nAChR subtype selective agonist, protects against a partial 6-hydroxydopamine lesion of the rat median forebrain bundle. Neuropharmacology 2006, 51, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.E.; Ross, S.A.; Drago, J.; Loiacono, R.E. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br. J. Pharmacol. 2001, 132, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Exley, R.; McIntosh, J.M.; Marks, M.J.; Maskos, U.; Cragg, S.J. Striatal α5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J. Neurosci. 2012, 32, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Exley, R.; Maubourguet, N.; David, V.; Eddine, R.; Evrard, A.; Pons, S.; Marti, F.; Threlfell, S.; Cazala, P.; McIntosh, J.M.; et al. Distinct contributions of nicotinic acetylcholine receptor subunit α4 and subunit α6 to the reinforcing effects of nicotine. Proc. Natl. Acad. Sci. USA 2011, 108, 7577–7582. [Google Scholar] [CrossRef]
- Shen, J.-x.; Yakel, J.L. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 2009, 30, 673–680. [Google Scholar] [CrossRef]
- Kawamata, J.; Suzuki, S.; Shimohama, S. Enhancement of nicotinic receptors alleviates cytotoxicity in neurological disease models. Ther. Adv. Chronic Dis. 2011, 2, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hai, S.; Zhou, Y.; Dong, B.R. Cholinesterase inhibitors for rarer dementias associated with neurological conditions. Cochrane Database Syst. Rev. 2015, 3, Cd009444. [Google Scholar] [CrossRef] [PubMed]
- Petiet, A.; Aigrot, M.-S.; Stankoff, B. Gray and White Matter Demyelination and Remyelination Detected with Multimodal Quantitative MRI Analysis at 11.7T in a Chronic Mouse Model of Multiple Sclerosis. Front. Neurosci. 2016, 10, 491. [Google Scholar] [CrossRef]
- Aktas, O.; Waiczies, S.; Zipp, F. Neurodegeneration in autoimmune demyelination: Recent mechanistic insights reveal novel therapeutic targets. J. Neuroimmunol. 2007, 184, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.; Halabi, A.; Ertel, M.; Petasis, N. Neuroinflammation. In Basic Neurochemistry: Molecular, Cellular, and Medical Aspects, 8th ed.; Brady, S., Siegel, G., Albers, R.W., Price, D., Eds.; Elsevier: New York, NY, USA, 2012; pp. 610–620. [Google Scholar]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple Sclerosis and Neuroinflammation: The Overview of Current and Prospective Therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B.; Kerschensteiner, M.; Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet. Neurol. 2015, 14, 406–419. [Google Scholar] [CrossRef]
- Darvesh, S.; Leblanc, A.M.; Macdonald, I.R.; Reid, G.A.; Bhan, V.; Macaulay, R.J.; Fisk, J.D. Butyrylcholinesterase activity in multiple sclerosis neuropathology. Chem. Biol. Interact. 2010, 187, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Zhao, J.; Kobori, N.; Redell, J.B.; Hylin, M.J.; Hood, K.N.; Moore, A.N. Activation of Alpha 7 Cholinergic Nicotinic Receptors Reduce Blood–Brain Barrier Permeability following Experimental Traumatic Brain Injury. J. Neurosci. 2016, 36, 2809–2818. [Google Scholar] [CrossRef]
- Anglister, L.; Etlin, A.; Finkel, E.; Durrant, A.R.; Lev-Tov, A. Cholinesterases in development and disease. Chem. Biol. Interact. 2008, 175, 92–100. [Google Scholar] [CrossRef]
- Gao, Z.; Nissen, J.C.; Ji, K.; Tsirka, S.E. The experimental autoimmune encephalomyelitis disease course is modulated by nicotine and other cigarette smoke components. PLoS ONE 2014, 9, e107979. [Google Scholar] [CrossRef]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef]
- Nicolussi, E.M.; Huck, S.; Lassmann, H.; Bradl, M. The cholinergic anti-inflammatory system limits T cell infiltration into the neurodegenerative CNS, but cannot counteract complex CNS inflammation. Neurobiol. Dis. 2009, 35, 24–31. [Google Scholar] [CrossRef]
- Treinin, M.; Papke, R.L.; Nizri, E.; Ben-David, Y.; Mizrachi, T.; Brenner, T. Role of the α7 Nicotinic Acetylcholine Receptor and RIC-3 in the Cholinergic Anti-inflammatory Pathway. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, E.; Agrawal, R.; Nath, C.; Shukla, R. Inhibitory role of cholinergic system mediated via alpha7 nicotinic acetylcholine receptor in LPS-induced neuro-inflammation. Innate Immun. 2010, 16, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nizri, E.; Hamra-Amitay, Y.; Sicsic, C.; Lavon, I.; Brenner, T. Anti-inflammatory properties of cholinergic up-regulation: A new role for acetylcholinesterase inhibitors. Neuropharmacology 2006, 50, 540–547. [Google Scholar] [CrossRef]
- Zabrodskii, P.F. Effect of acetylcholine on mortality of mice from sepsis and proinflammatory cytokine production. Bull. Exp. Biol. Med. 2011, 150, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Wiendl, H.; Marcenaro, E.; Kerlero de Rosbo, N.; Uccelli, A.; Laroni, A. Regulatory Functions of Natural Killer Cells in Multiple Sclerosis. Front. Immunol. 2016, 7, 606. [Google Scholar] [CrossRef] [PubMed]
- Frebel, H.; Nindl, V.; Schuepbach, R.A.; Braunschweiler, T.; Richter, K.; Vogel, J.; Wagner, C.A.; Loffing-Cueni, D.; Kurrer, M.; Ludewig, B.; et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 2012, 209, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, D.; Han, R.; Zhang, C.; Jin, W.-N.; Wood, K.; Liu, Q.; Shi, F.-D.; Hao, J. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2017, 114, 6202–6211. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.P.; Alstrup, A.K.; Mortensen, F.V.; Knudsen, K.; Jakobsen, S.; Madsen, L.B.; Bender, D.; Breining, P.; Petersen, M.S.; Schleimann, M.H.; et al. Cholinergic PET imaging in infections and inflammation using (11)C-donepezil and (18)F-FEOBV. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 449–458. [Google Scholar] [CrossRef]
- Nizri, E.; Brenner, T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids 2013, 45, 73–85. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. ImmunoTher. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Kayani, N.A.; Homan, S.; Yun, S.; Zhu, B.P. Health and economic burden of traumatic brain injury: Missouri, 2001–2005. Public Health Rep. 2009, 124, 551–560. [Google Scholar] [CrossRef]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.E.; Bao, J.; Bergmann, J.S.; Johnson, K.M. Traumatic brain injury reduces hippocampal high-affinity [3H]choline uptake but not extracellular choline levels in rats. Neurosci. Lett. 1994, 180, 127–130. [Google Scholar] [CrossRef]
- Dixon, C.E.; Flinn, P.; Bao, J.; Venya, R.; Hayes, R.L. Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp. Neurol. 1997, 146, 479–490. [Google Scholar] [CrossRef]
- Donat, C.K.; Schuhmann, M.U.; Voigt, C.; Nieber, K.; Deuther-Conrad, W.; Brust, P. Time-dependent alterations of cholinergic markers after experimental traumatic brain injury. Brain Res. 2008, 1246, 167–177. [Google Scholar] [CrossRef]
- Titus, D.J.; Johnstone, T.; Johnson, N.H.; London, S.H.; Chapalamadugu, M.; Hogenkamp, D.; Gee, K.W.; Atkins, C.M. Positive allosteric modulation of the α7 nicotinic acetylcholine receptor as a treatment for cognitive deficits after traumatic brain injury. PLoS ONE 2019, 14, e0223180. [Google Scholar] [CrossRef]
- Shin, S.S.; Dixon, C.E. Alterations in Cholinergic Pathways and Therapeutic Strategies Targeting Cholinergic System after Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, I.; Nicoll, J.A.; Graham, D.I.; Dewar, D. Nucleus basalis of Meynert pathology in the human brain after fatal head injury. J. Neurotrauma 2002, 19, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Östberg, A.; Virta, J.; Rinne, J.O.; Oikonen, V.; Luoto, P.; Någren, K.; Arponen, E.; Tenovuo, O. Cholinergic dysfunction after traumatic brain injury: Preliminary findings from a PET study. Neurology 2011, 76, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Gatson, J.W.; Simpkins, J.W.; Uteshev, V.V. High therapeutic potential of positive allosteric modulation of α7 nAChRs in a rat model of traumatic brain injury: Proof-of-concept. Brain Res. Bull. 2015, 112, 35–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verbois, S.L.; Scheff, S.W.; Pauly, J.R. Time-dependent changes in rat brain cholinergic receptor expression after experimental brain injury. J. Neurotrauma 2002, 19, 1569–1585. [Google Scholar] [CrossRef]
- Han, Z.; Li, L.; Wang, L.; Degos, V.; Maze, M.; Su, H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J. Neurochem. 2014, 131, 498–508. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Wang, A.; Zheng, Y.; Gao, Y.; Zhou, J. Evolutions in fragment-based drug design: The deconstruction-reconstruction approach. Drug Discov. Today 2015, 20, 105–113. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Peters, L.; König, G.M.; Terlau, H.; Wright, A.D. Four new bromotryptamine derivatives from the marine bryozoan Flustra foliacea. J. Nat. Prod. 2002, 65, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Weltzin, M.M.; Schulte, M.K. Pharmacological characterization of the allosteric modulator desformylflustrabromine and its interaction with alpha4beta2 neuronal nicotinic acetylcholine receptor orthosteric ligands. J. Pharmacol. Exp. Ther. 2010, 334, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Padnya, A.; Weltzin, M.; Edmonds, B.W.; Schulte, M.K.; Glennon, R.A. Synthesis of desformylflustrabromine and its evaluation as an alpha4beta2 and alpha7 nACh receptor modulator. Bioorg. Med. Chem. Lett. 2007, 17, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Positive allosteric modulation of α4β2 nicotinic acetylcholine receptors as a new approach to smoking reduction: Evidence from a rat model of nicotine self-administration. Psychopharmacology 2013, 230, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Mucha, M.; Khatri, S.N.; Glenon, R.; Schulte, M.K.; Bult-Ito, A. Attenuation of Compulsive-Like Behavior Through Positive Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors in Non-Induced Compulsive-Like Mice. Front. Behav. Neurosci. 2016, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.K.; Glennon, R.A.; Bult-Ito, A.; Khatri, S.; Mitra, S. Compositions and Methods for Treating Compulsive-Like Behavior in a Subject. Google Patent 16/084291, 21 February 2019. [Google Scholar]
- Bagdas, D.; Ergun, D.; Jackson, A.; Toma, W.; Schulte, M.K.; Damaj, M.I. Allosteric modulation of alpha4beta2* nicotinic acetylcholine receptors: Desformylflustrabromine potentiates antiallodynic response of nicotine in a mouse model of neuropathic pain. Eur. J. Pain 2018, 22, 84–93. [Google Scholar] [CrossRef]
- Saika, F.; Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Peripheral alpha4beta2 nicotinic acetylcholine receptor signalling attenuates tactile allodynia and thermal hyperalgesia after nerve injury in mice. Acta Physiol. 2015, 213, 462–471. [Google Scholar] [CrossRef]
- Pandya, A.; Yakel, J.L. Allosteric modulator Desformylflustrabromine relieves the inhibition of α2β2 and α4β2 nicotinic acetylcholine receptors by β-amyloid(1-42) peptide. J. Mol. Neurosci. 2011, 45, 42–47. [Google Scholar] [CrossRef]
- Jin, X.; Bermudez, I.; Steinbach, J.H. The nicotinic α5 subunit can replace either an acetylcholine-binding or nonbinding subunit in the α4β2* neuronal nicotinic receptor. Mol. Pharmacol. 2014, 85, 11–17. [Google Scholar] [CrossRef]
- Anderson, D.J.; Malysz, J.; Grønlien, J.H.; El Kouhen, R.; Håkerud, M.; Wetterstrand, C.; Briggs, C.A.; Gopalakrishnan, M. Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combination. Biochem. Pharmacol. 2009, 78, 844–851. [Google Scholar] [CrossRef]
- Olsen, J.; Kastrup, J.; Peters, D.; Gajhede, M.; Balle, T.; Ahring, P. Two Distinct Allosteric Binding Sites at α4β2 Nicotinic Acetylcholine Receptors Revealed by NS206 and NS9283 Give Unique Insights to Binding-Activity Associated Linkage at Cys-Loop Receptors. J. Biol. Chem. 2013, 288. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Cerdeira, R.M.; Burandt, C.L., Jr.; Bastos, J.K.; Nanayakkara, D.; Mikell, J.; Thurn, J.; McChesney, J.D. Evaluation of four Narcissus cultivars as potential sources for galanthamine production. Planta Med. 1997, 63, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Samochocki, M.; Höffle, A.; Fehrenbacher, A.; Jostock, R.; Ludwig, J.; Christner, C.; Radina, M.; Zerlin, M.; Ullmer, C.; Pereira, E.F.; et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2003, 305, 1024–1036. [Google Scholar] [CrossRef]
- Pereira, E.F.; Alkondon, M.; Reinhardt, S.; Maelicke, A.; Peng, X.; Lindstrom, J.; Whiting, P.; Albuquerque, E.X. Physostigmine and galanthamine: Probes for a novel binding site on the alpha 4 beta 2 subtype of neuronal nicotinic acetylcholine receptors stably expressed in fibroblast cells. J. Pharmacol. Exp. Ther. 1994, 270, 768–778. [Google Scholar] [PubMed]
- Maelicke, A.; Samochocki, M.; Jostock, R.; Fehrenbacher, A.; Ludwig, J.; Albuquerque, E.X.; Zerlin, M. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol. Psychiatry 2001, 49, 279–288. [Google Scholar] [CrossRef]
- Niederhofer, H.; Staffen, W.; Mair, A. Galantamine may be effective in treating autistic disorder. BMJ 2002, 325, 1422. [Google Scholar] [CrossRef] [PubMed]
- Kowal, N.M.; Ahring, P.K.; Liao, V.W.Y.; Indurti, D.C.; Harvey, B.S.; O’Connor, S.M.; Chebib, M.; Olafsdottir, E.S.; Balle, T. Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018, 175, 2911–2925. [Google Scholar] [CrossRef] [PubMed]
- Texidó, L.; Ros, E.; Martín-Satué, M.; López, S.; Aleu, J.; Marsal, J.; Solsona, C. Effect of galantamine on the human alpha7 neuronal nicotinic acetylcholine receptor, the Torpedo nicotinic acetylcholine receptor and spontaneous cholinergic synaptic activity. Br. J. Pharmacol. 2005, 145, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Wazea, S.A.; Wadie, W.; Bahgat, A.K.; El-Abhar, H.S. Galantamine anti-colitic effect: Role of alpha-7 nicotinic acetylcholine receptor in modulating Jak/STAT3, NF-κB/HMGB1/RAGE and p-AKT/Bcl-2 pathways. Sci. Rep. 2018, 8, 5110. [Google Scholar] [CrossRef]
- Godin, J.R.; Roy, P.; Quadri, M.; Bagdas, D.; Toma, W.; Narendrula-Kotha, R.; Kishta, O.A.; Damaj, M.I.; Horenstein, N.A.; Papke, R.L.; et al. A silent agonist of alpha7 nicotinic acetylcholine receptors modulates inflammation ex vivo and attenuates EAE. Brain Behav. Immun. 2020, 87, 286–300. [Google Scholar] [CrossRef]
- Triggle, D.J.; Mitchell, J.M.; Filler, R. The Pharmacology of Physostigmine. CNS Drug Rev. 1998, 4, 87–136. [Google Scholar] [CrossRef]
- Freitas, K.; Negus, S.S.; Carroll, F.I.; Damaj, M.I. In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br. J. Pharmacol. 2013, 169, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Freitas, K.; Ghosh, S.; Ivy Carroll, F.; Lichtman, A.H.; Imad Damaj, M. Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 2013, 65, 156–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freitas, K.; Carroll, F.I.; Damaj, M.I. The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J. Pharmacol. Exp. Ther. 2013, 344, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Wilkerson, J.L.; Kulkarni, A.; Toma, W.; AlSharari, S.; Gul, Z.; Lichtman, A.H.; Papke, R.L.; Thakur, G.A.; Damaj, M.I. The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br. J. Pharmacol. 2016, 173, 2506–2520. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.J.; Martin, R.J. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br. J. Pharmacol. 1993, 108, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.J.; Pennington, A.J.; Evans, A.M.; Martin, R.J. The action of pyrantel as an agonist and an open channel blocker at acetylcholine receptors in isolated Ascaris suum muscle vesicles. Eur. J. Pharmacol. 1994, 271, 273–282. [Google Scholar] [CrossRef]
- Martin, R.J. Modes of action of anthelmintic drugs. Vet. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Macdonald, J.S. Adjuvant therapy of colon cancer. CA Cancer J. Clin. 1999, 49, 202–219. [Google Scholar] [CrossRef]
- Gaertner, E.M.; Switlyk, S.A. Dermatologic complications from levamisole-contaminated cocaine: A case report and review of the literature. Cutis 2014, 93, 102–106. [Google Scholar]
- Ching, J.A.; Smith, D.J., Jr. Levamisole-induced necrosis of skin, soft tissue, and bone: Case report and review of literature. J. Burn Care Res. 2012, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Levandoski, M.M.; Piket, B.; Chang, J. The anthelmintic levamisole is an allosteric modulator of human neuronal nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2003, 471, 9–20. [Google Scholar] [CrossRef]
- Emil, N.S.; Cisneros, D.R.; Penmetsa, S.; Duchesne, J.H.; Sibbitt, W.L., Jr.; Gibb, J.I.; Noronha, L.E.; Fangtham, M.; Fields, R.A.; Bankhurst, A.D. Atypical Chronic Inflammatory ANCA-Positive Deforming Arthritis After Cocaine-Levamisole Exposure. J. Clin. Rheumatol. 2020, 26, 24–32. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, S.; Khatri, S.N.; Maulik, M.; Bult-Ito, A.; Schulte, M. Allosterism of Nicotinic Acetylcholine Receptors: Therapeutic Potential for Neuroinflammation Underlying Brain Trauma and Degenerative Disorders. Int. J. Mol. Sci. 2020, 21, 4918. https://doi.org/10.3390/ijms21144918
Mitra S, Khatri SN, Maulik M, Bult-Ito A, Schulte M. Allosterism of Nicotinic Acetylcholine Receptors: Therapeutic Potential for Neuroinflammation Underlying Brain Trauma and Degenerative Disorders. International Journal of Molecular Sciences. 2020; 21(14):4918. https://doi.org/10.3390/ijms21144918
Chicago/Turabian StyleMitra, Swarup, Shailesh N. Khatri, Malabika Maulik, Abel Bult-Ito, and Marvin Schulte. 2020. "Allosterism of Nicotinic Acetylcholine Receptors: Therapeutic Potential for Neuroinflammation Underlying Brain Trauma and Degenerative Disorders" International Journal of Molecular Sciences 21, no. 14: 4918. https://doi.org/10.3390/ijms21144918
APA StyleMitra, S., Khatri, S. N., Maulik, M., Bult-Ito, A., & Schulte, M. (2020). Allosterism of Nicotinic Acetylcholine Receptors: Therapeutic Potential for Neuroinflammation Underlying Brain Trauma and Degenerative Disorders. International Journal of Molecular Sciences, 21(14), 4918. https://doi.org/10.3390/ijms21144918



