Is p38 MAPK Associated to Drugs of Abuse-Induced Abnormal Behaviors?
Abstract
1. Introduction
2. Role of p38 MAPK in the Rewarding Effects of Drugs of Abuse
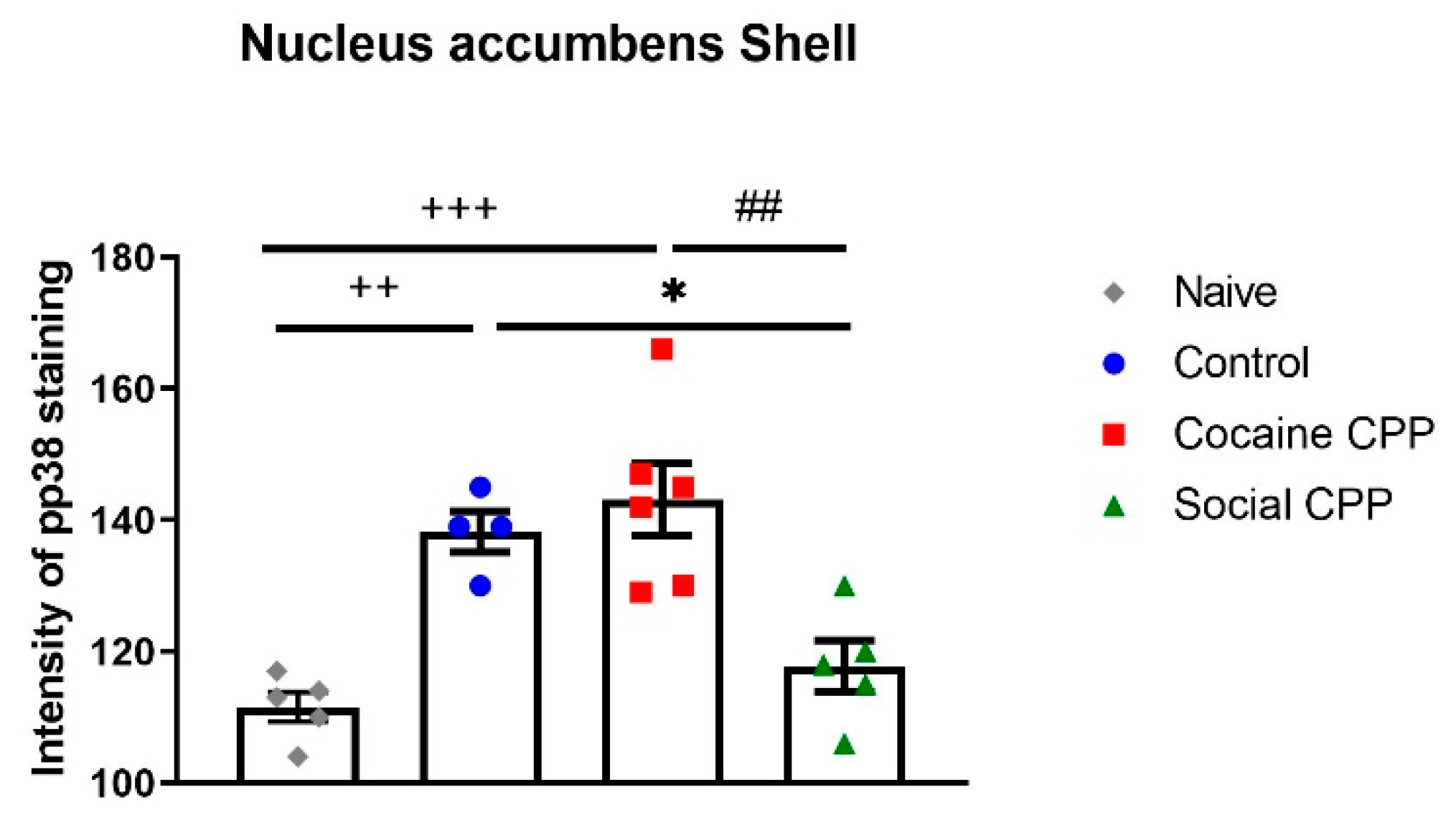
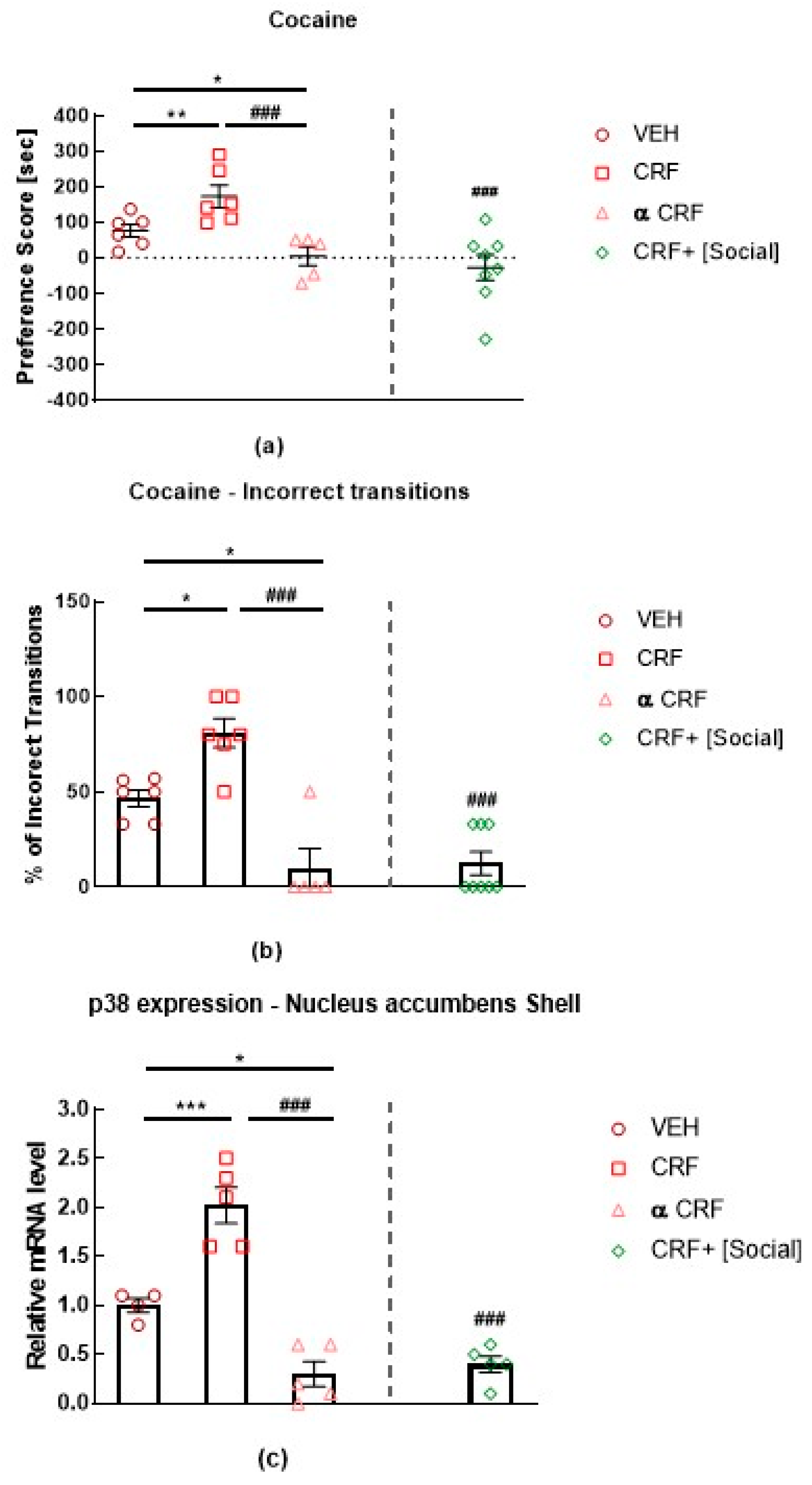
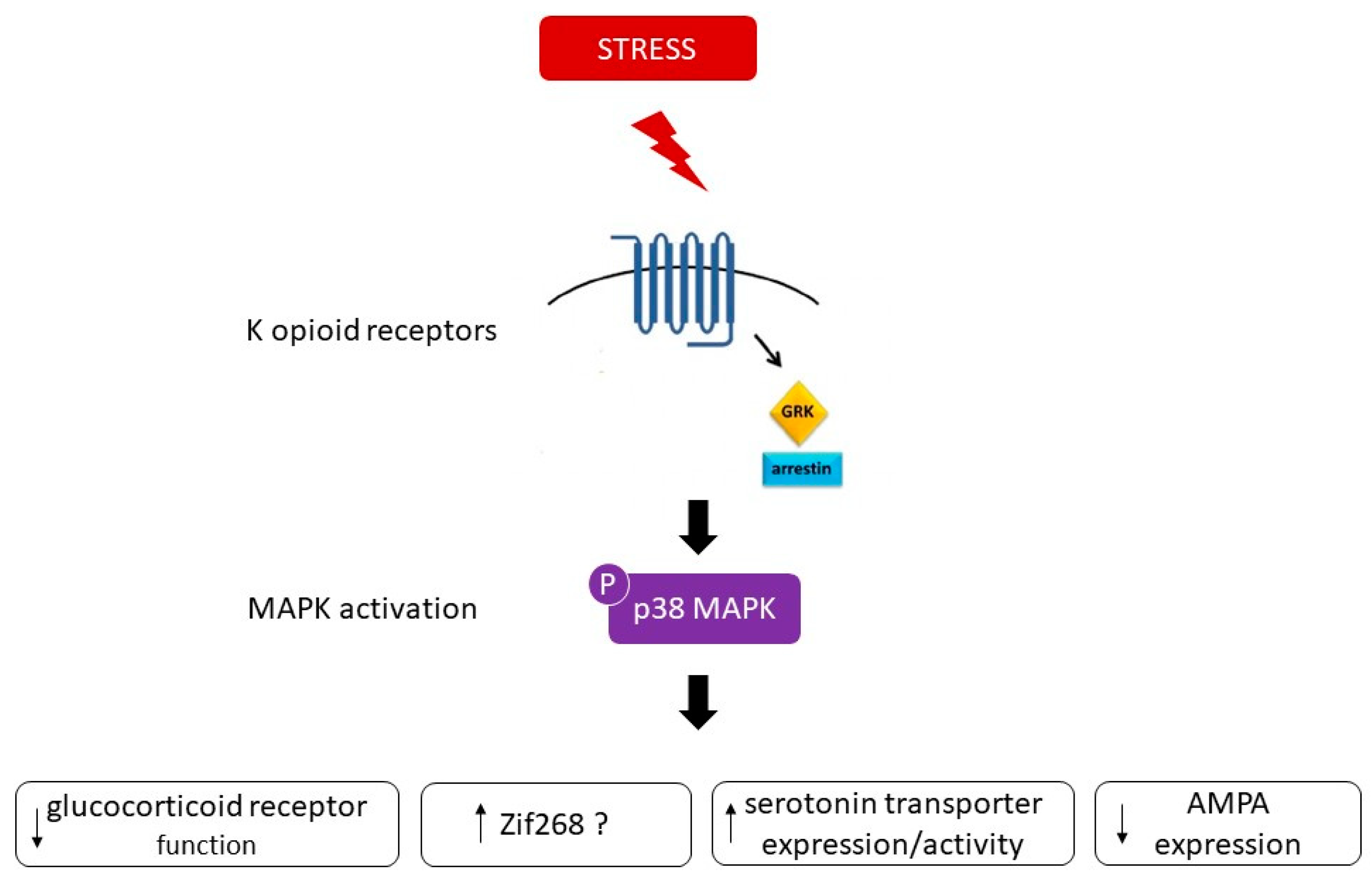
3. Role of p38 MAPK in Stress, Anxiety, and Depression
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ERK | Extracellular signal-regulated protein kinases |
| JNK | c-Jun N-terminal kinases |
| MAPK | Mitogen-activated protein kinases |
| MKK | MAP kinase kinase |
| NET | Norepinephrine transporter |
| pp38 | Phosphorylated p38 |
| CHOP | CCAAT/enhancer-binding protein-homologous protein |
| MAPKAP | MAPK-activated protein |
| MEF2C | Myocyte enhancer factor 2C |
| GS | Glycogen synthase |
| GRK3 | G-protein-coupled receptor kinase 3 |
| CPP | Conditioned place preference |
| KO | Knock out |
| NAc | Nucleus accumbens |
| i.p. | Intraperitoneal |
| icv | Intracerebroventricular |
| DRN | Dorsal raphe nucleus |
| VTA | Ventral tegmental area |
| CRF | Corticotropin-releasing factor |
| KOR | κ opioid receptor |
| αCRF | Alpha helical CRF |
| VEH | Vehicle |
| PFC | Prefrontal cortex |
| LPS | Lipopolysaccharide |
| CPA | Conditioned place aversion |
| SERT | Serotonin transporter |
| GR | Glucocorticoid receptor |
| MOR | µ opioid receptor |
| DOR | δ opioid receptor |
References
- Cowan, K.J.; Storey, K.B. Mitogen-activated Protein Kinases: New Signaling Pathways Functioning in Cellular Responses to Environmental Stress. J. Exp. Biol. 2003, 206, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- New, L.; Han, J. The p38 MAP kinase pathway and its biological function. Trends Cardiovasc. Med. 1998, 8, 220–228. [Google Scholar] [CrossRef]
- Wang, Z.; Harkins, P.C.; Ulevitch, R.J.; Han, J.; Cobb, M.H.; Goldsmith, E.J. The Structure of Mitogen-Activated Protein Kinase p38 at 2.1-A Resolution. Proc. Natl. Acad. Sci. USA 1997, 94, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, S.A.; Eales, K.L. The Role of p38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease. J. Signal Transduct. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.; Che, Y.; Han, P.L.; Lee, J.K. Constitutive activity and differential localization of p38alpha and p38beta MAPKs in adult mouse brain. J. Neurosci. Res. 2000, 60, 623–631. [Google Scholar] [CrossRef]
- Lee, J.C.; Kumar, S.; Griswold, D.E.; Underwood, D.C.; Votta, B.J.; Adams, J.L. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology 2000, 47, 185–201. [Google Scholar] [CrossRef]
- Lee, J.C.; Kassis, S.; Kumar, S.; Badger, A.; Adams, J.L. p38 mitogen-activated protein kinase inhibitors--mechanisms and therapeutic potentials. Pharmacol. Ther. 1999, 82, 389–397. [Google Scholar] [CrossRef]
- Fisk, M.; Gajendragadkar, P.R.; Mäki-Petäjä, K.M.; Wilkinson, I.B.; Cheriyan, J. Therapeutic Potential of p38 MAP Kinase Inhibition in the Management of Cardiovascular Disease. Am. J. Cardiovasc. Drugs 2014, 14, 155–165. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK Signalling Pathways as Molecular Targets for Anti-Inflammatory Therapy--From Molecular Mechanisms to Therapeutic Benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Bardo, M.T.; Bevins, R.A. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology 2000, 153, 31–43. [Google Scholar] [CrossRef]
- Tzschentke, T.M. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007, 12, 227–462. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T.M. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998, 56, 613–672. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Thomas, M.J. Biological Substrates of Reward and Aversion: A Nucleus Accumbens Activity Hypothesis. Neuropharmacology 2009, 56 (Suppl. 1), 122–132. [Google Scholar] [CrossRef]
- Gerdjikov, T.V.; Ross, G.M.; Beninger, R.J. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav. Neurosci. 2004, 118, 740–750. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Y.; Jing, J.; Cui, Y.; Xin, W.; Liu, X. Involvement of p38/NF-κB signaling pathway in the nucleus accumbens in the rewarding effects of morphine in rats. Behav. Brain Res. 2011, 218, 184–189. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Cui, Y.; Cui, Y.; Chen, Y.; Na, X.-D.; Chen, F.-Y.; Wei, X.-H.; Li, Y.-Y.; Liu, X.-G.; Xin, W.-J. Activation of p38 signaling in the microglia in the nucleus accumbens contributes to the acquisition and maintenance of morphine-induced conditioned place preference. Brain. Behav. Immun. 2012, 26, 318–325. [Google Scholar] [CrossRef]
- Hong, S.-I.; Nguyen, T.-L.; Ma, S.-X.; Kim, H.-C.; Lee, S.-Y.; Jang, C.-G. TRPV1 modulates morphine-induced conditioned place preference via p38 MAPK in the nucleus accumbens. Behav. Brain Res. 2017, 334, 26–33. [Google Scholar] [CrossRef]
- Mannangatti, P.; NarasimhaNaidu, K.; Damaj, M.I.; Ramamoorthy, S.; Jayanthi, L.D. A Role for p38 Mitogen-activated Protein Kinase-mediated Threonine 30-dependent Norepinephrine Transporter Regulation in Cocaine Sensitization and Conditioned Place Preference. J. Biol. Chem. 2015, 290, 10814–10827. [Google Scholar] [CrossRef]
- Bruchas, M.R.; Land, B.B.; Aita, M.; Xu, M.; Barot, S.K.; Li, S.; Chavkin, C. Stress-Induced p38 Mitogen-Activated Protein Kinase Activation Mediates -Opioid-Dependent Dysphoria. J. Neurosci. 2007, 27, 11614–11623. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.; Salti, A.; Amaral, I.M.; Fontebasso, V.; Singewald, N.; Dechant, G.; Hofer, A.; El Rawas, R. Social interaction reward in rats has anti-stress effects. Addict. Biol. 2020, e12878. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.R.; Schindler, A.G.; Shankar, H.; Messinger, D.I.; Miyatake, M.; Land, B.B.; Lemos, J.C.; Hagan, C.E.; Neumaier, J.F.; Quintana, A.; et al. Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 2011, 71, 498–511. [Google Scholar] [CrossRef]
- Ehrich, J.M.; Messinger, D.I.; Knakal, C.R.; Kuhar, J.R.; Schattauer, S.S.; Bruchas, M.R.; Zweifel, L.; Kieffer, B.L.; Phillips, P.E.M.; Chavkin, C. Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons. J. Neurosci. 2015, 35, 12917–12931. [Google Scholar] [CrossRef]
- Calcagnetti, D.J.; Schechter, M.D. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol. Behav. 1992, 51, 667–672. [Google Scholar] [CrossRef]
- Douglas, L.A.; Varlinskaya, E.I.; Spear, L.P. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004, 45, 153–162. [Google Scholar] [CrossRef]
- Thiel, K.J.; Okun, A.C.; Neisewander, J.L. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008, 96, 202–212. [Google Scholar] [CrossRef]
- Trezza, V.; Damsteegt, R.; Vanderschuren, L.J.M.J. Conditioned place preference induced by social play behavior: Parametrics, extinction, reinstatement and disruption by methylphenidate. Eur. Neuropsychopharmacol. 2009, 19, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; El Rawas, R.; Salti, A.; Klement, S.; Bardo, M.T.; Kemmler, G.; Dechant, G.; Saria, A.; Zernig, G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict. Biol. 2011, 16, 273–284. [Google Scholar] [CrossRef]
- Kummer, K.; Klement, S.; Eggart, V.; Mayr, M.J.; Saria, A.; Zernig, G. Conditioned place preference for social interaction in rats: Contribution of sensory components. Front. Behav. Neurosci. 2011, 5, 80. [Google Scholar] [CrossRef]
- El Rawas, R.; Klement, S.; Kummer, K.K.; Fritz, M.; Dechant, G.; Saria, A.; Zernig, G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front. Behav. Neurosci. 2012, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- El Rawas, R.; Saria, A. The Two Faces of Social Interaction Reward in Animal Models of Drug Dependence. Neurochem. Res. 2016, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.R.; Beckmann, J.S.; Meyer, A.C.; Bardo, M.T. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: Effects of age and housing condition. Drug Alcohol Depend. 2013, 129, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Salti, A.; Kummer, K.K.; Sadangi, C.; Dechant, G.; Saria, A.; El Rawas, R. Social interaction reward decreases p38 activation in the nucleus accumbens shell of rats. Neuropharmacology 2015, 99, 510–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalueff, A.V.; Tuohimaa, P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: Potential utility for neurobehavioural stress research. J. Neurosci. Methods 2005, 143, 169–177. [Google Scholar] [CrossRef]
- Land, B.B.; Bruchas, M.R.; Lemos, J.C.; Xu, M.; Melief, E.J.; Chavkin, C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008, 28, 407–414. [Google Scholar] [CrossRef]
- Bruchas, M.R.; Macey, T.A.; Lowe, J.D.; Chavkin, C. Kappa Opioid Receptor Activation of p38 MAPK Is GRK3- and Arrestin-dependent in Neurons and Astrocytes. J. Biol. Chem. 2006, 281, 18081–18089. [Google Scholar] [CrossRef]
- Al-Hasani, R.; McCall, J.G.; Shin, G.; Gomez, A.M.; Schmitz, G.P.; Bernardi, J.M.; Pyo, C.-O.; Park, S.; Marcinkiewcz, C.M.; Crowley, N.A.; et al. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 2015, 87, 1063–1077. [Google Scholar] [CrossRef]
- Sinha, R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008, 1141, 105–130. [Google Scholar] [CrossRef]
- Sousa, N. The Dynamics of the Stress Neuromatrix. Mol. Psychiatry 2016, 21, 302–312. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, Y.; Zhang, X.; Cai, T.; Liu, M.; Zhao, F.; Luo, W.; Chen, J. Acute cold exposure and rewarming enhanced spatial memory and activated the MAPK cascades in the rat brain. Brain Res. 2008, 1239, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, H.; Zhang, R.; Chen, Y.; Xue, F.; Nie, H.; Wu, D.; Wang, Y.; Tan, Q. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol. Res. 2013, 62, 537–545. [Google Scholar]
- Sanchez, M.M.; Alagbe, O.; Felger, J.C.; Zhang, J.; Graff, A.E.; Grand, A.P.; Maestripieri, D.; Miller, A.H. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol. Psychiatry 2007, 12, 895–897. [Google Scholar] [CrossRef]
- Zhao, Y.-W.; Pan, Y.-Q.; Tang, M.-M.; Lin, W.-J. Blocking p38 Signaling Reduces the Activation of Pro-inflammatory Cytokines and the Phosphorylation of p38 in the Habenula and Reverses Depressive-Like Behaviors Induced by Neuroinflammation. Front. Pharmacol. 2018, 9, 511. [Google Scholar] [CrossRef]
- Stefanoska, K.; Bertz, J.; Volkerling, A.M.; Van der Hoven, J.; Ittner, L.M.; Ittner, A. Neuronal MAP kinase p38α inhibits c-Jun N-terminal kinase to modulate anxiety-related behaviour. Sci. Rep. 2018, 8, 14296. [Google Scholar] [CrossRef]
- Zan, G.-Y.; Wang, Q.; Wang, Y.-J.; Chen, J.-C.; Wu, X.; Yang, C.-H.; Chai, J.-R.; Li, M.; Liu, Y.; Hu, X.-W.; et al. p38 Mitogen-Activated Protein Kinase Activation in Amygdala Mediates κ Opioid Receptor Agonist U50,488H-induced Conditioned Place Aversion. Neuroscience 2016, 320, 122–128. [Google Scholar] [CrossRef]
- Land, B.B.; Bruchas, M.R.; Schattauer, S.; Giardino, W.J.; Aita, M.; Messinger, D.; Hnasko, T.S.; Palmiter, R.D.; Chavkin, C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci. USA 2009, 106, 19168–19173. [Google Scholar] [CrossRef]
- Rolli, M.; Kotlyarov, A.; Sakamoto, K.M.; Gaestel, M.; Neininger, A. Stress-induced Stimulation of Early Growth Response gene-1 by p38/stress-activated Protein Kinase 2 Is Mediated by a cAMP-responsive Promoter Element in a MAPKAP Kinase 2-independent Manner. J. Biol. Chem. 1999, 274, 19559–19564. [Google Scholar] [CrossRef]
- Zhu, C.-B.; Carneiro, A.M.; Dostmann, W.R.; Hewlett, W.A.; Blakely, R.D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005, 280, 15649–15658. [Google Scholar] [CrossRef]
- Samuvel, D.J.; Jayanthi, L.D.; Bhat, N.R.; Ramamoorthy, S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: Evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci. 2005, 25, 29–41. [Google Scholar] [CrossRef]
- Zhu, C.-B.; Lindler, K.M.; Owens, A.W.; Daws, L.C.; Blakely, R.D.; Hewlett, W.A. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology 2010, 35, 2510–2520. [Google Scholar] [CrossRef] [PubMed]
- Baganz, N.L.; Lindler, K.M.; Zhu, C.B.; Smith, J.T.; Robson, M.J.; Iwamoto, H.; Deneris, E.S.; Hewlett, W.A.; Blakely, R.D. A requirement of serotonergic p38α mitogen-activated protein kinase for peripheral immune system activation of CNS serotonin uptake and serotonin-linked behaviors. Transl. Psychiatry 2015, 5, e671. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; You, J.-L.; Wu, M.-Y.; Hsu, K.-S. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J. Biol. Chem. 2004, 279, 12286–12292. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Huang, C.-C.; Hsu, K.-S. A Role of p38 Mitogen-Activated Protein Kinase in Adenosine A₁ Receptor-Mediated Synaptic Depotentiation in Area CA1 of the Rat Hippocampus. Mol. Brain 2008, 1, 13. [Google Scholar] [CrossRef]
- Bleakman, D.; Alt, A.; Witkin, J.M. AMPA Receptors in the Therapeutic Management of Depression. CNS Neurol. Disord. Drug Targets 2007, 6, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Miller, A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Miller, A.H. Interleukin 1alpha (IL-1alpha) Induced Activation of p38 Mitogen-Activated Protein Kinase Inhibits Glucocorticoid Receptor Function. Mol. Psychiatry 2004, 9, 65–75. [Google Scholar] [CrossRef][Green Version]
- Pace, T.W.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain. Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef]
- Schindler, A.G.; Li, S.; Chavkin, C. Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology 2010, 35, 1932–1942. [Google Scholar] [CrossRef]
- Yamada, H.; Shimoyama, N.; Sora, I.; Uhl, G.R.; Fukuda, Y.; Moriya, H.; Shimoyama, M. Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors. Brain Res. 2006, 1083, 61–69. [Google Scholar] [CrossRef]
- Pan, Z.Z.; Tershner, S.A.; Fields, H.L. Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature 1997, 389, 382–385. [Google Scholar] [CrossRef]
- Neisewander, J.L.; Pierce, R.C.; Bardo, M.T. Naloxone enhances the expression of morphine-induced conditioned place preference. Psychopharmacology 1990, 100, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Matthes, H.W.; Maldonado, R.; Simonin, F.; Valverde, O.; Slowe, S.; Kitchen, I.; Befort, K.; Dierich, A.; Le Meur, M.; Dollé, P.; et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 1996, 383, 819–823. [Google Scholar] [CrossRef]
- Wu, G.; Huang, W.; Zhang, H.; Li, Q.; Zhou, J.; Shu, H. Inhibitory Effects of Processed Aconiti Tuber on Morphine-Induced Conditioned Place Preference in Rats. J. Ethnopharmacol. 2011, 136, 254–259. [Google Scholar] [CrossRef]
- Billa, S.K.; Xia, Y.; Morón, J.A. Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: Study of mu-opioid and delta-opioid receptor expression at the synapse. Eur. J. Neurosci. 2010, 32, 625–631. [Google Scholar] [CrossRef]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef]
- Li, Z.-H.; Chu, N.; Shan, L.-D.; Gong, S.; Yin, Q.-Z.; Jiang, X. Inducible Expression of Functional Mu Opioid Receptors in Murine Dendritic Cells. J. Neuroimmune Pharmacol. 2009, 4, 359–367. [Google Scholar] [CrossRef]
- Rozenfeld-Granot, G.; Toren, A.; Amariglio, N.; Nagler, A.; Rosenthal, E.; Biniaminov, M.; Brok-Simoni, F.; Rechavi, G. MAP kinase activation by mu opioid receptor in cord blood CD34+CD38- cells. Exp. Hematol. 2002, 30, 473–480. [Google Scholar] [CrossRef]
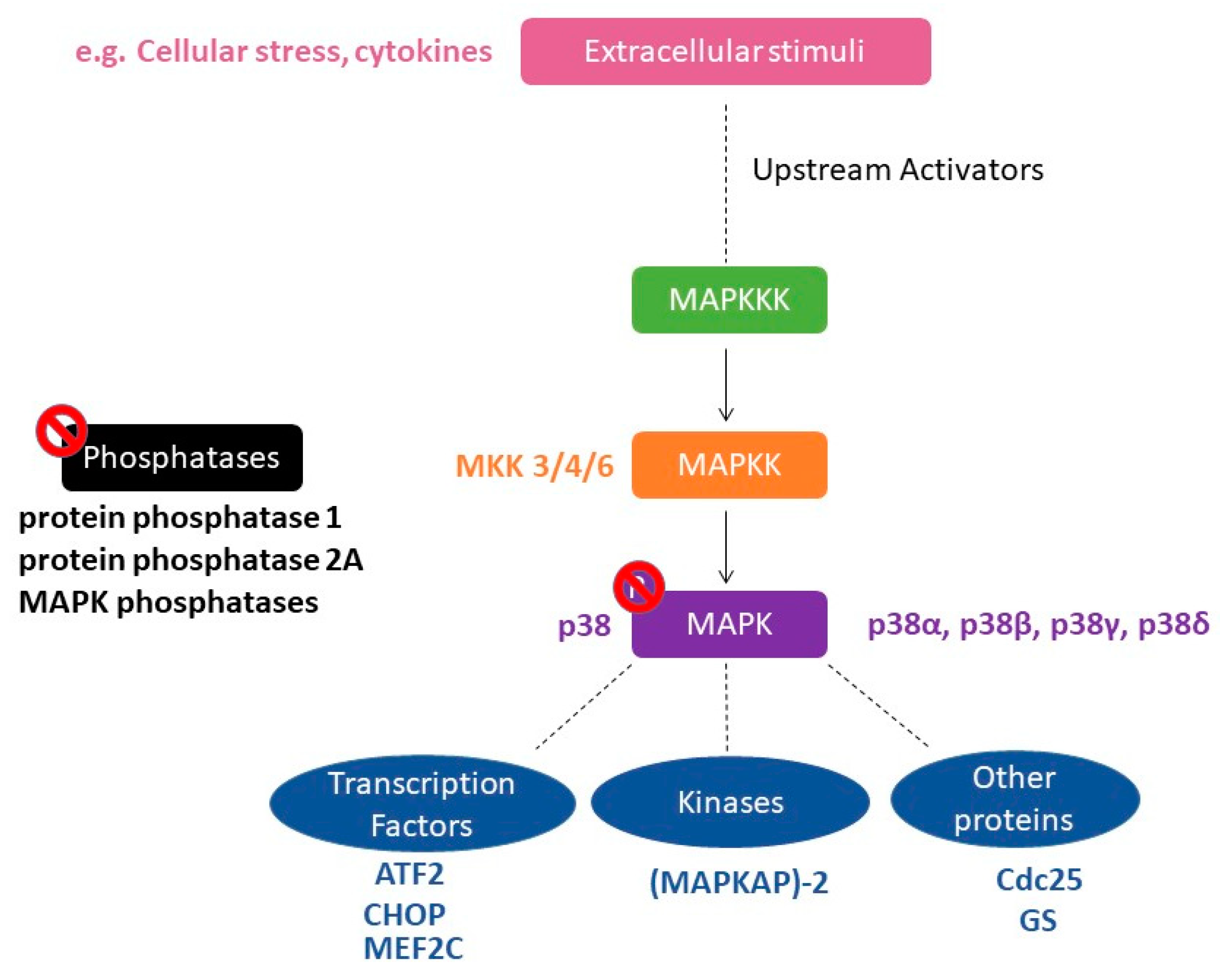

| Drug | P38 Blockade | Results | References |
|---|---|---|---|
| Amphetamine | SB203580 into the NAc | impaired amphetamine conditioned place preference (CPP) | [16] |
| Morphine | SB203580 into the NAc | prevented the acquisition but not the expression of morphine CPP | [17,18] |
| Morphine | SB203580 i.p. | dampened the acquisition of morphine-induced CPP | [19] |
| Morphine | SB203580 i.p. | failed to block the expression of morphine CPP | [20] |
| Cocaine | SB203580 i.p. | blocked the expression of cocaine CPP | [20] |
| Cocaine | SB203580 icv | did not affect acquisition to cocaine CPP | [21,22] |
| Cocaine | deletion of p38α MAPK in the serotonergic neurons | did not affect cocaine CPP | [23] |
| Cocaine | conditional knock-out of p38α MAPK in the dopaminergic neurons | did not affect cocaine CPP | [24] |
| Cocaine | serotonergic p38α MAPK deletion | blocked the reinstatement of cocaine preference induced by stress | [23] |
| Cocaine | serotonergic p38α MAPK deletion | did not affect the reinstatement of cocaine preference induced by cocaine priming | [23] |
| Stimuli/Protocol | Regions | Animals | References |
|---|---|---|---|
| morphine CPP | NAc | rats | [17,18] |
| mice | [19] | ||
| repeated forced swim stress | cortex, hippocampus, NAc | mice | [21] |
| cold exposure | PFC | rats | [41] |
| enhanced single prolonged stress | hippocampus | rats | [42] |
| social defeat stress | dorsal raphe nucleus (DRN) | mice | [23] |
| early life stress | monocytes staining for pp38 | monkeys | [43] |
| bacterial endotoxin LPS | habenula | rats | [44] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Rawas, R.; Amaral, I.M.; Hofer, A. Is p38 MAPK Associated to Drugs of Abuse-Induced Abnormal Behaviors? Int. J. Mol. Sci. 2020, 21, 4833. https://doi.org/10.3390/ijms21144833
El Rawas R, Amaral IM, Hofer A. Is p38 MAPK Associated to Drugs of Abuse-Induced Abnormal Behaviors? International Journal of Molecular Sciences. 2020; 21(14):4833. https://doi.org/10.3390/ijms21144833
Chicago/Turabian StyleEl Rawas, Rana, Inês M. Amaral, and Alex Hofer. 2020. "Is p38 MAPK Associated to Drugs of Abuse-Induced Abnormal Behaviors?" International Journal of Molecular Sciences 21, no. 14: 4833. https://doi.org/10.3390/ijms21144833
APA StyleEl Rawas, R., Amaral, I. M., & Hofer, A. (2020). Is p38 MAPK Associated to Drugs of Abuse-Induced Abnormal Behaviors? International Journal of Molecular Sciences, 21(14), 4833. https://doi.org/10.3390/ijms21144833






