The Novel gem-Dihydroperoxide 12AC3O Suppresses High Phosphate-Induced Calcification via Antioxidant Effects in p53LMAco1 Smooth Muscle Cells
Abstract
1. Introduction
2. Results
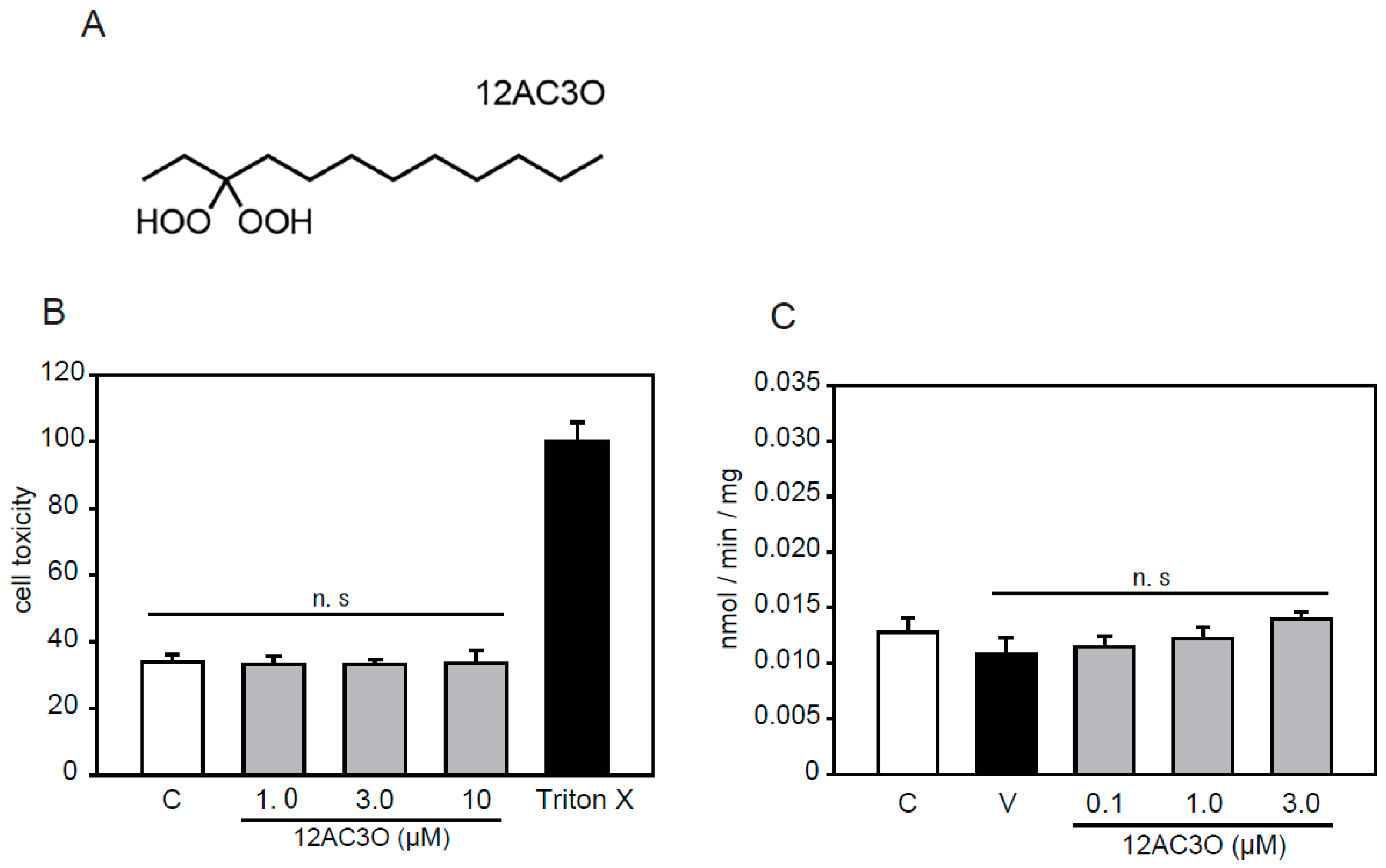
2.1. Selection of DHPs and Cell Toxicity Assay
2.2. Establishment of an Experimental Model of High Concentrations of Pi-Induced Calcification in p53LMAco1 Cells
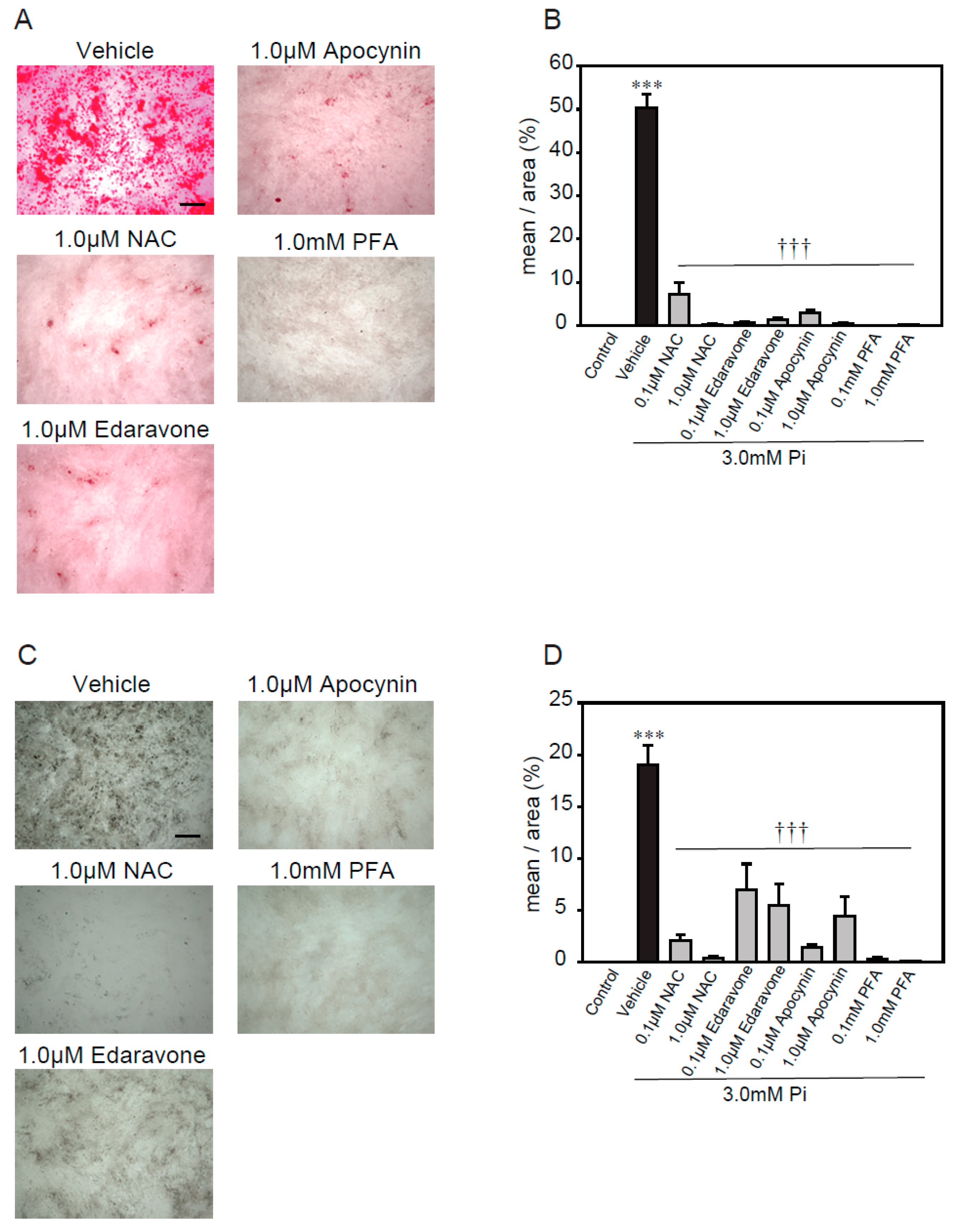
2.3. Antioxidants Suppress High Concentrations of Pi-Induced Calcification
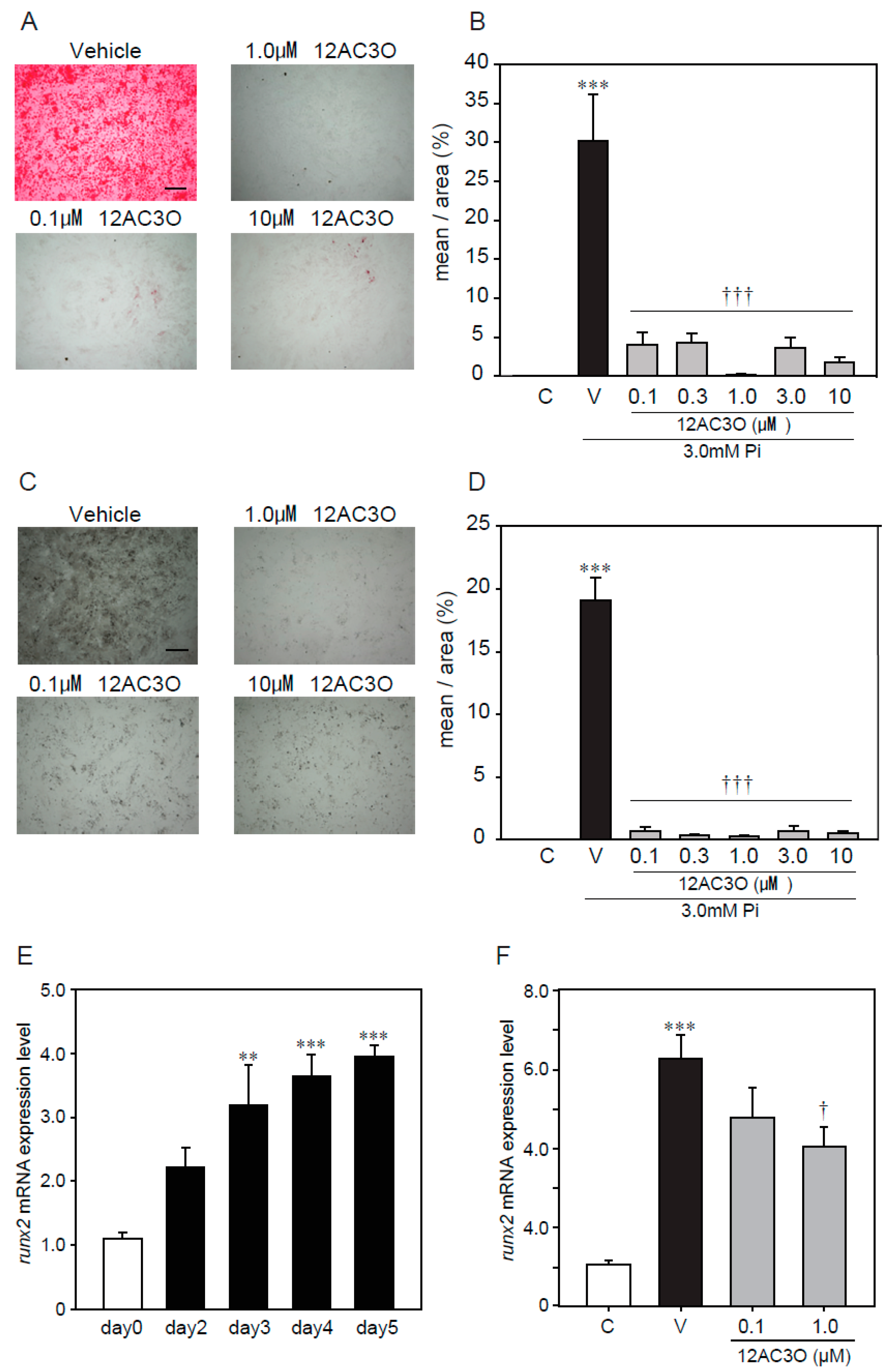
2.4. 12AC3O Suppresses High Concentrations of Pi-Induced Calcification
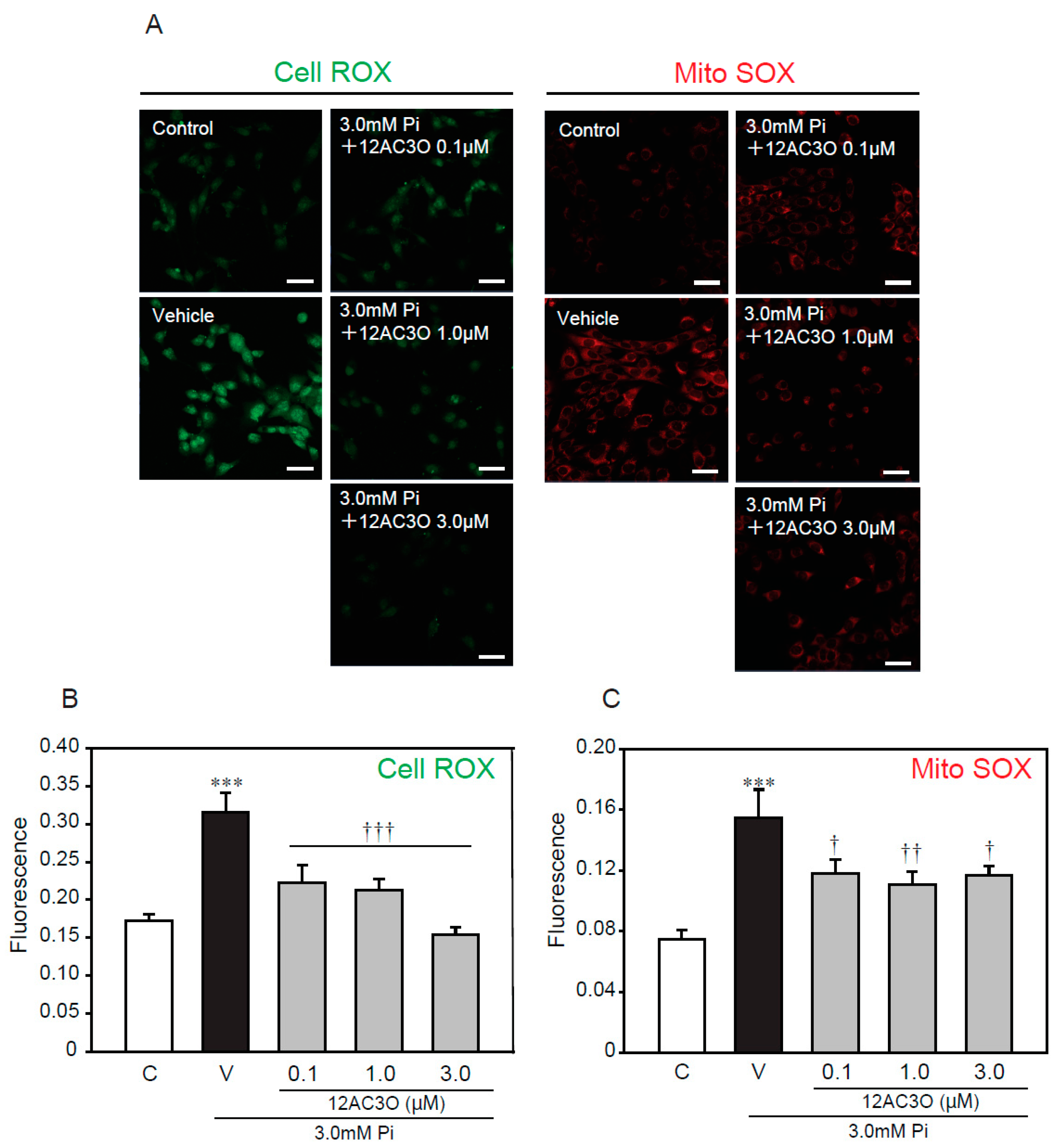
2.5. 12AC3O Suppresses High Concentrations of Pi-Induced Oxidative Stress
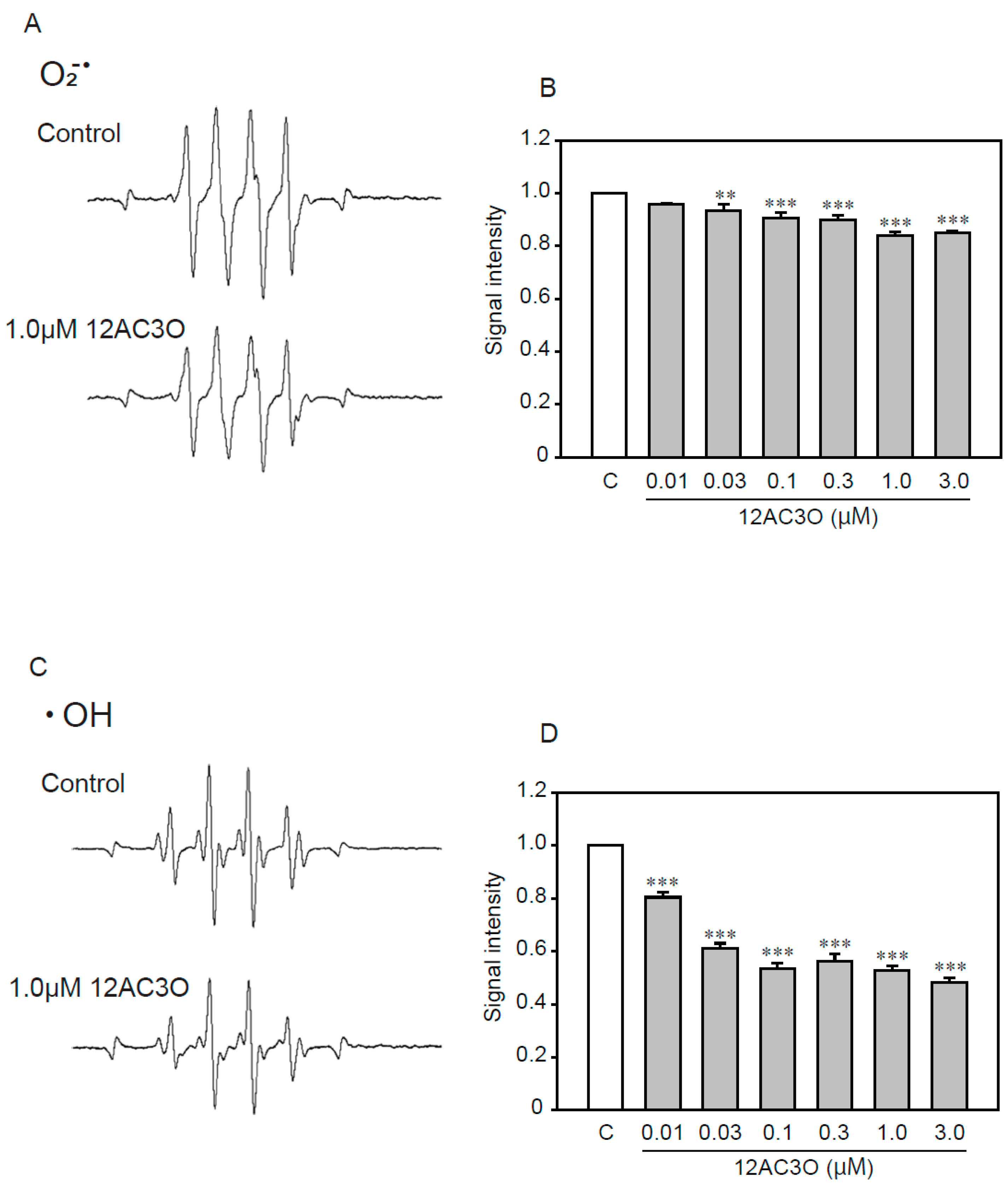
2.6. 12AC3O Directly Traps Superoxide Anion and Hydroxyl Radical
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Neurotoxicity Assays
4.3. 32Pi Transport Assays
4.4. qRT-PCR
4.5. Alizarin Red Staining
4.6. Von Kossa Staining
4.7. ROS Detection
4.8. ESR Analysis
4.9. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Pi | Phosphate |
| CKD | Chronic kidney disease |
| ROS | Reactive oxygen species |
| DHPs | Gem-dihydroperoxides |
| ESR | Electron spin resonance |
| TNF-α | Tumor necrosis factor-α |
| IBGC | Idiopathic basal ganglia calcification |
| CSF | Cerebral spinal fluid |
| VSMC | Vascular smooth muscle cells |
| BMP | Bone morphogenetic protein |
| NO | Nitric oxide |
| PMBCs | Peripheral blood monocytes |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| NAC | N-acetylcysteine |
| PFA | Phosphonoformic acid |
| NF-kB | Nuclear factor-kappa-B |
| MAPK | Mitogen-activated protein kinase |
| Runx2 | Runt-related transcription factor 2 |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| Msx2 | Muscle segment homeobox 2 |
References
- Keith, D.S.; Nichols, G.A.; Gullion, C.M.; Brown, J.B.; Smith, D.H. Longitudinal Follow-up and Outcomes among a Population with Chronic Kidney Disease in a Large Managed Care Organization. Arch. Intern. Med. 2004, 164, 659–663. [Google Scholar] [CrossRef]
- Ninomiya, T.; Kiyohara, Y.; Kubo, M.; Tanizaki, Y.; Doi, Y.; Okubo, K.; Wakugawa, Y.; Hata, J.; Oishi, Y.; Shikata, K.; et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: The Hisayama Study. Kidney Int. 2005, 68, 228–236. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guérin, A.P.; Marchais, S.J.; Métivier, F.; Pannier, B.; Adda, H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transpl. 2003, 18, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, M.; Bungay, P.; Taal, M.W.; McIntyre, C.W. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol. Dial. Transpl. 2006, 21, 707–714. [Google Scholar] [CrossRef]
- Foley, R.N. Clinical epidemiology of cardiovascular disease in chronic kidney disease. J. Ren. Care 1998, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.G.; Goldin, J.G.; Kuizon, B.D.; Yoon, C.; Gales, B.; Sider, D.; Wang, Y.; Chung, J.; Emerick, A.; Greaser, L.E.; et al. Coronary-Artery Calcification in Young Adults with End-Stage Renal Disease Who Are Undergoing Dialysis. N. Engl. J. Med. 2000, 342, 1478–1483. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 520–528. [Google Scholar] [CrossRef]
- Manyam, B.V. What is and what is not “Fahr’s disease”. Park. Relat. Disord. 2005, 11, 73–80. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Shi, L.; Ren, J.; Patti, M.; Wang, T.; De Oliveira, J.R.M.; Sobrido, M.J.; Quintáns, B.; Baquero, M.; et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat. Genet. 2012, 44, 254–256. [Google Scholar] [CrossRef]
- Yamada, M.; Tanaka, M.; Takagi, M.; Kobayashi, S.; Taguchi, Y.; Takashima, S.; Tanaka, K.; Touge, T.; Hatsuta, H.; Murayama, S.; et al. Evaluation of SLC20A2 mutations that cause idiopathic basal ganglia calcification in Japan. Neurology 2014, 82, 705–712. [Google Scholar] [CrossRef]
- Hozumi, I.; Kurita, H.; Ozawa, K.; Furuta, N.; Inden, M.; Sekine, S.I.; Yamada, M.; Hayashi, Y.; Kimura, A.; Inuzuka, T.; et al. Inorganic phosphorus (Pi) in CSF is a biomarker for SLC20A2-associated idiopathic basal ganglia calcification (IBGC1). J. Neurol. Sci. 2018, 388, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Jane, A.; Leopold, M. Vascular Calcification: Mechanisms of Vascular Smooth Muscle Cell Calcification. Physiol. Behav. 2015, 176, 139–148. [Google Scholar]
- Lau, W.L.; Pai, A.; Moe, S.M.; Giachelli, C.M. Direct Effects of Phosphate on Vascular Cell Function. Adv. Chronic Kidney Dis. 2011, 18, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef]
- Giachelli, C.M. The emerging role of phosphate in vascular calcification. Kidney Int. 2009, 75, 890–897. [Google Scholar] [CrossRef]
- Lau, W.L.; Festing, M.H.; Giachelli, C.M. Phosphate and vascular calcification: Emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb. Haemost. 2010, 104, 464–470. [Google Scholar] [CrossRef]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor necrosis factor-α promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef]
- Dalfino, G.; Simone, S.; Porreca, S.; Cosola, C.; Balestra, C.; Manno, C.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Bone morphogenetic protein-2 may represent the molecular link between oxidative stress and vascular stiffness in chronic kidney disease. Atherosclerosis 2010, 211, 418–423. [Google Scholar] [CrossRef]
- Agharazii, M.; St-Louis, R.; Gautier-Bastien, A.; Ung, R.V.; Mokas, S.; Larivière, R.; Richard, D.E. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am. J. Hypertens. 2015, 28, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Byon, C.H.; Sun, Y.; Chen, J.; Yuan, K.; Mao, X.; Heath, J.M.; Anderson, P.G.; Tintut, Y.; Demer, L.L.; Wang, D.; et al. Runx2-upregulated receptor activator of nuclear factor κb ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Deuell, K.A.; Callegari, A.; Giachelli, C.M.; Rosenfeld, M.E.; Scatena, M. RANKL enhances macrophage paracrine pro-calcific activity in high phosphate-treated smooth muscle cells: Dependence on IL-6 and TNF-α. J. Vasc. Res. 2012, 49, 510–521. [Google Scholar] [CrossRef]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-α and oncostatin M derived from macrophages. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef]
- Tintut, Y.; Demer, L. Role of osteoprotegerin and its ligands and competing receptors in atherosclerotic calcification. J. Investig. Med. 2006, 54, 395–401. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Shao, J.S.; Lai, C.F.; Huang, E.; Cai, J.; Behrmann, A.; Cheng, S.L.; Towler, D.A. Aortic Msx2-Wnt calcification cascade is regulated by TNF-α-dependent signals in diabetic Ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2589–2596. [Google Scholar] [CrossRef]
- Shinohara, H.; Taniguchi, K.; Kumazaki, M.; Yamada, N.; Ito, Y.; Otsuki, Y.; Uno, B.; Hayakawa, F.; Minami, Y.; Naoe, T.; et al. Anti-cancer fatty-acid derivative induces autophagic cell death through modulation of PKM isoform expression profile mediated by bcr-abl in chronic myeloid leukemia. Cancer Lett. 2015, 360, 28–38. [Google Scholar] [CrossRef]
- Zhang, M.; Harashima, N.; Moritani, T.; Huang, W.; Harada, M. The roles of ROS and caspases in TRAIL-induced apoptosis and necroptosis in human pancreatic cancer cells. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Chang, H.B.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar]
- Sudo, R.; Sato, F.; Azechi, T.; Wachi, H. 7-Ketocholesterol-induced lysosomal dysfunction exacerbates vascular smooth muscle cell calcification via oxidative stress. Genes Cells 2015, 20, 982–991. [Google Scholar] [CrossRef]
- Luong, T.T.D.; Schelski, N.; Boehme, B.; Makridakis, M.; Vlahou, A.; Lang, F.; Pieske, B.; Alesutan, I.; Voelkl, J. Fibulin-3 Attenuates Phosphate-Induced Vascular Smooth Muscle Cell Calcification by Inhibition of Oxidative Stress. Cell. Physiol. Biochem. 2018, 46, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Ortmann, R.; Jomaa, H.; Schlitzer, M. New Antimalarial Drugs. Angew. Chem. Int. Ed. 2003, 42, 5274–5293. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, M.; Zhang, R.; Wang, H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J. Cell. Mol. Med. 2009, 13, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Tada, N.; Cui, L.; Okubo, H.; Miura, T.; Itoh, A. A facile catalyst-free synthesis of gem-dihydroperoxides with aqueous hydrogen peroxide. Chem. Commun. 2010, 46, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Žmitek, K.; Zupan, M.; Stavber, S.; Iskra, J. Iodine as a catalyst for efficient conversion of ketones to gem-dihydroperoxides by aqueous hydrogen peroxide. Org. Lett. 2006, 8, 2491–2494. [Google Scholar] [CrossRef]
- Kuranaga, Y.; Yamada, N.; Kashiwaya, M.; Nakamura, M.; Cui, L.; Kumazaki, M.; Shinohara, H.; Sugito, N.; Taniguchi, K.; Ito, Y.; et al. Anti-Oncogenic gem-dihydroperoxides induce apoptosis in cancer cells by trapping reactive oxygen species. Int. J. Mol. Sci. 2016, 17, 177–184. [Google Scholar] [CrossRef]
- Ueda, T.; Inden, M.; Asaka, Y.; Masaki, Y.; Kurita, H.; Tanaka, W.; Yamaguchi, E.; Itoh, A.; Hozumi, I. Effects of gem-dihydroperoxides against mutant copper-zinc superoxide dismutase-mediated neurotoxicity. Mol. Cell. Neurosci. 2018, 92, 177–184. [Google Scholar] [CrossRef]
- Wang, H.; Yan, S.; Chai, H.; Riha, G.M.; Li, M.; Yao, Q.; Chen, C. Shear stress induces endothelial transdifferentiation from mouse smooth muscle cells. Biochem. Biophys. Res. Commun. 2006, 346, 860–865. [Google Scholar] [CrossRef]
- Lu, H.; Enosawa, S.; Ohmi, K.; Suzuki, S. The proliferative response of p53 knock-out mouse-derived vascular smooth muscle cell line, P53LMAC01, to PDGF, when compared with human aortic smooth muscle cells. Transpl. Immunol. 2001, 8, 253–257. [Google Scholar] [CrossRef]
- Saito, T.; Itoh, H.; Yamashita, J.; Doi, K.; Chun, T.H.; Tanaka, T.; Inoue, M.; Masatsugu, K.; Fukunaga, Y.; Sawada, N.; et al. Angiotensin II suppresses growth arrest specific homeobox (Gax) expression via redox-sensitive mitogen-activated protein kinase (MAPK). Regul. Pept. 2005, 127, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Morita, I.; Nishi, H.; Murota, S.I. Preventive effect of MCI-186 on 15-HPETE induced vascular endothelial cell injury in vitro. Prostaglandins Leukot. Essent. Fat. Acids 1988, 33, 81–87. [Google Scholar] [CrossRef]
- Tóth, A.E.; Walter, F.R.; Bocsik, A.; Sántha, P.; Veszelka, S.; Nagy, L.; Puskás, L.G.; Couraud, P.O.; Takata, F.; Dohgu, S.; et al. Edaravone protects against methylglyoxal-induced barrier damage in human brain endothelial cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- De Batista, P.R.; Palacios, R.; Martín, A.; Hernanz, R.; Médici, C.T.; Silva, M.A.S.C.; Rossi, E.M.; Aguado, A.; Vassallo, D.V.; Salaices, M.; et al. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Hernanz, R.; Martínez-Revelles, S.; Palacios, R.; Martín, A.; Cachofeiro, V.; Aguado, A.; García-Redondo, L.; Barrús, M.T.; De Batista, P.R.; Briones, A.M.; et al. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br. J. Pharmacol. 2015, 172, 3159–3176. [Google Scholar] [CrossRef]
- Mune, S.; Shibata, M.; Hatamura, I.; Saji, F.; Okada, T.; Maeda, Y.; Sakaguchi, T.; Negi, S.; Shigematsu, T. Mechanism of phosphate-induced calcification in rat aortic tissue culture: Possible involvement of Pit-1 and apoptosis. Clin. Exp. Nephrol. 2009, 13, 571–577. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Distinctive molecular signature and activated signaling pathways in aortic smooth muscle cells of patients with myocardial infarction. Atherosclerosis 2018, 271, 237–244. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Transcriptome alterations of vascular smooth muscle cells in aortic wall of myocardial infarction patients. Data Br. 2018, 17, 1112–1135. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, P.; Chen, W.; Peng, D.A.N. Combined effects of hyperphosphatemia and hyperglycemia on the calcification of cultured human aortic smooth muscle cells. Exp. Ther. Med. 2019, 17, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; O’Neill, K.D.; Moe, S.M. Matrix vesicles induce calcification of recipient vascular smooth muscle cells through multiple signaling pathways. Kidney Int. 2018, 93, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.J.; Zhang, Y.M.; Qi, J.P.; Liu, R.; Zhang, H.; He, L.C. Ferulic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NADPH oxidase and NF-κB pathway. Int. Immunopharmacol. 2015, 28, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Xu, M.J.; Cai, Y.; Zhao, G.; Guan, Y.; Kong, W.; Tang, C.; Wang, X. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int. 2011, 79, 1071–1079. [Google Scholar] [CrossRef]
- Almajdoob, S.; Hossain, E.; Anand-Srivastava, M.B. Resveratrol attenuates hyperproliferation of vascular smooth muscle cells from spontaneously hypertensive rats: Role of ROS and ROS-mediated cell signaling. Vascul. Pharmacol. 2018, 101, 48–56. [Google Scholar] [CrossRef]
- Sun, Y.; Byon, C.H.; Yuan, K.; Chen, J.; Mao, X.; Heath, J.M.; Javed, A.; Zhang, K.; Anderson, P.G.; Chen, Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ. Res. 2012, 111, 543–552. [Google Scholar] [CrossRef]
- Okamura, D.M.; Pennathur, S. The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol. 2015, 6, 495–504. [Google Scholar] [CrossRef]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramírez, R. Mechanisms of Cardiovascular Disorders in Patients With Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Takase, N.; Inden, M.; Sekine, S.I.; Ishii, Y.; Yonemitsu, H.; Iwashita, W.; Kurita, H.; Taketani, Y.; Hozumi, I. Neuroprotective effect of 5-Aminolevulinic acid against low inorganic phosphate in neuroblastoma SH-SY5Y cells. Sci. Rep. 2017, 7, 5768. [Google Scholar] [CrossRef]
- Lu, Y.; Bian, Y.; Wang, Y.; Bai, R.; Wang, J.; Xiao, C. Globular adiponectin reduces vascular calcification via inhibition of ER-stress-mediated smooth muscle cell apoptosis. Int. J. Clin. Exp. Pathol. 2015, 8, 2545–2554. [Google Scholar]
- Olah, Z.; Lehel, C.; Anderson, W.B.; Eiden, M.V.; Wilson, C.A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J. Biol. Chem. 1994, 269, 25426–25431. [Google Scholar] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takase, N.; Inden, M.; Hirai, S.; Yamada, Y.; Kurita, H.; Takeda, M.; Yamaguchi, E.; Itoh, A.; Hozumi, I. The Novel gem-Dihydroperoxide 12AC3O Suppresses High Phosphate-Induced Calcification via Antioxidant Effects in p53LMAco1 Smooth Muscle Cells. Int. J. Mol. Sci. 2020, 21, 4628. https://doi.org/10.3390/ijms21134628
Takase N, Inden M, Hirai S, Yamada Y, Kurita H, Takeda M, Yamaguchi E, Itoh A, Hozumi I. The Novel gem-Dihydroperoxide 12AC3O Suppresses High Phosphate-Induced Calcification via Antioxidant Effects in p53LMAco1 Smooth Muscle Cells. International Journal of Molecular Sciences. 2020; 21(13):4628. https://doi.org/10.3390/ijms21134628
Chicago/Turabian StyleTakase, Naoko, Masatoshi Inden, Shunsuke Hirai, Yumeka Yamada, Hisaka Kurita, Mitsumi Takeda, Eiji Yamaguchi, Akichika Itoh, and Isao Hozumi. 2020. "The Novel gem-Dihydroperoxide 12AC3O Suppresses High Phosphate-Induced Calcification via Antioxidant Effects in p53LMAco1 Smooth Muscle Cells" International Journal of Molecular Sciences 21, no. 13: 4628. https://doi.org/10.3390/ijms21134628
APA StyleTakase, N., Inden, M., Hirai, S., Yamada, Y., Kurita, H., Takeda, M., Yamaguchi, E., Itoh, A., & Hozumi, I. (2020). The Novel gem-Dihydroperoxide 12AC3O Suppresses High Phosphate-Induced Calcification via Antioxidant Effects in p53LMAco1 Smooth Muscle Cells. International Journal of Molecular Sciences, 21(13), 4628. https://doi.org/10.3390/ijms21134628





