Influence of Intermittent Cold Stimulations on CREB and Its Targeting Genes in Muscle: Investigations into Molecular Mechanisms of Local Cryotherapy
Abstract
1. Introduction
2. Results
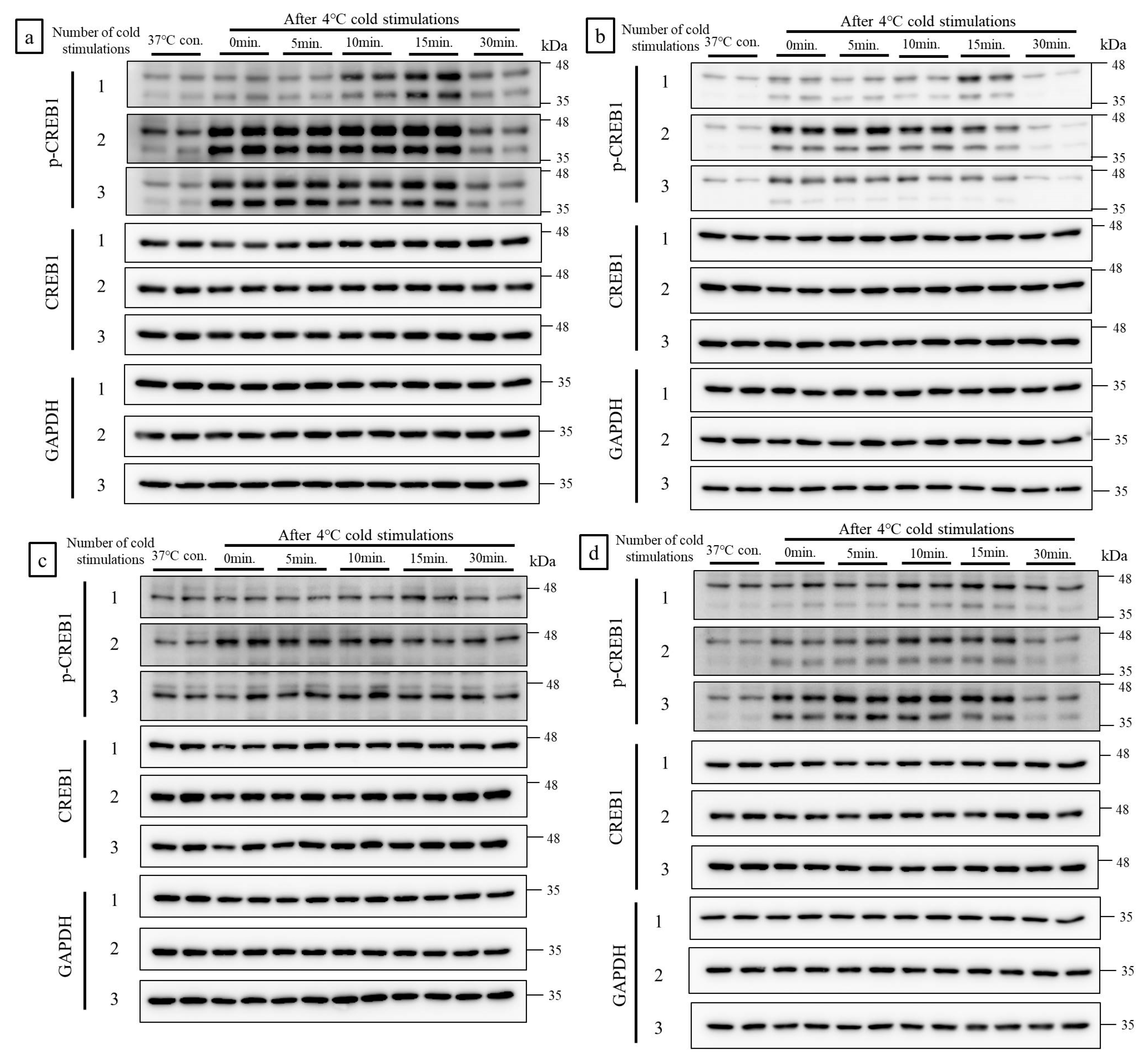
2.1. Cold Stimulation Induced CREB1 Phosphorylation in a Frequency- and Duration-Dependent Manner In Vitro
2.2. Cold Stimulation Did Not Cause Cell Damage In Vitro
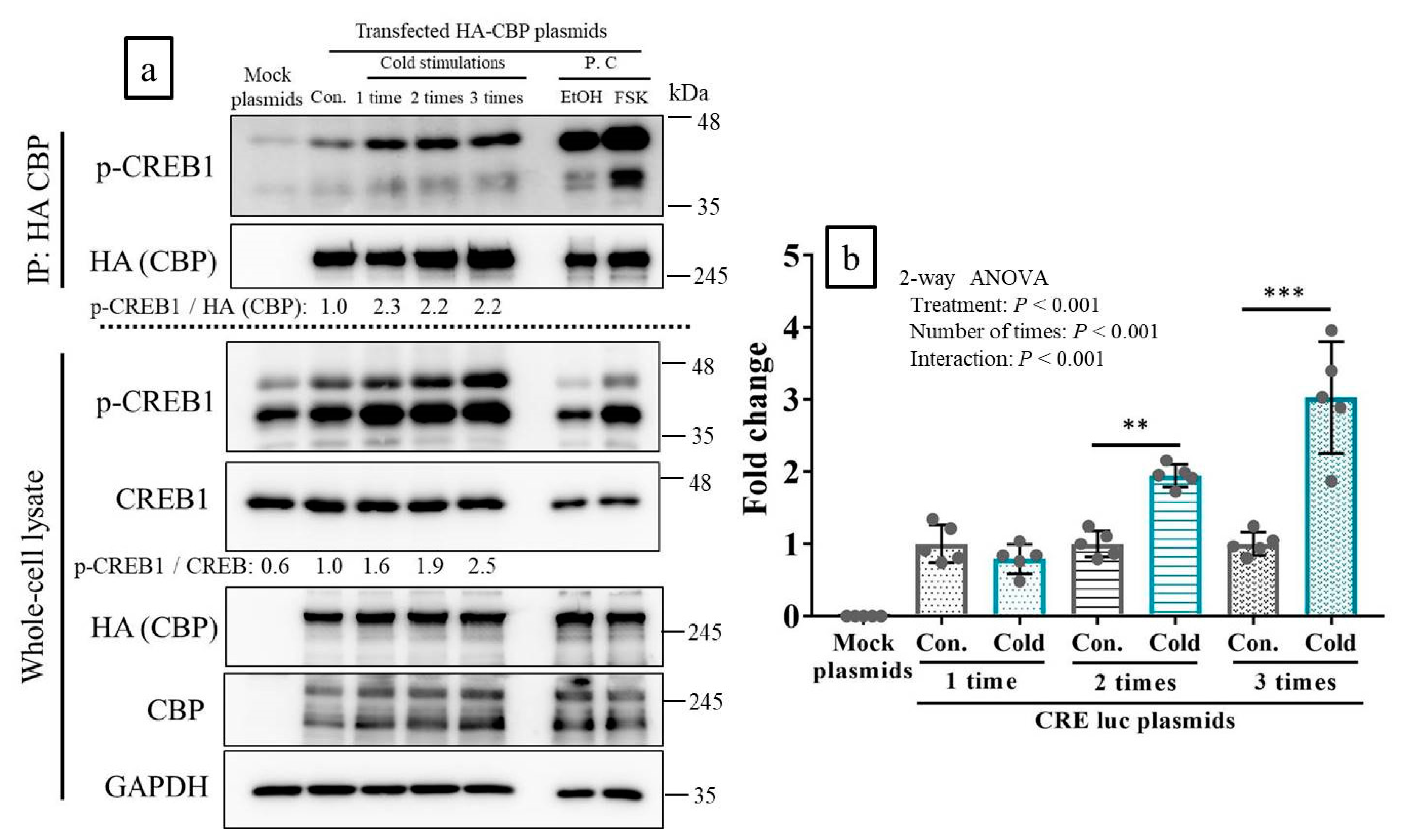
2.3. CREB-Binding Protein (CBP) Was Recruited to p-CREB in Response to Cold Stimulation In Vitro
2.4. Cold Stimulation Activated CREB Transcription In Vitro
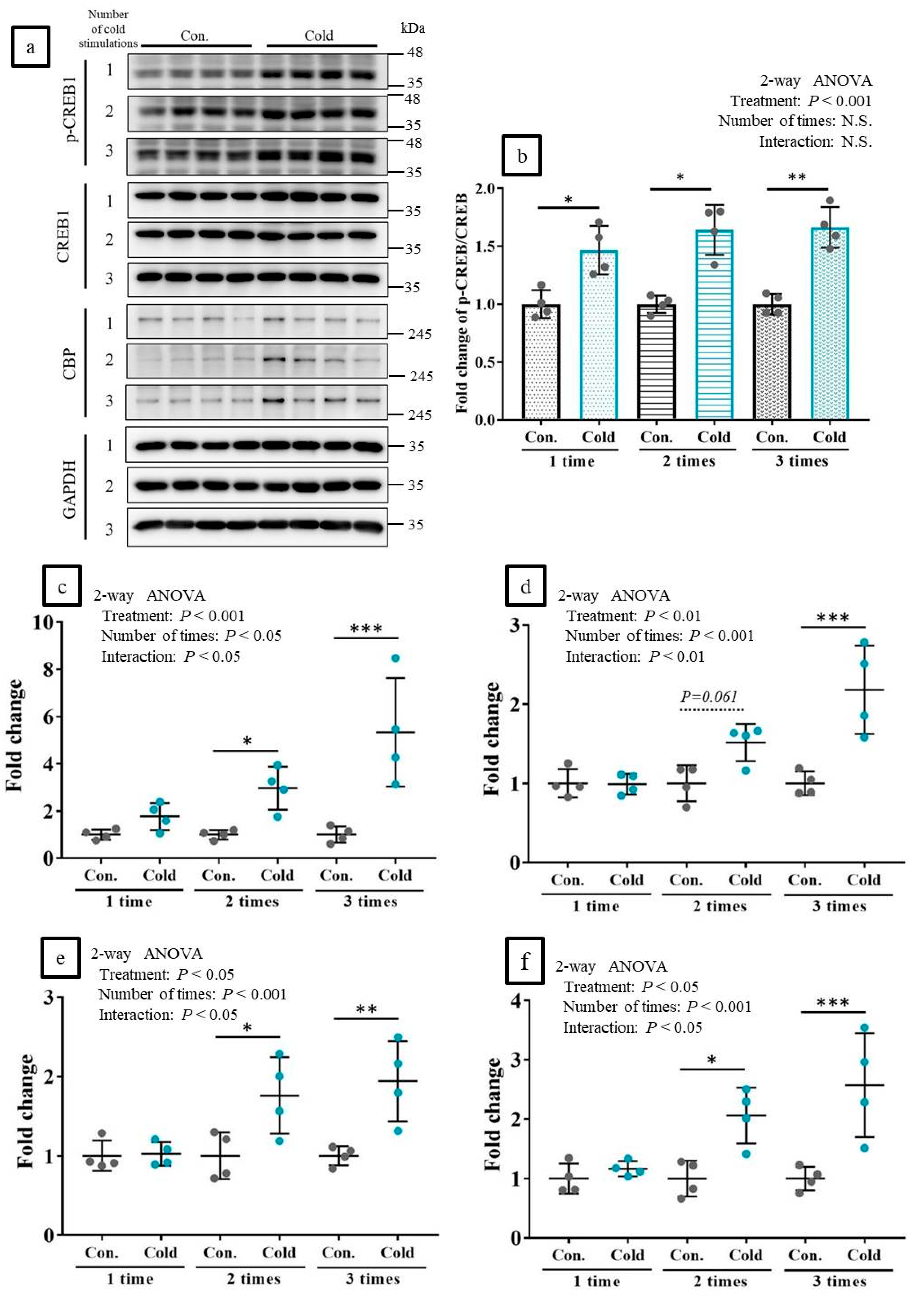
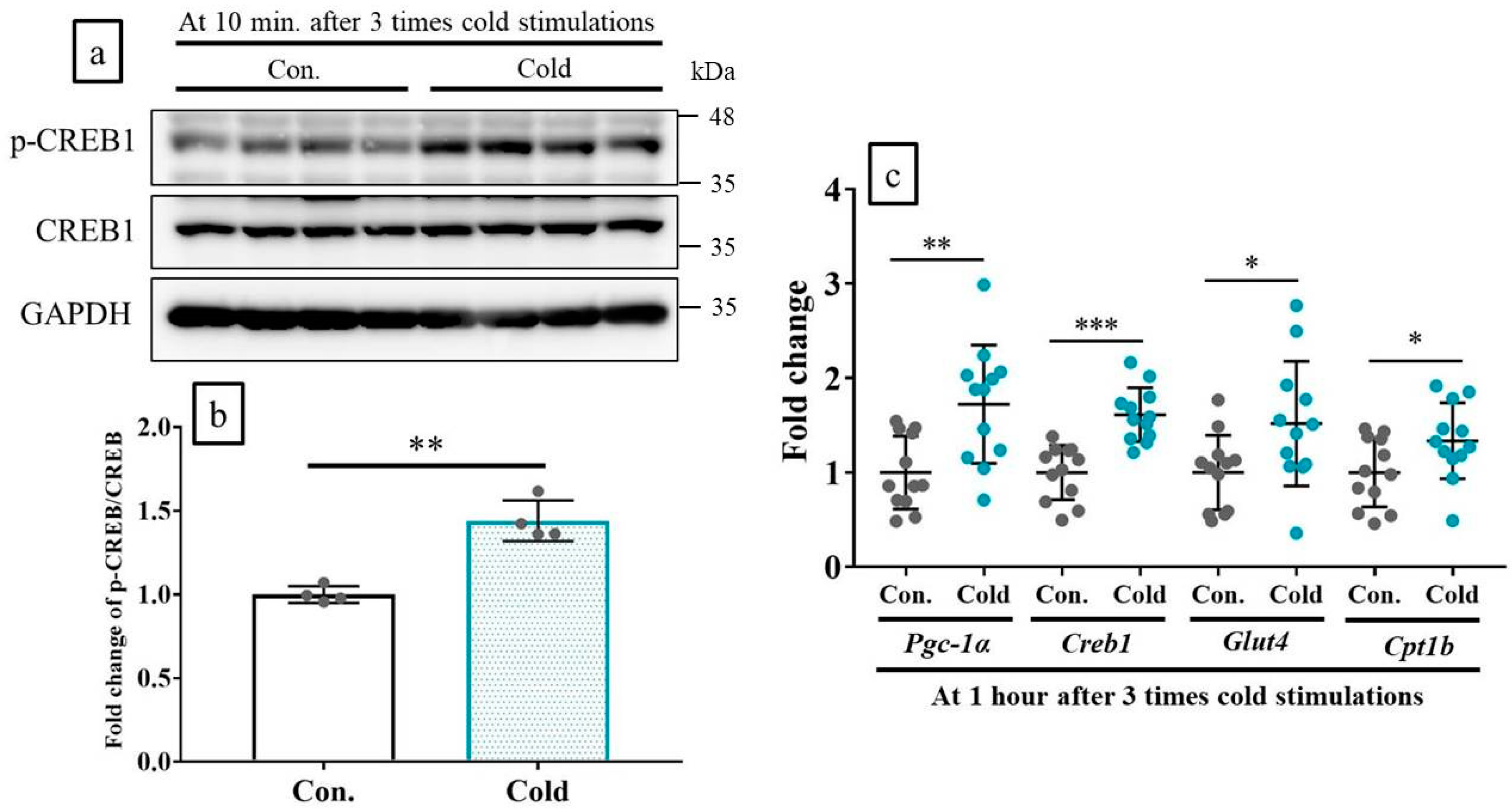
2.5. Cold Stimulations Upregulated the CREB-Targeting Gene and Induced CREB1 Phosphorylation in Skeltal Muscle Ex Vivo
2.6. Cold Stimulations Also Upregulated the CREB-Targeting Gene and Induced CREB1 Phosphorylation on Stkeltal Muscle In Vivo
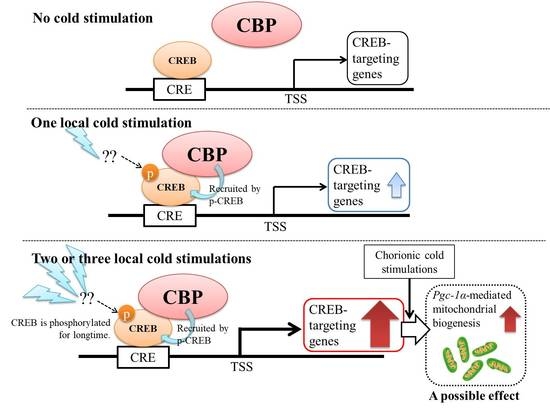
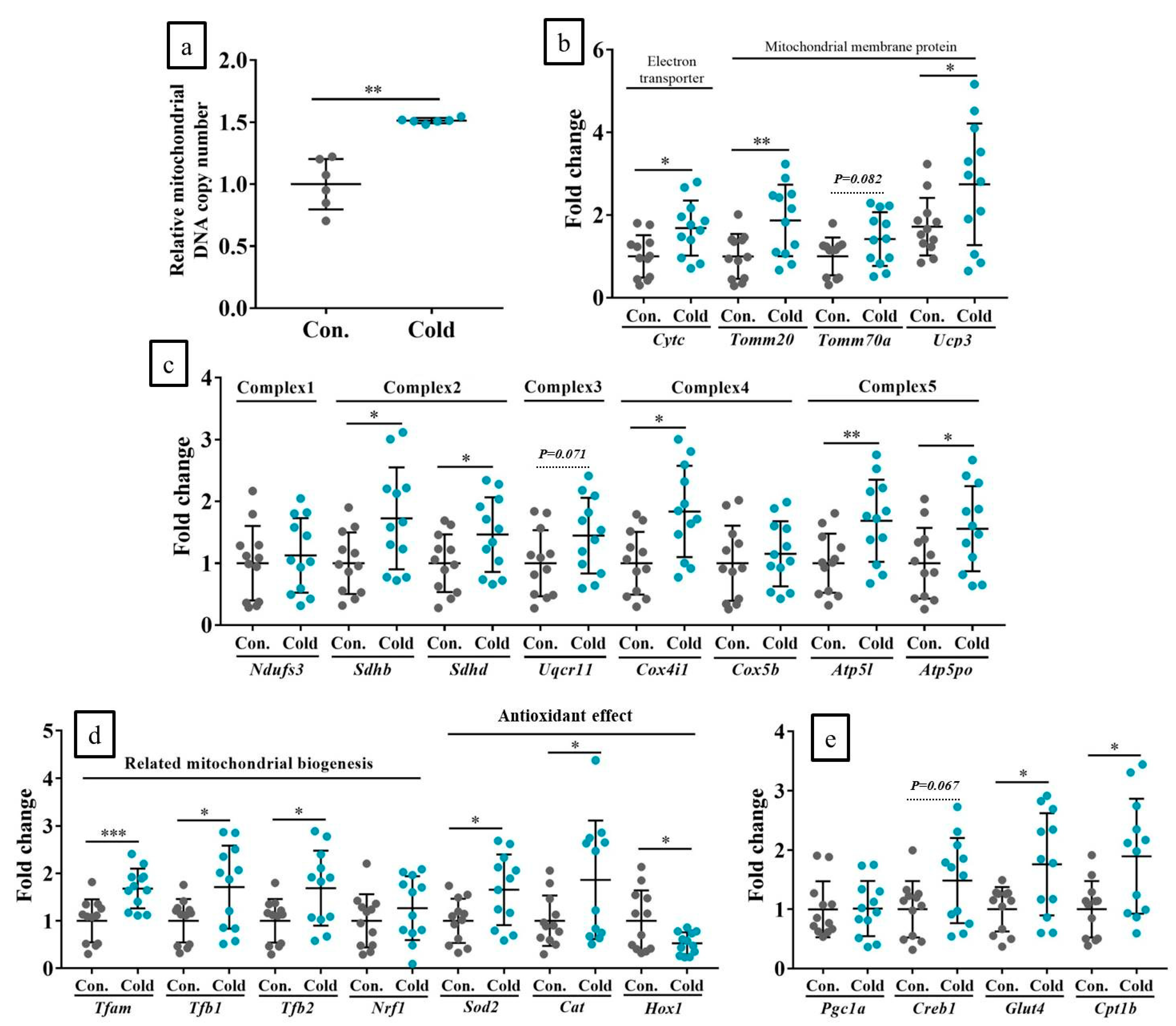
2.7. Chronic Cold Stimulation Increased Mitochondrial DNA Copy Number, Mitochondrial Biogenesis, and Its Component Gene Expression Levels in the Skeletal Muscle, Whereas Acute Cold Stimulation Had No Such Effects
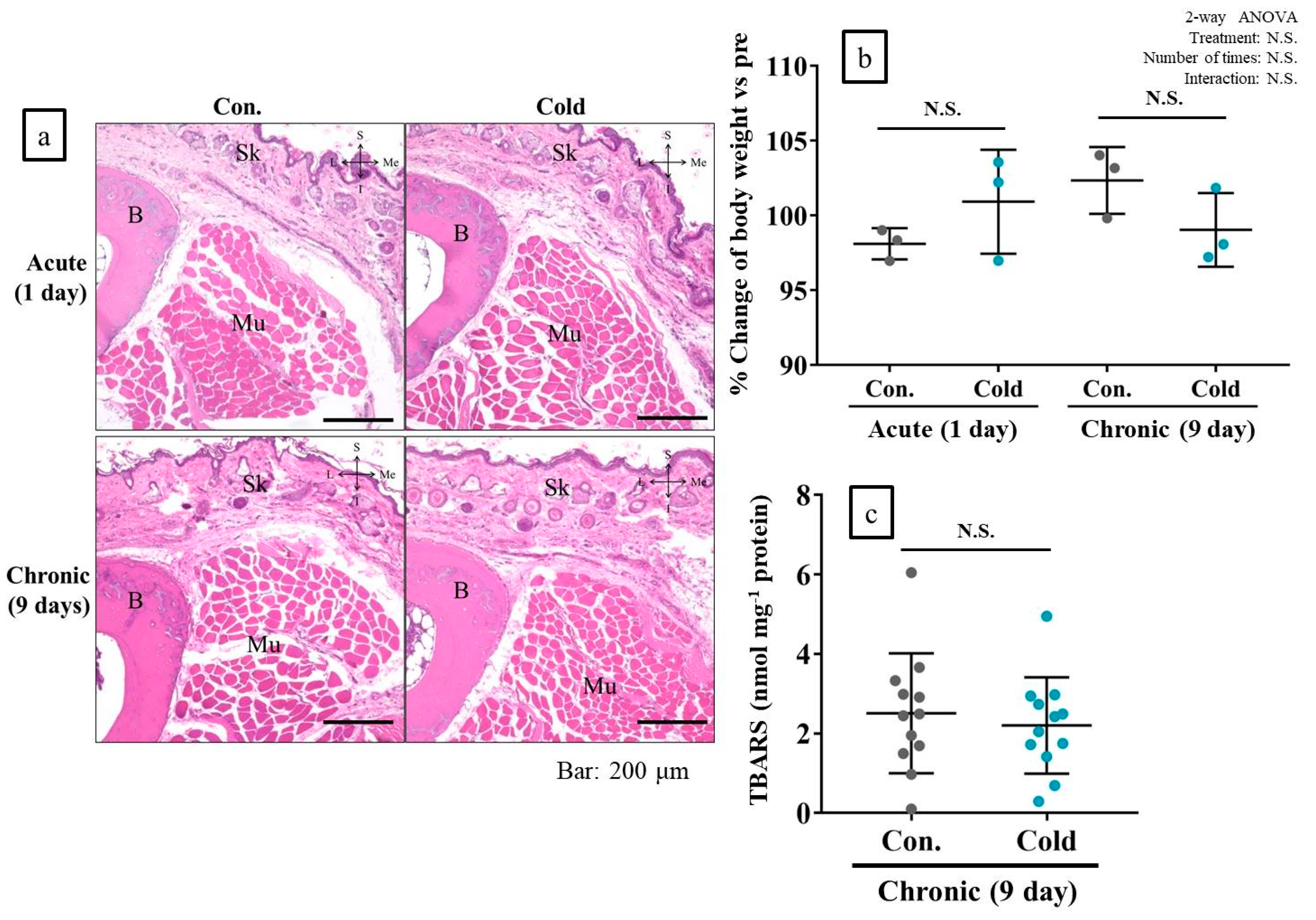
2.8. Cold Stimulation Had No Adverse Effect
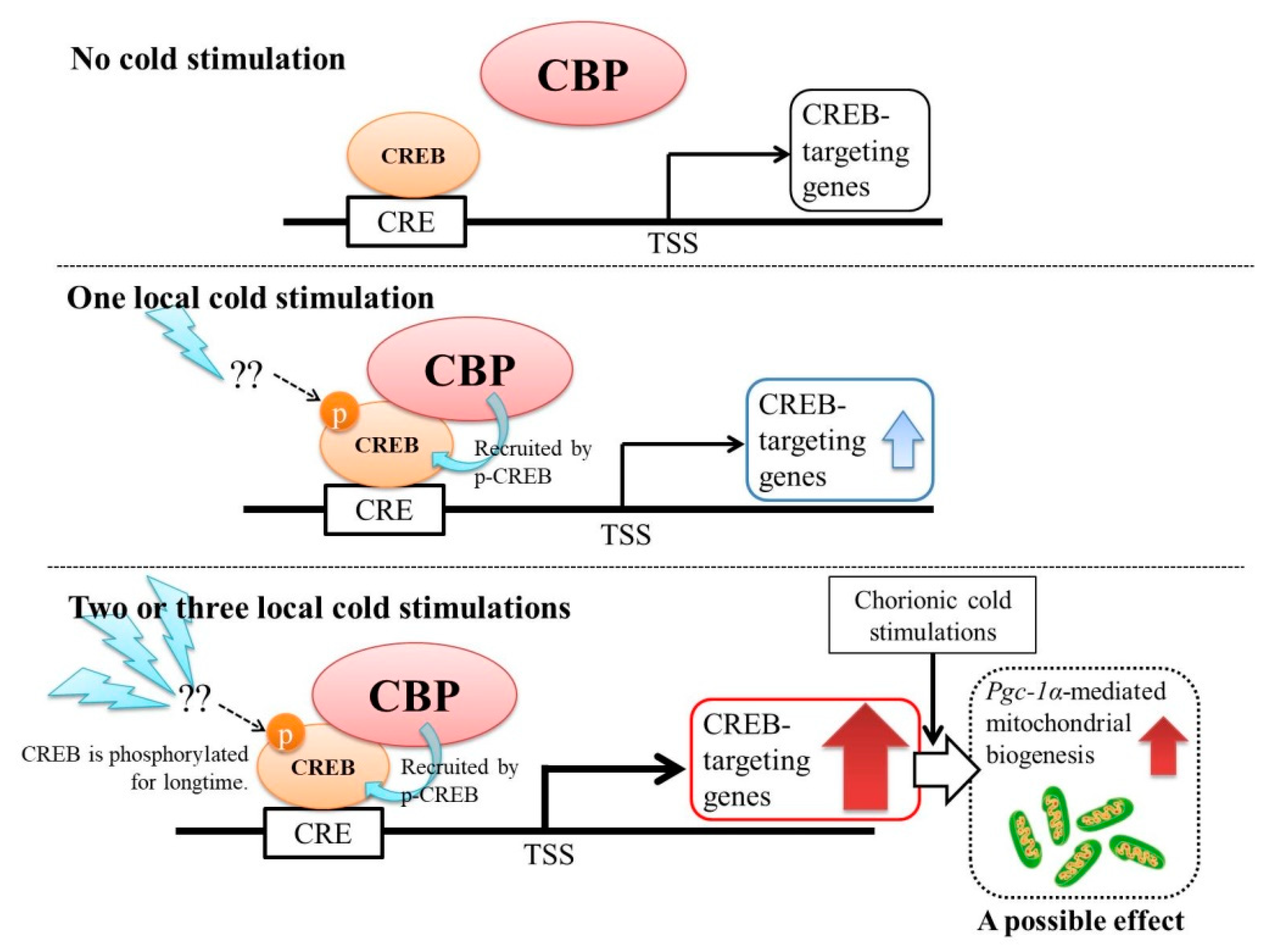
3. Discussion
4. Materials and Methods
4.1. In Vitro Experiments
4.1.1. Cell Lines
4.1.2. Cold Stimulation of Cells
4.1.3. Western Blotting (WB)
4.1.4. Cell Damage Assessment
4.1.5. Immunoprecipitation (IP)
4.1.6. Reporter Assay Using cAMP Response Elements
4.2. Ex Vivo and In Vivo Experiments
4.2.1. Animals
4.2.2. Cold Stimulations for Skeletal Muscle Ex Vivo
4.2.3. Cold Stimulations for In Vivo Experiments
Cold Exposure of Legs
Cold Exposure Experiments to Confirm CREB Phosphorylation and Induced Expressions of CREB-Targeting Genes
Acute and Chronic Cold Exposure for Legs Followed Each Analysis
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Knight, K.L. Historical perspective. In Cryotherapy in Sport Injury Management; Drews, C., Roselund, D., Wentworth, J., Thomas, J., Barker, D., Eds.; Human Kinetics: Champaign, IL, USA, 1995. [Google Scholar]
- Puntel, G.O.; Carvalho, N.R.; Dobrachinski, F.; Salgueiro, A.C.; Puntel, R.L.; Folmer, V.; Barbosa, N.B.; Royes, L.F.; Rocha, J.B.; Doares, F.A. Cryotherapy reduces skeletal muscle damage after ischemia/reperfusion in rats. J. Anat. 2012, 222, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, T.; Tanba, T.; Iwasawa, Y.; Shibuya, T.; Hayakawa, K.; Oshiro, S. The effect of icing on fracture healing in rats. Riryo 2015, 43, 59–65. [Google Scholar]
- Siqueira, A.F.; Vieira, A.; Ramos, G.V.; Marqueti, R.C.; Salvini, T.F.; Puntel, G.O.; Durigan, J.L.Q. Multiple cryotherapy applications attenuate oxidative stress following skeletal muscle injury. Redox Rep. 2016, 22, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Nusair, Y.M. Local application of ice bags did not affect postoperative facial swelling after oral surgery in rabbits. Br. J. Oral Maxillofac. Surg. 2007, 45, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Fujita, N.; Arakawa, T.; Kawada, S.; Ishii, N.; Miki, A. Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats. J. Appl. Physiol. 2011, 110, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; McDonough, S.; MacAuley, D. The use of ice in the treatment of acute soft-tissue injury: A systematic review of randomized controlled trials. Am. J. Sports Med. 2004, 32, 251–261. [Google Scholar] [CrossRef]
- Hubbard, T.J.; Denegar, C.R. Does cryotherapy improve outcomes with soft tissue injury? J. Athl. Train. 2004, 39, 278–279. [Google Scholar]
- Hohenauer, E.; Taeymans, J.; Baeyens, J.P.; Clarys, P.; Clijsen, R. The effect of post-exercise cryotherapy on recovery characteristics: A systematic review and meta-analysis. PLoS. ONE 2015, 10, e0139028. [Google Scholar] [CrossRef]
- Sugasawa, T.; Mukai, N.; Tamura, K.; Tamba, T.; Mori, S.; Miyashiro, Y.; Yamaguchi, M.; Nissato, S.; Ra, S.; Yoshida, Y.; et al. Effects of cold stimulation on mitochondrial activity and VEGF expression in vitro. Int. J. Sports Med. 2016, 37, 766–778. [Google Scholar] [CrossRef]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–990S. [Google Scholar] [CrossRef]
- Wenz, T. Regulation of mitochondrial biogenesis and PGC-1α under cellular stress. Mitochondrion 2013, 13, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Collins, S. Second messenger signaling mechanisms of the brown adipocyte thermogenic program: An integrative perspective. Horm. Mol. Biol. Clin. Investig. 2017, 26, 31. [Google Scholar] [CrossRef] [PubMed]
- Cardinaux, J.R.; Notis, J.C.; Zhang, Q.; Vo, N.; Craig, J.C.; Fass, D.M.; Brennan, R.G.; Goodman, R.H. Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol. Cell. Biol. 2000, 20, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Odom, D.T.; Koo, S.H.; Conkright, M.D.; Canettieri, G.; Best, J.; Chen, H.; Jenner, R.; Herbolsheimer, E.; Jacobsen, E.; et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 4459–4464. [Google Scholar] [CrossRef]
- Wang, F.; Marshall, C.B.; Ikura, M. Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis: Structural and functional versatility in target recognition. Cell. Mol. Life Sci. 2013, 70, 3989–4008. [Google Scholar] [CrossRef]
- Sarver, D.C.; Sugg, K.B.; Disser, N.P.; Enselman, E.R.S.; Awan, T.M.; Mendias, C.L. Local cryotherapy minimally impacts the metabolome and transcriptome of human skeletal muscle. Sci. Rep. 2017, 25, 2423. [Google Scholar] [CrossRef]
- Coutelle, O.; Hornig-Do, H.T.; Witt, A.; Andree, M.; Schiffmann, L.M.; Piekarek, M.; Brinkmann, K.; Seeger, J.M.; Liwschitz, M.; Miwa, S.; et al. Embelin inhibits endothelial mitochondrial respiration and impairs neoangiogenesis during tumor growth and wound healing. EMBO Mol. Med. 2014, 6, 624–639. [Google Scholar] [CrossRef]
- Quiros, M.; Nishio, H.; Neumann, P.A.; Siuda, D.; Brazil, J.C.; Azcutia, V.; Hilgarth, R.; O’Leary, M.N.; Garcia-Hernandez, V.; Leoni, G.; et al. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J. Clin. Investig. 2017, 127, 3510–3520. [Google Scholar] [CrossRef]
- Nishikai-Yan Shen, T.; Kanazawa, S.; Kado, M.; Okada, K.; Luo, L.; Hayashi, A.; Mizuno, H.; Tanaka, R. Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS ONE 2017, 23, e0178232. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- Pizanis, N.; Gillner, S.; Kamler, M.; de Groot, H.; Jakob, H.; Rauen, U. Cold-induced injury to lung epithelial cells can be inhibited by iron chelators—Implications for lung preservation. Eur. J. Cardiothorac. Surg. 2011, 40, 948–955. [Google Scholar] [CrossRef][Green Version]
- Neutelings, T.; Lambert, C.A.; Nusgens, B.V.; Colige, A.C. Effects of mild cold shock (25 °C) followed by warming up at 37 °C on the cellular stress response. PLoS ONE 2013, 8, e69687. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Pilegaard, H.; Litvintsev, A.; Leick, L.; Hood, D.A. Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem. 2006, 42, 13–29. [Google Scholar] [PubMed]
- Oki, S.; Ohta, T.; Shioi, G.; Hatanaka, H.; Ogasawara, O.; Okuda, Y.; Kawaji, H.; Nakaki, R.; Sese, J.; Meno, C. ChIP-Atlas: A data-mining suite powered by full integration of public ChIP-seq data. EMBO Rep. 2018, 19, e46255. [Google Scholar] [CrossRef]
- Obregon, M.J. Adipose tissues and thyroid hormones. Front. Physiol. 2014, 5, 479. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef]
- Hawley, J.A.; Morton, J.P. Ramping up the signal: Promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin. Exp. Pharmacol. Physiol. 2014, 41, 608–613. [Google Scholar] [CrossRef]
- Cherry, A.D.; Suliman, H.B.; Bartz, R.R.; Piantadosi, C.A. Peroxisome proliferator-activated receptor γ co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J. Biol. Chem. 2014, 289, 41–52. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugasawa, T.; Tome, Y.; Takeuchi, Y.; Yoshida, Y.; Yahagi, N.; Sharma, R.; Aita, Y.; Ueda, H.; Maruyama, R.; Takeuchi, K.; et al. Influence of Intermittent Cold Stimulations on CREB and Its Targeting Genes in Muscle: Investigations into Molecular Mechanisms of Local Cryotherapy. Int. J. Mol. Sci. 2020, 21, 4588. https://doi.org/10.3390/ijms21134588
Sugasawa T, Tome Y, Takeuchi Y, Yoshida Y, Yahagi N, Sharma R, Aita Y, Ueda H, Maruyama R, Takeuchi K, et al. Influence of Intermittent Cold Stimulations on CREB and Its Targeting Genes in Muscle: Investigations into Molecular Mechanisms of Local Cryotherapy. International Journal of Molecular Sciences. 2020; 21(13):4588. https://doi.org/10.3390/ijms21134588
Chicago/Turabian StyleSugasawa, Takehito, Yoshiya Tome, Yoshinori Takeuchi, Yasuko Yoshida, Naoya Yahagi, Rahul Sharma, Yuichi Aita, Haruna Ueda, Reina Maruyama, Kaoru Takeuchi, and et al. 2020. "Influence of Intermittent Cold Stimulations on CREB and Its Targeting Genes in Muscle: Investigations into Molecular Mechanisms of Local Cryotherapy" International Journal of Molecular Sciences 21, no. 13: 4588. https://doi.org/10.3390/ijms21134588
APA StyleSugasawa, T., Tome, Y., Takeuchi, Y., Yoshida, Y., Yahagi, N., Sharma, R., Aita, Y., Ueda, H., Maruyama, R., Takeuchi, K., Morita, S., Kawamai, Y., & Takekoshi, K. (2020). Influence of Intermittent Cold Stimulations on CREB and Its Targeting Genes in Muscle: Investigations into Molecular Mechanisms of Local Cryotherapy. International Journal of Molecular Sciences, 21(13), 4588. https://doi.org/10.3390/ijms21134588