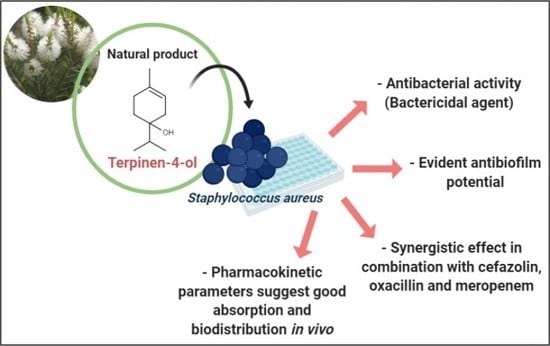
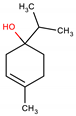
Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Terpinen-4-ol Against S. aureus
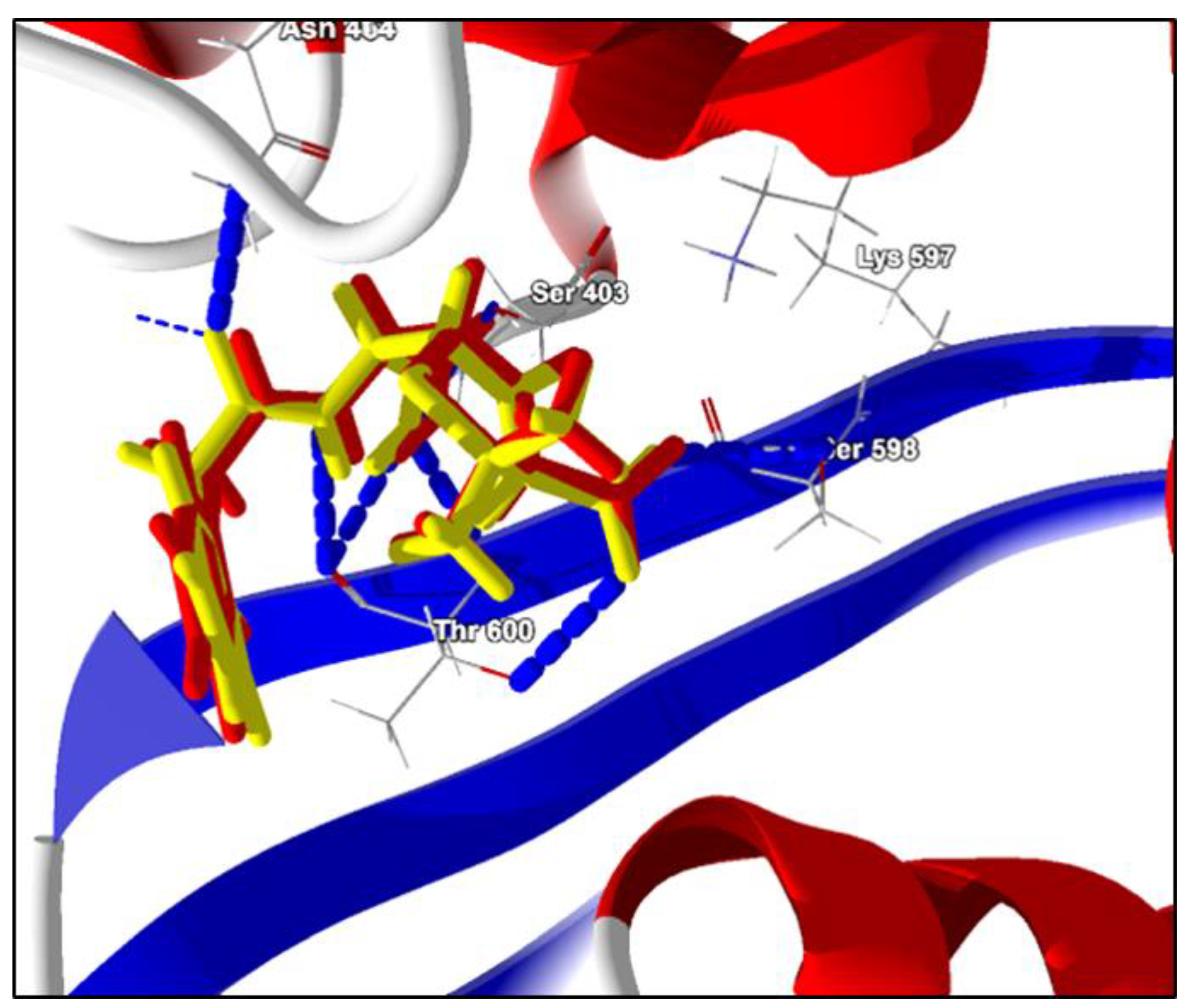
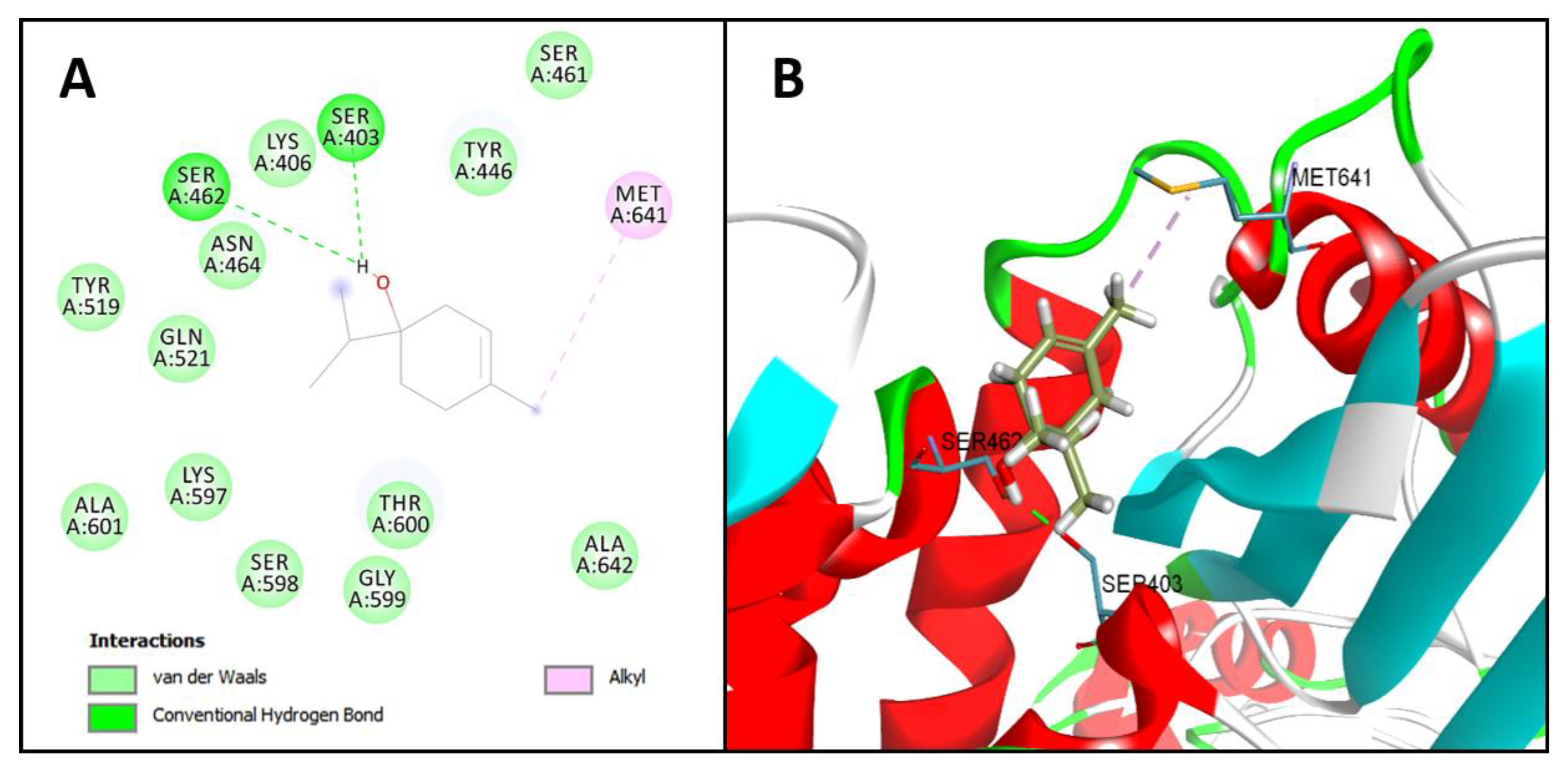
2.2. Molecular Docking Analysis
2.3. Association Test
2.4. Antibiofilm Effect
2.5. In Silico Analysis of Pharmacokinetic Parameters
3. Materials and Methods
3.1. Substances
3.2. Strains
3.3. Minimum Inhibitory Concentration (MIC)
3.4. Minimum Bactericidal Concentration (MBC)
3.5. Molecular Docking
3.6. Association Test
3.7. Antibiofilm Effect
3.8. In Silico Analysis of Pharmacokinetic Parameters
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| BHI | Cerebral Heart Infusion (Broth) |
| CFU | Colony-Forming Units |
| DMSO | Dimethyl Sulfoxide |
| FIC | Fractional Inhibitory Concentration |
| FICI | Fractional Inhibitory Concentration Index |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MVD | Molegro Virtual Docker |
| MW | Molecular Weight |
| PDB | Protein Data Bank |
| RMSD | Root-Mean Standard Deviation |
| TPSA | Topological polar surface area |
References
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Al-Mebairik, N.F.; El-Kersh, T.A.; Al-Sheikh, Y.A.; Marie, M.A.M. A review of virulence factors, pathogenesis, and antibiotic resistance in Staphylococcus aureus. Rev. Med. Microbiol. 2016, 27, 50–56. [Google Scholar] [CrossRef]
- Venkatesh, V. Staphylococcus aureus and MRSA: Do we know the true burden? Clin. Epidemiol. Glob. Health 2018, 6, 103–104. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 4 March 2020).
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, M.R.; Whitchurch, C.B.; Burrows, L.L. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr. Opin. Microbiol. 2018, 45, 164–169. [Google Scholar] [CrossRef]
- Simões, M.; Bennett, R.N.; Rosa, E.A. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—A review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; SharifI-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca genus as antimicrobial agents: From farm to pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Toron, C.C.; Francisconi, R.S.; Magalhães, F.A.C.; Huacho, P.M.M.; Bedran, T.L.; Pratavieira, S.; Spolidorio, L.C.; Spolidorio, D.P. Antimicrobial effects of terpinen-4-ol against oral pathogens and its capacity for the modulation of gene expression. Biofouling 2018, 34, 815–825. [Google Scholar] [CrossRef]
- Oliva, A.; Costantini, S.; Angelis, M.; Garzoli, S.; Božovic, M.; Mascellino, M.T.; Vullo, V.; Ragno, R. High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 2584. [Google Scholar] [CrossRef]
- Brun, P.; Bernabè, G.; Filippini, R.; Piovan, A. In vitro antimicrobial activities of commercially available tea tree (Melaleuca alternifolia) essential oils. Curr. Microbiol. 2019, 76, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Unlu, A.; Sar, T.; Seker, G.; Erman, A.G.; Kalpar, E.; Akbas, M.Y. Biofilm formation by Staphylococcus aureus strains and their control by selected phytochemicals. Int. J. Dairy Technol. 2018, 71, 637–646. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono-and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Determining Bactericidal Activity of Antimicrobial Agents. Approved Guideline M26-A; (1st ed. CLSI document M26-A); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999; pp. 1–29. Available online: https://clsi.org/standards/products/microbiology/documents/m26/ (accessed on 2 May 2020).
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Loughlin, R.; Gilmore, B.F.; McCarron, P.A.; Tunney, M.M. Comparison of the cidal activity of tea tree oil and terpinen-4-ol against clinical bacterial skin isolates and human fibroblast cells. Lett. Appl. Microbiol. 2008, 46, 428–433. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single-and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2012, 56, 909–915. [Google Scholar] [CrossRef]
- Shalaby, M.A.W.; Dokla, E.M.; Serya, R.A.; Abouzid, K.A. Penicillin binding protein 2a: An overview and a medicinal chemistry perspective. Eur. J. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 1–23. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Lim, D.; Strynadka, N.C.J. Structural basis for the β-lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Mol. Biol. 2002, 9, 870–876. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Maquera-Huacho, P.M.; Tonon, C.C.; Correia, M.F.; Francisconi, R.S.; Bordini, E.A.F.; Marcantonio, É.; Spolidorio, D.M.P. In vitro antibacterial and cytotoxic activities of carvacrol and terpinen-4-ol against biofilm formation on titanium implant surfaces. Biofouling 2018, 34, 699–709. [Google Scholar] [CrossRef]
- Maquera-Huacho, P.M.; Rodriguez Herrero, E.; Verspecht, T.; Pauwels, M.; Marcantonio Jr, E.; Palomari Spolidorio, D.M.; Teughels, W. Terpinen-4-ol and carvacrol affect multi-species biofilm composition. Biofouling 2019, 35, 561–572. [Google Scholar] [CrossRef]
- Bose, S.K.; Chauhan, M.; Dhingra, N.; Chhibber, S.; Harjai, K. Terpinen-4-ol attenuates quorum sensing regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Future Microbiol. 2020, 15, 127–142. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio 2012, 3, e00198–e00212. [Google Scholar] [CrossRef]
- Kuehl, R.; Al-Bataineh, S.; Gordon, O.; Luginbuehl, R.; Otto, M.; Textor, M.; Landmann, R. Furanone at subinhibitory concentrations enhances staphylococcal biofilm formation by luxS repression. Antimicrob. Agents Chemother. 2009, 53, 4159–4166. [Google Scholar] [CrossRef]
- Weiser, J.; Henke, H.A.; Hector, N.; Both, A.; Christner, M.; Büttner, H.; Kaplan, J.B.; Rohde, H. Sub-inhibitory tigecycline concentrations induce extracellular matrix binding protein Embp dependent Staphylococcus epidermidis biofilm formation and immune evasion. Int. J. Med. Microbiol. 2016, 306, 471–478. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, Y.; Zhan, Q.; Shang, Y.; Qu, D.; Yu, F. Sub-inhibitory concentrations of mupirocin stimulate Staphylococcus aureus biofilm formation by up-regulating cidA. Antimicrob. Agents Chemother. 2020, 3, e1912–e1919. [Google Scholar]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral. Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Evren, E.; Yurtcu, E. In vitro effects on biofilm viability and antibacterial and antiadherent activities of silymarin. Folia. Microbiol. 2015, 60, 351–356. [Google Scholar] [CrossRef]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.U.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral Bioavailabillity of Drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug absorption using multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In Silico Prediction of Aqueous Solubility Using Simple QSPR Models: The importance of Phenol and Phenol-like moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard M07; (11th ed. Approved Standard M07); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 1–91. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 2 May 2020).
- Silva, D.; Diniz-Neto, H.; Cordeiro, L.; Silva-Neta, M.; Silva, S.; Andrade-Júnior, F.; Leite, M.; Nóbrega, J.; Morais, M.; Souza, J.; et al. (R)-(+)-β-Citronellol and (S)-(−)-β-Citronellol in Combination with Amphotericin B against Candida spp. Int. J. Mol. Sci. 2020, 21, 1785. [Google Scholar] [CrossRef]
- Dewar, M.J. AMI: A New General Purpose Quantum Mechanical Molecular Model. J. Am. Chem. Soc. 1985, 7, 3902–3909. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Li, X.; Tian, Y.; Fan, Y.; Yu, C.; Zhou, B.; Liu, Y.; Xiang, R.; Yang, L. Synergistic effects of antimicrobial peptide DP7 combined with antibiotics against multidrug-resistant bacteria. Drug Des. Dev. Ther. 2017, 11, 939–946. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Merighi, M.; Smith, R.; Narasimhan, G.; Lory, S.; Mathee, K. The Regulatory Repertoire of Pseudomonas aeruginosa AmpC ß-Lactamase Regulator AmpR Includes Virulence Genes. PLoS ONE 2012, 7, e34067. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Ramesh, S.; Satish, A.S.; Lee, J. Antibiofilm and Anti-β-Lactamase Activities of Burdock Root Extract and Chlorogenic Acid against Klebsiella pneumoniae. J. Microbiol. Biotechnol. 2017, 27, 542–551. [Google Scholar] [CrossRef]


| S. aureus | Terpinen-4-ol | |||
|---|---|---|---|---|
| MIC | MBC | MIC/MBC | Effect | |
| ATCC-25923 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| ATCC-13150 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-02 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-40 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-45 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-116 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-222 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-232 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-297 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| LM-314 | 0.25% (v/v) | 0.5% (v/v) | 1:2 | Bactericidal |
| Moldock Score | Targeting Residues | |
|---|---|---|
 Open Form, Penicillin G (PDB ligand) | −125.7 kcal/mol | Hydrogen bonds: Ser403, Thr600, Lys597, Ser598, Asn464 e Ser462 Hydrophobic interactions: Tyr446 Van Der Walls: Lys406, Lys597, Gly599, Ala601, Ala642 e Met641. |
 Terpinen-4-ol | −54.8 kcal/mol | Hydrogen bond: Ser403 e Ser462. Hydrophobic interactions: Met641. Van Der Walls: Lys406, Asn464, Tyr446, Gln521, Thr600, Ser461, Lys406, Tyr519, Lys597, Ala601, Ser598, Gly599, Ala642 |
| Strains and Drugs | FICI | Effect 1 |
|---|---|---|
| ATCC-25923 | ||
| Gentamicin | 1.06 | Indifference |
| Cefazolin | 0.50 | Synergism |
| Vancomycin | 1.75 | Indifference |
| Oxacillin | 0.32 | Synergism |
| Meropenem | 0.50 | Synergism |
| ATCC-13150 | ||
| Gentamicin | 1.12 | Indifference |
| Cefazolin | 0.50 | Synergism |
| Vancomycin | 1.25 | Indifference |
| Oxacillin | 0.32 | Synergism |
| Meropenem | 0.50 | Synergism |
| LM-222 | ||
| Gentamicin | 1.06 | Indifference |
| Cefazolin | 0.32 | Synergism |
| Vancomycin | 1.25 | Indifference |
| Oxacillin | 0.32 | Synergism |
| Meropenem | 0.50 | Synergism |
| LM-314 | ||
| Gentamicin | 1.00 | Indifference |
| Cefazolin | 0.32 | Synergism |
| Vancomycin | 1.25 | Indifference |
| Oxacillin | 0.32 | Synergism |
| Meropenem | 0.50 | Synergism |
| Parameters | Terpinen-4-ol |
|---|---|
| Physicochemical Properties | |
| Formula | C10H18O |
| Molecular Weight | 154.25 g/mol |
| Num. Heavy atoms | 11 |
| Fraction Csp3 | 0.80 |
| Num. Rotatable Bonds | 1 |
| Num. H-bonds acceptors | 1 |
| Num. H-bonds donors | 1 |
| Molar Refractivity | 48.80 |
| TPSA 1 | 20.23 Å 2 |
| Lipophilicity | |
| Consensus 2 Log Po/w 3 | 2.60 |
| Water Solubility | |
| Log S (Ali) | −2.78 |
| Class 4 | Soluble |
| Druglikeness | |
| Lipinski 5 | Yes; 0 violation |
| Ghose 6 | No; 1 violation: MW < 160 |
| Veber 7 | Yes; 0 violation |
| Egan 8 | Yes; 0 violation |
| Bioavailability Score | 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. https://doi.org/10.3390/ijms21124531
Cordeiro L, Figueiredo P, Souza H, Sousa A, Andrade-Júnior F, Medeiros D, Nóbrega J, Silva D, Martins E, Barbosa-Filho J, et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. International Journal of Molecular Sciences. 2020; 21(12):4531. https://doi.org/10.3390/ijms21124531
Chicago/Turabian StyleCordeiro, Laísa, Pedro Figueiredo, Helivaldo Souza, Aleson Sousa, Francisco Andrade-Júnior, Daianne Medeiros, Jefferson Nóbrega, Daniele Silva, Evandro Martins, José Barbosa-Filho, and et al. 2020. "Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus" International Journal of Molecular Sciences 21, no. 12: 4531. https://doi.org/10.3390/ijms21124531
APA StyleCordeiro, L., Figueiredo, P., Souza, H., Sousa, A., Andrade-Júnior, F., Medeiros, D., Nóbrega, J., Silva, D., Martins, E., Barbosa-Filho, J., & Lima, E. (2020). Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. International Journal of Molecular Sciences, 21(12), 4531. https://doi.org/10.3390/ijms21124531