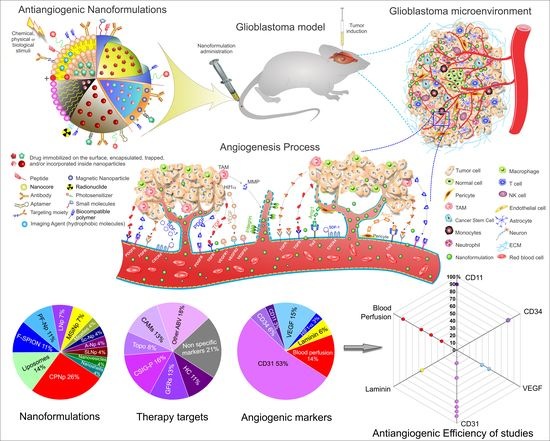
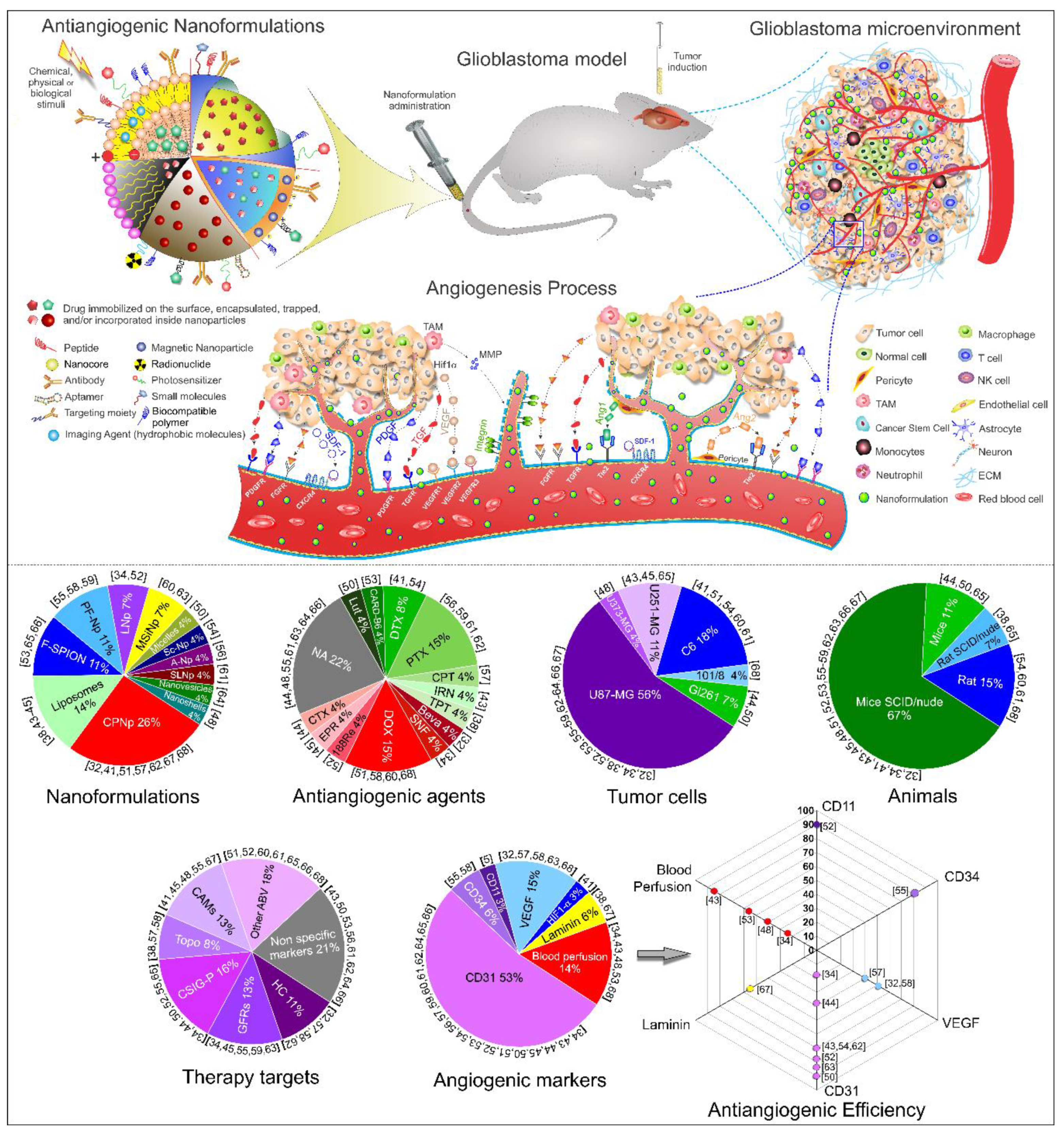
Antiangiogenic Targets for Glioblastoma Therapy from a Pre-Clinical Approach, Using Nanoformulations
Abstract
1. Introduction
2. Results
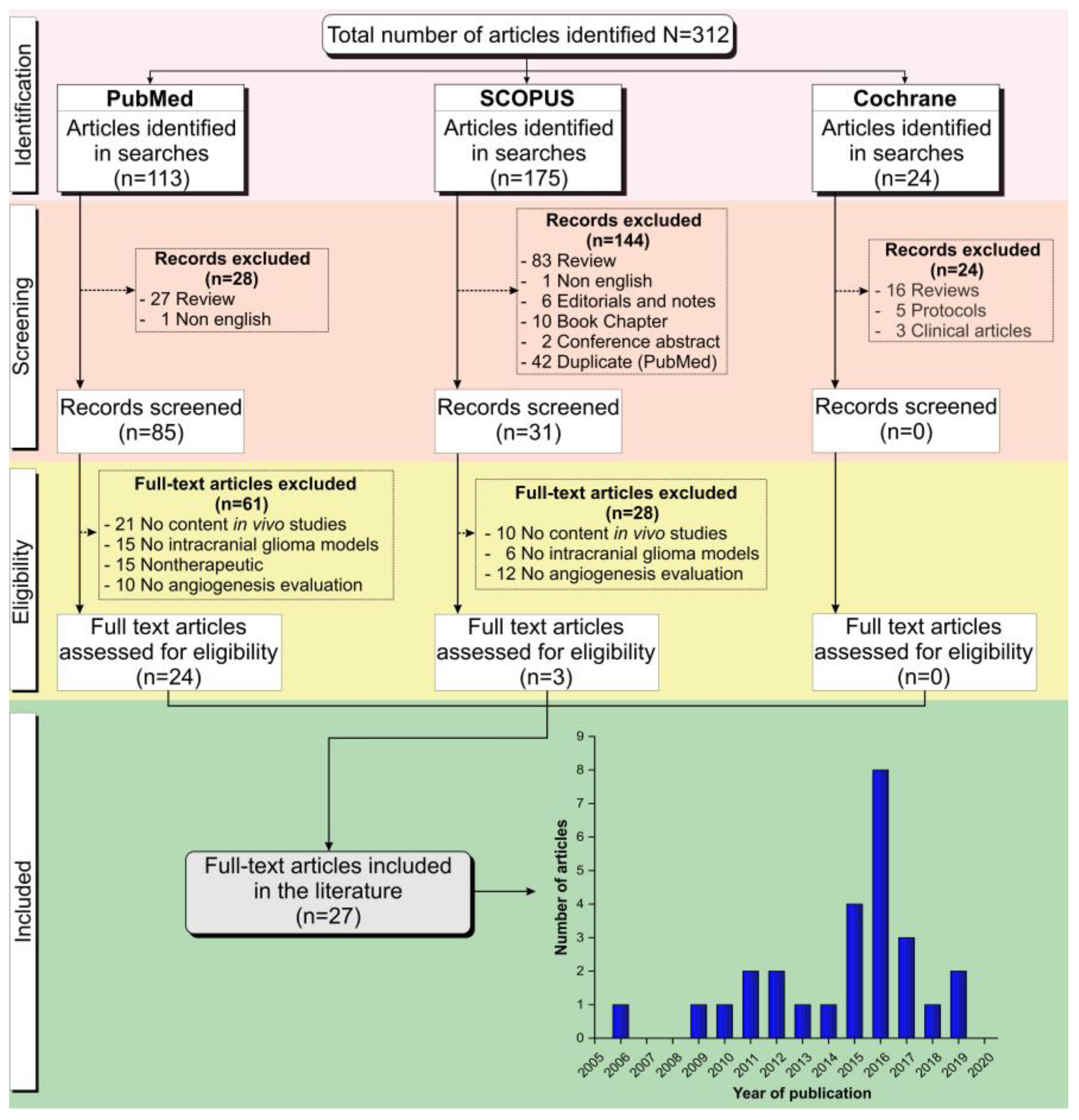
2.1. Study Selection
2.2. Cell Characteristics
2.3. Tumoral Induction
2.4. Nanoparticles Used in Studies of Angiogenesis
2.5. Antiangiogenic Therapeutic Process for Glioblastoma
2.6. Angiogenic Effects Evaluation
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion Criteria
4.3. Exclusion Criteria
4.4. Data Extraction, Data Collection, and Risk of Bias Assessment
4.5. Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, J.S.; Ko, K.W.; Kong, D.S.; Kang, C.M.; Kim, M.H.; Son, M.J.; Song, H.S.; Shin, H.J.; Lee, D.S.; et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol. Rep. 2006, 16, 33–39. [Google Scholar] [CrossRef][Green Version]
- Braun, K.; Ahluwalia, M.S. Treatment of Glioblastoma in Older Adults. Curr. Oncol. Rep. 2017, 19, 81. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, L.; Lv, S.; Li, Q.; Chen, G.; Luo, W.; Zhou, P.; Li, G. Efficacy of moderately hypofractionated simultaneous integrated boost intensity-modulated radiotherapy combined with temozolomide for the postoperative treatment of glioblastoma multiforme: A single-institution experience. Radiat. Oncol. (London, England) 2019, 14, 104. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Collins, V.P. Pathology and molecular genetics of astrocytic gliomas. J. Mol. Med. (Berl.) 2004, 82, 656–670. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivee, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef]
- Keller, S.; Schmidt, M.H.H. EGFR and EGFRvIII Promote Angiogenesis and Cell Invasion in Glioblastoma: Combination Therapies for an Effective Treatment. Int. J. Mol. Sci. 2017, 18, 1295. [Google Scholar] [CrossRef]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers (Basel) 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.M.; Alexandru, O.; Brindusa, C.; Purcaru, S.O.; Tache, D.E.; Tataranu, L.G.; Taisescu, C.; Dricu, A. Targeting the VEGF and PDGF signaling pathway in glioblastoma treatment. Int. J. Clin. Exp. Pathol. 2015, 8, 7825–7837. [Google Scholar] [PubMed]
- Camorani, S.; Esposito, C.L.; Rienzo, A.; Catuogno, S.; Iaboni, M.; Condorelli, G.; de Franciscis, V.; Cerchia, L. Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRbeta aptamer. Mol. Ther. 2014, 22, 828–841. [Google Scholar] [CrossRef]
- Cenciarelli, C.; Marei, H.E.; Felsani, A.; Casalbore, P.; Sica, G.; Puglisi, M.A.; Cameron, A.J.; Olivi, A.; Mangiola, A. PDGFRalpha depletion attenuates glioblastoma stem cells features by modulation of STAT3, RB1 and multiple oncogenic signals. Oncotarget 2016, 7, 53047–53063. [Google Scholar] [CrossRef]
- Tamura, R.; Morimoto, Y.; Kosugi, K.; Sato, M.; Oishi, Y.; Ueda, R.; Kikuchi, R.; Nagashima, H.; Hikichi, T.; Noji, S.; et al. Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma—A case series. BMC Cancer 2020, 20, 196. [Google Scholar] [CrossRef] [PubMed]
- Eskilsson, E.; Rosland, G.V.; Talasila, K.M.; Knappskog, S.; Keunen, O.; Sottoriva, A.; Foerster, S.; Solecki, G.; Taxt, T.; Jirik, R.; et al. EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro-Oncology 2016, 18, 1644–1655. [Google Scholar] [CrossRef]
- Li, G.; Wong, A.J. EGF receptor variant III as a target antigen for tumor immunotherapy. Expert Rev. Vaccines 2008, 7, 977–985. [Google Scholar] [CrossRef]
- Cummings, C.T.; Deryckere, D.; Earp, H.S.; Graham, D.K. Molecular pathways: MERTK signaling in cancer. Clin. Cancer Res. 2013, 19, 5275–5280. [Google Scholar] [CrossRef]
- Pierce, A.M.; Keating, A.K. TAM receptor tyrosine kinases: Expression, disease and oncogenesis in the central nervous system. Brain Res. 2014, 1542, 206–220. [Google Scholar] [CrossRef]
- Miller, M.A.; Sullivan, R.J.; Lauffenburger, D.A. Molecular Pathways: Receptor Ectodomain Shedding in Treatment, Resistance, and Monitoring of Cancer. Clin. Cancer Res. 2017, 23, 623–629. [Google Scholar] [CrossRef]
- Morisse, M.C.; Jouannet, S.; Dominguez-Villar, M.; Sanson, M.; Idbaih, A. Interactions between tumor-associated macrophages and tumor cells in glioblastoma: Unraveling promising targeted therapies. Expert Rev. Neurother. 2018, 18, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Thomas, R.; Nagpal, S.; Recht, L. Macrophage exclusion after radiation therapy (MERT): A new and effective way to increase the therapeutic ratio of radiotherapy. Radiother. Oncol. 2020, 144, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; An, T.; Liu, P.; Zhu, J.; Yang, H.; Zhang, W.; Dong, T.; Jiang, J.; Zhang, Y.; et al. Nuciferine inhibits the progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Chen, N.F.; Lin, P.Y.; Su, J.H.; Chen, B.H.; Kuo, H.M.; Sung, C.S.; Sung, P.J.; Wen, Z.H.; Chen, W.F. Anti-Invasion and Antiangiogenic Effects of Stellettin B through Inhibition of the Akt/Girdin Signaling Pathway and VEGF in Glioblastoma Cells. Cancers (Basel) 2019, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, W.N.A.; Yusoff, A.A.M.; Abdullah, J.M.; Osman, Z.F.; Ahmad, F. Roles of EphA2 Receptor in Angiogenesis Signaling Pathway of Glioblastoma Multiforme. Malays. J. Med. Sci. 2018, 25, 22–27. [Google Scholar] [CrossRef]
- Rajesh, Y.; Banerjee, A.; Pal, I.; Biswas, A.; Das, S.; Dey, K.K.; Kapoor, N.; Ghosh, A.K.; Mitra, P.; Mandal, M. Delineation of crosstalk between HSP27 and MMP-2/MMP-9: A synergistic therapeutic avenue for glioblastoma management. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1196–1209. [Google Scholar] [CrossRef]
- Cetin, A.; Biltekin, B. Combining Ellagic Acid with Temozolomide Mediates the Cadherin Switch and Angiogenesis in a Glioblastoma Model. World Neurosurg. 2019, 132, e178–e184. [Google Scholar] [CrossRef]
- Scaringi, C.; Minniti, G.; Caporello, P.; Enrici, R.M. Integrin inhibitor cilengitide for the treatment of glioblastoma: A brief overview of current clinical results. Anticancer Res. 2012, 32, 4213–4223. [Google Scholar]
- Zhang, M.; Ye, G.; Li, J.; Wang, Y. Recent advance in molecular angiogenesis in glioblastoma: The challenge and hope for anti-angiogenic therapy. Brain Tumor Pathol. 2015, 32, 229–236. [Google Scholar] [CrossRef]
- Paolillo, M.; Serra, M.; Schinelli, S. Integrins in glioblastoma: Still an attractive target? Pharmacol. Res. 2016, 113, 55–61. [Google Scholar] [CrossRef]
- Malric, L.; Monferran, S.; Gilhodes, J.; Boyrie, S.; Dahan, P.; Skuli, N.; Sesen, J.; Filleron, T.; Kowalski-Chauvel, A.; Cohen-Jonathan Moyal, E.; et al. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: An update. Oncotarget 2017, 8, 86947–86968. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Moura, R.P.; Moreira, E.; Martins, C.; Sarmento, B. Therapeutic Monoclonal Antibodies Delivery for the Glioblastoma Treatment. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 112, pp. 61–80. [Google Scholar]
- Clavreul, A.; Roger, E.; Pourbaghi-Masouleh, M.; Lemaire, L.; Tetaud, C.; Menei, P. Development and characterization of sorafenib-loaded lipid nanocapsules for the treatment of glioblastoma. Drug Deliv. 2018, 25, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Dancy, J.G.; Hersh, D.S.; Anastasiadis, P.; Tran, N.L.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Tumor-targeted nanotherapeutics: Overcoming treatment barriers for glioblastoma. WIREs Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Nano-therapies for glioblastoma treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef]
- Saito, R.; Krauze, M.T.; Noble, C.O.; Drummond, D.C.; Kirpotin, D.B.; Berger, M.S.; Park, J.W.; Bankiewicz, K.S. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro-Oncology 2006, 8, 205–214. [Google Scholar] [CrossRef]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Berger, M.S.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Greer, K.; Herndon, J.E., 2nd; Kunwar, S.; et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro-Oncology 2008, 10, 320–329. [Google Scholar] [CrossRef]
- Clavreul, A.; Pourbaghi-Masouleh, M.; Roger, E.; Menei, P. Nanocarriers and nonviral methods for delivering antiangiogenic factors for glioblastoma therapy: The story so far. Int. J. Nanomed. 2019, 14, 2497–2513. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.; Cao, S.; Xiong, Y.; Zhang, S.; Pang, Z.; Jiang, X. Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment. Biomaterials 2014, 35, 2374–2382. [Google Scholar] [CrossRef]
- Kurrikoff, K.; Aphkhazava, D.; Langel, Ü. The future of peptides in cancer treatment. Curr. Opin. Pharmacol. 2019, 47, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Verreault, M.; Strutt, D.; Masin, D.; Anantha, M.; Yung, A.; Kozlowski, P.; Waterhouse, D.; Bally, M.B.; Yapp, D.T. Vascular normalization in orthotopic glioblastoma following intravenous treatment with lipid-based nanoparticulate formulations of irinotecan (Irinophore C), doxorubicin (Caelyx(R)) or vincristine. BMC Cancer 2011, 11, 124. [Google Scholar] [CrossRef]
- Costa, P.M.; Cardoso, A.L.; Custodia, C.; Cunha, P.; Pereira de Almeida, L.; Pedroso de Lima, M.C. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. J. Control. Release 2015, 207, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Zhao, W.Y.; Liu, L.; Ju, R.J.; Mu, L.M.; Zhao, Y.; Zeng, F.; Xie, H.J.; Yan, Y.; Lu, W.L. A nanostructure of functional targeting epirubicin liposomes dually modified with aminophenyl glucose and cyclic pentapeptide used for brain glioblastoma treatment. Oncotarget 2015, 6, 32681–32700. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Ryan, G.M.; Leung, A.W.Y.; Firmino, N.S.; Bennewith, K.L.; Bally, M.B. Liposomal formulations to modulate the tumour microenvironment and antitumour immune response. Int. J. Mol. Sci. 2018, 19, 2922. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Lee, R.J. Tumour-selective drug delivery via folate receptor-targeted liposomes. Expert Opin. Drug Deliv. 2004, 1, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Day, E.S.; Zhang, L.; Thompson, P.A.; Zawaski, J.A.; Kaffes, C.C.; Gaber, M.W.; Blaney, S.M.; West, J.L. Vascular-targeted photothermal therapy of an orthotopic murine glioma model. Nanomedicine 2012, 7, 1133–1148. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.; Chen, X.; Liu, J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int. J. Nanomed. 2019, 14, 7515–7531. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Liu, Y.; Huang, L.; Kong, C.; Qu, X.; Wang, M.; Gao, R.; Qin, H. Application of dual targeting drug delivery system for the improvement of anti-glioma efficacy of doxorubicin. Oncotarget 2017, 8, 58823–58834. [Google Scholar] [CrossRef]
- Séhédic, D.; Chourpa, I.; Tétaud, C.; Griveau, A.; Loussouarn, C.; Avril, S.; Legendre, C.; Lepareur, N.; Wion, D.; Hindré, F.; et al. Locoregional confinement and major clinical benefit of 188re-loaded CXCR4-targeted nanocarriers in an orthotopic human to mouse model of glioblastoma. Theranostics 2017, 7, 4517–4536. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Shi, Y.; Li, Y.; Xiao, Z.; Zhang, X. Traceable Nanoparticles with Spatiotemporally Controlled Release Ability for Synergistic Glioblastoma Multiforme Treatment. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Xu, H.L.; Mao, K.L.; Lu, C.T.; Fan, Z.L.; Yang, J.J.; Xu, J.; Chen, P.P.; ZhuGe, D.L.; Shen, B.X.; Jin, B.H.; et al. An injectable acellular matrix scaffold with absorbable permeable nanoparticles improves the therapeutic effects of docetaxel on glioblastoma. Biomaterials 2016, 107, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Zhang, Y.; Huang, M.; Zhou, Z.; Luo, W.; Tang, J.; Wang, J.; Xiao, Q.; Chen, H.; et al. Dual-Targeting Heparin-Based Nanoparticles that Re-Assemble in Blood for Glioma Therapy through Both Anti-Proliferation and Anti-Angiogenesis. Adv. Funct. Mater. 2016, 26, 7873–7885. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Lin, C.J.; Lin, Y.L.; Luh, F.; Yen, Y.; Chen, R.M. Preclinical effects of CRLX101, an investigational camptothecin-containing nanoparticle drug conjugate, on treating glioblastoma multiforme via apoptosis and antiangiogenesis. Oncotarget 2016, 7, 42408–42421. [Google Scholar] [CrossRef]
- Kuang, Y.; Jiang, X.; Zhang, Y.; Lu, Y.; Ma, H.; Guo, Y.; Zhang, Y.; An, S.; Li, J.; Liu, L.; et al. Dual Functional Peptide-Driven Nanoparticles for Highly Efficient Glioma-Targeting and Drug Codelivery. Mol. Pharm. 2016, 13, 1599–1607. [Google Scholar] [CrossRef]
- Hu, Q.; Kang, T.; Feng, J.; Zhu, Q.; Jiang, T.; Yao, J.; Jiang, X.; Chen, J. Tumor Microenvironment and Angiogenic Blood Vessels Dual-Targeting for Enhanced Anti-Glioma Therapy. ACS Appl. Mater. Interfaces 2016, 8, 23568–23579. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Wen, Z.; Tan, Y.; Huang, N.; Cheng, S.; Zheng, H.; Cheng, Y. Asn-Gly-Arg-modified polydopamine-coated nanoparticles for dual-targeting therapy of brain glioma in rats. Oncotarget 2016, 7, 73681–73696. [Google Scholar] [CrossRef]
- Banerjee, I.; De, K.; Mukherjee, D.; Dey, G.; Chattopadhyay, S.; Mukherjee, M.; Mandal, M.; Bandyopadhyay, A.K.; Gupta, A.; Ganguly, S.; et al. Paclitaxel-loaded solid lipid nanoparticles modified with Tyr-3-octreotide for enhanced anti-angiogenic and anti-glioma therapy. Acta Biomater. 2016, 38, 69–81. [Google Scholar] [CrossRef]
- Feng, X.; Gao, X.; Kang, T.; Jiang, D.; Yao, J.; Jing, Y.; Song, Q.; Jiang, X.; Liang, J.; Chen, J. Mammary-Derived Growth Inhibitor Targeting Peptide-Modified PEG-PLA Nanoparticles for Enhanced Targeted Glioblastoma Therapy. Bioconjug. Chem. 2015, 26, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Bechet, D.; Auger, F.; Couleaud, P.; Marty, E.; Ravasi, L.; Durieux, N.; Bonnet, C.; Plenat, F.; Frochot, C.; Mordon, S.; et al. Multifunctional ultrasmall nanoplatforms for vascular-targeted interstitial photodynamic therapy of brain tumors guided by real-time MRI. Nanomedicine 2015, 11, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Wojton, J.; Chu, Z.; Mathsyaraja, H.; Meisen, W.H.; Denton, N.; Kwon, C.H.; Chow, L.M.; Palascak, M.; Franco, R.; Bourdeau, T.; et al. Systemic delivery of SapC-DOPS has antiangiogenic and antitumor effects against glioblastoma. Mol. Ther. 2013, 21, 1517–1525. [Google Scholar] [CrossRef]
- Janic, B.; Jafari-Khouzani, K.; Babajani-Feremi, A.; Iskander, A.S.M.; Varma, N.R.S.; Ali, M.M.; Knight, R.A.; Arbab, A.S. MRI tracking of FePro labeled fresh and cryopreserved long term in vitro expanded human cord blood AC133+ endothelial progenitor cells in rat glioma. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Agemy, L.; Friedmann-Morvinski, D.; Kotamraju, V.R.; Roth, L.; Sugahara, K.N.; Girard, O.M.; Mattrey, R.F.; Verma, I.M.; Ruoslahti, E. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17450–17455. [Google Scholar] [CrossRef]
- Ding, H.; Inoue, S.; Ljubimov, A.V.; Patil, R.; Portilla-Arias, J.; Hu, J.; Konda, B.; Wawrowsky, K.A.; Fujita, M.; Karabalin, N.; et al. Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release [corrected]. Proc. Natl. Acad. Sci. USA 2010, 107, 18143–18148. [Google Scholar] [CrossRef] [PubMed]
- Hekmatara, T.; Bernreuther, C.; Khalansky, A.S.; Theisen, A.; Weissenberger, J.; Matschke, J.; Gelperina, S.; Kreuter, J.; Glatzel, M. Efficient systemic therapy of rat glioblastoma by nanoparticle-bound doxorubicin is due to antiangiogenic effects. Clin. Neuropathol. 2009, 28, 153–164. [Google Scholar] [CrossRef]
- Arbab, A.S.; Janic, B.; Knight, R.A.; Anderson, S.A.; Pawelczyk, E.; Rad, A.M.; Read, E.J.; Pandit, S.D.; Frank, J.A. Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings. FASEB J. 2008, 22, 3234–3246. [Google Scholar] [CrossRef][Green Version]
- Lenting, K.; Verhaak, R.; Ter Laan, M.; Wesseling, P.; Leenders, W. Glioma: Experimental models and reality. Acta Neuropathol. 2017, 133, 263–282. [Google Scholar] [CrossRef]
- Barth, R.F.; Kaur, B. Rat brain tumor models in experimental neuro-oncology: The C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J. Neurooncol. 2009, 94, 299–312. [Google Scholar] [CrossRef]
- Rego, G.N.A.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; Faria, D.P.; Espinha, P.L.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hon, G.C.; Villa, G.R.; Turner, K.M.; Ikegami, S.; Yang, H.; Ye, Z.; Li, B.; Kuan, S.; Lee, A.Y.; et al. EGFR Mutation Promotes Glioblastoma through Epigenome and Transcription Factor Network Remodeling. Mol. Cell 2015, 60, 307–318. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Patiar, S.; Wigfield, S.; Li, J.L.; Ledaki, I.; Turley, H.; Leek, R.; Snell, C.; Gatter, K.; Sly, W.S.; et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin. Cancer Res. 2012, 18, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Driessen, A.; Landuyt, W.; Pastorekova, S.; Moons, J.; Goethals, L.; Haustermans, K.; Nafteux, P.; Penninckx, F.; Geboes, K.; Lerut, T.; et al. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann. Surg. 2006, 243, 334–340. [Google Scholar] [CrossRef]
- Zagzag, D.; Lukyanov, Y.; Lan, L.; Ali, M.A.; Esencay, M.; Mendez, O.; Yee, H.; Voura, E.B.; Newcomb, E.W. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: Implications for angiogenesis and glioma cell invasion. Lab. Investig. 2006, 86, 1221–1232. [Google Scholar] [CrossRef]
- Singh, A.K.; Arya, R.K.; Trivedi, A.K.; Sanyal, S.; Baral, R.; Dormond, O.; Briscoe, D.M.; Datta, D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013, 24, 41–49. [Google Scholar] [CrossRef]
- Jadhao, C.S.; Bhatwadekar, A.D.; Jiang, Y.; Boulton, M.E.; Steinle, J.J.; Grant, M.B. Nerve growth factor promotes endothelial progenitor cell-mediated angiogenic responses. Invest. Ophthalmol. Vis. Sci. 2012, 53, 2030–2037. [Google Scholar] [CrossRef]
- Patry, C.; Stamm, D.; Betzen, C.; Tonshoff, B.; Yard, B.A.; Beck, G.C.; Rafat, N. CXCR-4 expression by circulating endothelial progenitor cells and SDF-1 serum levels are elevated in septic patients. J. Inflamm. (Lond.) 2018, 15, 10. [Google Scholar] [CrossRef]
- Shan, C.; Ma, Y. MicroRNA-126/stromal cell-derived factor 1/C-X-C chemokine receptor type 7 signaling pathway promotes post-stroke angiogenesis of endothelial progenitor cell transplantation. Mol. Med. Rep. 2018, 17, 5300–5305. [Google Scholar] [CrossRef]
- Mahmoud, B.S.; Alamri, A.H.; McConville, C. Polymeric nanoparticles for the treatment of malignant gliomas. Cancers 2020, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, R.; Ganapathi, M. Mechanisms regulating resistance to inhibitors of topoisomerase II. Front. Pharmacol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Cikankowitz, A.; Clavreul, A.; Tetaud, C.; Lemaire, L.; Rousseau, A.; Lepareur, N.; Dabli, D.; Bouchet, F.; Garcion, E.; Menei, P.; et al. Characterization of the distribution, retention, and efficacy of internal radiation of (188)Re-lipid nanocapsules in an immunocompromised human glioblastoma model. J. Neurooncol. 2017, 131, 49–58. [Google Scholar] [CrossRef]
- Lollo, G.; Vincent, M.; Ullio-Gamboa, G.; Lemaire, L.; Franconi, F.; Couez, D.; Benoit, J.P. Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma. Int J. Pharm. 2015, 495, 972–980. [Google Scholar] [CrossRef]
- Danhier, F.; Messaoudi, K.; Lemaire, L.; Benoit, J.P.; Lagarce, F. Combined anti-Galectin-1 and anti-EGFR siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide resistance in glioblastoma: In vivo evaluation. Int. J. Pharm. 2015, 481, 154–161. [Google Scholar] [CrossRef]
- Mamer, S.B.; Chen, S.; Weddell, J.C.; Palasz, A.; Wittenkeller, A.; Kumar, M.; Imoukhuede, P.I. Discovery of High-Affinity PDGF-VEGFR Interactions: Redefining RTK Dynamics. Sci. Rep. 2017, 7, 16439. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chen, X.; He, X. Platelet-derived growth factors and their receptors: Structural and functional perspectives. Biochim. Biophys. Acta 2013, 1834, 2176–2186. [Google Scholar] [CrossRef]
- Starovasnik, M.A.; Christinger, H.W.; Wiesmann, C.; Champe, M.A.; de Vos, A.M.; Skelton, N.J. Solution structure of the VEGF-binding domain of Flt-1: Comparison of its free and bound states. J. Mol. Biol. 1999, 293, 531–544. [Google Scholar] [CrossRef]
- Goel, S.; Wong, A.H.; Jain, R.K. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006486. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.T.; Duda, D.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.J.; Zhu, M.; et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Ebos, J.M.; Kerbel, R.S. Antiangiogenic therapy: Impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 2011, 8, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Koh, W.; Stratman, A.N. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res. C Embryo Today 2007, 81, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Arosio, D.; Casagrande, C.; Manzoni, L. Integrin-mediated drug delivery in cancer and cardiovascular diseases with peptide-functionalized nanoparticles. Curr. Med. Chem. 2012, 19, 3128–3151. [Google Scholar] [CrossRef]
- Robinson, S.D.; Hodivala-Dilke, K.M. The role of beta3-integrins in tumor angiogenesis: Context is everything. Curr. Opin. Cell Biol. 2011, 23, 630–637. [Google Scholar] [CrossRef]
- Yao, X.; Ping, Y.; Liu, Y.; Chen, K.; Yoshimura, T.; Liu, M.; Gong, W.; Chen, C.; Niu, Q.; Guo, D.; et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PLoS ONE 2013, 8, e57188. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. AlphaV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef]
- Hadjimichael, A.C.; Foukas, A.F.; Savvidou, O.D.; Mavrogenis, A.F.; Psyrri, A.K.; Papagelopoulos, P.J. The anti-neoplastic effect of doxycycline in osteosarcoma as a metalloproteinase (MMP) inhibitor: A systematic review. Clin. Sarcoma Res. 2020, 10, 7. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Bicknell, R. Angiogenic signalling pathways. Methods Mol. Biol. 2009, 467, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.G.; Zhuang, G.; Brantley-Sieders, D.; Swat, W.; Cowan, C.W.; Chen, J. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol. Cell Biol. 2006, 26, 4830–4842. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.K.; Brobeil, A.; Planz, J.; Brauninger, A.; Gattenlohner, S.; Nestler, U.; Stenzinger, A.; Paradowska, A.; Wimmer, M. PTPIP51 levels in glioblastoma cells depend on inhibition of the EGF-receptor. J. Neurooncol. 2015, 123, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Li, L.; Xu, Z.; Bi, B.; Wang, Y.; Li, J.Y. MiR-7-5p is frequently downregulated in glioblastoma microvasculature and inhibits vascular endothelial cell proliferation by targeting RAF1. Tumour Biol. 2014, 35, 10177–10184. [Google Scholar] [CrossRef]
- Herbert, J.M.; Stekel, D.J.; Mura, M.; Sychev, M.; Bicknell, R. Bioinformatic methods for finding differentially expressed genes in cDNA libraries, applied to the identification of tumour vascular targets. Methods Mol. Biol. 2011, 729, 99–119. [Google Scholar] [CrossRef]
- Desai, V.; Bhushan, A. Natural Bioactive Compounds: Alternative Approach to the Treatment of Glioblastoma Multiforme. BioMed. Res. Int. 2017, 2017, 9363040. [Google Scholar] [CrossRef]
- Hess, S.M.; Mounce, A.; Sequeira, R.; Anderson, J.G.; Blackstock, A.W.; Lesser, G.J. The in vitro cytotoxic and radiation sensitizing effects of docetaxel (Taxotere) and a novel taxane RPR116258 on Glioblastoma cell lines. Cancer Res. 2005, 65, 341. [Google Scholar]
- Fan, T.P.; Yeh, J.C.; Leung, K.W.; Yue, P.Y.; Wong, R.N. Angiogenesis: From plants to blood vessels. Trends Pharmacol. Sci. 2006, 27, 297–309. [Google Scholar] [CrossRef]
- Park, M.N.; Song, H.S.; Kim, M.; Lee, M.J.; Cho, W.; Lee, H.J.; Hwang, C.H.; Kim, S.; Hwang, Y.; Kang, B.; et al. Review of Natural Product-Derived Compounds as Potent Antiglioblastoma Drugs. Biomed. Res. Int. 2017, 2017, 8139848. [Google Scholar] [CrossRef]
- Lu, K.; Bhat, M.; Basu, S. Plants and their active compounds: Natural molecules to target angiogenesis. Angiogenesis 2016, 19, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, M.C.; Vella-Zarb, L. Evolution of Nanocarrier Drug-Delivery Systems and Recent Advancements in Covalent Organic Framework–Drug Systems. ACS Appl. Nano Mater. 2020, 3, 3097–3115. [Google Scholar] [CrossRef]
- Chowdhary, S.A.; Ryken, T.; Newton, H.B. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: A meta-analysis. J. Neurooncol. 2015, 122, 367–382. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Year | Tumor Cell | Source of Cells | Medium Culture | Supplement | Cell Modifications |
|---|---|---|---|---|---|---|
| Wu et al. [50] | 2019 | GL261 | Mouse | DMEM | 10%FBS | NA |
| Sousa et al. [32] | 2019 | U87-MG | Human | DMEM | 10%FBS; 10 μg/mL Blast | Luciferase expression |
| Clavreul et al. [34] | 2018 | U87-MG | Human | DMEM-HG | 10%FBS; 1% antibiotics | NA |
| Sun et al. [51] | 2017 | C6 | Rattus norvegicus | DMEM | 10%FBS; 2 mM Glut; 100 U/mL Pen; 100 mg/mL Strep | NA |
| Séhédic et al. [52] | 2017 | U87-MG | Human | DMEM-HG | 10%FBS; l-Glut; 10 U/mL Pen; 10 mg/mL Strep; 25 μg/mL Amp-B | Expression of CXCR4 and RFP |
| Lu et al. [53] | 2017 | U87-MG | Human | DMEM | 10%FBS; 1% Pen/Strep | NA |
| Xu et al. [54] | 2016 | C6 | Rattus norvegicus | DMEM-HG | FBS | NA |
| Wang et al. [55] | 2016 | U87-MG | Human | DMEM | 10%FBS | Luciferase expression |
| Lin et al. [56] | 2016 | U87-MG | Human | DMEM | 10%FBS; 1% antibiotics | Luciferase expression |
| Lin et al. [57] | 2016 | U87-MG | Human | MEM | 10%FBS; 2 mM l-Glut; 100 U/mL Pen; 100 mg/mL Strep; 1 mM SP; 1 mM NAA | Luciferase expression |
| Kuang et al. [58] | 2016 | U87-MG | Human | NR | NR | Luciferase expression |
| Hu et al. [59] | 2016 | U87-MG | Human | DMEM | FBS; Pen/Strep | NA |
| Hu et al. [60] | 2016 | C6 | Rattus norvegicus | NR | NR | NA |
| Banerjee et al. [61] | 2016 | C6 | Rattus norvegicus | DMEM | 10%FBS; 1 mM Glut; 100 U/mL Pen; 100 ng/mL Strep | NA |
| Zhang et al. [45] | 2015 | U251-MG | Human | MEM-EBSS | 10%FBS | NA |
| Feng et al. [62] | 2015 | U87-MG | Human | DMEM-HG | 10%FBS; 100 U/mL Pen; 100 µg/mL Strep | NA |
| Costa et al. [44] | 2015 | GL261 | Mouse | DMEM-HG | 10%FBS; 100 U/mL Pen; 100 µg/mL Strep; 100 mM HEPES; 12 mM NaHCO3 | NA |
| Bechet et al. [63] | 2015 | U87-MG | Human | NR | NR | NA |
| Gao et al. [41] | 2014 | C6 | Rattus norvegicus | DMEM-HG | FBS | Expression RFP |
| Wojton et al. [64] | 2013 | U87-MG | Human | DMEM | 10%FBS; 100 U/mL Pen; 10 mg/mL Strep | U87ΔEGFR; U87ΔEGFR-Luc |
| Janic et al. [65] | 2012 | U251-MG | Human | DMEM | 10%FBS | NA |
| Day et al. [48] | 2012 | U373-MG | Human | RPMI 1640 | 10%FBS; 1% Glut | Luciferase expression |
| Verreault et al. [43] | 2011 | U251-MG | Human | DMEM | 10%FBS; 1% l-Glut; 1% Pen; 1% Strep | NA |
| Agemy et al. [66] | 2011 | U87-MG | Human | DMEM-F12 | 10%FBS; 1% Glut; 1% Pen; 1% Strep | Luciferase expression |
| 005 | Mouse | DMEM-F12 | 1%N2; 20 ng/mL FGF-2; 20 ng/mL EGF; 40 μg/mL heparin | Luciferase expression | ||
| Spheres# | Human | DMEM-F12 | l-Glut; 0.3% Gluc; 50 μg/mL Pen/Strep; 0.1 mg/mL Apo-transf; 20 nM Prog; 30 nM Na2SeO3, 60 μM Put; 25 μM/mL Ins; 3 mM NaHCO3; 10 mM HEPES; 20 ng/mL EGF, 10 ng/mL LIF; 20 ng/mL FGF | NA | ||
| H-RasV12-shp53 lentivirus | NA | NA | NA | Luciferase expression | ||
| Ding et al. [67] | 2010 | U87-MG | Human | MEM | 10% FBS | NA |
| Hekmatara et al. [68] | 2009 | 101/8* | Rattus norvegicus | NA | NA | NA |
| Saito et al. [38] | 2006 | U87-MG | Human | MEM | 10% FBS; 100 U/mL; 0.1 mg/mL Strep | NA |
| Ref. | Animal Description | Glioblastoma Induction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Specie | Sex | Weight (g) | Age (week) | n/N | Cell Type | Cell Number (cell/mL) | AV (μL) | Vehicle | AT (min) | Local Administration | Coordinates (AP; ML; DV:mm) | |
| Wu et al. [50] | Mice | C57BL/6 | F | NR | 6–8 | 7/35 | GL261 | 2 × 107 | 5 | DMEM | NR | NR | NR |
| Sousa et al. [32] | Mice | Nude athymic | NR | 26–32 | 6–8 | 3–4/12–16 | U87-MG | 5 × 105 | 5 | NR | NR | L. Cerebral hemisphere | 1.5; 2; 3.5 |
| Clavreul et al. [34] | Mice | Swiss nude | F | 22–23 | 8–10 | 5–7/22 | U87-MG | 5 × 104 | 5 | HBSS+ | NR | R. Striatum | 0.5; 2.1; 3 |
| Sun et al. [51] | Mice | Nude athymic | NR | NR | NR | 7/28 | C6 | 1 × 104 | 5 | PBS | NR | R. Striatum | NR |
| Séhédic et al. [52] | Mice | CB17-SCID | F | NR | 8 | 6–9/47 | U87-MG | 5 × 104 | 5 | EMEM | NR | R. Striatum | 0.5; 2; 3 |
| Lu et al. [53] | Mice | BALB/C nude | F | 22–23 | NR | NR/NR | U87-MG | 5 × 105 | 5 | MEM | NR | NR | 3; 3; 3 |
| Xu et al. [54] | Rat | Sprague-Dawley | M | 250–350 | NR | 12/60 | C6 | 1 × 106 | 10 | NR | 10 | NR | 1; 3; 5 |
| Wang et al. [55] | Mice | Nude athymic | NR | NR | 4–6 | 11/30 | U87-MG | 2.4×105 | 8 | PBS | >1 | NR | 1; 2; 3 |
| Lin et al. [56] | Mice | BALB/C nude | NR | NR | 3–4 | 4/40 | U87-MG | NR | NR | NR | NR | L. Cerebral hemisphere | NR |
| Lin et al. [57] | Mice | BALB/C nude | F | NR | 6 | 5/25 | U87-MG | 2 × 105 | 3 | PBS | NR | R. Frontal lobe | 1; 2; 3 |
| Kuang et al. [58] | Mice | BALB/C nude | M | 20–25 | NR | 15/105 | U87-MG | NR | NR | DMEM | NR | NR | NR |
| Hu et al. [59] | Mice | BALB/C nude | M | NR | 4 | 3/12 | U87-MG | 5 × 105 | 5 | PBS | NR | R. Striatum | NR; 1.8; 3 |
| Hu et al. [60] | Rat | Sprague-Dawley | M | NR | 4 | 16/64 | C6 | 1 × 106 | 10 | PBS | 10 | R. Cerebral hemisphere | NR; 2; 5 |
| Banerjee et al. [61] | Rat | Sprague-Dawley | F | 200–220 | 27 | 9/36 | C6 | 1 × 106 | 5 | NR | NR | R. Cerebral hemisphere | 2; 2; 3 |
| Zhang et al. [45] | Mice | BALB/C nude | M | 18–20 | NR | 3/15 | U251-MG | 6 × 105 | 3 | MEM-EBSS | 3 | R. Cerebral hemisphere | 1.5; 1.8; 3 |
| Feng et al. [62] | Mice | BALB/C nude | M | 18–22 | NR | 6/20 | U87-MG | 5 × 105 | 5 | PBS | NR | R. Striatum | NR |
| Costa et al. [44] | Mice | C57BL/6 | M | NR | 8 | 6–8/NR | GL261 | 1.25 × 105 | 3 | NR | 15 | R. Cerebral hemisphere | −1.06; 3; 3 |
| Bechet et al. [63] | Mice | Nude athymic | M | 150–180 | 8 | NR/NR | U87-MG | 5 × 104 | 5 | HBSS+ | 25 | Parenchyma | 0.5; 2.7; 4.4 |
| Gao et al. [41] | Mice | BALB/C nude | M | 18–22 | 4–5 | 13/78 | C6 | 5 × 105 | 5 | NR | 3 | R. Striatum | NR |
| Wojton et al. [64] | Mice | Mut6¥ /cKO | F; M | NR | NR | 3–4/NR | U87-MG | 1 × 105 | NR | NR | NR | NR | NR; 2; 3 |
| Janic et al. [65] | Rat | Nude athymic* | NR | 150–170 | 6–8 | 10/NR | U251-MG | 4 × 105 | 5 | DMEM | 5 | R. Cerebral hemisphere | 1; 3; 2.5–3.5 |
| Day et al. [48] | Mice | ICR-PrkdcSCID | M | NR | NR | 3/9 | U373-MG | 1 × 105 | NR | RPMI-1640 | NR | NR | 2; 1; 3 |
| Verreault et al. [43] | Mice | Rag2M | F | NR | 7–10 | 5–6/9 | U251-MG | 7.5 × 104 | NR | NR | NR | R. Caudate nucleus-putamen | 1; −1.5; −3.5 |
| Agemy et al. [66] | Mice | NOD-SCID | NR | NR | NR | 8–10/16–20 | U87-MG | NR | NR | NR | NR | R. Hippocampus | NR |
| Mice | NOD-SCID | NR | NR | NR | 8–10/16–20 | 005 | 3 × 105 | 1.5 | PBS | NR | R. Hippocampus | NR | |
| Mice | NOD-SCID | NR | NR | NR | 8–10/16–20 | Spheres# | 5 × 105 | 1.5 | PBS | NR | R. Hippocampus | NR | |
| Mice | NOD-SCID | NR | NR | NR | 8–10/16–20 | H-RasV12-shp53 lentivirus £ | NA | NR | NR | NR | R. Hippocampus | NR | |
| Ding et al. [67] | Mice | Nude Athymic$ | NR | NR | NR | 8/24 | U87-MG | 5 × 104 | NR | NR | NR | R. Basal ganglia field | NR |
| Hekmatara et al. [68] | Rat | Wistar | M | 200–250 | NR | 18–20/58 | 101/8 | 1 × 106 | NR | NR | NR | R. Lateral ventricle | 2; 2; 4 |
| Saito et al. [38] | Rat | Nude athymic* | M | 250 | NR | 7/35 | U87-MG | 5 × 105 | 2 × 5 | HBSS- | 2 × 2 | Striatum | 0.5; 3; 4–4.5 |
| Ref. | Particle | Drug | Formulations | Manufacture | Size (nm) | ζ (mV) | PDI | EE (%) | DLE (%) | Release of the Drugs |
|---|---|---|---|---|---|---|---|---|---|---|
| Wu et al. [50] | Micelles | Luteolin | (Lut/Fa-PEG-PCL) | Synthesized | 34.7 | −9.2 | 0.12 | 98.5 | 5 | Luteolin: 46%(PBS 0.5% Tween-80, 10 h, 37 °C) |
| Sousa et al. [32] | Polymeric Nanoparticles | Bevacizumab | (Beva-loaded PLGA) | Synthesized | 185 | −1.6 | 0.056 | 82.47 | 1.62 | Bevacizumab: 14%(after 7 days in vitro study, pH 7.4) |
| Clavreul et al. [34] | Lipid nanocapsules | Sorafenib | (SFN-LNCs) | Synthesized | 54 | −7.8 | 0.15 | 105 | NR | SFN: 11%(DPBS, 8 h) and 20% (DPBS, 120 h) |
| Sun et al. [51] | Polymeric Nanoparticles | DOX | DOX-NP | 110 | −29.7 | 56.33 | 1.43 | DOX:~70%(PBS, pH7.4, 72 h, 37 °C) and ~80%(10% rat plasma, 72 h, 37 °C) | ||
| (AP1-DOX-NP) | 120 | −26.3 | 53.74 | 1.37 | ||||||
| Séhédic et al. [52] | Lipid nanocapsules Nanocarriers | 188Re | LNC | Synthesized | 55.41 | −4.51 | 0.03 | NA | NA | Antibodies per LNC: |
| 12G5-LNC | 60.44 | −13.87 | 0.24 | 35% | ||||||
| IgG2a-LNC | 63.48 | −14.95 | 0.26 | 15% | ||||||
| LNC188Re | 58.12 | −8.37 | 0.05 | - | ||||||
| (12G5-LNC188Re) | 77.25 | −24.77 | 0.21 | 13% | ||||||
| IgG2a-LNC188Re | 74.81 | −26.23 | 0.21 | 10% | ||||||
| Lu et al. [53] | Functionalized SPIONs | ATRA; CA4; DOX | (CARD-B6) | Synthesized | <100 | −0.5–0.9 | NR | CA4: 75.3 ATRA: 77.8 DOX: 78.4 | PAD: 58.37 PAAA: 14.53 PABA: 16.23 | CA4:64.47%(pH 6.5, 12 h, 37°C DOX:68.37%(pH5.0, 48 h, 37 °C) ATRA: 85.11%(hypoxic condition, pH 5.0, 12 h, 37 °C) DOX: 66.39%(hypoxic condition, pH 5.0, 48 h, 37 °C) |
| Xu et al. [54] | DTX-NPs-adsorbing dBECM scaffold | DTX | (DTX-NPs) | Synthesized | 32.0 | 17.7 | NR | 73.37 | 3.99 | DTX: 38%(pH 7.4, 24 h, 37 °C) |
| Wang et al. [55] | Multi-functional nanoparticles | NA | H-S-R NPs1 | Synthesized | 164 | −31.77 | 0.119 | NA | NA | NA |
| H-S-R NPs2 | 190 | −29 | 0.128 | |||||||
| Lin et al. [56] | LMWP-modified albumin nanoparticles | PTX; 4-HPR | BSA NPs | Synthesized | 140 | −30 | 0.089 | 51.63 | 3.6 | PTX: 70%(PBS, pH 7.4, 0.5% w/v SDS, 96 h, 37 °C) |
| (L-BSA NPs) | 145 | −16 | 0.074 | 53.24 | 3.7 | PTX: 73%(PBS, pH 7.4, 0.5% w/v SDS, 96 h, 37 °C) | ||||
| Lin et al. [57] | Polymeric Nanoparticles | CPT | (CRLX101) | Cerulean Pharma, Cambridge, MA | 20–30 | −6 | NR | NR | 10 | NR |
| Kuang et al. [58] | Dual Functional peptide-driven nanoparticles | DOX; shVEGF | (DGL-PEG-T7/shVEGF-DOX) | Synthesized | 142.9 | NR | NR | NR | NR | NR |
| Hu et al. [59] | Peptide dual-decorated nanoparticle | PTX | NP | 102 | −37.5 | 0.13 | NA | NA | PTX: 78.3%(1 mL of PBS/39 mL of release medium, 72 h) | |
| ATWLPPR-NP | 113 | −31.6 | 0.14 | PTX: 77.1%(1 mL of PBS/39 mL of release medium, 72 h) | ||||||
| CGKRK-NP | 119 | −14.6 | 0.17 | PTX: 79.4%(1 mL of PBS/39 mL of release medium, 72 h) | ||||||
| (AC-NP) | 123 | −11.4 | 0.15 | PTX: 75.6%(1 mL of PBS/39 mL of release medium, 72 h) | ||||||
| Hu et al. [60] | Functionalized mesoporous nanoparticles | DOX | MSN-DOX-PDA | Synthesized | 156.1 | −24 | 44.84 | 19.02 | DOX:50%(Acetate, pH 4.5, 24 h, 37 °C) | |
| (MSN-DOX-PDA-NGR) | 168 | −22 | ||||||||
| Banerjee et al. [61] | Solid lipid Nanoparticles | PTX | PSLN | Synthesized | 158 | −24.8 | 0.16 | 88 | 5.18 | NR |
| (PSM) | 178 | −17.4 | 0.19 | 86 | 5.06 | |||||
| Zhang et al. [45] | Liposomes | Epirubicin | Epirubicin liposomes | Synthesized | 97.92 | −14.3 | 0.24 | 96.88 | NR | Epirubicin:<1%(PBS 10% FBS, Ph 7.4,2 h, 37 °C) ~2%(PBS 10% FBS, Ph 7.4, 36 h, 37 °C) |
| Glu-targeting epirubicin liposomes | 108.87 | −14.6 | 0.20 | 97.81 | ||||||
| cRGD-targeting epirubicin liposomes | 110.91 | −9.63 | 0.21 | 98.30 | ||||||
| (Functional targeting epirubicin liposomes) | 108.97 | −15.2 | 0.23 | 98.68 | ||||||
| Feng et al. [62] | Polymeric Nanoparticles | PTX | NP-PTX | Synthesized | 109.76 | −33.35 | 0.092 | 47.07 | 1.49 | PTX: 69.25%(PBS 0.5% Tween 80, Ph 7.4, 96 h, 37 °C) and 82.91%(10% rat plasma, 96 h, 37 °C) |
| (CooP-NP-PTX) | 118.95 | −27.59 | 0.157 | 44 | 1.31 | PTX: 73.52%(PBS 0.5% Tween 80, pH7.4, 96 h, 37 °C) and 84.53% (10% rat plasma, 96 h, 37 °C) | ||||
| Costa et al. [44] | Liposomes | NA | (CTX-coupled SNALPs) | Synthesized | <190 | NR | 0.3 | 85-95 | NA | NA |
| Bechet et al. [63] | Multifunctional Silica-based nanoparticles | Chlorin (photosensitizer) | NP-TPC | Synthesized | 2.9 | 42.9 | NR | NR | NR | NR |
| (NP-TPC-ATWLPPR) | 22.6 | |||||||||
| Gao et al. [41] | Polymeric nanoparticles | Coumarin-6-NPs | Synthesized | 101.3 | −9.07 | 0.191 | NA | NA | NA | |
| Coumarin-6-ILNPs | 105.6 | −10.12 | 0.201 | |||||||
| Coumarin-6-IRNPs | 112.4 | −9.86 | 0.224 | |||||||
| Coumarin-6-RNPs | 111.8 | −11.21 | 0.199 | |||||||
| DTX | DTX-NPs | 120.1 | −8.77 | 0.173 | ||||||
| DTX-ILNPs | 131.2 | −8.89 | 0.181 | |||||||
| (DTX-IRNPs) | 137.9 | −9.76 | 0.192 | |||||||
| DTX-RNPs | 127.6 | −7.81 | 0.187 | |||||||
| Wojton et al. [64] | Nanovesicles | NA | (SapC-DOPS nanovesicles) | Synthesized | NR | NR | NR | NA | NA | NA |
| Janic et al. [65] | SPIONs | NA | (FePro) | Feridex IV; Bayer-Schering Pharma, Wayne, NJ, USA | 141.8 | −31.30 | 0.285 | NA | NA | NA |
| Day et al. [48] | Nanoshells | NA | Bare NS | Synthesized | 162.4 | −57.9 | NR | NA | NA | NA |
| (VEGF-NS) | 188.0 | −33.4 | ||||||||
| PEG-NS | 196.8 | −32.7 | ||||||||
| Verreault et al. [43] | Liposomes | Irinotecan, DOX and vincristine | (Liposomal Irinophore CTM, Caelyx® and Vincristine) | Caelyx®, Schering-Plough, QC, Canada | NR | NR | NR | NR | NR | NR |
| Agemy et al. [66] | Functionalized SPIONs | Mitochondria-targeted D[KLAKLAK]2 peptide | (Iron Oxide Nanoworms-CGKRKD(KLAKLAK)2) | Synthesized | NR | NR | NR | NA | NA | NA |
| Ding et al. [67] | Polymeric Nanoparticles | NA | PMLA-→ (P/LLL/AON/Hu/Ms, P/LOEt/AON/Hu/Ms) | Synthesized | 6.6→18 22 | −27→ −9.4–5.2 | NR | NA | NA | NA |
| Hekmatara et al. [68] | Polymeric Nanoparticles | DOX | (DOX-np) | Synthesized | 260 | −19 | 0.02 | 70 | NR | NR |
| Saito et al. [38] | Liposomes | TPT | (Ls-TPT) | Hermes Bioscience, Inc. (South San Francisco, Calif.) | NR | NR | NR | >95 | NR | NR |
| Ref. | Therapy Type (NP: Formulations) | Therapeutic Target | Route/Local of Administration | Frequency- Dose (mg/Kg) | Vehicle | Time Point of Therapy | Tumoral Reduction | Follow-Up Evaluation after Induction | Therapeutic Evaluation Techniques |
|---|---|---|---|---|---|---|---|---|---|
| Wu et al. [50] | Drug delivery (Lut/Fa-PEG-PCL) | Signal transduction pathways thar regulates tumor activities | Tail vein | Daily 50 | Saline | 5th to 13th day | NR | 5th to 13th day (each 2 days) -Until cachexia of the mouse appeared | FLI; TUNNEL assay; Survival curve |
| Sousa et al. [32] | Drug delivery (Beva-loaded PLGA NP) | VEGF | Intranasal | Weekly 5 | NR | 10th; 17th day | ~46% at 24th day | 10th, 17th and 24th days | BLI |
| Clavreul et al. [34] | CED (SFN-LNCs) | RTKs (VEGFR-2; VEGRF-3, PDGFR-β, c-kit e Flt-3); Intracellular serine /threonine kinases (Raf-1; B-Raf; B-Raf-mut) | Intratumoral | Single 3.5 µg/mouse | Transcutol®HP (0.7 g) | 9th day | No reduction at 13th and 16th day | 13th and 16th days | MRI; H&E |
| Sun et al. [51] | Dual-targeting drug delivery (AP1-DOX-NP) | IL-4R | Tail vein | Every other day 10 | PBS | 10th, 12th, 14th, 16th days | NR | 3 and 24 h and 47 days | FLI; H&E; Survival curve |
| Séhédic et al. [52] | CED (12G5-LNC188Re) | CXCR4; Signaling pathways (PI3K/Akt and MAP-kinases); Activation of MMPs; CD11b+ myeloid cells | Intratumoral | Single 10 µL/mouse | Saline | 12th day | ~100% after 24 days | 12th, 17th, 19th to 100 days | MRI; IF; Western Blot; Survival curve |
| Lu et al. [53] | Drug delivery (CARD-B6) | GBM microenvironment; Transferrin receptors; Telomerase activity | Intravenous | Every other day 0.5 CA4+2.5 DOX+ 0.5 ATRA | PBS | 16th to 32nd day | NR | 12, 24, 36, 48 h, 30th and 36th day | MRI; LSCA; TUNNEL assay; Survival curve |
| Xu et al. [54] | Drug delivery (DTX-NPs-dBECM) | GBM microenvironment | Intratumoral | Single 800 µg/mL-DTX+ 10 mg/mL-dBECM | Saline | 7th day | ~98% at 28th day | 7th to 28th days (weekly) | MRI; FLI-ex vivo; H&E; TUNNEL assay; Survival curve |
| Wang et al. [55] | Systemic therapy (H–S–R NPs1) | Integrin αvβ3 on endothelium; EphA2 Tyrosine kinase receptor on tumor cells and tumor vasculature | Tail vein | Every 2 days NR | Saline | Started when the tumors were visible by BLI | ~99% at 12th day | 0, 4th, 8th, 12th day; 26th day | BLI; FLI Survival curve; |
| Lin et al. [56] | Drug delivery (L-BSA NPs) | SPARC; gp60 | Tail vein | Daily PTX/ 4-HPR, 2 each | PBS | Started with tumor size (100−200 mm3) for 2 days | ~93% at 16th day | 0, 7 and 16 days; 37th day | BLI; Western Blot; TUNNEL assay; Survival curve |
| Lin et al. [57] | Systemic therapy (CRLX101) | Topo I inhibition; Hypoxia cascade (CA IX, HIF-1α, VEGF) | NR | Weekly 10 | NR | 4th and 11th day | NR | 20th and 32nd day | H&E; TUNNEL assay; Survival curve |
| Kuang et al. [58] | Targeted drug delivery (DGL-PEG-T7/shVEGF-DOX) | Transferrin receptor; VEGF gene; Topo II inhibition | Intravenous | Every 2 days 50 µg+8 µg DOX/mouse | Saline | 12th, 15th, 18th day | ~80% after 18th day | 12th and 21st day | BLI; TUNNEL assay; Survival curve |
| Hu et al. [59] | Dual-targeting drug delivery (AC-NP-PTX) | HSPG; NRP-1 | Intravenous | Every 3 days 5 | Saline | NR | NR | 51st day | FLI; IF; Survival curve |
| Hu et al. [60] | Dual-targeting drug delivery (MSN-DOX-PDA-NGR) | CD13 | Tail vein | Every 3 days 5 | Saline | 5, 8, 11, 14 days | NR | 5th, 10th, 17th, 32nd day | H&E; TUNNEL assay; FLI; MVD; Survival curve |
| Banerjee et al. [61] | Targeted drug delivery (PSM) | SSTR2 | Intravenous | Daily 2 | Saline | 2 weeks | NR | 15th and 36th day | H&E; Survival curve |
| Zhang et al. [45] | Targeted drug delivery (Functional targeting epirubicin liposomes) | Glut1 on BBB; GBM integrin receptors and neovasculature | Tail vein | Every 3 days 100 µg/Kg | Saline | 14th, 18th day | NR | 20th and 28th day | FM; Survival curve |
| Feng et al. [62] | Drug delivery (CooP-NP-PTX) | MDGI (H-FABP/ FABP3) | Intravenous | Every 3 days 5 | Saline | 2 weeks | NR | 47.5 | H&E; Survival curve |
| Costa et al. [44] | Multimodal gene therapy(CTX-coupled SNALPs) | miR-21 (inhibits PDCD4); RhoB; p53; TGF-β; mitochondrial apoptotic networks | Tail vein/ oral | Single 2.5/ 3 days 30 | Saline | 13th day/ 13th, 14th, 15th day | ~45% at 17th day | 17th and 30th day | H&E; Western blot; Survival curve |
| Bechet et al. [63] | Photodynamic therapy (NP-TPC-ATWLPPR) | VEGF receptor; NRP-1 | Tail vein | Single 2.8 (1.75 µmol/kg) | NR | NR | ~50% after 6 days of iPDT | 4th, 6th, 10th day after iPDT | MRI; PET-CT; H&E |
| Gao et al. [41] | Dual-targeting drug delivery (DTX-IRNPs) | Integrin αvβ3 on endothelium; IL13Rα2 | Tail vein | Every 3 days 6 | Saline | 10th, 11th, 12th day | ~71% at 17th day | 13tn, 17th, 35th day | IF; H&E; Survavil curve |
| Wojton et al. [64] | Systemic therapy (SapC-DOPS nanovesicles) | PtdSer | Tail vein | Single 12-SapC 4.6-DOPS | PBS | 10th day | NR | 11th, 12th, 17th day | IF; H&E; Survival currve |
| Janic et al. [65] | Cell therapy (FePro) | CD34; AC133; SDF-1-CXCR4 signaling pathway | Intravenous | Single 10 × 106 | PBS | 12th day | NR | 18th day | MRI; Prussian Blue |
| Day et al. [48] | Photothermal therapy (VEGF-NS) | Integrin αvβ3 on endothelium; VEGFR-2 | Tail vein | Single 4.35 × 1010 VEGF-NS/mouse | Saline | When tumor reached 3–5 mm | NR | 24 h and 3 days after treatment | Intravital microscopy images; H&E; Survival curve |
| Verreault et al. [43] | Drug delivery (Liposomal Irinophore CTM) | GBM microenvironment; GBM vasculature | Intravenous | Weekly 25 Irinophore CTM; 15 Caelyx®; 2 liposomal vincristine | PBS | 21st; 28th; 35th day | ~70% at 42nd day | 42nd day | H&E |
| Agemy et al. [66] | Systemic therapy (Iron Oxide Nanoworms- CGKRKD(KLAKLAK)2) | Peptides homing to epidermal tumors; GBM vasculature; mitochondrial membrane | Intravenous (U87-MG) | Every other day 5 | PBS | 3 weeks | NR | 5–6 h after the injection | IF; Survival curve |
| Intravenous (005) | Every other day 5 | PBS | 10th, 12th, 14th, 16th, 18th, 20th, 22nd 24th, 26th, 28th, 30th day | NR | 5–6 h after the injection | IF; Survival curve | |||
| Intravenous (Sphere) | Every other day 5 | PBS | 3 weeks | NR | 5–6 h after the injection | IF | |||
| Intravenous (Lentiviral) | Every other day 5 | PSB | 21st, 23th, 25th, 27th, 29th, 31st, 33th, 35th, 37th day | NR | 21st, 28th, 35th day | BLI; H&E; IF; Survival curve | |||
| Ding et al. [67] | Systemic therapy (P/LLL/AON/Hu/Ms, P/LOEt/AON/Hu/Ms) | Laminin α4 and β1 chain | Intravenous | Single 5 | PBS | 21st day | ~91% NR day | NR | IF; H&E |
| Hekmatara et al. [68] | Drug delivery (DOX-np) | Endothelial cells | Tail vein | Dayly 1.5 | NR | 2nd, 5th, 8th day | ~100% at 14th day | 10th, 14th, 18th day | H&E; IMC |
| Saito et al. [38] | CED (Ls-TPT) | Topo I inhibition; GBM vasculature | Intratumoral | Single (0.5 mg /mL; 20 μL) | NR | 10th day | NR | 17th, 19th day | H&E; Survival curve |
| Ref. | Angiogenic Markers | Technique Evaluation | Expression of Control Groups | Expression of Treatment Groups | Efficiency of Therapy and Time (d) | Conclusions |
|---|---|---|---|---|---|---|
| Wu et al. [50] | CD31 | IHC | Number of microvessels: 37.8 ± 7.3 (NS); 33.4 ± 7.2 (EM) | 17.3 ± 5.2 (F-Lut), 11.3 ± 3.1 (Lut-M); 4.1 ± 2.2 (Lut/Fa-PEG-PCL) | ~89%/ NR | Lut/Fa-PEG-PCL significantly inhibit the NV of GL261 tumor, play an important role in inhibiting tumor cellular growth |
| Sousa et al. [32] | VEGF mRNA; | qPCR | 8 × 10−5 (U87 MG) | 2 × 10−5 (Beva-loaded PLGA); 1 × 10−5(Free Beva) | ~49% at 24th day | Beva significantly decrease both extracellular and intracellular VEGF levels, having a higher anti-angiogenic effect compared to the free Beva |
| VEGF protein level | ELISA | 2000 ng/mL (U87 MG) | 1000 ng/mL (Beva-loaded PLGA); 1250 ng/mL (Free Beva) | ~38% at 24th day | ||
| Clavreul et al. [34] | CD31 | IF | 130 ± 9 µm2 (HBSS) | 124 ± 6 µm2 (B-LNC), 128 ± 6 µm2 (SFN); 105 ± 5 µm2 (SFN-LNC) | ~19% at 16th day | SFN-LNCs decreased the proportion of proliferating cells and tumor vessel area, inducing an early increase in tumor blood flow and a vascular normalization process. |
| Blood Perfusion | Perfusion MRI | 50 ± 3 mL/100 g/min (HBSS) | 51 ± 2 mL/100 g/min (B-LNC); 49 ± 3 mL/100 g/ min (SFN); 62 ± 4 mL/100 g/min (SFN-LNC) | ~24% (-) at 16th day | ||
| Sun et al. [51] | CD31 | IF | NP IF expression < AP1-NP IF expression | NP IF expression < AP1-NP IF expression | NA | AP1-NP has high affinity with vascular endothelial cells. |
| Séhédic et al. [52] | CD31 | IHC | ~12.5% (PBS)* | ~7.5% (LNC188Re)*; ~5% (IgG2a-Re-LNC)*; ~2.5% (12G5-LNC188Re)* | ~80% at 19th day | The clinical improvement was accompanied by locoregional effects on tumor development including hipovascularization and stimulation of the recruitment of bone marrow derived CD11b- or CD68-positive cells. NOS-II analysis from inside to the external part of the tumor while Arg1 was exclusively present in the peripheral part of the tumor |
| CD11b | ~1.5% (PBS)* | ~8% (LNC188Re)*; ~25% (IgG2a-Re-LNC)*; ~22.5% (12G5-LNC188Re)* | ~93% (-) at 19th day | |||
| CD68 | NA | CD68+/NOSII+ M1 | NA | |||
| NA | CD68+/Arg1+ M2 | NA | ||||
| MMP9 | NR | NR | NA | |||
| Lu et al. [53] | Blood Perfusion; | LSCI; | 1.76 UA (PBS) | 1.03 UA (CARD); 1.24 UA (CARD-B6); 1.23 UA (CA4 + ATRA + DOX); 0.74 UA (CARD-B6) | ~58% after 16th day | Almost no blood flow existed in the tumor region following treatment with CARD-B6 |
| CD31 | IHC | NR | NR | NA | ||
| Xu et al. [54] | CD31 | IHC | 100% (Control) | 78.1 ± 1.9% (DTX), 58.0 ± 3.9% (DTX-NPs), 30.2 ± 2.8% (DTX-NPs-dBECM) | ~70% after 8th day | DTX-NPs-dBECM complex display effective anti-angiogenesis |
| Wang et al. [55] | CD34+(endothelial lined vessel); | IHC | ~45 UA (Control) | ~7 UA (H-S); ~20 UA (H-S-R) | ~56% at 12th day | H–S–R NPs exerted a significant synergic anti-tumor effect through anti-angiogenic therapy |
| CD34-/PAS+ (VM) | ~62.33 UA (Control) | ~30.67 UA (H-S); ~11.33 UA (H-S-R) | ~82% at 12th day | |||
| Lin et al. [56] | CD31, $ SPARC and gp60 | IF; WB | NR | NR | NA | Decreased vessel size and number by IHC and reduced CD31 levels by WB in PTX/4-HPR treatment group |
| Lin et al. [57] | CD31 | IHC | Control | > CRLX101 and CPT | NA | In vivo results indicate that CRLX101 was more effective than CPT in inducing apoptosis and suppressing angiogenesis due to CRLX101′s improved drug delivery profile and enhanced permeability and retention effect |
| CA IX | NR | CRLX101 > CPT | NA | |||
| VEGF | IHC; WB | 1.0 (Vehicle) | ~0.1 CRLX101 ~0.6 CPT | ~40% at 14th day | ||
| Kuang et al. [58] | CD34/Lectin# | IF | NR | NR | NA | DGL-PEG-T7/shVEGF could inhibit VEGF mRNA much better than DGL-PEG/shVEGF. This could be explained as the nanoparticles bind to TfR on the surface of the tumor cells via the T7 peptide. shVEGF and DOX delivered by DGL-PEG-T7 could inhibit tumor growth and angiogenesis |
| VEGF mRNA; | RT-PCR | 100% Saline | 69.2% (DGL-PEG/shVEGF); 41.6% (DLG-PEG-T7/shVEGF); 49.0% DGL-PEG-T7/shVEGF-DOX | ~51% at 21st day | ||
| Hu et al. [59] | CD31 | IF | NR | NR | NA | The abundant extracellular matrix-derived HSPG and enhanced tumor penetration ability mediated by NRP-1 protein, allowed the AC-NP to achieve angiogenic blood vessels and tumor microenvironment with dual-targeting effect. |
| Hu et al. [60] | CD31 | IHC | NR | NR | NA | Delivered the drugs into the glioma cells was more efficiently, induced more cell apoptosis and necrosis with fewer MV in the MSN-DOX-PDA-NGR group |
| Banerjee et al. [61] | CD31 (MV density) | IHC | ~180 UA | ~150 UA (Taxol); ~90 UA (PSLN); ~25 UA (PSM) | ~86% at 15th day | PSM holds high potential dual-targeting for tumor neovasculature and tumor cells due to TOC (in PSM surface) interaction with SSTR2 expressed in EC NV, PTX improves AA effects when encapsuladed. |
| Zhang et al. [45] | CD3, DiI | IF | NR | NR | NA | FTEL are able to destroy brain glioblastoma NV and to extend the survival of brain glioblastoma-bearing mice |
| Feng et al. [62] | CD31 | IF | ~97% (Taxol) | ~65% (NP-PTX); ~30% (CooP-NP-PTX) | ~69% at 1 week after treatment | CooP-NP-PTX led to an effective tumor angiogenic blood vessel and glioma cell, holds great potential to improve anticancer activity and avoid the drawbacks of anti-angiogenic therapy alone. |
| Costa et al. [44] | CD31 | IHC | 145 ± 63 cells | 113 ± 79 cells (Mismatch + Sunitinib); 88 ± 69 cells/ (Anti-miRNA-21 + Sunitinib) | ~39% at 17th day | CTX-coupled SNALP formulated anti-miR-21 OG reduction of the number of vascular EC |
| Bechet et al. [63] | VEGF | IHC | NR | NR | NA | Vascular disruption and edema into both tumor and BAT areas; Intense decrease of VEGF expression after iPDT |
| Gao et al. [41] | HIF1α | IF | Low HIF1α expression (Saline) | HIF1α expression (DTX-ILNPs)>(DTX-RNPs) | NR (+) at 17th day | DTX-ILNPs increased the expression of HIF1a in tumor and could be effectively for antiangiogenesis problems |
| Wojton et al. [64] | CD31 | IF | NR | NR | NA | SapC-DOPS targets glioma cells (DAPI) and tumor vasculature (CD31), but not normal brain tissue. |
| Janic et al. [65] | CD31, vWF | IHC | NR | NR | NA | Strong expression of vWF and CD31 in iron-labeled CB AC 133+ EPC positive cells overlapped with tumor vasculature |
| Day et al. [48] | Vessel morphology | Intravital microscopy; H&E | Increase of 18% of VD (Saline) | Decreade of 24% of VD (VEGF-NSs) | ~42% after 3 days of treatment | Treatment with VEGF-NS, following laser exposure disrupts tumor vessels, majorly in tumor and at its periphery, but not in the adjacent normal brain. (Intravital microscopy); vessel dilation and hemorrhaging within the tumor exposed to VEGF-NSs and PEG-NSs (H&E). |
| Verreault et al. [43] | CD31, Collagen IV, NG2 | IHC, IF | Collagen IV-free CD31: ~12 pixels (Control tumor) | Collagen IV-free CD31: ~9.5 pixels (Irinophore CTM, Caelyx®) | ~21% at 42nd day | Irinophore CTM restored the BMA and reduced BVD of the tumor vasculature, suggesting a restoration of the vessel architecture to a more normal state. In addition, it increased the quantity of vessel staining in the center of tumors, suggesting a more homogenous distribution of blood across the entire tumor, as well as reduced K trans values. No changes in ECD in the TTA or the periphery of tumors treated with Caelyx® or liposomal vincristine |
| BVD: ~11 pixels (Control tumor) | BVD: ~6.5 pixels (Irinophore CTM, Caelyx®) | ~39% at 42nd day | ||||
| NG2-free CD31: ~2.5 pixels (Control tumor) | NG2-free CD31: ~0.75 pixels (Irinophore CTM, Caelyx®) | 70% at 42nd day | ||||
| CD31-free Collagen IV: ~0.9 pixels (Control tumor) | CD31-free Collagen IV: ~0.9 pixels (Irinophore CTM, Caelyx®) | ~0% at 42nd day | ||||
| Ktrans | DCE-MRI | 0.0232 mL/g/min (Control) | 0.0034 mL/g/min (Irinophore CTM) | ~85% at 42nd day | ||
| Agemy et al. [66] | CD31 | IF | NR | NR | NA | NWs coinjected with iRGD had spread into the extravascular tumor tissue, whereas NWs coinjected with CRGDC mainly accumulated in tumor vessels; Vascular structures were filled with CGKRKD (KLAKLAK)2-NWs; destruction of the BV by the NWs |
| Ding et al. [67] | Laminin 411 (α1 and β4 chains) | IHC | Vessel area: 5.5% (PBS) | Vessel area: 3.75% (LOEt); 2.5% (LLL) | ~55% after 21st day | Antitumor efficacy of LOEt and LLL due to reduced production of laminin-411 chains and decreased angiogenesis |
| Hekmatara et al. [68] | VEGF; | IHC | NR | 2 score (Dox-sol); 1 score (Dox-np) | NA at 18th day | Dox-sol led to a slight decrease of necrosis and MVP whereas Dox-np drastically decreases necrosis and led to the complete disappearance of MVP. |
| Isolectin B4 | NR | ~7% (Dox-sol); ~1%(Dos-np) | ~86% at 18th day | |||
| Microvascular proliferation: | H&E | NR | 1 score (Dox-sol); 0 score (Dox-np) | NA at 18th day | ||
| Saito et al. [38] | Laminin | IHC; WB | BV: control ≈ free-TPT | BV: Ls-TPT< free-TPT | NA at 14th day | Marked decrease in blood vessels in Ls-TPT group, as well as hypophosphoriylated Akt, whereas control and free TPT shower high density of blood vessels |
| p-Akt | WB | NR | Ls-TPT< free-TPT or control | NA at 14th day |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nery de Albuquerque Rego, G.; da Hora Alves, A.; Penteado Nucci, M.; Bustamante Mamani, J.; Anselmo de Oliveira, F.; Gamarra, L.F. Antiangiogenic Targets for Glioblastoma Therapy from a Pre-Clinical Approach, Using Nanoformulations. Int. J. Mol. Sci. 2020, 21, 4490. https://doi.org/10.3390/ijms21124490
Nery de Albuquerque Rego G, da Hora Alves A, Penteado Nucci M, Bustamante Mamani J, Anselmo de Oliveira F, Gamarra LF. Antiangiogenic Targets for Glioblastoma Therapy from a Pre-Clinical Approach, Using Nanoformulations. International Journal of Molecular Sciences. 2020; 21(12):4490. https://doi.org/10.3390/ijms21124490
Chicago/Turabian StyleNery de Albuquerque Rego, Gabriel, Arielly da Hora Alves, Mariana Penteado Nucci, Javier Bustamante Mamani, Fernando Anselmo de Oliveira, and Lionel Fernel Gamarra. 2020. "Antiangiogenic Targets for Glioblastoma Therapy from a Pre-Clinical Approach, Using Nanoformulations" International Journal of Molecular Sciences 21, no. 12: 4490. https://doi.org/10.3390/ijms21124490
APA StyleNery de Albuquerque Rego, G., da Hora Alves, A., Penteado Nucci, M., Bustamante Mamani, J., Anselmo de Oliveira, F., & Gamarra, L. F. (2020). Antiangiogenic Targets for Glioblastoma Therapy from a Pre-Clinical Approach, Using Nanoformulations. International Journal of Molecular Sciences, 21(12), 4490. https://doi.org/10.3390/ijms21124490