Biotechnological Strategies for Chitosan Production by Mucoralean Strains and Dimorphism Using Renewable Substrates
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis of Cassava Waste Water (CWW) and Corn Steep Liquor (CSL)
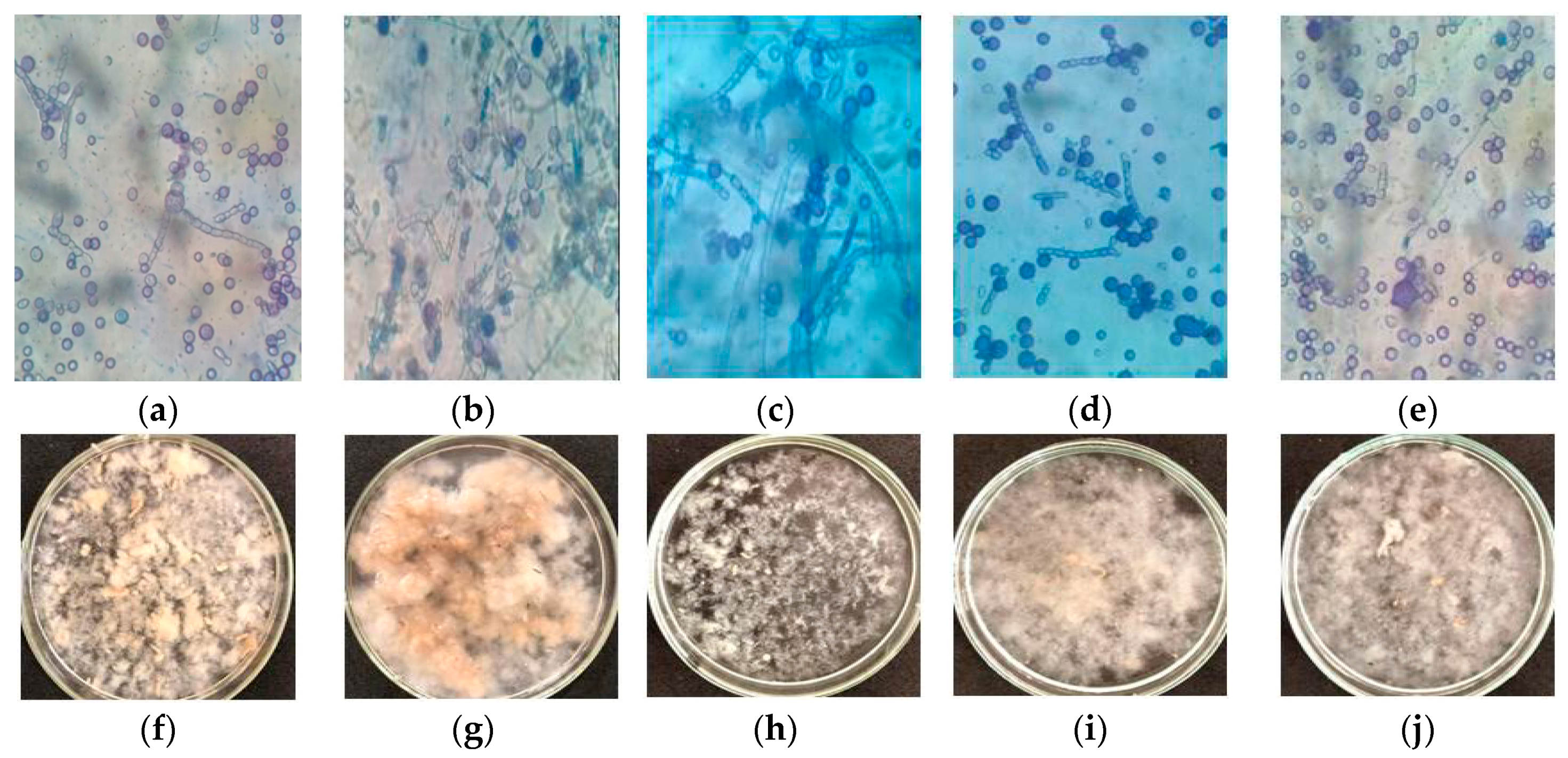
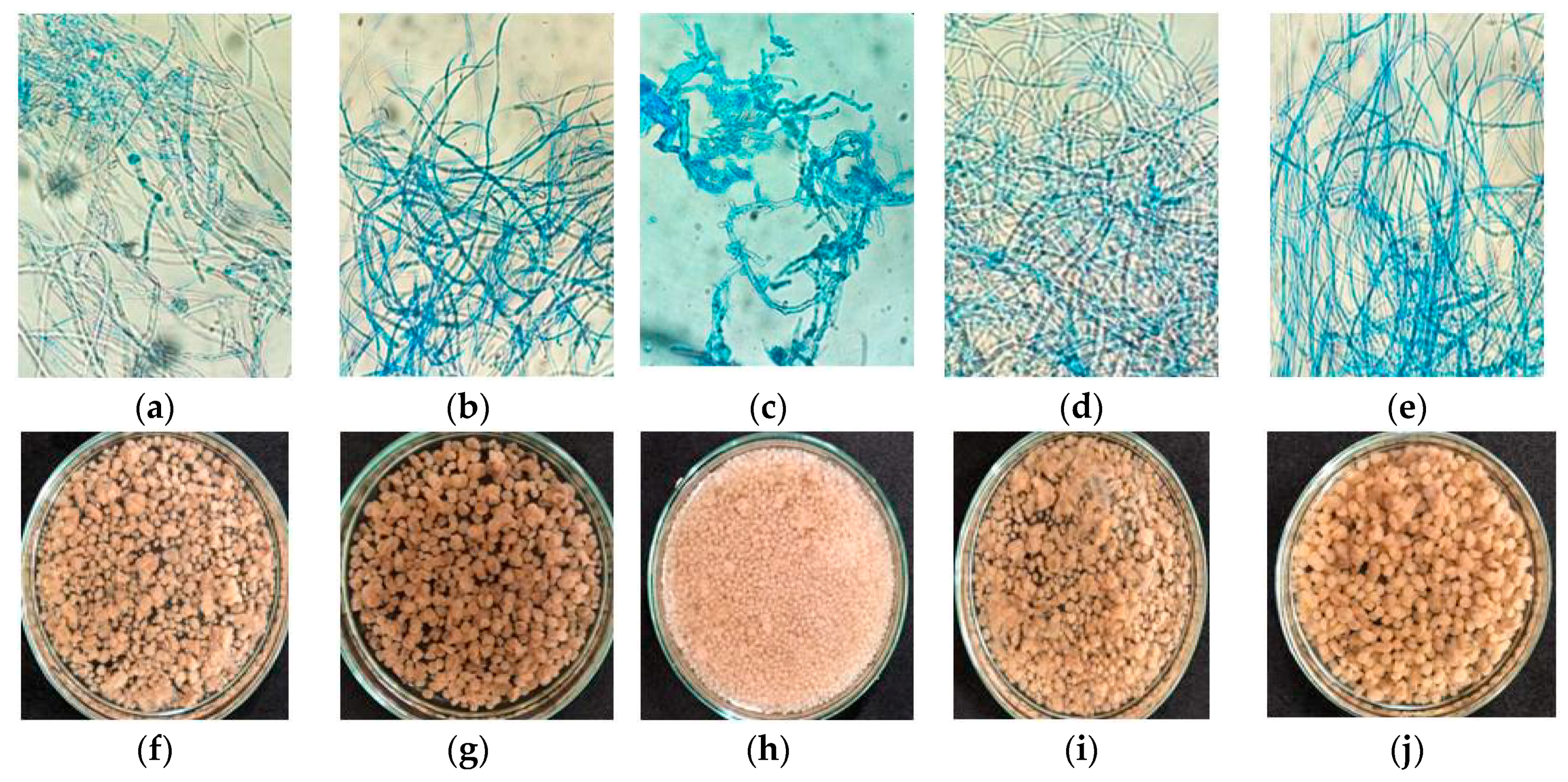
2.2. Effect of Substrate Concentrations on Fungal Morphology
2.3. Effect of Substrates on Biomass and Chitosan Production by M. subtilissimus (UCP 1262) and L. hyalospora (UCP 1266)
2.4. Characterization of Chitosan Extracted from M. subtilissimus (UCP 1262) and L. hyalospora (UCP 1266)
Degree of Deacetylation
2.5. Optimization of Chitosan Production by L. hyalospora (UCP 1266)
3. Materials and Methods
3.1. Microorganisms
3.2. Substrates
3.3. Conditions of Culture and Biomass Production
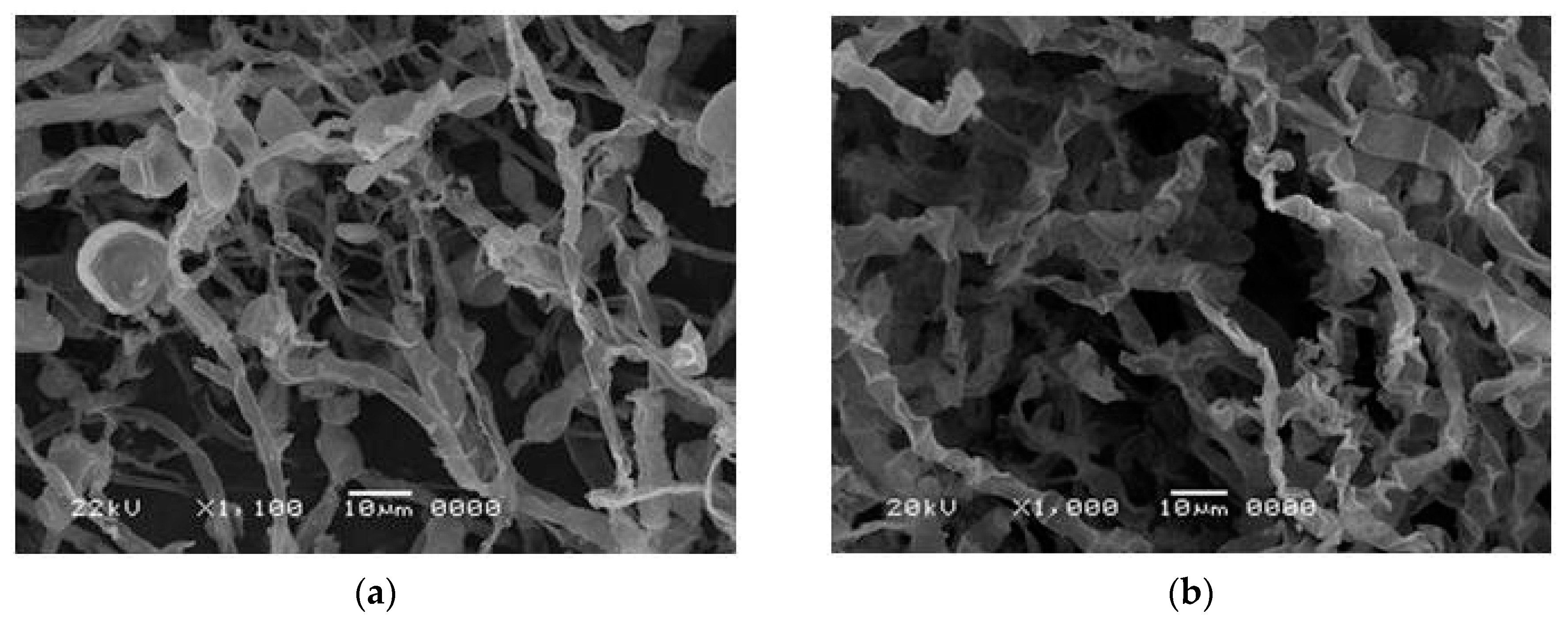
3.4. Morphological Analysis in Scanning Electron Microscopy (SEM)
3.5. Extration Chitosan
3.6. Characterization of Chitosan
3.6.1. Infrared Spectroscopy (FTIR)
3.6.2. Deacetylation Degree
3.6.3. Viscosity Determination
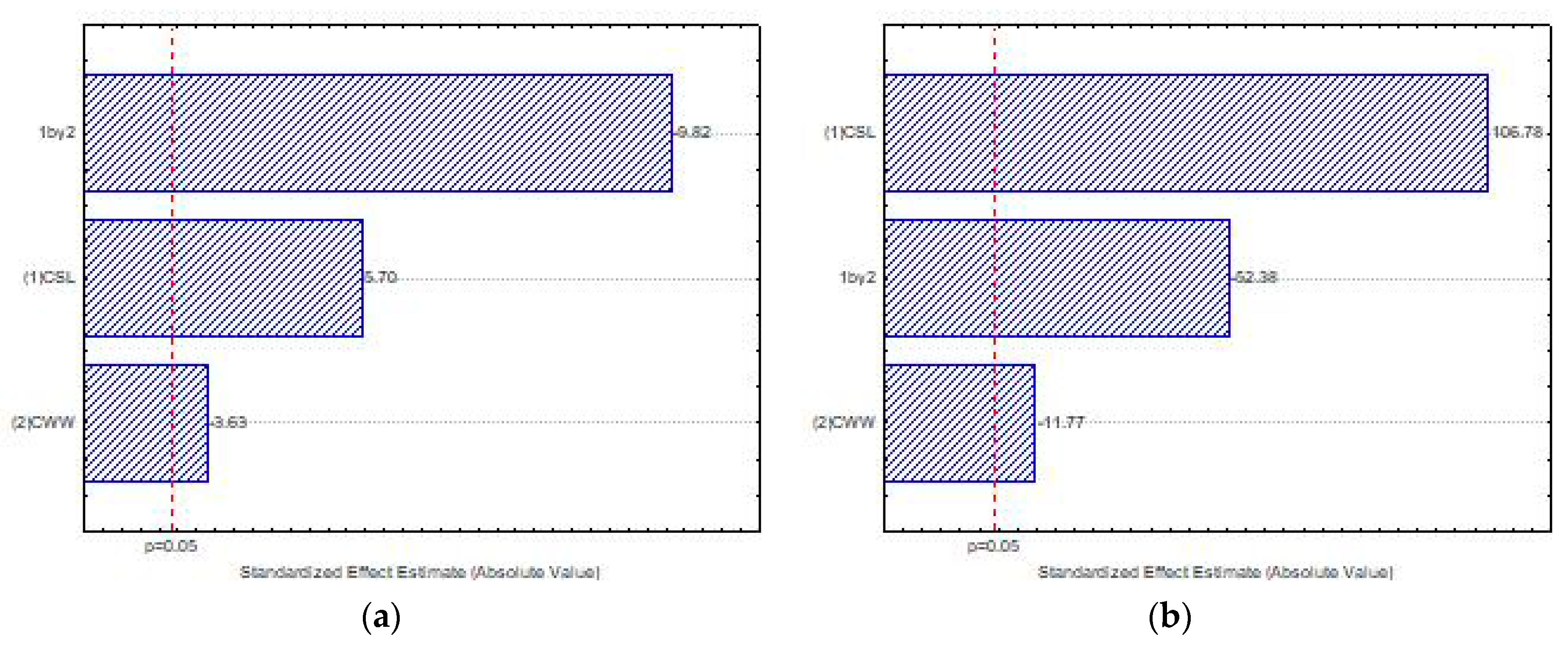
3.7. Selection of Waste Concentrations in Culture Using Factory Design
3.8. Central Composite Rotational Design (CCRD) for Optimization of Chitosan Production by Lichtheimia hyalospora (UCP 1266)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Sobczak-Kupiec, A. Sustainable Production of Chitosan. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems; Springer: Cham, Switzerland, 2020; pp. 45–60. [Google Scholar]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, Properties and Applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Badawy, M.E.; Rabea, E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011, 2011, 460381. [Google Scholar] [CrossRef]
- Mondala, A.; Al-Mubarak, R.; Atkinson, J.; Shields, S.; Young, B.; Senger, Y.D.S.; Pekarovic, J. Direct Solid-State Fermentation of Soybean Processing Residues for the Production of Fungal Chitosan by Mucorrouxii. J. Mater. Sci. Chem. Eng. 2015, 3, 11. [Google Scholar]
- Vaingankar, P.N.; Juvekar, A.R. Fermentative production of mycelial chitosan from zygomycetes: Media optimization and physico-chemical characterization. Adv. Biosci. Biotechnol. 2014, 5, 940. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; James, T.Y. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Amorim, R.V.S.; Souza, W.; Fukushima, K.; Campos-Takaki, G.M. Faster chitosan production by Mucorelean strains in submerged culture. Braz. J. Microbiol. 2001, 32, 20–23. [Google Scholar] [CrossRef]
- Naghdi, M.; Zamani, A.; Karimi, K. A sulfuric-lactic acid process for efficient purification of fungal chitosan with intact molecular weight. Int. J. Biol. Macromol. 2014, 63, 158–162. [Google Scholar] [CrossRef]
- Amorim, R.V.S.; Ledingham, W.M.; Kennedy, J.F.; Campos-Takaki, G.M. Chitosan from Syncephalastrum racemosum using sugar cane substrates as inexpensive carbon sources. Food Biotechnol. 2006, 20, 43–53. [Google Scholar] [CrossRef]
- Akila, R.M. Fermentative production of fungal Chitosan, a versatile biopolymer (perspectives and its applications). Adv. Appl. Sci. Res. 2014, 5, 157–170. [Google Scholar]
- Satari, B.; Karimi, K.; Zamani, A. Oil, chitosan, and ethanol production by dimorphic fungus Mucorindicus from different lignocelluloses. J. Chem. Technol. Biotechnol. 2016, 91, 1835–1843. [Google Scholar] [CrossRef]
- Zininga, J.T.; Puri, A.K.; Govender, A.; Singh, S.; Permaul, K. Concomitant production of chitosan and lipids from a newly isolated Mucor circinelloides ZSKP for biodiesel production. Bioresour. Technol. 2019, 272, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Dhania, G. Cadmium as an Environmental Pollutant: Ecotoxicological Effects, Health Hazards, and Bioremediation Approaches for Its Detoxification from Contaminated Sites. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020; pp. 357–387. [Google Scholar]
- Tan, S.C.; Tan, T.K.; Wong, S.M.; Khor, E. The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr. Polym. 1996, 30, 239–242. [Google Scholar] [CrossRef]
- White, S.A.; Farina, P.R.; Fulton, I. Production and isolation of chitosan from Mucorrouxii. Appl. Environ. Microbiol. 1979, 38, 323–328. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J. Fungal Cell Wall: Structure, Synthesis, and Assembly; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Karimi, K.; Zamani, A. Mucorindicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Bartnicki-Garcia, S.; Nickerson, W. JInduction of yeastlike development in Mucor by carbon dioxide. J. Bacteriol. 1962, 84, 829–840. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S.; Nickerson, W.J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucorrouxii. Biochim. Biophys. 1962, 58, 102–119. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S. Control of dimorphism in Mucor by hexoses: Inhibition of hyphal morphogenesis. J. Bacteriol. 1968, 96, 1586–1594. [Google Scholar] [CrossRef]
- Sharifia, M.; Karimi, K.; Taherzadeh, M.J. Production of ethanol by filamentous and yeast-like forms of Mucorindicus from fructose, glucose, sucrose, and molasses. J. Ind. Microbiol. Biotechnol. 2008, 35, 1253–1259. [Google Scholar] [CrossRef]
- Berger, L.; Stamford, T.; Stamford-Arnaud, T.; de Oliveira Franco, L.; do Nascimento, A.; Cavalcante, H.; de Campos-Takaki, G. Effect of corn steep liquor (CSL) and cassava waste water (CWW) on chitin and chitosan production by Cunninghamella elegans and their physicochemical characteristics and cytotoxicity. Molecules 2014, 19, 2771–2792. [Google Scholar] [CrossRef]
- Araújo, N.C.D.; Lima, V.L.A.; Ramos, J.G.; Andrade, E.M.G.; Lima, G.S.D.; Oliveira, S.J.C. Contents of macronutrients and growth of ‘BRS Marataoã’cowpeafertigated with yellow water and cassava waste water. Environ. Water 2019, 14. [Google Scholar] [CrossRef]
- Metz, B.; Kossen, N.W.F. The growth of molds in the form of pellets—A literature review. Biotechnol. Bioeng. 1977, 19, 781–799. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Lee, W.G.; Theodore, K.; Chang, H.N. Optimization of culture conditions and continuous production of chitosan by the fungi, Absidiacoerulea. Biotechnol. Bioprocess Eng. 2001, 6, 6–10. [Google Scholar] [CrossRef]
- Gow, N.A.; Gadd, G.M. (Eds.) Growing Fungus; Chapman & Hall: London, UK, 2007. [Google Scholar]
- Sitanggang, A.B.; Wu, H.S.; Wang, S.S.; Ho, Y.C. Effect of pellet size and stimulating factor on the glucosamine production using Aspergillus sp. BCRC 31742. Bioresour. Technol. 2010, 101, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Pirt, S.J.; Callow, D.S. Continuous-flow culture of the filamentous mould Penicillium chrysogenum and the control of its morphology. Nature 1959, 184, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.R.R.; Cardoso, A.; Stamford, T.C.M.; Cavalcante, H.M.M.; Macedo, R.O.; Campos-Takaki, G.M. Agroindustrial waste as alternative medium in the production of chitin and chitosan by Rhizopus arrhizus—A factorial design. Asian Chitin J. 2011, 7, 83–90. [Google Scholar]
- Souza, A.; Rodriguez, D.; Ribeaux, D.; Luna, M.; Lima e Silva, T.; Andrade, R.; Campos-Takaki, G. Waste soybean oil and corn steep liquor as economic substrates for bioemulsifier and biodiesel production by Candida lipolytica UCP 0998. Int. J. Mol. Sci. 2016, 17, 1608. [Google Scholar] [CrossRef]
- Cardoso, A.; Lins, C.I.M.; dos Santos, E.R.; Silva, M.C.F.; Campos-Takaki, G.M. Microbial enhance of chitosan production by Rhizopusarrhizus using agroindustrial substrates. Molecules 2012, 17, 4904–4914. [Google Scholar] [CrossRef]
- Cardoso, A.; Marques, A.; Campos Marinho, P.H.; Shiosaki, R.K.; Campos Takaki, G.M. Influence of the heavy metals on chitosan production by Absidiacorymbifera UCP 0134. In Microorganisms in Industry and Environment: From Scientific and Industrial Research to Consumer Products; World Scientific: Singapore, 2011; pp. 176–180. [Google Scholar]
- Santos, E.; da Silva, M.; de Souza, P.; da Silva, A.; de Paiva, S.; Albuquerque, C.; Campos-Takaki, G. Enhancementof Cunninghamella elegans UCP/WFCC 0542 biomassa and chitosanwith amino acidsupply. Molecules 2013, 18, 10095–10107. [Google Scholar] [CrossRef]
- Berger, L.; Stamford, T.; Stamford-Arnaud, T.; de Alcântara, S.; da Silva, A.; da Silva, A.; Campos-Takaki, G. Green conversion of agroindustrial wastes into chitin and chitosan by Rhizopusarrhizus and Cunninghamella elegans strains. Int. J. Mol. Sci. 2014, 15, 9082–9102. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Chandrkrachang, S.; Stevens, W.F.; Maw, T.; Tan, T.K.; Khor, E.; Wong, S.M. Production of fungal chitosan by solid state and submerged fermentation. Carbohydr. Polym. 2002, 49, 235–237. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef]
- Oliveira, C.E.V.; Magnani, M.; de Sales, C.V.; de Souza Pontes, A.L.; Campos-Takaki, G.M.; Stamford, T.C.M.; de Souza, E.L. Effects of chitosan from Cunninghamella elegans on virulence of post-harvest pathogenic fungi in table grapes (Vitislabrusca L.). Int. J. Food Microbiol. 2014, 171, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.V.; Stamford, T.C.; Stamford-Arnaud, T.M.; Lima, J.M.N.; Silva, A.M.; Okada, K.; Campos-Takaki, G.M. Conversion of agro-industrial wastes to chitosan production by Syncephalastrum racemosum UCP 1302. Int. J. Appl. Res. Nat. Prod. 2008, 8, 5–11. [Google Scholar]
- Lins, C.I.M.; Cardoso, A.; Silva, M.C.F.; Batista, A.C.L.; Jara, A.M.A.T.; Berger, L.R.R.; Campos-Takaki, G.M. Evaluation of chitin and chitosan by different extraction methods from mucoralean fungi biomass. Asian Chitin J. 2010, 6, 5–8. [Google Scholar]
- Sparringa, R.A.; Owens, J.D. Glucosamine content of tempemould, Rhizopusoligosporus. Int. J. Food Microbiol. 1999, 47, 153–157. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S. Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material–dairy manure–using a pelletized filamentous fungus Rhizopusoryzae ATCC 20344. Bioresour. Technol. 2008, 99, 5859–5866. [Google Scholar] [CrossRef]
- Denardi-Souza, T.; Massarolo, K.C.; Tralamazza, S.M.; Badiale-Furlong, E. Monitoring of fungal biomass changed by Rhizopusoryzae in relation to amino acid and essential fatty acids profile in soybean meal, wheat and rice. CyTA-J. Food 2018, 16, 156–164. [Google Scholar] [CrossRef]
- Kroll, K.; Paehtz, V.; Kniemeyer, O. Elucidating the fungal stress response by proteomics. J. Proteom. 2014, 97, 151–163. [Google Scholar] [CrossRef]
- Berger, L.R.R.; Stamford, T.C.M.; de Oliveira, K.Á.R.; Pessoa, A.D.M.P.; de Lima, M.A.B.; Pintado, M.M.E.; de Souza, E.L. Chitosan produced from Mucorales fungi using agroindustrial by-products and its efficacy to inhibit Colletotrichum species. Int. J. Biol. Macromol. 2018, 108, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Fai, A.E.C.; Stamford, T.; Stamford-Arnaud, T.M.; Santa-Cruz, P.D.; Silva, M.C.; Campos-Takaki, G.M.; Stamford, T.L. Physico-chemical characteristics and functional properties of chitin and chitosan produced by Mucorcircinelloides using yam bean as substrate. Molecules 2011, 16, 7143–7154. [Google Scholar] [CrossRef] [PubMed]
- Tajdini, F.; Amini, M.A.; Nafissi-Varcheh, N.; Faramarzi, M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucormiehei and Mucorracemosus. Int. J. Biol. Macromol. 2010, 47, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rath, P.K. Extraction and characterization of chitin and chitosan from (Labeorohit) fish scales. Procedia Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef]
- Zvezdova, D. Synthesis and characterization of chitosan from marine sources in Black Sea. Annu. Proc. Angel Kanchev Univ. Ruse 2010, 49, 65–69. [Google Scholar]
- Dakia, P.A.; Blecker, C.; Robert, C.; Wathelet, B.; Paquot, M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocoll. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.; Opwis, K.; Knittel, D.; Schollmeyer, E.; Nickisch-Hartfiel, A. Inhibition of microbial pathogens by fungal chitosan. Int. J. Biol. Macromol. 2010, 47, 10–14. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Sarma, S.J.; Brar, S.K. Integrated process for fungal citric acid fermentation using apple processing wastes and sequential extraction of chitosan from waste stream. Ind. Crops Prod. 2013, 50, 346–351. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Lai, C.; Fan, Y.; Ouyang, J.; Yong, Q. Fungal chitosan production using xylose rich of corn stoverprehydrolysate by Rhizopusoryzae. Biotechnol. Biotechnol. Equip. 2017, 31, 1160–1166. [Google Scholar] [CrossRef]
- Latha, S.; Suresh, G. Studies on chitosan production from different fungal mycelium. Growth 2013, 564, 189. [Google Scholar]
- Hu, K.J.; Hu, J.L.; Ho, K.P.; Yeung, K.W. Screening of fungi for chitosan producers, and copper adsorption capacity of fungal chitosan and chitosanaceous materials. Carbohydr. Polym. 2004, 58, 45–52. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
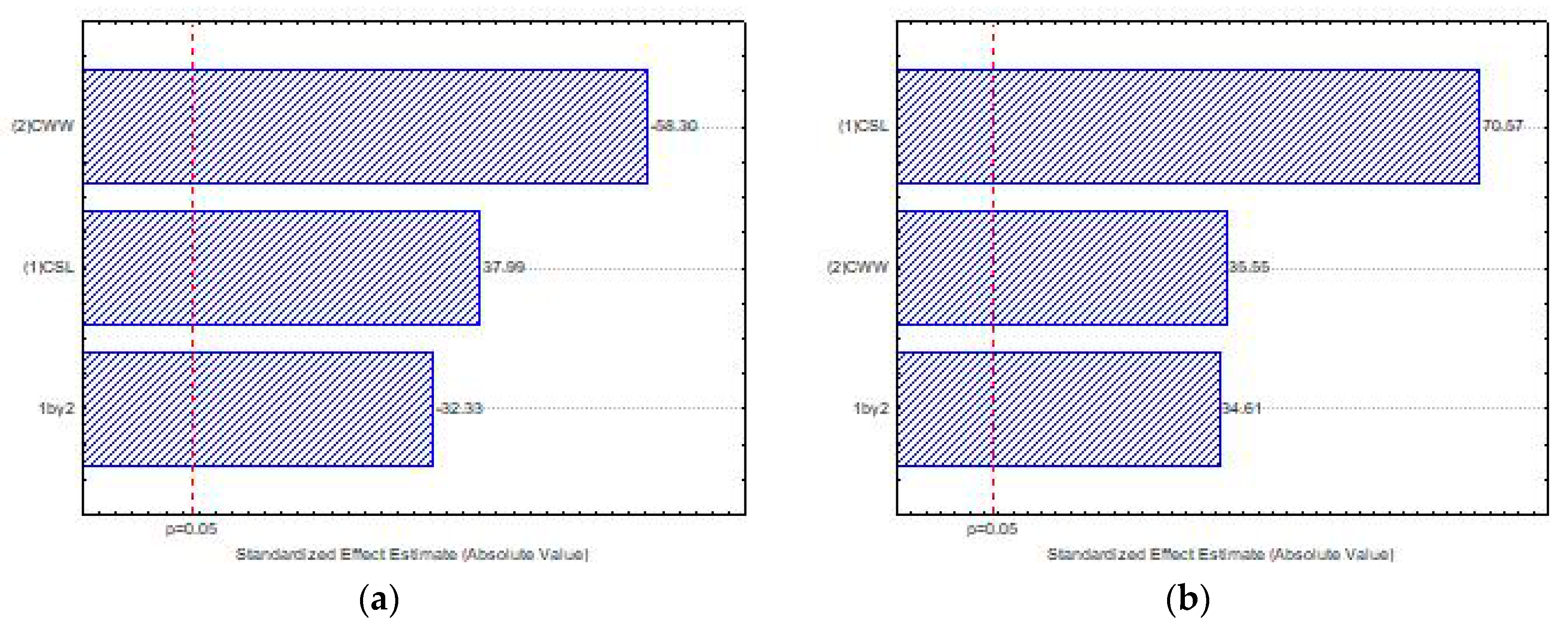

| Substrate | Carbon | Nitrogen | Oxygen | Sulfur |
|---|---|---|---|---|
| Cassava Waste Water (CWW) | 33.35 | 2.04 | 6.74 | 0 |
| Corn Steep Liquor (CSL) | 34.84 | 7.06 | 6.59 | 1.18 |
| Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Corn Steep Liquor—CSL (%, v/v) | 2 | 4 | 6 |
| Cassava Waste Water—CWW (%, v/v) | 4 | 6 | 8 |
| Assays | Substrates | Mucor subtilissimus (UCP 1262) | Lichtheimia hyalospora (UCP 1266) | |||||
|---|---|---|---|---|---|---|---|---|
| CSL | CWW | pH | Biomass (g/L) | Chitosan (mg/g) | pH | Biomass (g/L) | Chitosan(mg/g) | |
| 1 | 2 | 4 | 7.1 | 1.725 | 18.87 | 5.8 | 2.675 | 23.31 |
| 2 | 6 | 4 | 6.2 | 4.832 * | 32.41 * | 5.2 | 6.540 * | 29.84 |
| 3 | 2 | 8 | 6.9 | 2.963 | 13.87 | 5.8 | 3.661 | 23.49 |
| 4 | 6 | 8 | 6.9 | 2.140 | 14.96 | 5.6 | 4.982 | 42.56 |
| 5 | 4 | 6 | 6.4 | 4.752 | 19.36 | 5.1 | 6.345 | 44.60 |
| 6 | 4 | 6 | 6.2 | 4.527 | 19.02 | 5.2 | 6.298 | 44.91 |
| 7 | 4 | 6 | 6.4 | 4.269 | 18.92 | 5.2 | 6.291 | 44.86 |
| 8 | 4 | 6 | 6.4 | 4.582 | 19.18 | 5.1 | 6.319 | 45.03 * |
| Fungal Strain | Medium Composition | Cultural Conditions | Biomass (g/L) | Chitosan (mg/g) | References |
|---|---|---|---|---|---|
| Mucor subtilissimus UCP 1262 | 6% CSL and 4% CWW | SmF, 28 °C, 150 rpm, 120 h | 4.83 | 32.41 | Present study |
| Lichtheimia hyalospora UCP 1266 | 4% CSL and 6% CWW | SmF, 28 °C, 150 rpm, 120 h | 6.298 | 44.91 | Present study |
| L. hyalospora UCP 1266 | 1% CSL and 25% papaya peel juice | SmF, 28 °C 150 rpm, 96 h | - | 12.04 | Kroll et al. [46] |
| Cunninghamella elegans UCP 0542 | 9.43% CSL and 42.5% papaya peel juice | SmF, 28 °C, 150 rpm, 96 h | - | 37.25 | Kroll et al. [46] |
| C. elegans UCP 0542 | 10% CWW and 4% CSL | SmF, 28 °C, 150 rpm, 72 h | 5.67 | 57.82 | Sharifia et al. [23] |
| Rhizopu sarrhizus UCP 0402 | 6% CSL and 13.24% honey | SmF, 28 °C, 150 rpm, 96 h | 11.71 | 29.30 | Berger et al. [32] |
| Syncephalastrum racemosum UCP 1302 | 8% CSL and 2% sugarcane bagasse | SSF, 28 °C, 96 h | 32.0 | 25.0 | Oliveira et al. [40] |
| Mucor circinelloides UCP 0050 | Yam bean medium | SmF, 28 °C, 150 rpm, 96 h | 20.7 | 64.00 | Berger et al. [47] |
| Mucor rouxii ATCC 24905 | Soybean meal | SSF, 25 °C, 144 h | - | 34.40 | Mondala et al. [5] |
| Rhizomucor miehei (ATCC 26282) | Sabouraud broth | SmF, 28 °C, 120 rpm, 168 h | 4.1 | 13.67 | Fai et al. [48] |
| Mucor racemosus | Sabouraud broth | SmF, 28 °C, 120 rpm, 168 h | 3.8 | 11.72 | Fai et al. [48] |
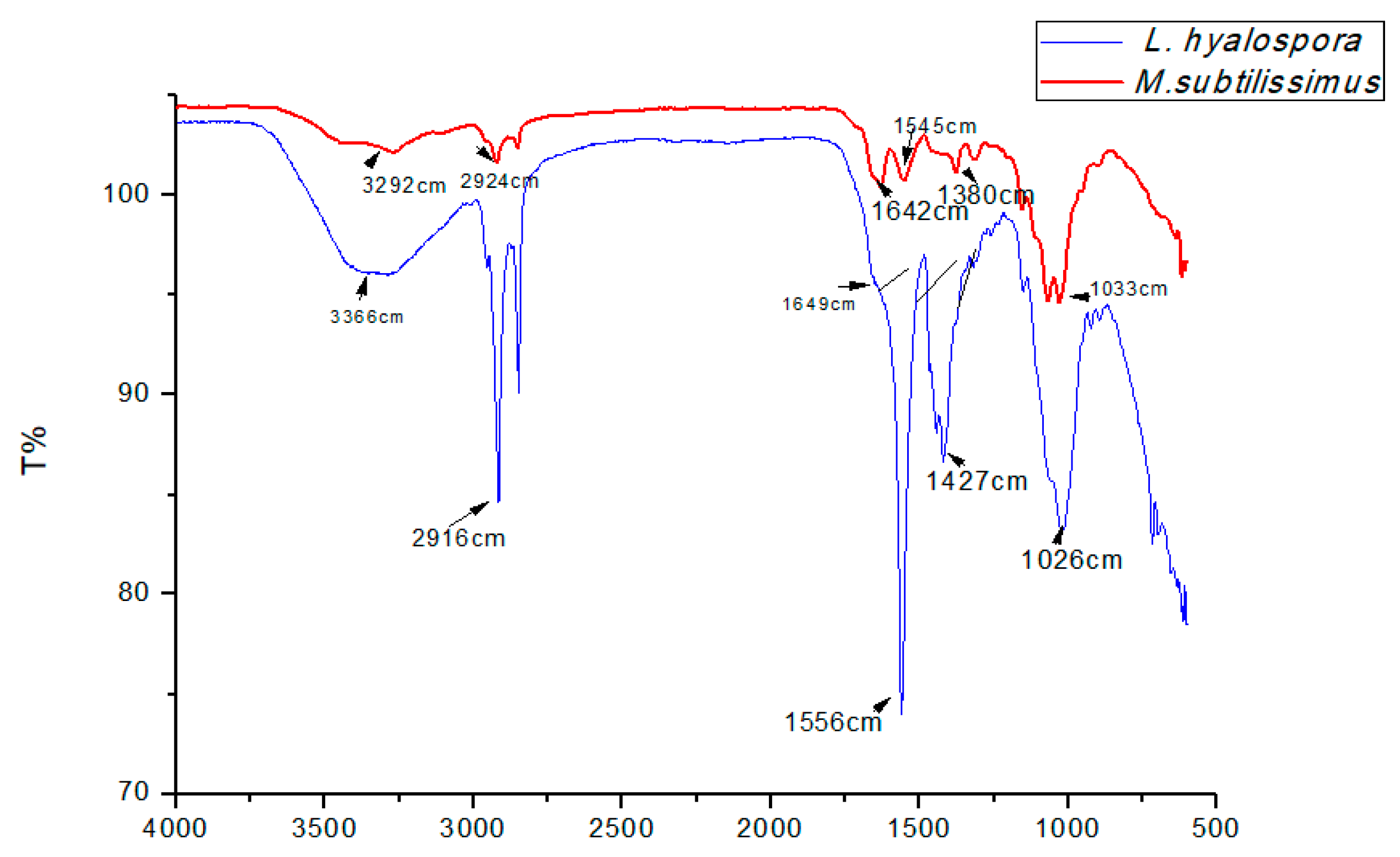
| Biopolymers | Infrared Spectroscopy | Degree of Deacetylation (DD%) | Viscosity (cP) |
|---|---|---|---|
| Chitosan from L. hyalospora (UCP 1266) | 1649–1556 | 83.61 | 2.78 |
| Chitosan from M. subtilissimus (UCP 1262) | 1642–1545 | 80.28 | 3.06 |
| Assays | CSL (%) | CWW (%) | pH | Biomass (g/L) | Chitosan (mg/g) | Chitosan (mg/L) |
|---|---|---|---|---|---|---|
| 1 | 4 | 6 | 5.4 | 6.32 | 44.88 | 283.64 |
| 2 | 4 | 8 | 5.6 | 5.59 | 46.83 | 261.78 |
| 3 | 8 | 6 | 5.4 | 9.34 | 51.98 | 485.49 |
| 4 | 8 | 8 | 5.4 | 8.79 | 54.03 | 474.92 |
| 5 | 3.17 | 7 | 5.8 | 5.48 | 46.89 | 256.96 |
| 6 | 8.82 | 7 | 4.8 | 11.87 | 63.18 | 749.95 |
| 7 | 6 | 5.58 | 5.4 | 7.81 | 48.09 | 375.58 |
| 8 | 6 | 8.41 | 5.4 | 7.26 | 57.81 | 437.04 |
| 9 | 6 | 7 | 4.8 | 9.14 | 58.46 | 534.32 |
| 10 | 6 | 7 | 5.1 | 9.09 | 58.71 | 533.67 |
| 11 | 6 | 7 | 5.2 | 8.91 | 58.42 | 520.52 |
| 12 | 6 | 7 | 4.8 | 9.19 | 58.90 | 541.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.F.; Galindo, H.M.; de Lima, M.A.B.; Ribeaux, D.R.; Rodríguez, D.M.; da Silva Andrade, R.F.; Gusmão, N.B.; de Campos-Takaki, G.M. Biotechnological Strategies for Chitosan Production by Mucoralean Strains and Dimorphism Using Renewable Substrates. Int. J. Mol. Sci. 2020, 21, 4286. https://doi.org/10.3390/ijms21124286
de Souza AF, Galindo HM, de Lima MAB, Ribeaux DR, Rodríguez DM, da Silva Andrade RF, Gusmão NB, de Campos-Takaki GM. Biotechnological Strategies for Chitosan Production by Mucoralean Strains and Dimorphism Using Renewable Substrates. International Journal of Molecular Sciences. 2020; 21(12):4286. https://doi.org/10.3390/ijms21124286
Chicago/Turabian Stylede Souza, Adriana Ferreira, Hugo Marques Galindo, Marcos Antônio Barbosa de Lima, Daylin Rubio Ribeaux, Dayana Montero Rodríguez, Rosileide Fontenele da Silva Andrade, Norma Buarque Gusmão, and Galba Maria de Campos-Takaki. 2020. "Biotechnological Strategies for Chitosan Production by Mucoralean Strains and Dimorphism Using Renewable Substrates" International Journal of Molecular Sciences 21, no. 12: 4286. https://doi.org/10.3390/ijms21124286
APA Stylede Souza, A. F., Galindo, H. M., de Lima, M. A. B., Ribeaux, D. R., Rodríguez, D. M., da Silva Andrade, R. F., Gusmão, N. B., & de Campos-Takaki, G. M. (2020). Biotechnological Strategies for Chitosan Production by Mucoralean Strains and Dimorphism Using Renewable Substrates. International Journal of Molecular Sciences, 21(12), 4286. https://doi.org/10.3390/ijms21124286






