Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K+ Current
Abstract
1. Introduction
2. Results
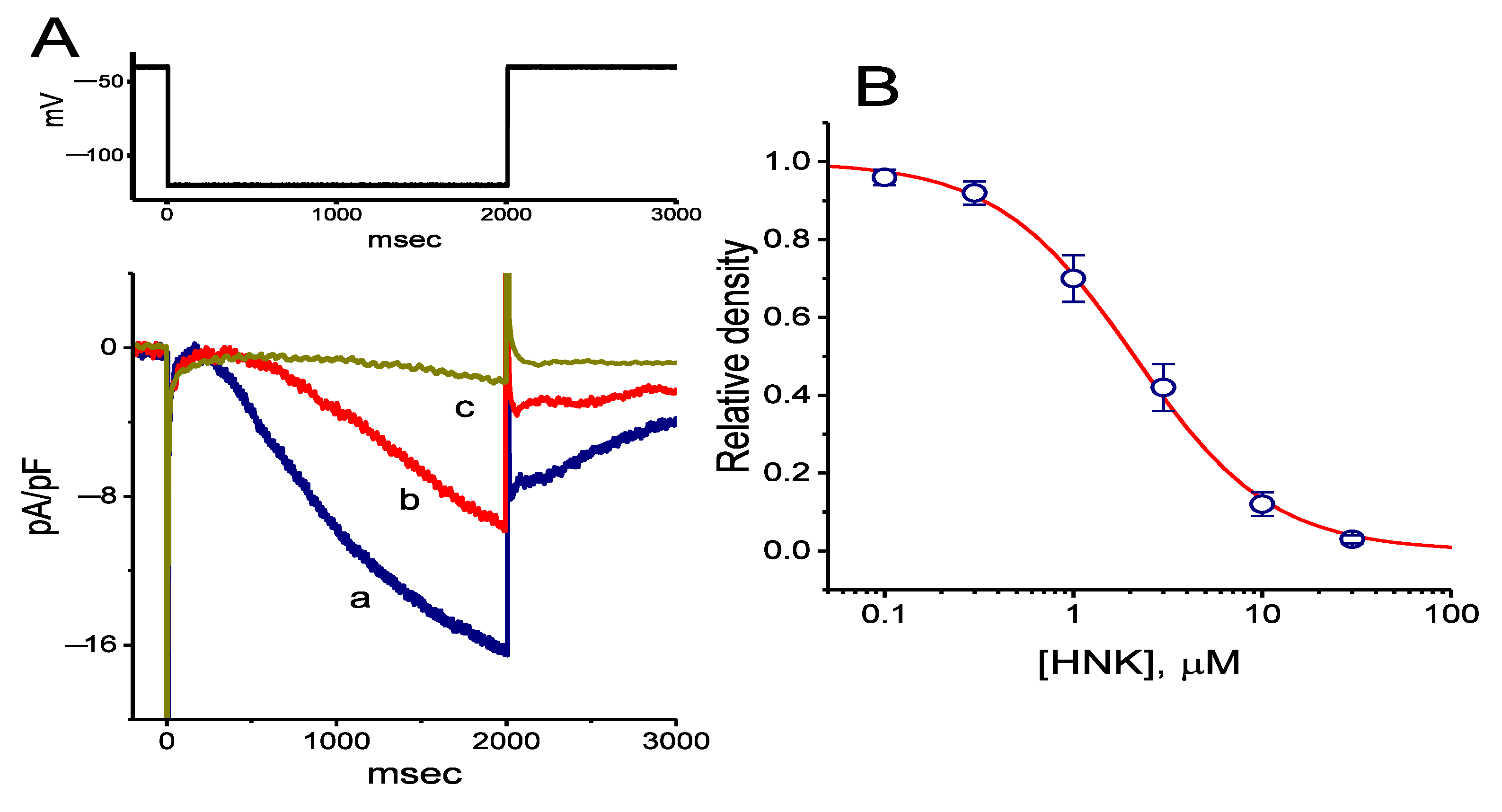
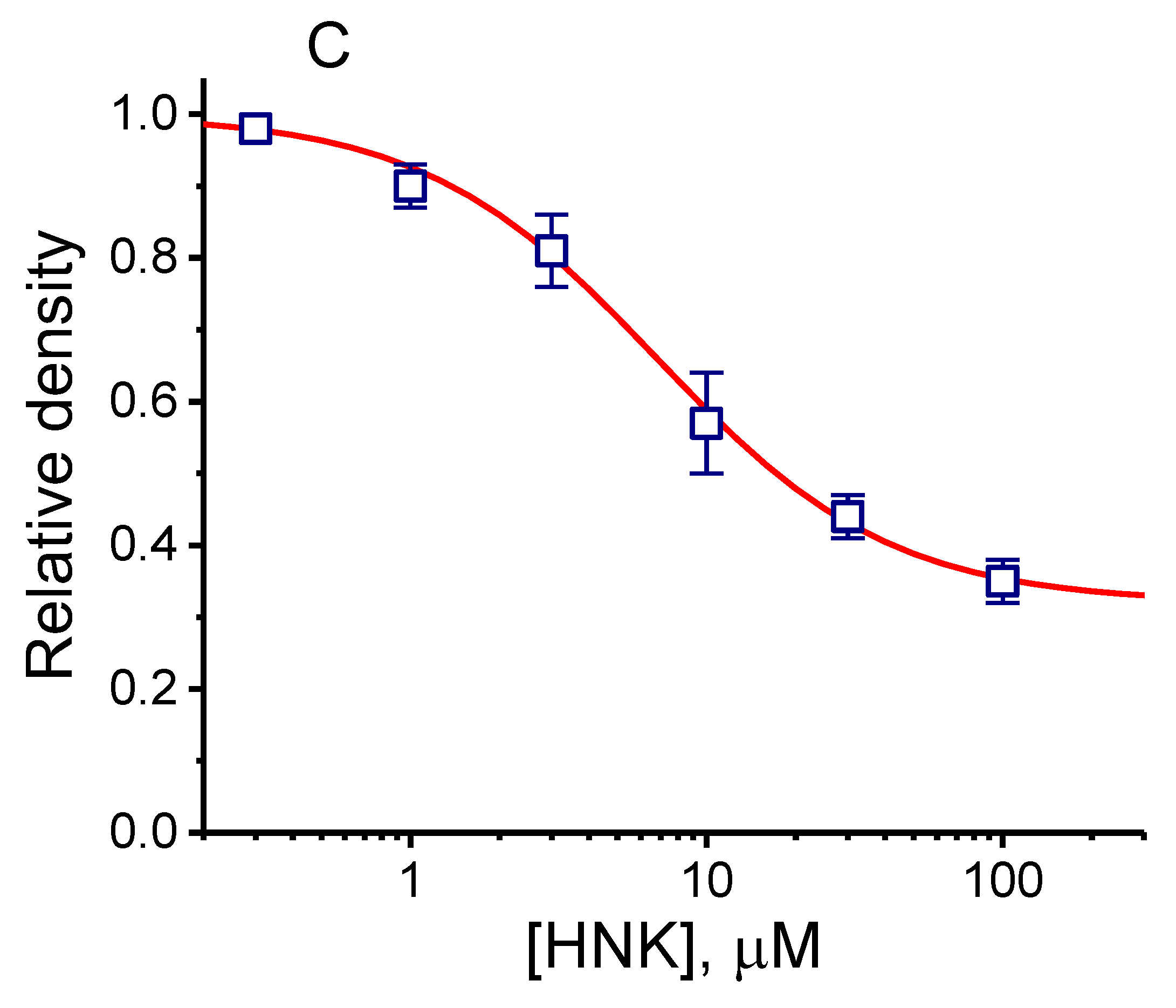
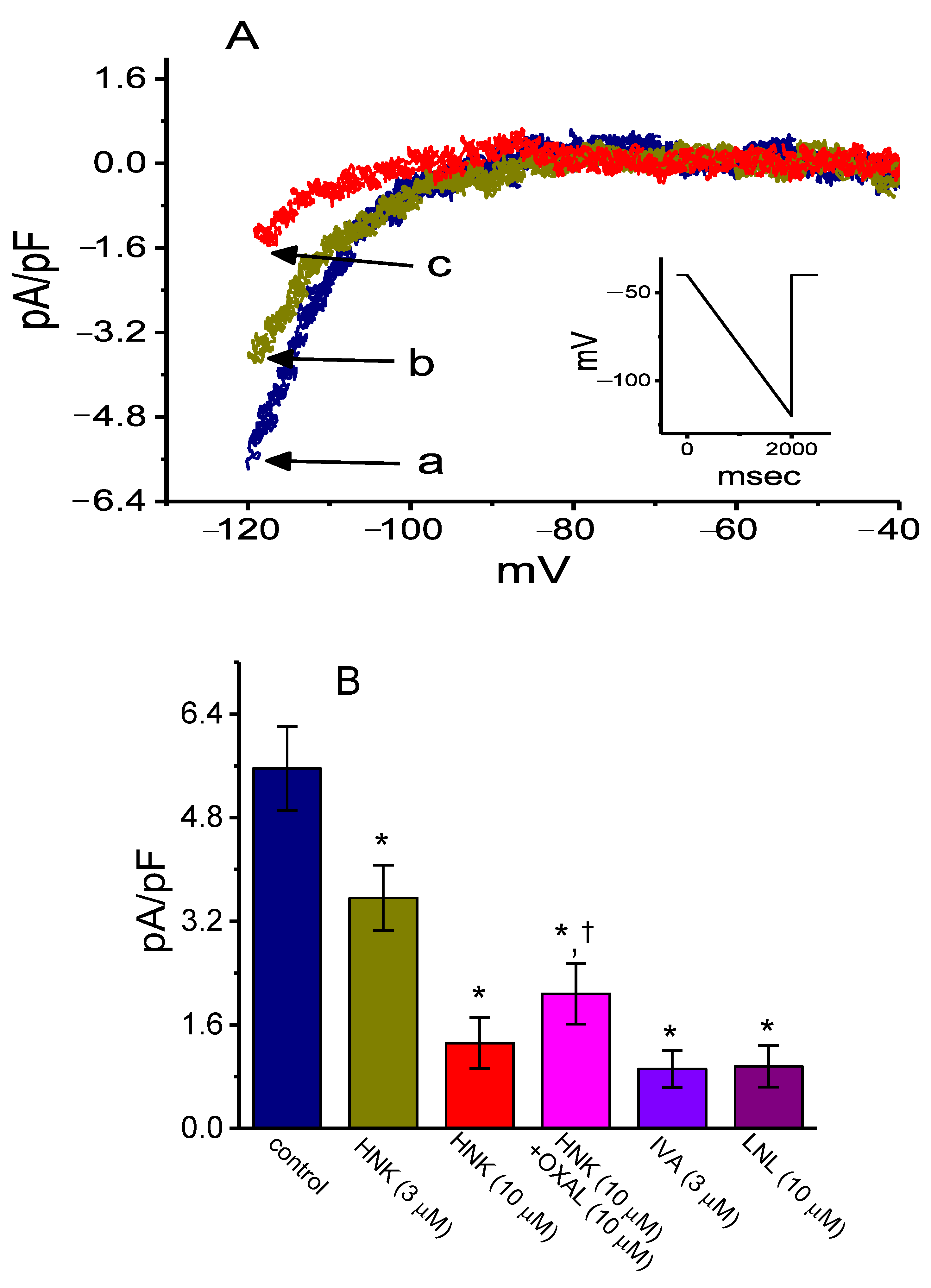
2.1. Effect of HNK on the Density of Hyperpolarization-Activated Cation Current (Ih) in GH3 Cells
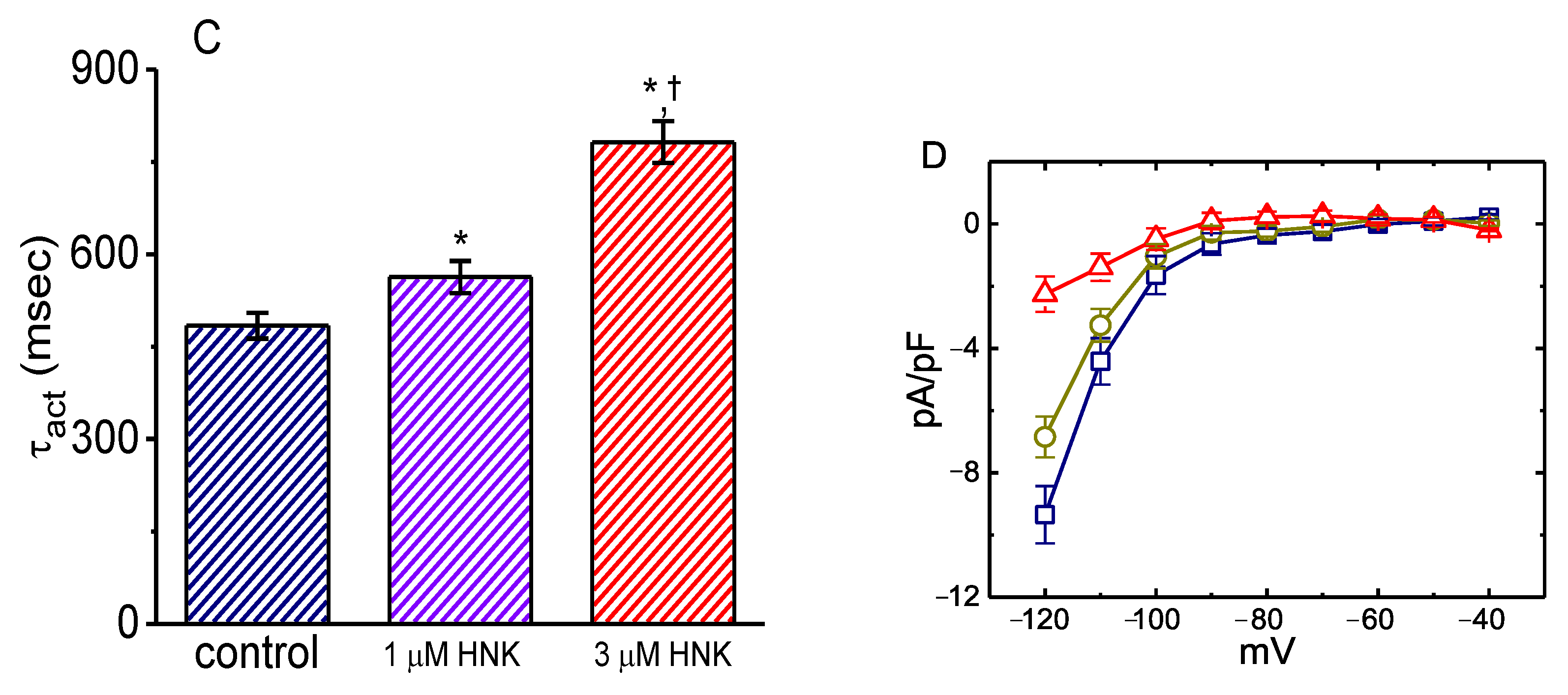
2.2. Modification of the Steady-State Activation Curve of Ih Produced by the Presence of HNK
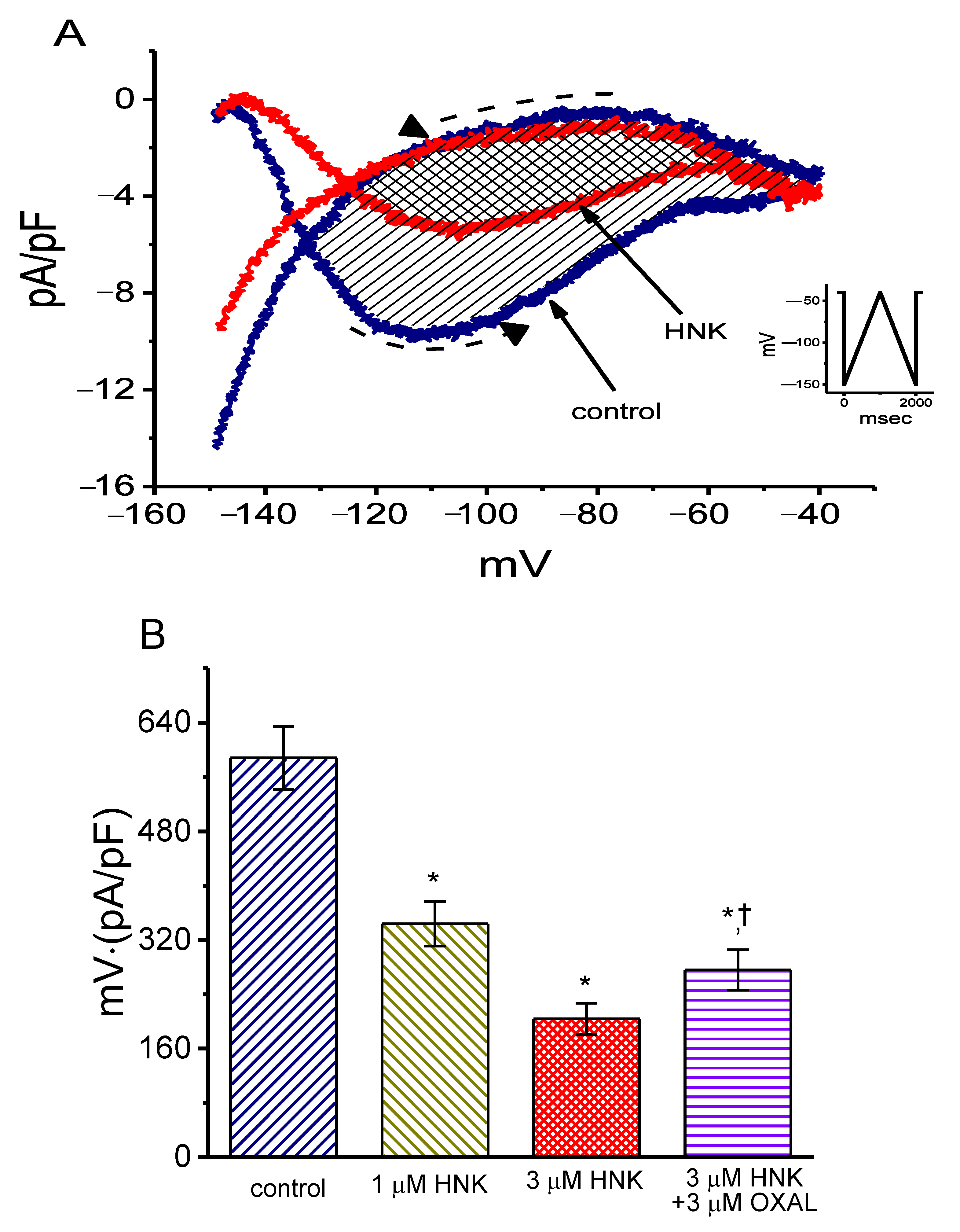
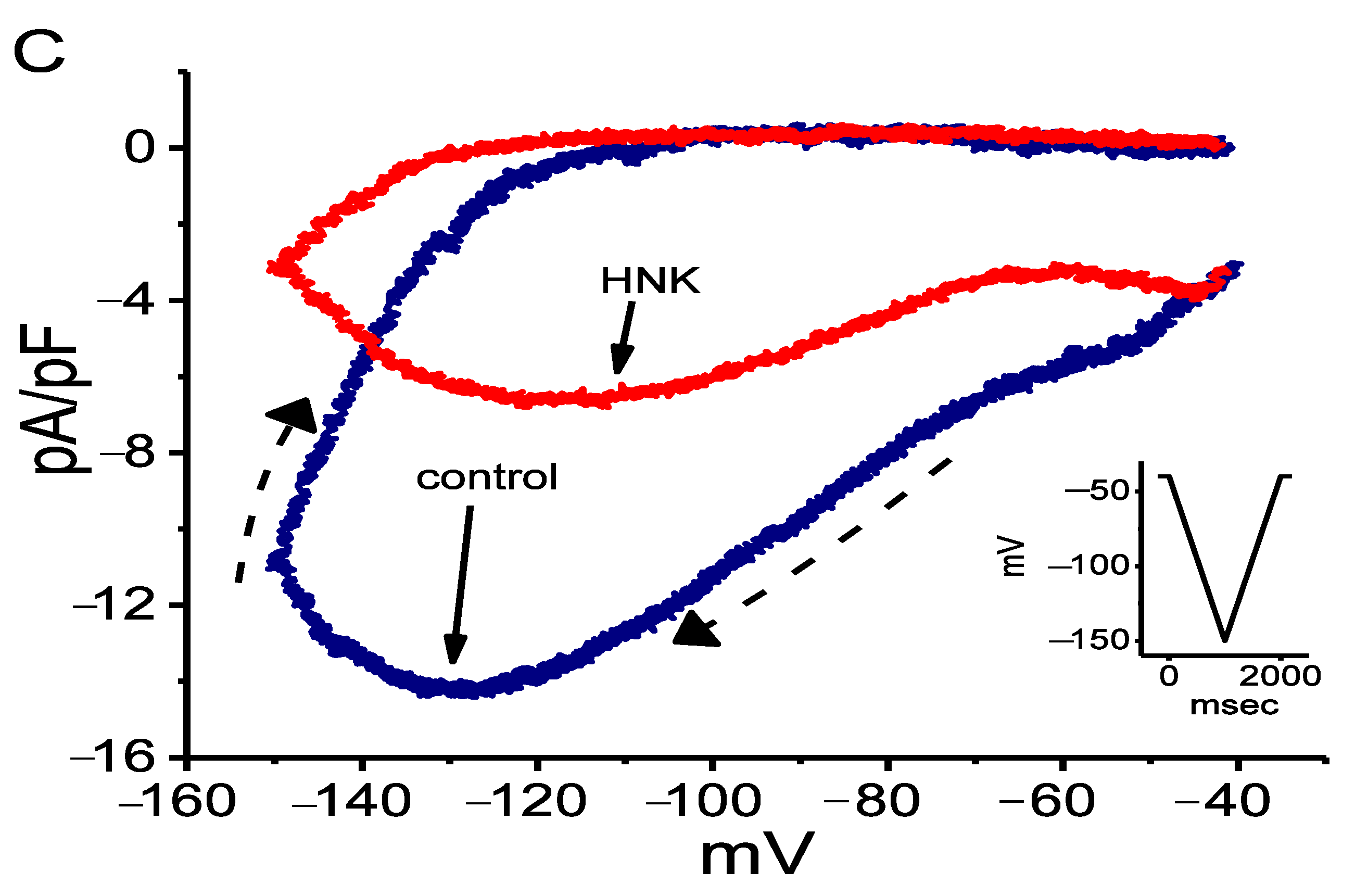
2.3. Effect of HNK on Voltage-Dependent Hysteresis of Ih Elicited in Response to Long Triangular Ramp Pulse
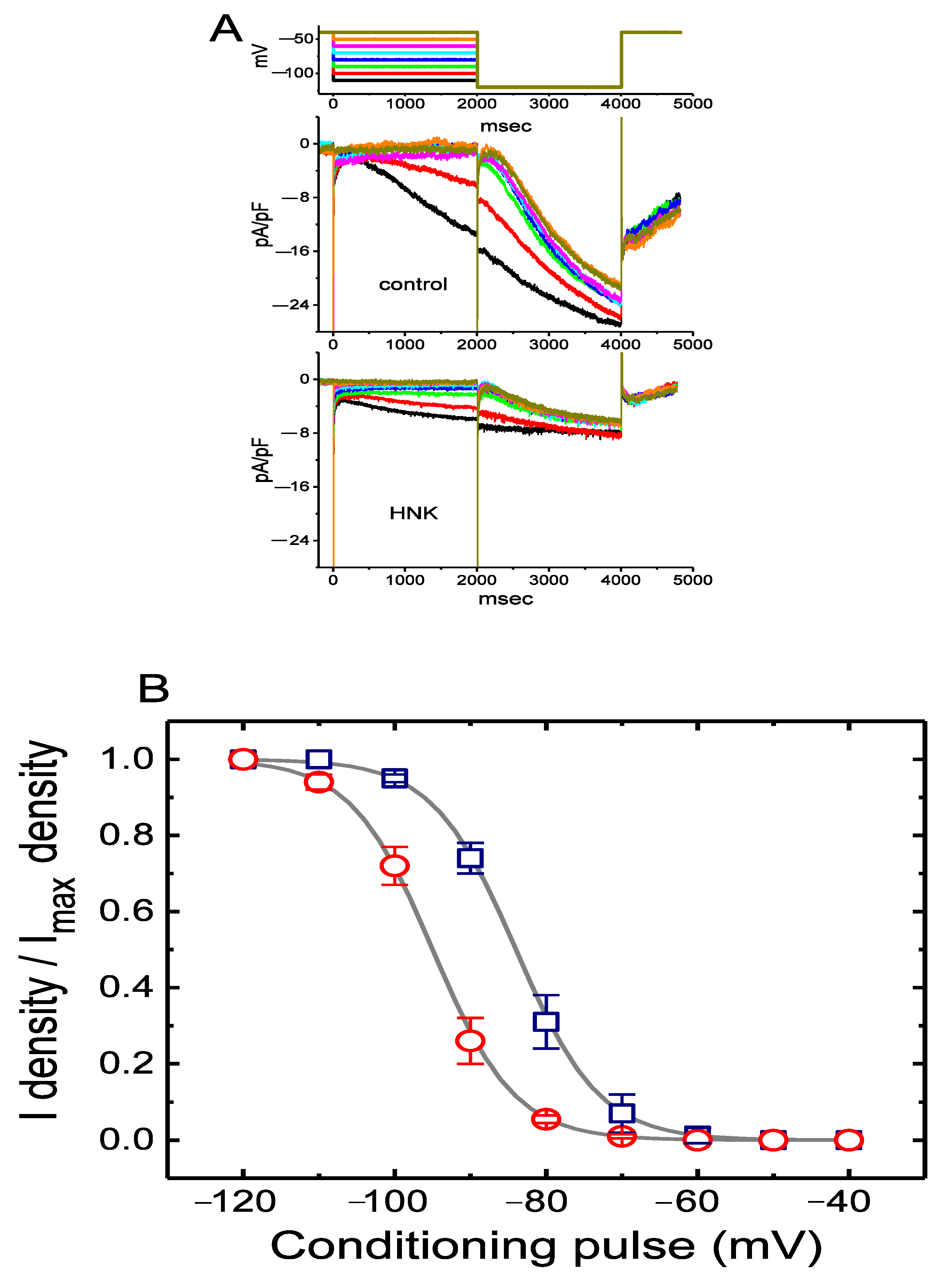
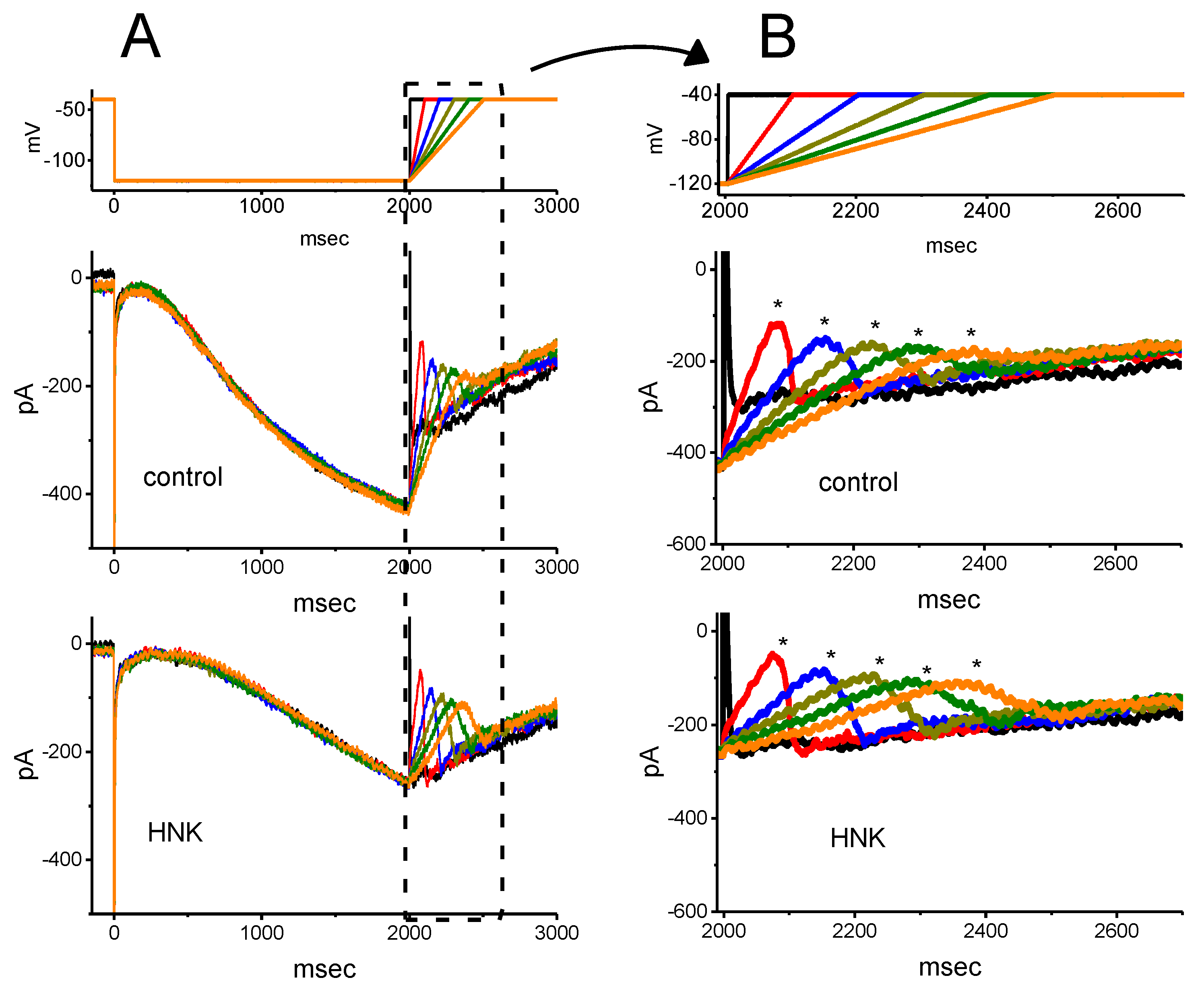
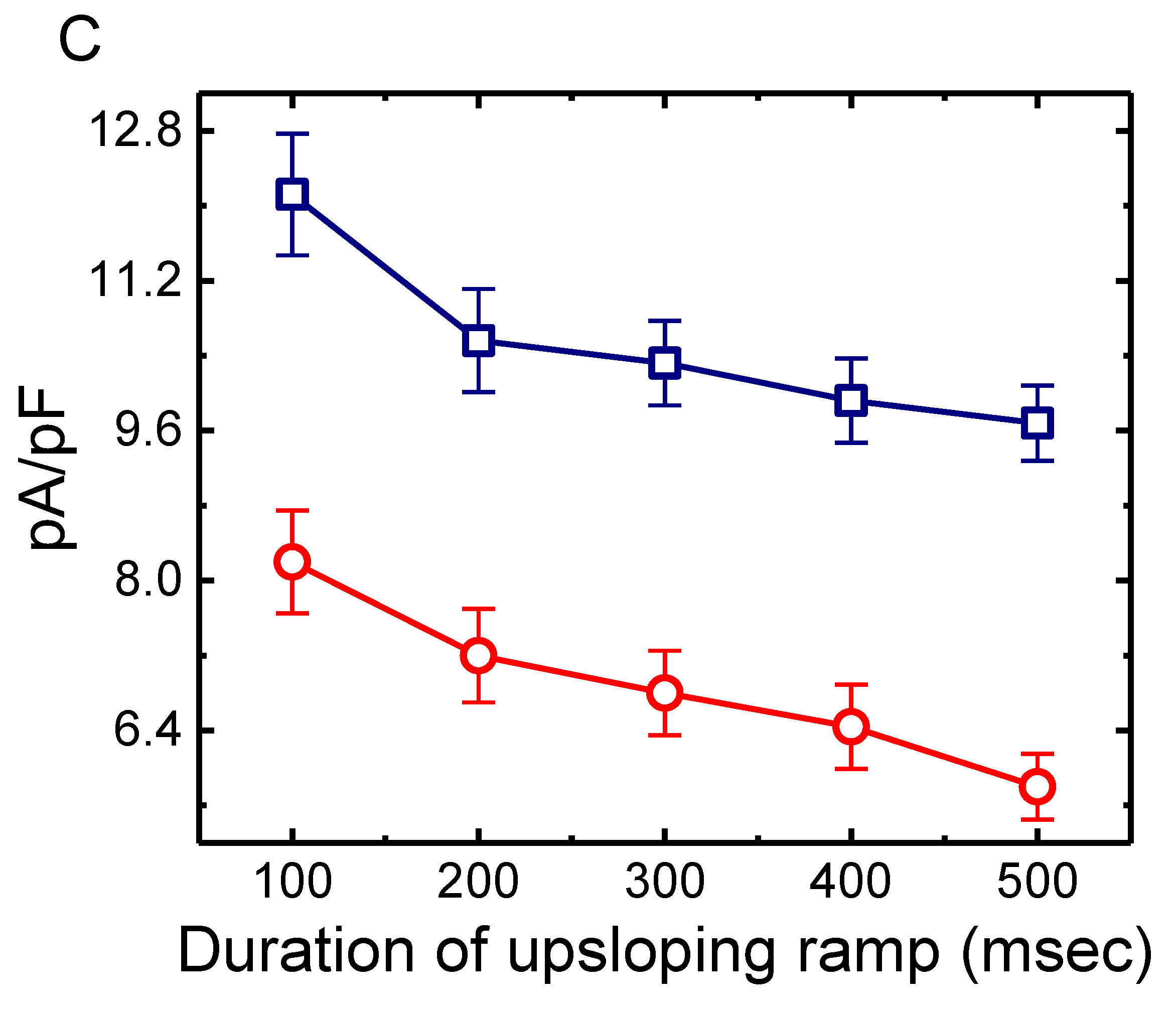
2.4. Effect of HNK on Deactivating Ih Elicited upon Return to Membrane Depolarization with Varying Duration
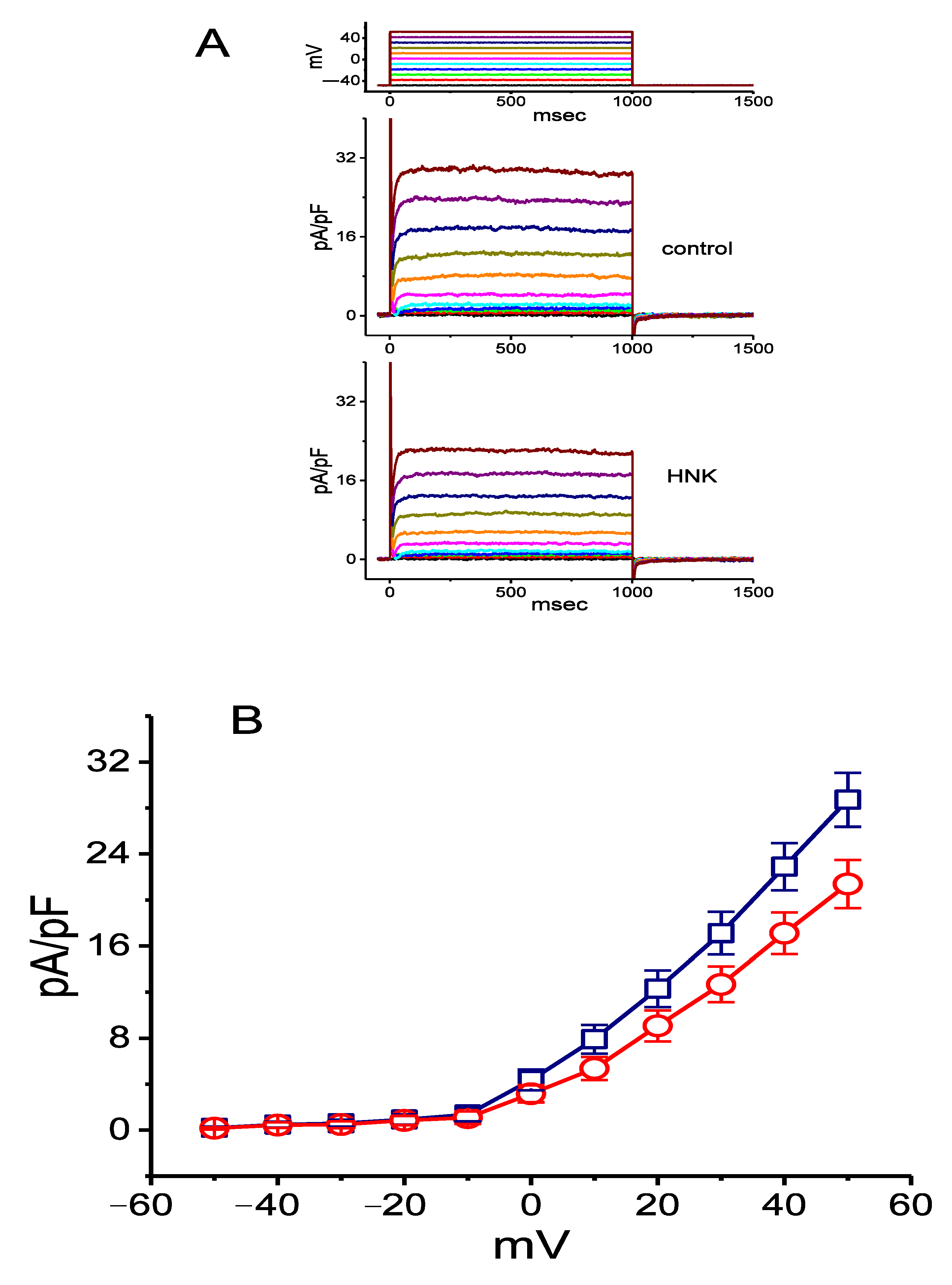
2.5. Inhibitory Effect of HNK on Delayed-Rectifier K+ Current (IK(DR)) in GH3 Cells
2.6. Inhibitory Effect of HNK on Spontaneous Action Currents (ACs) in GH3 Cells
2.7. Inhibitory Effect of HNK on Ih Density Identified in Rolf B1.T Olfactory Neurons
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs and Solutions
4.2. Cell Preparations
4.3. Electrophysiological Measurements
4.4. Data Recordings
4.5. Data Analyses
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | action current; |
| HCN channel | hyperpolarization-activated cyclic nucleotide-gated channel; |
| HNK | honokiol (2-(4-hydroxy-3-prop-2-enyl-phenyl)-4-prop-2-enyl-phenol); |
| Ih | hyperpolarization-activated cation current; |
| IC50 | the concentration required for 50% inhibition; |
| IK(DR) | delayed-rectifier K+ current; |
| SEM | standard error of the mean; |
| τact | activation time constant. |
References
- Fujita, M.; Itokawa, H.; Sashida, Y. Studies on the components of Magnolia obovata THUNB. III. Occurrence of magnolol and honokiol in M. obovata and other allied plants. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1973, 93, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Yu, Z.; Wu, J.; Yu, K.; Hong, G.; Lu, Z.-Q.; Gao, S. Honokiol inhibits constitutive and inducible STAT3 signaling via PU.1-induced SHP1 expression in acute myeloid leukemia cells. Tohoku J. Exp. Med. 2015, 237, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Chen, S.-H.; Chang, Y.-S.; Liu, Y.-W.; Wu, J.-Y.; Lim, Y.-P.; Yu, H.-I.; Lee, Y.-R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017, 115, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lee, Y.; Zhang, Q.; Xiong, D.; Wan, T.C.; Wang, Y.; You, M. Honokiol decreases lung cancer metastasis through inhibition of the STAT3 signaling pathway. Cancer Prev. Res. 2016, 10, 133–141. [Google Scholar] [CrossRef]
- Halasi, M.; Hitchinson, B.; Shah, B.N.; Váraljai, R.; Khan, I.; Benevolenskaya, E.V.; Gaponenko, V.; Arbiser, J.L.; Gartel, A.L. Honokiol is a FOXM1 antagonist. Cell Death Dis. 2018, 9, 84. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: a review of its anticancer potential and mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Liu, P.-S.; Chen, C.C.; Kao, L.S. Multiple effects of honokiol on catecholamine secretion from adrenal chromaffin cells. Proc. Natl. Sci. Counc. Repub. China Part B Life Sci. 1989, 13, 307–313. [Google Scholar]
- Tachikawa, E.; Takahashi, M.; Kashimoto, T. Effects of extract and ingredients isolated from Magnolia obovata thunberg on catecholamine secretion from bovine adrenal chromaffin cells. Biochem. Pharmacol. 2000, 60, 433–440. [Google Scholar] [CrossRef]
- Xu, Q.; Yi, L.-T.; Pan, Y.; Wang, X.; Li, Y.-C.; Li, J.-M.; Wang, C.-P.; Kong, L.-D. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 715–725. [Google Scholar] [CrossRef]
- Wang, C.; Gan, D.; Wu, J.; Liao, M.; Liao, X.; Ai, W. Honokiol exerts antidepressant effects in rats exposed to chronic unpredictable mild stress by regulating brain derived neurotrophic factor level and hypothalamus–pituitary–adrenal axis activity. Neurochem. Res. 2018, 43, 1519–1528. [Google Scholar] [CrossRef]
- Woodbury, A.; Yu, S.P.; Wei, L.; Garcia, P. Neuro-modulating effects of honokiol: a review. Front. Neurol. 2013, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and toxicology of magnolol and honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Vega-García, A.; Santana-Gómez, C.; Rocha, L.; Magdaleno-Madrigal, V.M.; Morales-Otal, A.; Buzoianu-Anguiano, V.; Feria-Romero, I.; Orozco-Suarez, S. Magnolia officinalis reduces the long-term effects of the status epilepticus induced by kainic acid in immature rats. Brain Res. Bull. 2019, 149, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-P.; Chen, H.-T.; Liang, Y.-C.; Wang, T.-E.; Huang, K.-H.; Hsu, C.-C.; Liang, H.-J.; Huang, C.-H.; Jan, T.-R. Development of nanosome-encapsulated honokiol for intravenous therapy against experimental autoimmune encephalomyelitis. Int. J. Nanomed. 2020, 15, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, X.; Zhang, Z.; Lv, H. Preparation and characterization of honokiol nanosuspensions and preliminary evaluation of anti-inflammatory effect. AAPS PharmSciTech 2020, 21, 1–7. [Google Scholar] [CrossRef]
- Zhai, H.; Nakade, K.; Mitsumoto, Y.; Fukuyama, Y. Honokiol and magnolol induce Ca2+ mobilization in rat cortical neurons and human neuroblastoma SH-SY5Y cells. Eur. J. Pharmacol. 2003, 474, 199–204. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Chen, H.-H.; Ko, C.-H.; Chan, M.-H. Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur. J. Pharmacol. 2006, 537, 64–69. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Chen, H.-H.; Lin, Y.-C.; Ko, C.-H.; Chan, M.-H. Antinociceptive actions of honokiol and magnolol on glutamatergic and inflammatory pain. J. Biomed. Sci. 2009, 16, 94. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Chen, H.-H.; Ko, C.-H.; Chan, M.-H. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci. 2007, 81, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic Properties of Honokiol in Inflammatory Pain Models by Targeting of NF-κB and Nrf2 Signaling. Front. Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef]
- Khalid, S.; Khan, A.; Shal, B.; Ali, H.; Kim, Y.S.; Khan, S. Suppression of TRPV1 and P2Y nociceptors by honokiol isolated from Magnolia officinalis in 3rd degree burn mice by inhibiting inflammatory mediators. Biomed. Pharmacother. 2019, 114, 108777. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-S.; Chen, L.; Lu, Y.-Y.; Lei, S.; Peng, M.; Xia, Z.-Y. Honokiol-Mediated Mitophagy Ameliorates Postoperative Cognitive Impairment Induced by Surgery/Sevoflurane via Inhibiting the Activation of NLRP3 Inflammasome in the Hippocampus. Oxidative Med. Cell. Longev. 2019, 2019, 8639618. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.R.; West, A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Simasko, S.; Sankaranarayanan, S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am. J. Physiol. Metab. 1997, 272, E405–E414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Wang, Y.-J.; Wu, P.-Y.; Wu, S.-N. Tramadol-induced block of hyperpolarization-activated cation current in rat pituitary lactotrophs. Naunyn Schmiedeberg Arch. Pharmacol. 2008, 379, 127–135. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, F.; Li, B.; Hu, Z. Neurophysiology of HCN channels: From cellular functions to multiple regulations. Prog. Neurobiol. 2014, 112, 1–23. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef]
- Spinelli, V.; Sartiani, L.; Mugelli, A.; Romanelli, M.N.; Cerbai, E. Hyperpolarization-activated cyclic-nucleotide-gated channels: Pathophysiological, developmental, and pharmacological insights into their function in cellular excitability. Can. J. Physiol. Pharmacol. 2018, 96, 977–984. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Liu, Y.-C.; Lo, Y.-C.; Wu, S.-N. Characterization of inhibitory effectiveness in hyperpolarization-activated cation currents by a group of ent-kaurane-type diterpenoids from Croton tonkinensis. Int. J. Mol. Sci. 2020, 21, 1268. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Cerbai, E.; Dei, S.; Guandalini, L.; Martelli, C.; Martini, E.; Scapecchi, S.; Teodori, E.; Mugelli, A. Design, synthesis and preliminary biological evaluation of zatebradine analogues as potential blockers of the hyperpolarization-activated current. Bioorganic Med. Chem. 2005, 13, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Postea, O.; Biel, M. Exploring HCN channels as novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, M.N.; Sartiani, L.; Masi, A.; Mannaioni, G.; Manetti, D.; Mugelli, A.; Cerbai, E. HCN channels modulators: The need for selectivity. Curr. Top. Med. Chem. 2016, 16, 1764–1791. [Google Scholar] [CrossRef]
- Santoro, B.; Shah, M.M. Hyperpolarization-activated cyclic nucleotide-gated channels as drug targets for neurological disorders. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 109–131. [Google Scholar] [CrossRef]
- DiFrancesco, D. Serious workings of the funny current. Prog. Biophys. Mol. Biol. 2006, 90, 13–25. [Google Scholar] [CrossRef]
- Resta, F.; Micheli, L.; Laurino, A.; Spinelli, V.; Mello, T.; Sartiani, L.; Mannelli, L.D.C.; Cerbai, E.; Ghelardini, C.; Romanelli, M.N.; et al. Selective HCN1 block as a strategy to control oxaliplatin-induced neuropathy. Neuropharmacology 2018, 131, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Chen, C.-C.; Li, H.-F.; Lo, Y.-K.; Chen, S.-A.; Chiang, H.-T. Stimulation of the BKCa channel in cultured smooth muscle cells of human trachea by magnolol. Thorax 2002, 57, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Han, X.; Tang, S.; Xiao, W.; Tan, Z.; Zhou, C.; Wang, M.; Kang, J. Magnolol and honokiol regulate the calcium-activated potassium channels signaling pathway in Enterotoxigenic Escherichia coli-induced diarrhea mice. Eur. J. Pharmacol. 2015, 755, 66–73. [Google Scholar] [CrossRef]
- Gong, C.-L.; Wong, K.-L.; Cheng, K.-S.; Kuo, C.-S.; Chao, C.-C.; Tsai, M.-F.; Leung, Y.-M. Inhibitory effects of magnolol on voltage-gated Na+ and K+ channels of NG108-15 cells. Eur. J. Pharmacol. 2012, 682, 73–78. [Google Scholar] [CrossRef]
- Sheng, A.; Zhang, Y.; Li, G.; Zhang, G. Inhibitory effects of honokiol on the voltage-gated potassium channels in freshly isolated mouse dorsal root ganglion neurons. Neurochem. Res. 2017, 43, 450–457. [Google Scholar] [CrossRef]
- Chang, W.-T.; Gao, Z.-H.; Li, S.-W.; Liu, P.-Y.; Lo, Y.-C.; Wu, S.-N. Characterization in dual activation by oxaliplatin, a platinum-based chemotherapeutic agent of hyperpolarization-activated cation and electroporation-induced currents. Int. J. Mol. Sci. 2020, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-T.; Liu, Y.-C.; Liu, P.-Y.; Wu, S.-N. Concerted suppression of Ih and activation of IK(M) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 2019, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Gao, Z.-H.; Ko, S.Y.; Wu, S.-N. Characterization of effectiveness in concerted Ih inhibition and IK(Ca) stimulation by pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene), a stilbenoid. Int. J. Mol. Sci. 2020, 21, 357. [Google Scholar] [CrossRef] [PubMed]
- Männikkö, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef]
- Fürst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef]
- Barthel, L.; Reetz, O.; Strauss, U. Use dependent attenuation of rat HCN1-mediated Ih in intact HEK293 cells. Cell. Physiol. Biochem. 2016, 38, 2079–2093. [Google Scholar] [CrossRef]
- Dougalis, A.; Matthews, G.A.C.; Liss, B.; Ungless, M.A. Ionic currents influencing spontaneous firing and pacemaker frequency in dopamine neurons of the ventrolateral periaqueductal gray and dorsal raphe nucleus (vlPAG/DRN): A voltage-clamp and computational modelling study. J. Comput. Neurosci. 2017, 42, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Amarillo, Y.; Tissone, A.I.; Mato, G.; Nadal, M.S. Inward rectifier potassium current IKir promotes intrinsic pacemaker activity of thalamocortical neurons. J. Neurophysiol. 2018, 119, 2358–2372. [Google Scholar] [CrossRef]
- Ghaffari, B.V.; Kouhnavard, M.; Aihara, T.; Kitajima, T. Mathematical modeling of subthreshold resonant properties in pyloric dilator neurons. BioMed Res. Int. 2015, 2015, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mellon, D. Electrophysiological evidence for intrinsic pacemaker currents in crayfish parasol cells. PLoS ONE 2016, 11, e0146091. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A.M. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef] [PubMed]
- Kasimova, M.A.; Tewari, D.; Cowgill, J.B.; Ursuleaz, W.C.; Lin, J.L.; Delemotte, L.; Chanda, B. Helix breaking transition in the S4 of HCN channel is critical for hyperpolarization-dependent gating. ELife 2019, 8, e53400. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Nagasawa, Y.; Obara, Y.; Ishii, K.; Takagi, D.; Ono, K. Molecular identification of HSPA8 as an accessory protein of a hyperpolarization-activated chloride channel from rat pulmonary vein cardiomyocytes. J. Biol. Chem. 2019, 294, 16049–16061. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.B.; Siegelbaum, S.A. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 2003, 65, 453–480. [Google Scholar] [CrossRef]
- Wang, H.-J.; Martin, A.G.; Chao, P.-K.; Reichard, R.A.; Martin, A.L.; Huang, Y.; Chan, M.-H.; Aronstam, R.S. Honokiol blocks store operated calcium entry in CHO cells expressing the M3 muscarinic receptor: Honokiol and muscarinic signaling. J. Biomed. Sci. 2013, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Jun-Jun, W.; Xiao-Lei, M.; Jing-Ya, C.; Yong, C. The pharmacokinetics and tissue distribution of honokiol and its metabolites in rats. Eur. J. Drug Metab. Pharmacokinet. 2015, 41, 587–594. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Yang, G.; Shi, Q.; Feng, F. Comparative pharmacokinetics and brain distribution of magnolol and honokiol after oral administration of Magnolia officinalis cortex extract and its compatibility with other herbal medicines in Zhi-Zi-Hou-Po Decoction to rats. Biomed. Chromatogr. 2015, 30, 369–375. [Google Scholar] [CrossRef]
- Frace, A.M.; Maruoka, F.; Noma, A. Control of the hyperpolarization-activated cation current by external anions in rabbit sino-atrial node cells. J. Physiol. 1992, 453, 307–318. [Google Scholar] [CrossRef]
- Wu, S.-N.; Chern, J.-H.; Shen, S.; Chen, H.-H.; Hsu, Y.-T.; Lee, C.-C.; Chan, M.-H.; Lai, M.-C.; Shie, F.-S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell. Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef]
- Sonigra, R.J.; Kandiah, S.S.; Wigley, C.B. Spontaneous immortalisation of ensheathing cells from adult rat olfactory nerve. Glia 1996, 16, 247–256. [Google Scholar] [CrossRef]
- So, E.C.; Wang, Y.; Yang, L.; So, K.H.; Lo, Y.-C.; Wu, S.-N. Multiple regulatory actions of 2-guanidine-4-methylquinazoline (GMQ), an agonist of acid-sensing ion channel type 3, on ionic currents in pituitary GH3 cells and in olfactory sensory (Rolf B1.T) neurons. Biochem. Pharmacol. 2018, 151, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Wu, Y.-H.; Chen, B.-S.; Lo, Y.-C.; Liu, Y.-C. Underlying mechanism of actions of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.-H.; Chen, H.-H.; Lo, Y.-C.; Wu, S.-N. Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K+ Current. Int. J. Mol. Sci. 2020, 21, 4260. https://doi.org/10.3390/ijms21124260
Chan M-H, Chen H-H, Lo Y-C, Wu S-N. Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K+ Current. International Journal of Molecular Sciences. 2020; 21(12):4260. https://doi.org/10.3390/ijms21124260
Chicago/Turabian StyleChan, Ming-Huan, Hwei-Hsien Chen, Yi-Ching Lo, and Sheng-Nan Wu. 2020. "Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K+ Current" International Journal of Molecular Sciences 21, no. 12: 4260. https://doi.org/10.3390/ijms21124260
APA StyleChan, M.-H., Chen, H.-H., Lo, Y.-C., & Wu, S.-N. (2020). Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K+ Current. International Journal of Molecular Sciences, 21(12), 4260. https://doi.org/10.3390/ijms21124260




