Back to the Future: Genetically Encoded Fluorescent Proteins as Inert Tracers of the Intracellular Environment
Abstract
1. Introduction
2. GFPs as Inert Tracers of Cytoplasmic Architecture
3. GFPs as Inert Tracers of Nuclear Architecture
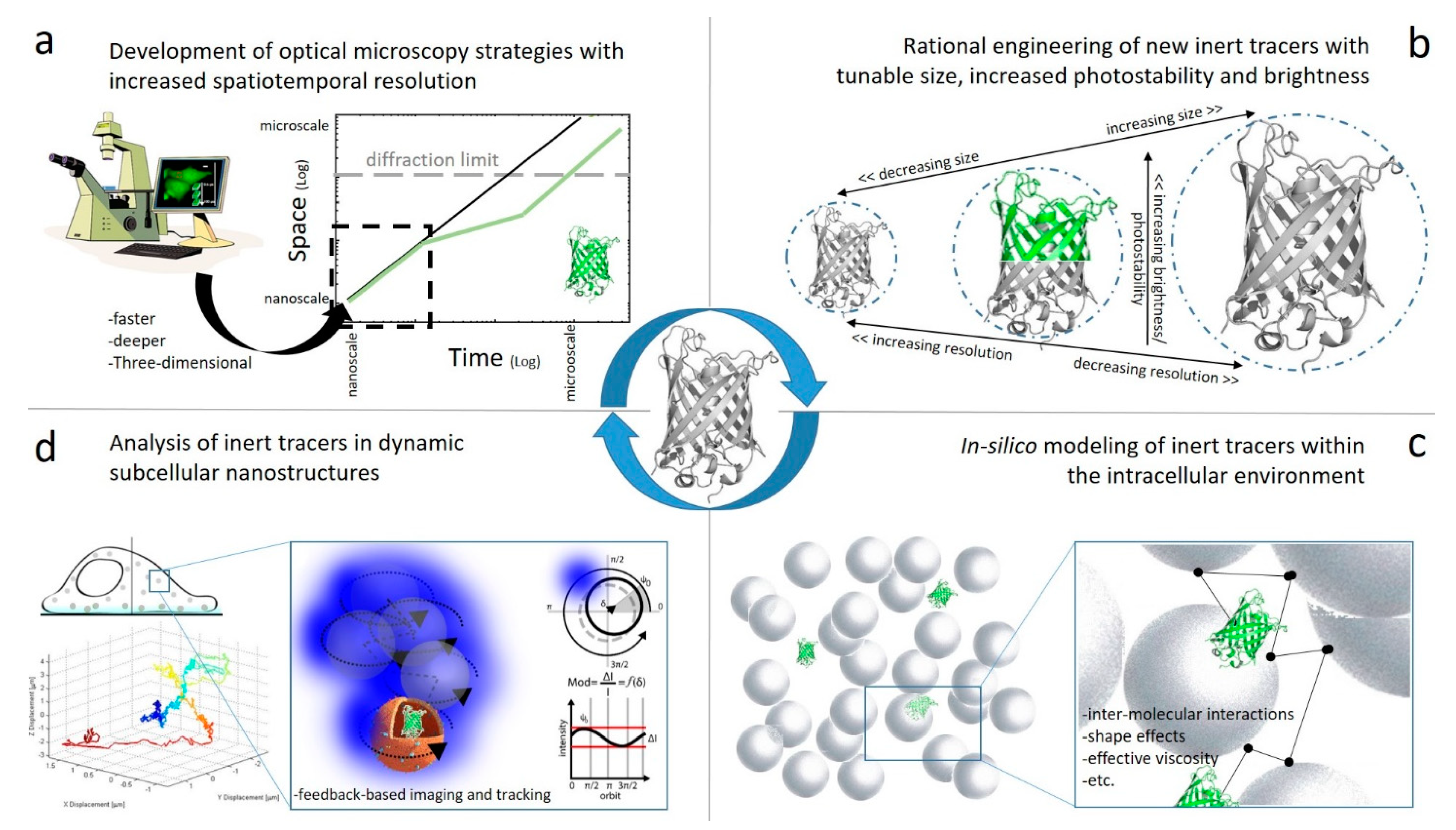
4. Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Chalfie, M. GFP: Lighting up life (Nobel Lecture). Angew. Chem. Int. Ed. 2009, 48, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Prasher, D.C.; Eckenrode, V.K.; Ward, W.W.; Prendergast, F.G.; Cormier, M.J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 1992, 111, 229–233. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.; Prasher, D. Green fluorescent protein as a marker for gene expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef]
- Wiedenmann, J.; Oswald, F.; Nienhaus, G.U. Fluorescent proteins for live cell imaging: Opportunities, limitations, and challenges. IUBMB Life 2009, 61, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Lippincott-Schwartz, J.; Gardel, M.L.; Shin, J.H.; Mackintosh, F.C.; Mahadevan, L.; Matsudaira, P.; Weitz, D.A. Development and use of fluorescent protein markers in living cells. Science 2003, 300, 87–91. [Google Scholar] [CrossRef]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef]
- Nifosì, R.; Amat, P.; Tozzini, V. Variation of spectral, structural, and vibrational properties within the intrinsically fluorescent proteins family: A density functional study. J. Comput. Chem. 2007, 28, 2366–2377. [Google Scholar] [CrossRef]
- Cubitt, A.B.; Heim, R.; Adams, S.R.; Boyd, A.E.; Gross, L.A.; Tsien, R.Y. Understanding, improving and using green fluorescent proteins. Trends Biochem. Sci. 1995, 20, 448–455. [Google Scholar] [CrossRef]
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef]
- Aoki, K.; Kamioka, Y.; Matsuda, M. Fluorescence resonance energy transfer imaging of cell signaling from in vitro to in vivo: Basis of biosensor construction, live imaging, and image processing. Dev. Growth Differ. 2013, 55, 515–522. [Google Scholar] [CrossRef]
- Bizzarri, R.; Serresi, M.; Cardarelli, F.; Abbruzzetti, S.; Campanini, B.; Viappiani, C.; Beltram, F. Single amino acid replacement makes aequorea victoria fluorescent proteins reversibly photoswitchable. J. Am. Chem. Soc. 2010, 132, 85–95. [Google Scholar] [CrossRef]
- Sample, V.; Mehta, S.; Zhang, J. Genetically encoded molecular probes to visualize and perturb signaling dynamics in living biological systems. J. Cell Sci. 2014, 127, 1151–1160. [Google Scholar] [CrossRef][Green Version]
- Arosio, D.; Ricci, F.; Marchetti, L.; Gualdani, R.; Albertazzi, L.; Beltram, F. Simultaneous intracellular chloride and pH measurements using a GFP-based sensor. Nat. Methods 2010, 7, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Arosio, D.; Garau, G.; Ricci, F.; Marchetti, L.; Bizzarri, R.; Nifosì, R.; Beltram, F. Spectroscopic and structural study of proton and halide ion cooperative binding to GFP. Biophys. J. 2007, 93, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, R.; Serresi, M.; Luin, S.; Beltram, F. Green fluorescent protein based pH indicators for in vivo use: A review. Anal. Bioanal. Chem. 2008, 393, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, R.; Arcangeli, C.; Arosio, D.; Ricci, F.; Faraci, P.; Cardarelli, F.; Beltram, F. Development of a novel GFP-based ratiometric excitation and emission pH indicator for intracellular studies. Biophys. J. 2006, 90, 3300–3314. [Google Scholar] [CrossRef]
- Germond, A.; Fujita, H.; Ichimura, T.; Watanabe, T.M. Design and development of genetically encoded fluorescent sensors to monitor intracellular chemical and physical parameters. Biophys. Rev. 2016, 8, 121–138. [Google Scholar] [CrossRef]
- Luby-Phelps, K.; Castle, P.E.; Taylor, D.L.; Lanni, F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc. Natl. Acad. Sci. USA 1987, 84, 4910–4913. [Google Scholar] [CrossRef]
- Luby-Phelps, K.; Taylor, D.L.; Lanni, F. Probing the structure of cytoplasm. J. Cell Boil. 1986, 102, 2015–2022. [Google Scholar] [CrossRef]
- Fushimi, K.; Verkman, A.S. Low viscosity in the aqueous domain of cell cytoplasm measured by picosecond polarization microfluorimetry. J. Cell Boil. 1991, 112, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R.; Hoang, C.; Verkman, A. Photobleaching recovery and anisotropy decay of green fluorescent protein GFP-S65T in solution and cells: Cytoplasmic viscosity probed by green fluorescent protein translational and rotational diffusion. Biophys. J. 1997, 72, 1900–1907. [Google Scholar] [CrossRef]
- Braeckmans, K.; Remaut, K.; Vandenbroucke, R.E.; Lucas, B.; De Smedt, S.C.; Demeester, J. Line FRAP with the confocal laser scanning microscope for diffusion measurements in small regions of 3-D samples. Biophys. J. 2007, 92, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gelman, H.; Gruebele, M. Coupled protein diffusion and folding in the cell. PLoS ONE 2014, 9, e113040. [Google Scholar] [CrossRef]
- Seksek, O.; Biwersi, J.; Verkman, A. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Boil. 1997, 138, 131–142. [Google Scholar] [CrossRef]
- Sprague, B.L.; Pego, R.L.; Stavreva, D.A.; McNally, J.G. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 2004, 86, 3473–3495. [Google Scholar] [CrossRef]
- Sadovsky, R.G.; Brielle, S.; Kaganovich, D.; England, J.L. Measurement of rapid protein diffusion in the cytoplasm by photo-converted intensity profile expansion. Cell Rep. 2017, 18, 2795–2806. [Google Scholar] [CrossRef]
- Mazza, D.; Braeckmans, K.; Cella, F.; Testa, I.; Vercauteren, I.; Demeester, J.; De Smedt, S.C.; Diaspro, A. A new FRAP/FRAPa method for three-dimensional diffusion measurements based on multiphoton excitation microscopy. Biophys. J. 2008, 95, 3457–3469. [Google Scholar] [CrossRef]
- Höfling, F.; Franosch, T. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 2013, 76, 46602. [Google Scholar] [CrossRef]
- Caspi, A.; Granek, R.; Elbaum, M. Diffusion and directed motion in cellular transport. Phys. Rev. E 2002, 66, 011916. [Google Scholar] [CrossRef]
- Schwille, P.; Haupts, U.; Maiti, S.; Webb, W.W. Molecular dynamics in living cells observed by fluorescence correlation spectroscopy with one- and two-photon excitation. Biophys. J. 1999, 77, 2251–2265. [Google Scholar] [CrossRef]
- Elsner, M.; Hashimoto, H.; Simpson, J.C.; Cassel, D.; Nilsson, T.; Weiss, M. Spatiotemporal dynamics of the COPI vesicle machinery. EMBO Rep. 2003, 4, 1000–1004. [Google Scholar] [CrossRef]
- Ruan, Q.; Chen, Y.; Gratton, E.; Glaser, M.; Mantulin, W.W. Cellular characterization of adenylate kinase and its isoform: Two-photon excitation fluorescence imaging and fluorescence correlation spectroscopy. Biophys. J. 2002, 83, 3177–3187. [Google Scholar] [CrossRef]
- Lenne, P.-F.; Wawrezinieck, L.; Conchonaud, F.; Wurtz, O.; Boned, A.; Guo, X.-J.; Rigneault, H.; He, H.-T.; Marguet, D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006, 25, 3245–3256. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, V.; Wieser, S.; Marguet, D.; Schütz, G.J. Spot variation fluorescence correlation spectroscopy allows for superresolution chronoscopy of confinement times in membranes. Biophys. J. 2011, 100, 2839–2845. [Google Scholar] [CrossRef]
- Wawrezinieck, L.; Rigneault, H.; Marguet, D.; Lenne, P.-F. Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys. J. 2005, 89, 4029–4042. [Google Scholar] [CrossRef]
- Eggeling, C.; Ringemann, C.; Medda, R.; Schwarzmann, G.; Sandhoff, K.; Polyakova, S.; Belov, V.N.; Hein, B.; Von Middendorff, C.; Schönle, A.; et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 2008, 457, 1159–1162. [Google Scholar] [CrossRef]
- Hell, S.W. Far-field optical nanoscopy. Science 2007, 316, 1153–1158. [Google Scholar] [CrossRef]
- Hedde, P.N.; Dörlich, R.M.; Blomley, R.; Gradl, D.; Oppong, E.; Cato, A.; Nienhaus, G.U. Stimulated emission depletion-based raster image correlation spectroscopy reveals biomolecular dynamics in live cells. Nat. Commun. 2013, 4, 2093. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, P.; Cardarelli, F.; Di Luca, M.; Diaspro, A.; Bizzarri, R. Nanoscale protein diffusion by STED-based pair correlation analysis. PLoS ONE 2014, 9, e99619. [Google Scholar] [CrossRef]
- Lanzanó, L.; Scipioni, L.; Di Bona, M.; Bianchini, P.; Bizzarri, R.; Cardarelli, F.; Diaspro, A.; Vicidomini, G. Measurement of nanoscale three-dimensional diffusion in the interior of living cells by STED-FCS. Nat. Commun. 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.; Erdel, F.; Wachsmuth, M.; Rippe, K. Retrieving the intracellular topology from multi-scale protein mobility mapping in living cells. Nat. Commun. 2014, 5, 4494. [Google Scholar] [CrossRef] [PubMed]
- Digman, M.A.; Gratton, E. Imaging barriers to diffusion by pair correlation functions. Biophys. J. 2009, 97, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Digman, M.A.; Sengupta, P.; Wiseman, P.W.; Brown, C.M.; Horwitz, A.R.; Gratton, E. Fluctuation correlation spectroscopy with a laser-scanning microscope: Exploiting the hidden time structure. Biophys. J. 2005, 88, L33–L36. [Google Scholar] [CrossRef] [PubMed]
- Digman, M.A.; Brown, C.M.; Sengupta, P.; Wiseman, P.W.; Horwitz, A.R.; Gratton, E. Measuring fast dynamics in solutions and cells with a laser scanning microscope. Biophys. J. 2005, 89, 1317–1327. [Google Scholar] [CrossRef]
- Gröner, N.; Capoulade, J.; Cremer, C.; Wachsmuth, M. Measuring and imaging diffusion with multiple scan speed image correlation spectroscopy. Opt. Express 2010, 18, 21225–21237. [Google Scholar] [CrossRef][Green Version]
- Di Rienzo, C.; Gratton, E.; Beltram, F.; Cardarelli, F. Fast spatiotemporal correlation spectroscopy to determine protein lateral diffusion laws in live cell membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 12307–12312. [Google Scholar] [CrossRef]
- Di Rienzo, C.; Piazza, V.; Gratton, E.; Beltram, F.; Cardarelli, F. Probing short-range protein Brownian motion in the cytoplasm of living cells. Nat. Commun. 2014, 5, 5891. [Google Scholar] [CrossRef]
- Saxton, M. Anomalous diffusion due to obstacles: A Monte Carlo study. Biophys. J. 1994, 66, 394–401. [Google Scholar] [CrossRef]
- Di Rienzo, C.; Gratton, E.; Beltram, F.; Cardarelli, F. From fast fluorescence imaging to molecular diffusion law on live cell membranes in a commercial microscope. J. Vis. Exp. 2014, e51994. [Google Scholar] [CrossRef]
- Kusumi, A.; Nakada, C.; Ritchie, K.; Murase, K.; Suzuki, K.; Murakoshi, H.; Kasai, R.S.; Kondo, J.; Fujiwara, T.K. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: High-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.L.; Kraikivski, P.; Slepchenko, B.M. Diffusion in cytoplasm: Effects of excluded volume due to internal membranes and cytoskeletal structures. Biophys. J. 2009, 97, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Boersma, A.J.; Zuhorn, I.S.; Poolman, B. A sensor for quantification of macromolecular crowding in living cells. Nat. Methods 2015, 12, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.J.; Fujita, H.; Kitamura, A.; Horio, T.; Yamamoto, J.; Kinjo, M.; Sasaki, A.; Machiyama, H.; Yoshizawa, K.; Ichimura, T.; et al. Dependence of fluorescent protein brightness on protein concentration in solution and enhancement of it. Sci. Rep. 2016, 6, 22342. [Google Scholar] [CrossRef]
- Wachsmuth, M.; Waldeck, W.; Langowski, J. Anomalous diffusion of fluorescent probes inside living cell nuclei investigated by spatially-resolved fluorescence correlation spectroscopy. J. Mol. Boil. 2000, 298, 677–689. [Google Scholar] [CrossRef]
- Hedde, P.N.; Stakic, M.; Gratton, E. Rapid measurement of molecular transport and interaction inside living cells using single plane illumination. Sci. Rep. 2014, 4, 7048. [Google Scholar] [CrossRef] [PubMed]
- Dross, N.; Spriet, C.; Zwerger, M.; Muller, G.; Waldeck, W.; Langowski, J. Mapping eGFP oligomer mobility in living cell nuclei. PLoS ONE 2009, 4, e5041. [Google Scholar] [CrossRef]
- Hinde, E.; Cardarelli, F.; Digman, M.A.; Gratton, E. In vivo pair correlation analysis of EGFP intranuclear diffusion reveals DNA-dependent molecular flow. Proc. Natl. Acad. Sci. USA 2010, 107, 16560–16565. [Google Scholar] [CrossRef]
- Malacrida, L.; Hedde, P.N.; Ranjit, S.; Cardarelli, F.; Gratton, E. Visualization of barriers and obstacles to molecular diffusion in live cells by spatial pair-cross-correlation in two dimensions. Biomed. Opt. Express 2017, 9, 303–321. [Google Scholar] [CrossRef]
- Hinde, E.; Cardarelli, F. Measuring the flow of molecules in cells. Biophys. Rev. 2011, 3, 119–129. [Google Scholar] [CrossRef]
- Hinde, E.; Cardarelli, F.; Digman, M.A.; Gratton, E. Changes in chromatin compaction during the cell cycle revealed by micrometer-scale measurement of molecular flow in the nucleus. Biophys. J. 2012, 102, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Hinde, E.; Cardarelli, F.; Digman, M.A.; Kershner, A.; Kimble, J.; Gratton, E. The impact of mitotic versus interphase chromatin architecture on the molecular flow of EGFP by pair correlation analysis. Biophys. J. 2011, 100, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Kurz, A.; Zirbel, R.; Dietzel, S.; Rinke, B.; Schröck, E.; Speicher, M.; Mathieu, U.; Jauch, A.; Emmerich, P.; et al. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb. Symp. Quant. Boil. 1993, 58, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Münkel, C.; Eils, R.; Dietzel, S.; Zink, D.; Mehring, C.; Wedemann, G.; Cremer, T.; Langowski, J. Compartmentalization of interphase chromosomes observed in simulation and experiment. J. Mol. Boil. 1999, 285, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Kühn, T.; Ihalainen, T.O.; Hyväluoma, J.; Dross, N.; Willman, S.F.; Langowski, J.; Vihinen-Ranta, M.; Timonen, J. Protein diffusion in mammalian cell cytoplasm. PLoS ONE 2011, 6, e22962. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Zahn, J.; Maglione, M.; Sigrist, S.J.; Marquard, J.; Chojnacki, J.; Kräusslich, H.-G.; Sahl, S.J.; Engelhardt, J.; Hell, S.W. Ultrafast, temporally stochastic STED nanoscopy of millisecond dynamics. Nat. Methods 2015, 12, 827–830. [Google Scholar] [CrossRef]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 2016, 355, 606–612. [Google Scholar] [CrossRef]
- Di Rienzo, C.; Gratton, E.; Beltram, F.; Cardarelli, F. Spatiotemporal fluctuation analysis: A powerful tool for the future nanoscopy of molecular processes. Biophys. J. 2016, 111, 679–685. [Google Scholar] [CrossRef]
- Di Rienzo, C.; Cardarelli, F.; Di Luca, M.; Beltram, F.; Gratton, E. Diffusion tensor analysis by two-dimensional pair correlation of fluorescence fluctuations in cells. Biophys. J. 2016, 111, 841–851. [Google Scholar] [CrossRef][Green Version]
- Enderlein, J.; Gregor, I.; Patra, D.; Fitter, J.; Fitter, J. Statistical analysis of diffusion coefficient determination by fluorescence correlation spectroscopy. J. Fluoresc. 2005, 15, 415–422. [Google Scholar] [CrossRef]
- Saffarian, S.; Elson, E.L. Statistical analysis of fluorescence correlation spectroscopy: The standard deviation and bias. Biophys. J. 2003, 84, 2030–2042. [Google Scholar] [CrossRef]
- Wohland, T.; Rigler, R.; Vogel, H. The standard deviation in fluorescence correlation spectroscopy. Biophys. J. 2001, 80, 2987–2999. [Google Scholar] [CrossRef]
- Dou, J.; Vorobieva, A.A.; Sheffler, W.; Doyle, L.A.; Park, H.; Bick, M.J.; Mao, B.; Foight, G.W.; Lee, M.Y.; Gagnon, L.A.; et al. De novo design of a fluorescence-activating β-barrel. Nature 2018, 561, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, F.; Serresi, M.; Bizzarri, R.; Giacca, M.; Beltram, F. In vivo study of HIV-1 tat arginine-rich motif unveils its transport properties. Mol. Ther. 2007, 15, 1313–1322. [Google Scholar] [CrossRef]
- Wang, R.; Brattain, M.G. The maximal size of protein to diffuse through the nuclear pore is larger than 60kDa. FEBS Lett. 2007, 581, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- A Nigg, E. Nucleocytoplasmic transport: Signals, mechanisms and regulation. Nature 1997, 386, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1986, 864, 305–359. [Google Scholar] [CrossRef]
- Trovato, F.; Fumagalli, G. Molecular simulations of cellular processes. Biophys. Rev. 2017, 9, 941–958. [Google Scholar] [CrossRef]
- Trovato, F.; Nifosì, R.; Di Fenza, A.; Tozzini, V. A minimalist model of protein diffusion and interactions: The green fluorescent protein within the cytoplasm. Macromolecules 2013, 46, 8311–8322. [Google Scholar] [CrossRef]
- Trovato, F.; Tozzini, V. Diffusion within the cytoplasm: A mesoscale model of interacting macromolecules. Biophys. J. 2014, 107, 2579–2591. [Google Scholar] [CrossRef]
- Welch, G.R.; Easterby, J.S. Metabolic channeling versus free diffusion: Transition-time analysis. Trends Biochem. Sci. 1994, 19, 193–197. [Google Scholar] [CrossRef]
- Partikian, A.; Ölveczky, B.; Swaminathan, R.; Li, Y.; Verkman, A. Rapid diffusion of green fluorescent protein in the mitochondrial matrix. J. Cell Boil. 1998, 140, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Dayel, M.; Hom, E.; Verkman, A. Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophys. J. 1999, 76, 2843–2851. [Google Scholar] [CrossRef]
- Levi, V.; Ruan, Q.; Gratton, E. 3-D particle tracking in a two-photon microscope: Application to the study of molecular dynamics in cells. Biophys. J. 2005, 88, 2919–2928. [Google Scholar] [CrossRef]
- Anzalone, A.; Annibale, P.; Gratton, E. 3D orbital tracking in a modified two-photon microscope: An application to the tracking of intracellular vesicles. J. Vis. Exp. 2014, 51794. [Google Scholar] [CrossRef]
- Begarani, F.; D’Autilia, F.; Signore, G.; Del Grosso, A.; Cecchini, M.; Gratton, E.; Beltram, F.; Cardarelli, F. Capturing metabolism-dependent solvent dynamics in the lumen of a trafficking lysosome. ACS Nano 2019, 8b07682. [Google Scholar] [CrossRef]
- Cardarelli, F.; Lanzano, L.; Gratton, E. Capturing directed molecular motion in the nuclear pore complex of live cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9863–9868. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, F.; Lanzano, L.; Gratton, E. Fluorescence correlation spectroscopy of intact nuclear pore complexes. Biophys. J. 2011, 101, L27–L29. [Google Scholar] [CrossRef]
- Coppola, S.; Cardarelli, F.; Pozzi, D.; Estrada, L.C.; A Digman, M.; Gratton, E.; Bifone, A.; Marianecci, C.; Caracciolo, G. The role of cytoskeleton networks on lipid-mediated delivery of DNA. Ther. Deliv. 2013, 4, 191–202. [Google Scholar] [CrossRef]
- Coppola, S.; Estrada, L.C.; Digman, M.A.; Pozzi, D.; Cardarelli, F.; Gratton, E.; Caracciolo, G. Intracellular trafficking of cationic liposome–DNA complexes in living cells†. Soft Matter 2012, 8, 7919–7927. [Google Scholar] [CrossRef]
- Wehnekamp, F.; Plucińska, G.; Thong, R.; Misgeld, T.; Lamb, D.C. Nanoresolution real-time 3D orbital tracking for studying mitochondrial trafficking in vertebrate axons in vivo. eLife 2019, 8, 46059. [Google Scholar] [CrossRef] [PubMed]
 | (i) Genetically encoded fluorescent marker of a molecule of interest | See for instance Refs [3,4,5,6,7,8,9,10] |
| (ii) Genetically encoded fluorescent biosensor | See for instance Refs [11,12,13,14,15,16,17,18] | |
(iii) Genetically encoded fluorescent tracer of the intracellular environment (+ time-resolved fluorescence microscopy) | This work |
| Spatial Scale (nm) | Temporal Scale (ms) | Inert Tracer | Biologic System | Method/Result | Ref. |
|---|---|---|---|---|---|
| >1 µm | milliseconds | GFP | CHO cell cytoplasm | FRAP: GFP diffusivity is estimated by fitting the recovery curve. Result: GFP diffusion is 3–5-fold more suppressed than dilute solutions. This is interpreted as the result of macromolecular crowding | Refs. [21,22] |
| >1 µm | milliseconds | Dendra2 | COS7 cell cytoplasm | PIPE: GFP diffusivity is estimated by fitting the time expansion of the photoactivated spot. Result: GFP diffusion is 3–5-fold more suppressed than dilute solutions | Ref. [27] |
| >1 µm | µ-to-milliseconds | GFP | CHO cell cytoplasm | RICS: GFP diffusion is extracted from raster-scan images averaging over the entire image. Result: GFP diffusion is 3–5 fold more suppressed than dilute solutions | Ref. [44,45] |
| >1 µm | milliseconds | GFP | CHO cell cytoplasm | SPIM-iMSD: GFP diffusion is measured on a grid of points simultaneously. iMSD analysis yields the average GFP diffusion law within the intracellular environment. Result: GFP diffusion is 3–5-fold more suppressed than dilute solutions | Ref. [56] |
| 200–300 nm | µseconds | GFP | Cell cytoplasm | Single point FCS: local measurement of GFP concentration and diffusion. Result: GFP diffusion is still 3–5-fold more suppressed than dilute solutions | Ref. [33] |
| 200–300 nm | µseconds | GFP, GFP multimers | Cell nucleus | Single point FCS in multiple locations: GFP diffusion is measured on a grid of points, consecutively. Result: GFP diffusion is suppressed; no correlation is found between GFP diffusivity and chromatin density | Ref. [57] |
| From 200–300 nm to several microns | Hundreds of µseconds | GFP | CHO cell nucleoplasm | pCF analysis on a line: GFP diffusion is measured on a grid of points along a scanned line. Result: cross-correlation of points highlights disconnected GFP flow across chromatin density barriers | Ref. [58] |
| 200 nm | 50 µs | GFP, GFP3, GFP5, RFP | U2OS cell cytoplasm and nucleus | Multiscale fluorescence cross-correlation spectroscopy: GFP diffusion is measured on a grid of points simultaneously. Cross-correlation of points is used to measure the GFP transit time to reach the different locations. Result: the regulation imparted by the intracellular structural organization on FPs diffusion is characterized | Ref. [42] |
| 80–100 nm | µseconds | GFP | CHO cell cytoplasm | Single point STED-FCS: GFP diffusion is measured, locally, at a sub-diffraction scale. Result: GFP diffusion is on the average only 2-fold more suppressed than dilute solutions, but spatial heterogeneity is highlighted | Ref. [41] |
| From 100 nm to whole cell | milliseconds | GFP | MB231 cell cytoplasm | 2D-pCF analysis on SPIM-based measurements: GFP diffusion is measured on a grid of points simultaneously. The 2D-pCF algorithm enables to draw GFP molecular paths across space. Result: a map of the intracellular connectivity/obstacles to diffusion can be drawn | Ref. [59] |
| From 20–30 nm to several micrometers | 1 µs | GFP, GFP2 | CHO cell cytoplasm and nucleus | RICS-iMSD at tunable timescales: GFP displacement is measured by averaging over many microns at tunable time scales. Result: GFP unobstructed (Brownian) motion is observed below 100 nm, anomalous and then suppressed diffusion (3–5-fold) above 100 nm | Ref. [48] |
| <5 nm | 5–50 ns | GFP | CHO cell cytoplasm | Anisotropy decay: GFP rotational diffusion is measured at the nanoscale. Result: GFP rotation is almost unhindered in the cell cytoplasm. | Refs. [21,22] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardarelli, F. Back to the Future: Genetically Encoded Fluorescent Proteins as Inert Tracers of the Intracellular Environment. Int. J. Mol. Sci. 2020, 21, 4164. https://doi.org/10.3390/ijms21114164
Cardarelli F. Back to the Future: Genetically Encoded Fluorescent Proteins as Inert Tracers of the Intracellular Environment. International Journal of Molecular Sciences. 2020; 21(11):4164. https://doi.org/10.3390/ijms21114164
Chicago/Turabian StyleCardarelli, Francesco. 2020. "Back to the Future: Genetically Encoded Fluorescent Proteins as Inert Tracers of the Intracellular Environment" International Journal of Molecular Sciences 21, no. 11: 4164. https://doi.org/10.3390/ijms21114164
APA StyleCardarelli, F. (2020). Back to the Future: Genetically Encoded Fluorescent Proteins as Inert Tracers of the Intracellular Environment. International Journal of Molecular Sciences, 21(11), 4164. https://doi.org/10.3390/ijms21114164





