The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer
Abstract
:1. Introduction
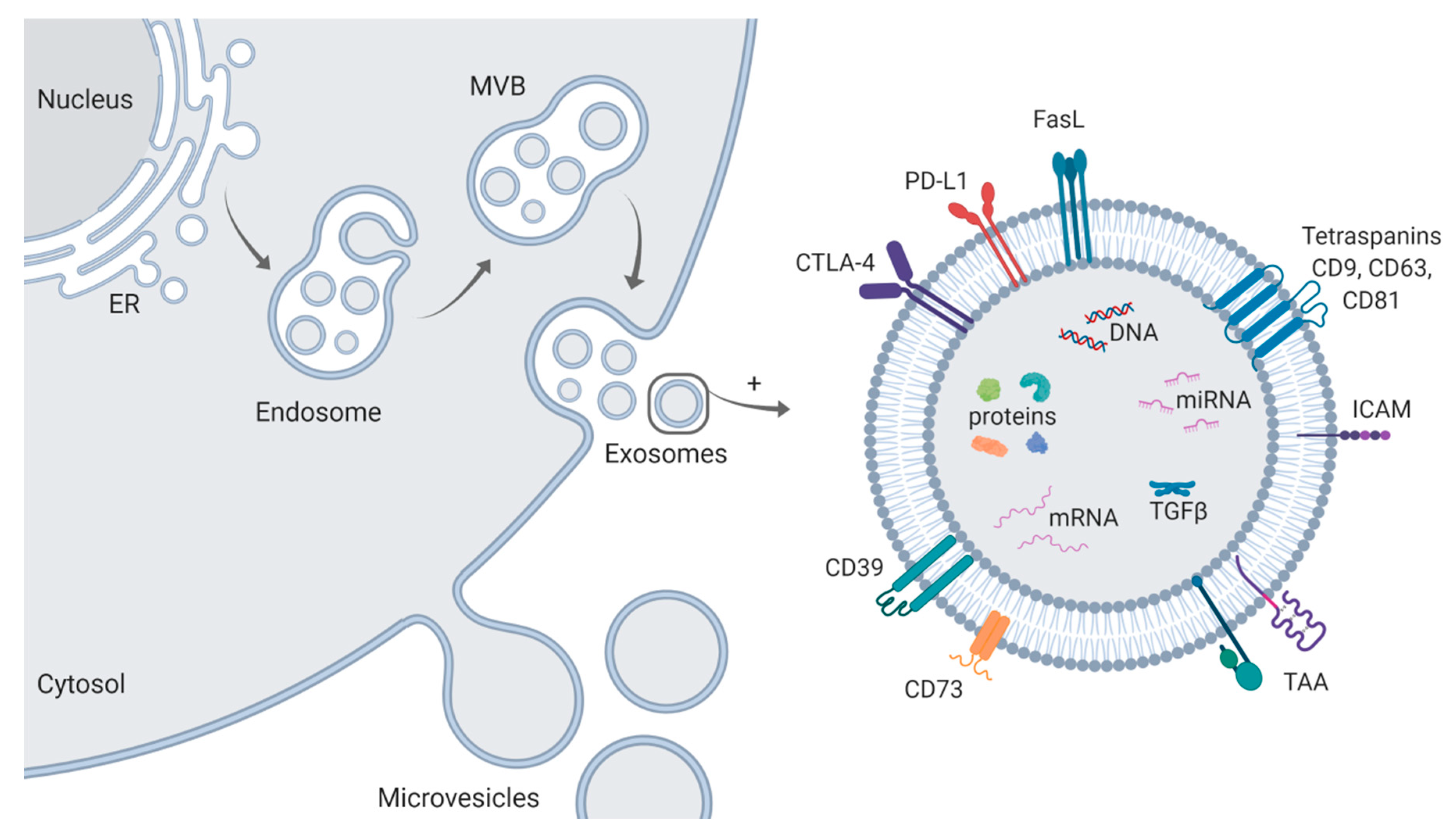
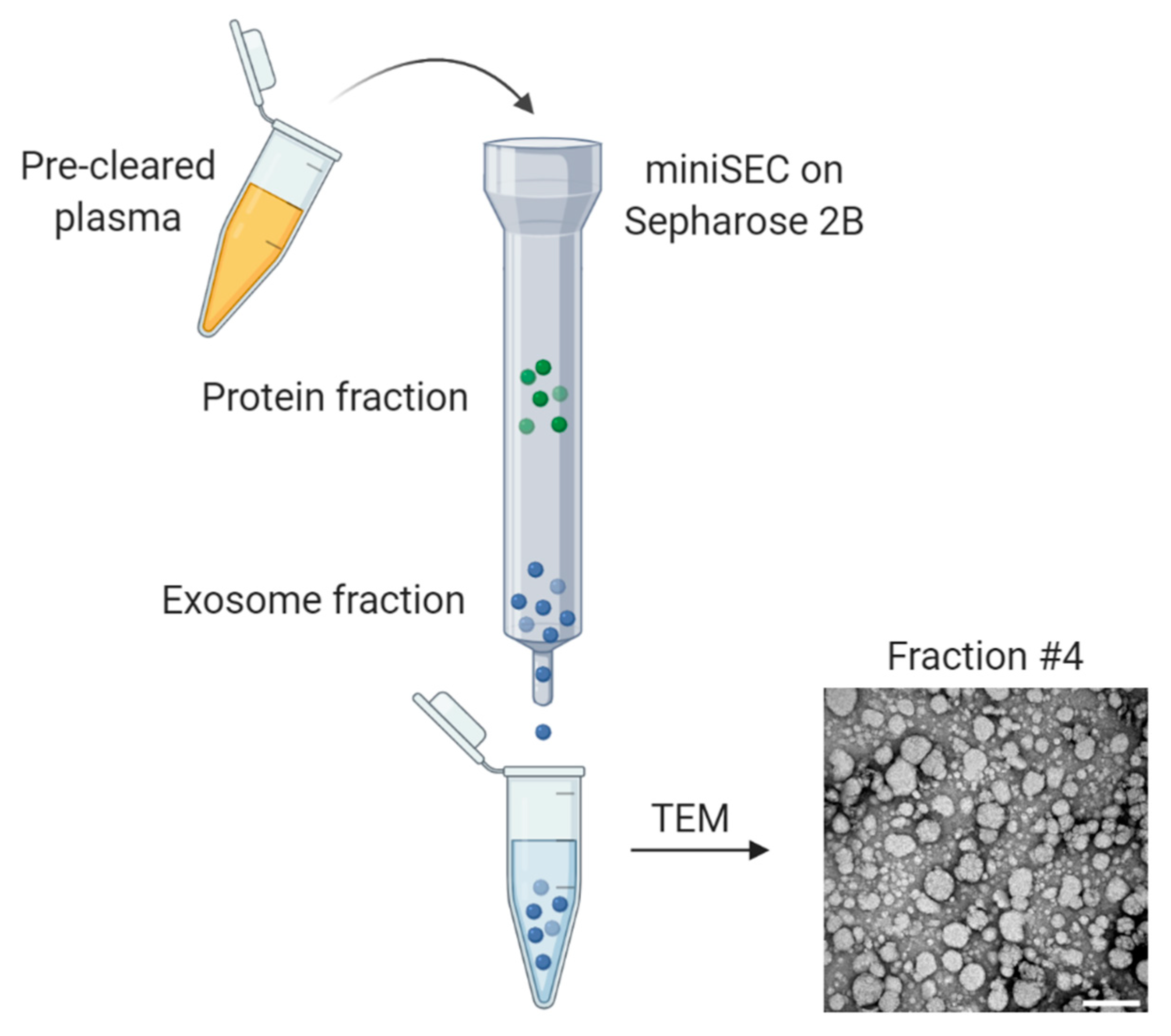
2. Isolation and Characterization of Exosomes
3. Exosomes Mediate Immune Suppression and Tumor Progression in HNSCC
| Exosome Source | Isolation Method | Outcome | Reference |
|---|---|---|---|
| PCI-13 HNSCC cell line | Differential centrifugation and mini-SEC | TEX induced apoptosis of activated CD8+ T cells and modulated Treg suppressor functions via cell surface signaling. | [33] |
| PCI-13 HNSCC cell line | SEC and ultracentrifugation | TEX inhibited signaling and proliferation of activated CD8+ T cells and induced expansion of Treg. | [31] |
| PCI-13 HNSCC cell line | SEC and ultracentrifugation | TEX induced generation, expansion, biologic activity, and resistance to apoptosis of Treg. | [35] |
| C15 and C17 PDX (patient-derived xenograft) NPC cell line | Differential centrifugation and sucrose gradient flotation | TEX facilitated Treg recruitment and expansion of CD25high FOXP3high Treg. | [34] |
| PCI-13 HNSCC cell line | Differential centrifugation, SEC, and ultracentrifugation | TEX regulated expression of immune-function related genes in T cell subsets translating into increased adenosine production and loss of CD69 expression on activated T cells. | [32] |
| UM-SCC-1, UM-SCC-19, UM-SCC-47, and 96-VU-147T-UP-6 HNSCC cell lines | Differential ultracentrifugation and iodixanol gradient centrifugation | TEX and exosomes from patients (both plasma and tumor) stimulated neurite outgrowth in PC12 neuronal model cells. | [36] |
| PCI-13 and UM-SCC47 HNSCC cell lines | Differential centrifugation and mini-SEC | TEX stimulated proliferation, migration, and tube formation of endothelial cells, thus promoting angiogenesis. | [37] |
| HOC313 OSCC cell line | SEC and ultracentrifugation | TEX from highly metastatic cells induced cell growth and promoted cell motility of poorly metastatic cells through the delivery of miR-1246. | [38] |
| HSC-3 and RT-7 OSCC cell lines | Differential centrifugation and Total Exosome Isolation Kit (Invitrogen) | EGFR-positive TEX transformed normal epithelial cells into a mesenchymal phenotype which was inhibited by cetuximab. | [39] |
| Ca1, CaLH2, SQCC/Y1, SVpgC2a, and SVFN8 OSCC cell lines | Differential centrifugation and ultracentrifugation | TEX changed transcriptome profile in oral keratinocytes regarding pathways involved in matrix remodeling and immune modulation. | [40] |
| SCC-9 and CAL-27 OSCC cell lines | ExoQuick Exosome Precipitation Kit (System Biosciences) | TEX derived from hypoxic cells increased migration and invasion of normoxic cells by delivery of miR-21. | [42] |
| HPV(+) UM-SCC-2, UM-SCC-47, UPCI-SCC-90, HPV(−) PCI-13, and PCI-30 HNSCC cell lines | Differential centrifugation and mini-SEC | HPV(+) and HPV(−) TEX carried immune modulatory proteins and inhibited T cell function. Only HPV(−) TEX suppressed dendritic cell function. | [48] |
| HPV(+) UM-SCC-2, UM-SCC-47, UPCI-SCC-90, HPV(−) PCI-13, and PCI-30 HNSCC cell lines | Differential centrifugation and mini-SEC | The proteomic cargo differed between HPV(+) and HPV(−) TEX. HPV(+) TEX were enriched in CD47 and CD276, whereas HPV(−) TEX contained tumor-protective/growth-promoting antigens, MUC-1 and HLA-DA. | [49] |
| HPV(+) SCC-90, SCC-47, SCC-104, HPV(−) SAS, CAL-27, and CAL-33 HNSCC cell lines | Differential centrifugation and ultracentrifugation | MiR-9-enriched TEX from HPV(+) HNSCC transformed macrophages into the M1 phenotype and increased the radiosensitivity of HPV(+) HNSCC. | [50] |
| HSC-3 and SCC-9 OSCC cell lines | Differential centrifugation and ultracentrifugation | TEX derived from cisplatin-resistant cells induced chemoresistance in platin-naive cells and decreased DNA damage signaling in response to cisplatin. | [51] |
| Primary, HNSCC patient-derived cancer-associated fibroblasts | Differential centrifugation and ultracentrifugation | TEX derived from cisplatin-resistant cancer-associated fibroblasts conferred chemoresistance and an aggressive phenotype in cancer cells by transfer of functional miR-196a. | [52] |
| KYSE30, KYSE70, and KYSE180 ESCC cell lines | Differential centrifugation and ultracentrifugation | Radioresistant cells showed a differential miRNA expression profile compared to normal cells and exosomal miR-339-5p mediated regulation of radiosensitivity. | [53] |
| UM-SCC-6 HNSCC cell line | Differential centrifugation and SEC | Proteomic analysis of TEX released from irradiated cells revealed overexpressed proteins involved in response to radiation, ROS metabolism, and DNA repair. | [54] |
| FaDu HNSCC cell line | Total Exosome Isolation Kit (Invitrogen) and ultracentrifugation | Proteomic profile of TEX released from irradiated cells was significantly altered compared to TEX from nonirradiated cells. | [55] |
| BHY and FaDu HNSCC cell lines | Differential centrifugation and ultracentrifugation | TEX derived from irradiated cells promoted survival and proliferation and conferred a migratory phenotype to recipient cancer cells. | [56,57] |
4. Molecular and Functional Profiles of Exosomes from HPV(+) and HPV(−) Tumors
5. Exosomes as Biomarkers for Disease Progression and Activity
6. TEX and Non-TEX as Biomarkers for Tumor Status and Immune Dysfunction
7. Exosomes as Biomarkers and Players in Response to Therapy and Outcome
8. Exosomes as Therapeutic Vesicles
9. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Active disease |
| ADA | Adenosine deaminase |
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| ATP | Adenosine triphosphate |
| CSPG4 | Chondroitin sulfate proteoglycan 4 |
| CTL | Cytotoxic T lymphocyte |
| DC | Dendritic cell |
| EC | Endothelial cell |
| EMT | Epithelial-mesenchymal transition |
| ESCC | Esophageal squamous cell carcinoma |
| EV | Extracellular vesicle |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papilloma virus |
| LSCC | Laryngeal squamous cell carcinoma |
| MISEV | Minimal information for studies of extracellular vesicles |
| MVB | Multivesicular body |
| NED | Nonevident disease |
| Nef | Negative regulatory factor |
| NK | Natural killer |
| OSCC | Oral squamous cell carcinoma |
| PDT | Photodynamic therapy |
| ROC | Receiver operating characteristic |
| SEC | Size exclusion chromatography |
| siRNA | Short interfering RNA |
| TEM | Transmission electron microscopy |
| TEX | Tumor-derived exosomes |
| TME | Tumor microenvironment |
| Treg | Regulatory T cells |
| UICC | Union for International Cancer Control |
| 4NQO | 4-nitroquinoline 1-oxide |
References
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteside, T.L. Head and Neck Carcinoma Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, M.D.; Devlin, M.-J. Immune Checkpoint Inhibition in Head and Neck Cancer. Front. Oncol. 2018, 8, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.-H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Xie, X.; O’Neill, W.; Pan, Q. Immunotherapy for head and neck cancer: The future of treatment? Expert Opin. Biol. Ther. 2017, 17, 701–708. [Google Scholar] [CrossRef]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control Release 2015, 219, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Ludwig, S.; Floros, T.; Theodoraki, M.-N.; Hong, C.-S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017, 13, 2583–2592. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin. Exp. Immunol. 2017, 189, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Ruivo, C.F.; Adem, B.; Silva, M.; Melo, S.A. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017, 77, 6480–6488. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. The potential of tumor-derived exosomes for noninvasive cancer monitoring: An update. Expert Rev. Mol. Diagn. 2018, 18, 1029–1040. [Google Scholar] [CrossRef]
- Whiteside, T.L. The emerging role of plasma exosomes in diagnosis, prognosis and therapies of patients with cancer. Contemp. Oncol. (Poznan, Poland) 2018, 22, 38–40. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoraki, M.-N.; Yerneni, S.S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019, 8, 1593805. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.-N.; Hoffmann, T.K.; Whiteside, T.L. Separation of plasma-derived exosomes into CD3((+)) and CD3((−)) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin. Exp. Immunol. 2018, 192, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Theodoraki, M.-N.; Yerneni, S.S.; Brunner, C.; Theodorakis, J.; Hoffmann, T.K.; Whiteside, T.L. Plasma-derived Exosomes Reverse Epithelial-to-Mesenchymal Transition after Photodynamic Therapy of Patients with Head and Neck Cancer. Oncoscience 2018, 5, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Hong, C.-S.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J. Extracell. Vesicles 2016, 5, 29289. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Tumor-Induced Immune Suppression. Vaccines 2016, 4, 35. [Google Scholar] [CrossRef]
- Ludwig, N.; Razzo, B.M.; Yerneni, S.S.; Whiteside, T.L. Optimization of cell culture conditions for exosome isolation using mini-size exclusion chromatography (mini-SEC). Exp. Cell Res. 2019, 378, 149–157. [Google Scholar] [CrossRef]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, L.; Mitsuhashi, M.; Simms, P.; Gooding, W.E.; Whiteside, T.L. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 2016, 6, 20254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, L.; Simms, P.; Hong, C.-S.; Nishimura, M.I.; Jackson, E.K.; Watkins, S.C.; Whiteside, T.L. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology 2017, 6, e1261243. [Google Scholar] [CrossRef] [PubMed]
- Mrizak, D.; Martin, N.; Barjon, C.; Jimenez-Pailhes, A.-S.; Mustapha, R.; Niki, T.; Guigay, J.; Pancré, V.; de Launoit, Y.; Busson, P.; et al. Effect of Nasopharyngeal Carcinoma-Derived Exosomes on Human Regulatory T Cells. JNCI J. Natl. Cancer Inst. 2014, 107, dju363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szajnik, M.; Czystowska, M.; Szczepanski, M.J.; Mandapathil, M.; Whiteside, T.L. Tumor-Derived Microvesicles Induce, Expand and Up-Regulate Biological Activities of Human Regulatory T Cells (Treg). PLoS ONE 2010, 5, e11469. [Google Scholar] [CrossRef] [Green Version]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, N.; Yerneni, S.S.; Razzo, B.M.; Whiteside, T.L. Exosomes from HNSCC Promote Angiogenesis through Reprogramming of Endothelial Cells. Mol. Cancer Res. 2018, 16. [Google Scholar] [CrossRef] [Green Version]
- Sakha, S.; Muramatsu, T.; Ueda, K.; Inazawa, J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci. Rep. 2016, 6, 38750. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Eguchi, T.; Sogawa, C.; Ono, K.; Murakami, J.; Ibaragi, S.; Asaumi, J.; Calderwood, S.K.; Okamoto, K.; Kozaki, K. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncol. 2018, 86, 251–257. [Google Scholar] [CrossRef]
- Qadir, F.; Aziz, M.A.; Sari, C.P.; Ma, H.; Dai, H.; Wang, X.; Raithatha, D.; Da Silva, L.G.L.; Hussain, M.; Poorkasreiy, S.P.; et al. Transcriptome reprogramming by cancer exosomes: Identification of novel molecular targets in matrix and immune modulation. Mol. Cancer 2018, 17, 97. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Kang, Y. Hypoxia and Hypoxia-Inducible Factors: Master Regulators of Metastasis. Clin. Cancer Res. 2010, 16, 5928–5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver miR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sento, S.; Sasabe, E.; Yamamoto, T. Application of a Persistent Heparin Treatment Inhibits the Malignant Potential of Oral Squamous Carcinoma Cells Induced by Tumor Cell-Derived Exosomes. PLoS ONE 2016, 11, e0148454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzo, B.M.; Ludwig, N.; Hong, C.-S.; Sharma, P.; Fabian, K.P.; Fecek, R.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma. Carcinogenesis 2019. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, D.; Vaidya, M.M. 4-Nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006, 42, 655–667. [Google Scholar] [CrossRef]
- Hawkins, B.L.; Heniford, B.W.; Ackermann, D.M.; Leonberger, M.; Martinez, S.A.; Hendler, F.J. 4NQO carcinogenesis: A mouse model of oral cavity squamous cell carcinoma. Head Neck 1994, 16, 424–432. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, B.; Men, T.; Fujimoto, J.; Xu, X. Comparable molecular alterations in 4-nitroquinoline 1-oxide-induced oral and esophageal cancer in mice and in human esophageal cancer, associated with poor prognosis of patients. In Vivo 2013, 27, 473–484. [Google Scholar]
- Ludwig, S.; Sharma, P.; Theodoraki, M.-N.; Pietrowska, M.; Yerneni, S.S.; Lang, S.; Ferrone, S.; Whiteside, T.L. Molecular and Functional Profiles of Exosomes From HPV(+) and HPV(−) Head and Neck Cancer Cell Lines. Front. Oncol. 2018, 8, 445. [Google Scholar] [CrossRef]
- Ludwig, S.; Marczak, L.; Sharma, P.; Abramowicz, A.; Gawin, M.; Widlak, P.; Whiteside, T.L.; Pietrowska, M. Proteomes of exosomes from HPV(+) or HPV(−) head and neck cancer cells: Differential enrichment in immunoregulatory proteins. Oncoimmunology 2019, 8, 1593808. [Google Scholar] [CrossRef]
- Tong, F.; Mao, X.; Zhang, S.; Xie, H.; Yan, B.; Wang, B.; Sun, J.; Wei, L. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020, 478, 34–44. [Google Scholar] [CrossRef]
- Liu, T.; Chen, G.; Sun, D.; Lei, M.; Li, Y.; Zhou, C.; Li, X.; Xue, W.; Wang, H.; Liu, C.; et al. Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Guo, H.; Wang, X.; Zhu, X.; Yan, M.; Wang, X.; Xu, Q.; Shi, J.; Lu, E.; Chen, W.; et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Zhou, X.; Shi, X.; Zhao, Y.; Men, Y.; Chang, X.; Chen, H.; Ding, F.; Li, Y.; Su, D.; et al. Exosome-derived miR-339-5p mediates radiosensitivity by targeting Cdc25A in locally advanced esophageal squamous cell carcinoma. Oncogene 2019, 38, 4990–5006. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, A.; Wojakowska, A.; Marczak, L.; Lysek-Gladysinska, M.; Smolarz, M.; Story, M.D.; Polanska, J.; Widlak, P.; Pietrowska, M. Ionizing radiation affects the composition of the proteome of extracellular vesicles released by head-and-neck cancer cells in vitro. J. Radiat. Res. 2019, 60, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Wojakowska, A.; Marczak, L.; Muer, A.; Tinhofer-Keilholz, I.; Lysek-Gladysinska, M.; Widlak, P.; Pietrowska, M. Ionizing radiation affects protein composition of exosomes secreted in vitro from head and neck squamous cell carcinoma. Acta Biochim. Pol. 2015, 62, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutschelknaus, L.; Azimzadeh, O.; Heider, T.; Winkler, K.; Vetter, M.; Kell, R.; Tapio, S.; Merl-Pham, J.; Huber, S.M.; Edalat, L.; et al. Radiation alters the cargo of exosomes released from squamous head and neck cancer cells to promote migration of recipient cells. Sci. Rep. 2017, 7, 12423. [Google Scholar] [CrossRef] [PubMed]
- Mutschelknaus, L.; Peters, C.; Winkler, K.; Yentrapalli, R.; Heider, T.; Atkinson, M.J.; Moertl, S. Exosomes Derived from Squamous Head and Neck Cancer Promote Cell Survival after Ionizing Radiation. PLoS ONE 2016, 11, e0152213. [Google Scholar] [CrossRef]
- Mork, J.; Lie, A.K.; Glattre, E.; Clark, S.; Hallmans, G.; Jellum, E.; Koskela, P.; Møller, B.; Pukkala, E.; Schiller, J.T.; et al. Human Papillomavirus Infection as a Risk Factor for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2001, 344, 1125–1131. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. JNCI J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. JNCI J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Del Mistro, A.; Bussu, F.; Lupato, V.; Baboci, L.; Almadori, G.; DA Mosto, M.C.; Paludetti, G. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2013, 33, 77–87. [Google Scholar]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients With Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. JNCI J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Economopoulou, P.; Kotsantis, I.; Psyrri, A. Special Issue about Head and Neck Cancers: HPV Positive Cancers. Int. J. Mol. Sci. 2020, 21, 3388. [Google Scholar] [CrossRef]
- Cordonnier, M.; Nardin, C.; Chanteloup, G.; Derangere, V.; Algros, M.-P.; Arnould, L.; Garrido, C.; Aubin, F.; Gobbo, J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J. Extracell. Vesicles 2020, 9, 1710899. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Zhi, C.; Liang, W.; Wang, X.; Chen, X.; Lv, T.; Shen, Q.; Song, Y.; Lin, D.; et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J. Transl. Med. 2019, 17, 355. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef]
- Irimie-Aghiorghiesei, A.I.; Pop-Bica, C.; Pintea, S.; Braicu, C.; Cojocneanu, R.; Zimța, A.-A.; Gulei, D.; Slabý, O.; Berindan-Neagoe, I. Prognostic Value of MiR-21: An Updated Meta-Analysis in Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Med. 2019, 8, 2041. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhou, Y.; Lu, J.; Sun, Y.; Xiao, H.; Liu, M.; Tian, L. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med. Oncol. 2014, 31, 1–8. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kamohara, H.; Kinoshita, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Watanabe, M.; Baba, H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer 2013, 119, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Y.; Liu, J.; Su, X.; Qin, H.; Huang, S.; Huang, X.; Zhou, N. Potential Markers from Serum-Purified Exosomes for Detecting Oral Squamous Cell Carcinoma Metastasis. Cancer Epidemiol. Prev. Biomark. 2019, 28, 1668–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Y.; Yu, L.; Sakakura, K.; Visus, C.; Schwab, J.H.; Ferrone, C.R.; Favoino, E.; Koya, Y.; Campoli, M.R.; et al. CSPG4 in Cancer: Multiple Roles. Curr. Mol. Med. 2010, 10, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Campoli, M.R.; Chang, C.-C.; Kageshita, T.; Wang, X.; McCarthy, J.B.; Ferrone, S. Human High Molecular Weight-Melanoma-Associated Antigen (HMW-MAA): A Melanoma Cell Surface Chondroitin Sulfate Proteoglycan (MSCP) with Biological and Clinical Significance. Crit. Rev. Immunol. 2004, 24, 267–296. [Google Scholar] [CrossRef]
- Sharma, P.; Ludwig, S.; Muller, L.; Hong, C.S.; Kirkwood, J.M.; Ferrone, S.; Whiteside, T.L. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J. Extracell. vesicles 2018, 7, 1435138. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Matsumoto, A.; Beccard, I.; Hoffmann, T.K.; Whiteside, T.L. CD44v3 protein-carrying tumor-derived exosomes in HNSCC patients’ plasma as potential noninvasive biomarkers of disease activity. Oncoimmunology 2020, 9, 1747732. [Google Scholar] [CrossRef] [Green Version]
- Sagawa, K.; Uwa, N.; Daimon, T.; Sakagami, M.; Tsujimura, T. Expression of CD44 variant isoforms, CD44v3 and CD44v6, are associated with prognosis in nasopharyngeal carcinoma. J. Laryngol. Otol. 2016, 130, 843–849. [Google Scholar] [CrossRef]
- Spiegelberg, D.; Kuku, G.; Selvaraju, R.; Nestor, M. Characterization of CD44 variant expression in head and neck squamous cell carcinomas. Tumour Biol. 2014, 35, 2053–2062. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Wreesmann, V.B.; Bourguignon, L.Y.W. Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck 2007, 29, 550–558. [Google Scholar] [CrossRef]
- Whiteside, T.L. Proteomic Analysis of Plasma-Derived Exosomes in Defining Their Role as Biomarkers of Disease Progression, Response to Therapy and Outcome. Proteomes 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Theodoraki, M.-N.; Hoffmann, T.K.; Jackson, E.K.; Whiteside, T.L. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin. Exp. Immunol. 2018, 194, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Schuler, P.J.; Saze, Z.; Hong, C.-S.; Muller, L.; Gillespie, D.G.; Cheng, D.; Harasymczuk, M.; Mandapathil, M.; Lang, S.; Jackson, E.K.; et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 2014, 177, 531–543. [Google Scholar] [CrossRef]
- Wulff, S.; Pries, R.; Börngen, K.; Trenkle, T.; Wollenberg, B. Decreased levels of circulating regulatory NK cells in patients with head and neck cancer throughout all tumor stages. Anticancer. Res. 2009, 29, 3053–3057. [Google Scholar]
- Watanabe, M.; Kono, K.; Kawaguchi, Y.; Mizukami, Y.; Mimura, K.; Maruyama, T.; Izawa, S.; Fujii, H. NK cell dysfunction with down-regulated CD16 and up-regulated CD56 molecules in patients with esophageal squamous cell carcinoma. Dis. Esophagus 2010, 23, 675–681. [Google Scholar] [CrossRef]
- Klöß, S.; Chambron, N.; Gardlowski, T.; Arseniev, L.; Koch, J.; Esser, R.; Glienke, W.; Seitz, O.; Köhl, U. Increased sMICA and TGFβ(1) levels in HNSCC patients impair NKG2D-dependent functionality of activated NK cells. Oncoimmunology 2015, 4, e1055993. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Hofmann, L.; Ludwig, S.; Schuler, P.J.; Hoffmann, T.K.; Brunner, C.; Theodoraki, M.-N. The Potential of CD16 on Plasma-Derived Exosomes as a Liquid Biomarker in Head and Neck Cancer. Int. J. Mol. Sci. 2020, 21, 3739. [Google Scholar] [CrossRef]
- Battke, C.; Ruiss, R.; Welsch, U.; Wimberger, P.; Lang, S.; Jochum, S.; Zeidler, R. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol. Immunother. 2011, 60, 639–648. [Google Scholar] [CrossRef]
- Rodrigues-Junior, D.M.; Tan, S.S.; de Souza Viana, L.; Carvalho, A.L.; Lim, S.K.; Iyer, N.G.; Vettore, A.L. A preliminary investigation of circulating extracellular vesicles and biomarker discovery associated with treatment response in head and neck squamous cell carcinoma. BMC Cancer 2019, 19, 373. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Gao, H.; Lv, P.; Liu, J.; Liu, G. Extracellular vesicles as an efficient nanoplatform for the delivery of therapeutics. Hum. Vaccin. Immunother. 2017, 13, 2678–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Yang, M.; Wu, S.Y. The Advances and Challenges in Utilizing Exosomes for Delivering Cancer Therapeutics. Front. Pharmacol. 2018, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Aspe, J.R.; Diaz Osterman, C.J.; Jutzy, J.M.S.; Deshields, S.; Whang, S.; Wall, N.R. Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J. Extracell. Vesicles 2014, 3, 23244. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; von der Ohe, J.; Hass, R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers (Basel) 2019, 11, 798. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-derived Exosomes Combined With GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef] [Green Version]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lu, S.; Liang, X.; Cao, B.; Wang, S.; Jiang, J.; Luo, H.; He, S.; Lang, J.; Zhu, G. γδTDEs: An Efficient Delivery System for miR-138 with Anti-tumoral and Immunostimulatory Roles on Oral Squamous Cell Carcinoma. Mol. Ther. Nucleic Acids 2019, 14, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Di Bonito, P.; Accardi, L.; Galati, L.; Ferrantelli, F.; Federico, M. Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes. Cancers (Basel) 2019, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Manfredi, F.; Di Bonito, P.; Arenaccio, C.; Anticoli, S.; Federico, M. Incorporation of Heterologous Proteins in Engineered Exosomes. Methods Mol. Biol. 2016, 1448, 249–260. [Google Scholar]
- Lattanzi, L.; Federico, M. A strategy of antigen incorporation into exosomes: Comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles. Vaccine 2012, 30, 7229–7237. [Google Scholar] [CrossRef]
- Di Bonito, P.; Ridolfi, B.; Columba-Cabezas, S.; Giovannelli, A.; Chiozzini, C.; Manfredi, F.; Anticoli, S.; Arenaccio, C.; Federico, M. HPV-E7 delivered by engineered exosomes elicits a protective CD8+ T cell-mediated immune response. Viruses 2015, 7, 1079–1099. [Google Scholar] [CrossRef] [Green Version]
- Di Bonito, P.; Chiozzini, C.; Arenaccio, C.; Anticoli, S.; Manfredi, F.; Olivetta, E.; Ferrantelli, F.; Falcone, E.; Ruggieri, A.; Federico, M. Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation. Int. J. Nanomed. 2017, 12, 4579–4591. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Kothandan, V.K.; Kim, H.W.; Kim, K.S.; Kim, J.Y.; Cho, H.J.; Lee, Y.-K.; Lee, D.-E.; Hwang, S.R. Noninvasive Assessment of Exosome Pharmacokinetics In Vivo: A Review. Pharmaceutics 2019, 11, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Exosome Source | Isolation Method | Mouse Model | Outcome | Reference |
|---|---|---|---|---|
| OSC-4 OSCC cell line | Total Exosome Isolation Kit (Invitrogen) | OSC-4 xenografts implanted into nude mice | TEX promoted growth rate of tumor xenografts, which could be inhibited by continuous administration of heparin. | [43] |
| SCC-9 and CAL-27 OSCC cell lines | ExoQuick Exosome Precipitation Kit (System Biosciences) | CAL-27 xenografts implanted into nude mice | Tumor-derived exosomal miR-21 induced tumor growth and metastasis in a xenograft OSCC model. | [42] |
| PCI-13 and UM-SCC-47 HNSCC cell lines | Differential centrifugation and mini-SEC | 4-NQO oral carcinogenesis mouse model | TEX promoted formation of defined vascular structures within the tumor and thus, promoted angiogenesis. | [37] |
| SCCVII, SCC-90, and PCI-13 HNSCC cell lines | Differential centrifugation and mini-SEC | 4-NQO oral carcinogenesis mouse model | TEX promoted tumor progression and reduced immune cell migration to the tumor. | [44] |
| Exosome Source | Isolation Method | Exosome Subset | Methods | Molecules | Outcome | Prediction | Reference |
|---|---|---|---|---|---|---|---|
| Plasma of HNSCC patients | Differential centrifugation and mini-SEC | Total exosomes | Nanoparticle tracking, western blot, functional coincubation assays | - (Establishment of mini-SEC) | Mini-SEC allows for simple and reproducible isolation from human plasma of exosomes retaining structural integrity and functional activity. | – | [27] |
| Plasma of HNSCC patients, n = 38 | Differential centrifugation and mini-SEC | Total exosomes | Western blot, functional coincubation assays | - (Exosome-mediated immune suppression) | Patients with active disease (AD) had significantly higher exosome levels compared to patients with nonevident disease (NED). Exosomes from patients with AD mediated stronger immune suppression than exosomes from patients with NED. | Tumor progression/disease activity and immune status | [15] |
| Plasma of HNSCC patients, n = 40 | Differential centrifugation and mini-SEC | Total exosomes | On-bead flow cytometry, and functional coincubation assays | PD-L1 | Levels of PD-L1 on exosomes correlated with disease activity, UICC stage, and the presence of lymph node metastasis. In contrast, plasma levels of soluble PD-L1 did not correlate with any clinicopathological data. High PD-L1 levels, but not low PD-L1 level, exosomes suppressed T cell activity, which could be attenuated with an anti-PD-1 antibody. | Tumor progression/disease activity | [21] |
| Plasma of OSCC patients, n = 108 | ExoQuick Exosome Precipitation Kit (System Biosciences) | Total exosomes | miRNA sequencing | miR-21 | Exosomal miR-21 levels correlated with advanced T classification, the presence of lymph node metastasis, and tumor HIF-1α/2α expression. | Tumor progression/disease activity | [42] |
| Serum of LSCC patients, n = 52 | ExoQuick Exosome Precipitation Kit (System Biosciences) | Total exosomes | miRNA analysis (RT-PCR) | miR-21 | Exosomal miR-21 and HOTAIR levels correlated with advanced T classification and UICC high stage. | Tumor progression/disease activity | [70] |
| Serum of ESCC patients, n = 51 | ExoQuick Exosome Precipitation Kit (System Biosciences) | Total exosomes | miRNA analysis (RT-PCR) | miR-21 | Exosomal miR-21 levels correlated with advanced T classification, positive lymph node status, and the presence of metastasis. | Tumor progression/disease activity | [71] |
| Serum of OSCC patients, n = 30 | ExoQuick Exosome Precipitation Kit (System Biosciences) | Total exosomes | Quantitative proteomics approach and bioinformatics | PF4V1, CXCL7, F13A1, and ApoA1 | PF4V1, CXCL7, F13A1, and ApoA1 were correlated to tumor differentiation level, the presence of lymph node metastasis, and the abusus of alcohol and tobacco. Combining these biomarkers improved diagnostic accuracy compared to a single biomarker. | Tumor progression/disease activity | [72] |
| Plasma of HNSCC patients, n = 44 | Differential centrifugation and mini-SEC | Total exosomes, T cell exosomes (CD3 separation), and TEX (CD44v3 capture) | On-bead flow cytometry | CD44v3 | CD44v3 levels on CD3(−) exosomes were higher in patients than in healthy donors and correlated with UICC stage and lymph node metastasis. The molecular profile of CD44v3(+) exosomes was strongly immune-suppressive and correlated with disease stage and lymph node metastasis. | Tumor progression/disease activity | [76] |
| Plasma of HNSCC patients, n = 22 | Differential centrifugation and mini-SEC | T cell exosomes and TEX (CD3 separation) | On-bead flow cytometry and functional coincubation assays | PD-L1, CTLA-4, COX-2, and CD15s | CD3(+) and CD3(−) exosomes carried immune regulatory proteins and induced apoptosis of activated T cells. The cargo of both subsets correlated with tumor stage and nodal status albeit the associations were weaker for the CD3(−) fraction. | Tumor progression/disease activity | [23] |
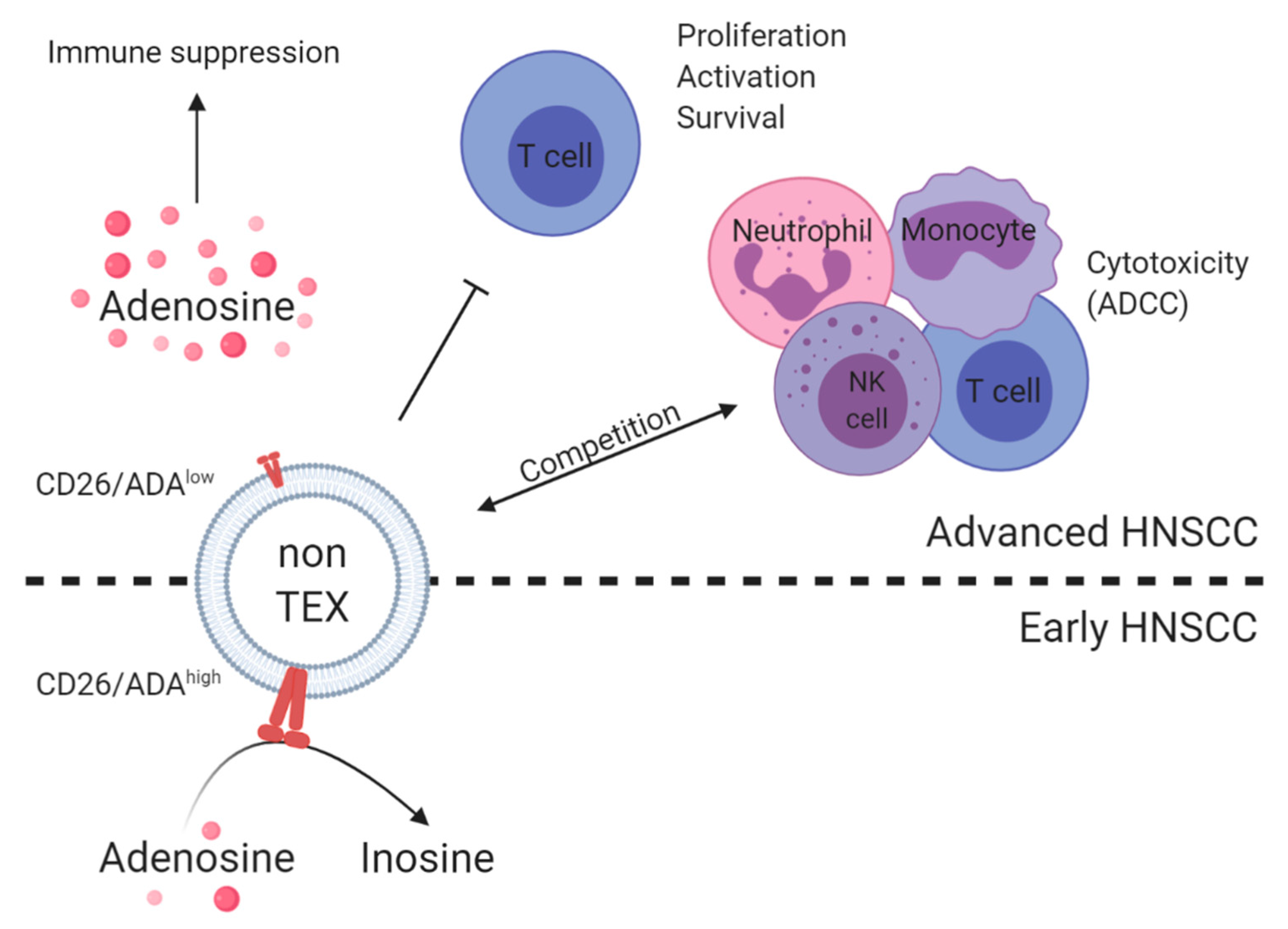
| Plasma of HNSCC patients, n = 14 | Differential centrifugation and mini-SEC | T cell exosomes and TEX (CD3 separation) | On-bead flow cytometry, functional coincubation assays, and mass spectrometry | CD39, CD73, ADA, CD26, and adenosine | High CD39/CD73 levels and adenosine production were found in patients with UICC high stage. ADA/CD26 levels on CD3(+) exosomes correlated with UICC low stage. | Tumor progression/disease activity and immune status | [81] |
| Plasma of HNSCC patients, n = 14 | Differential centrifugation, SEC, and ultracentrifugation | Total exosomes | Mass spectrometry and functional coincubation assays | CD39 and CD73 | Exosomes carried enzymatically active CD39 and CD73 and, when supplied with exogenous ATP, hydrolyzed it to adenosine. | Immune status | [82] |
| Plasma of HNSCC patients, n = 53 | Differential centrifugation and mini-SEC | Total exosomes and TEX (CD44v3 capture) | On-bead flow cytometry | CD16 | CD16 on total exosomes but not TEX, correlated with advanced T classification and UICC high stage. | Tumor progression/disease activity | [87] |
| Plasma of HNSCC patients undergoing chemoradiation therapy (CRT), n = 12 | Beads coated with cholera toxin chain B (CTB) and annexin V (AV) | CTB- and AV-exosomes | Antibody array | List of potential markers analyzed by the array | Exosomes from responders and nonresponders to CRT showed a different proteomic profile. Differentially present proteins in exosomes from responders and nonresponders were associated to FAS, p53, and apoptosis pathways or tumorigenesis and angiogenesis, respectively. | Therapy response/outcome | [89] |
| Plasma of HNSCC patients undergoing photodynamic therapy (PDT), n = 9 | Differential centrifugation and mini-SEC | Total exosomes | On-bead flow cytometry and functional coincubation assays | EMT-associated markers (TGFβ, E-cadherin, and N-cadherin) | Exosomes harvested before PDT had a mesenchymal profile and enhanced tumor proliferation, migration, and invasion. In contrast, exosomes harvested after PDT had an epithelial profile, restored the epithelial morphology of tumor cells, and inhibited their proliferation, migration, and invasion. | Therapy response/outcome | [24] |
| Plasma of HNSCC patients enrolled in a phase I clinical trial and receiving cetuximab, ipilimumab, and radiation, n = 18 | Differential centrifugation and mini-SEC | T cell exosomes and TEX (CD3 separation) | On-bead flow cytometry and antibody microarray | PD-L1, CTLA-4, and CD15s | In recurrent patients, TEX levels, total CD3(+), CD3(−) PD-L1+, and CD3(+) CD15s+ (Treg-derived) exosomes increased from baseline levels. In disease-free patients, TEX levels decreased, CD3(+) and CD3(+) CD15s+ exosomes stabilized and CD3(+) CTLA4+ exosomes declined after ipilimumab therapy. | Therapy response/outcome and disease recurrence | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, L.; Ludwig, S.; Vahl, J.M.; Brunner, C.; Hoffmann, T.K.; Theodoraki, M.-N. The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. Int. J. Mol. Sci. 2020, 21, 4072. https://doi.org/10.3390/ijms21114072
Hofmann L, Ludwig S, Vahl JM, Brunner C, Hoffmann TK, Theodoraki M-N. The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. International Journal of Molecular Sciences. 2020; 21(11):4072. https://doi.org/10.3390/ijms21114072
Chicago/Turabian StyleHofmann, Linda, Sonja Ludwig, Julius M. Vahl, Cornelia Brunner, Thomas K. Hoffmann, and Marie-Nicole Theodoraki. 2020. "The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer" International Journal of Molecular Sciences 21, no. 11: 4072. https://doi.org/10.3390/ijms21114072
APA StyleHofmann, L., Ludwig, S., Vahl, J. M., Brunner, C., Hoffmann, T. K., & Theodoraki, M.-N. (2020). The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. International Journal of Molecular Sciences, 21(11), 4072. https://doi.org/10.3390/ijms21114072






